Abstract
Cruciferous veggies are a varied group of vegetables of the family, brassicaceae that includes cauliflower, broccoli, cabbage, Brussels sprouts, bok choy, kale, arugula, etc., and play a vital part in the human diet. Apart from being a good source of nutrients, they contain various natural compounds that are valuable for human health. Consuming cruciferous veggies can, astonishingly, be helpful in the chemoprevention of cancers. Cruciferous plants contain many bioactive natural products like polyphenols, flavanoids, isothiocyanates, lignans, phytosterols, carotenoids, and indole-3-carbinol. The most studied bioactive phytocompounds found in cruciferous veggies include glucosinolates and indole-3-carbinol. Brassica vegetables with glucosinolates and their hydrolysis products exhibit several biological properties like antioxidant, chemopreventive, and anti-carcinogenic properties. In addition, they are found to be nontoxic with negligible adverse effects. Isothiocyanates (ITCs) and indoles inhibit carcinogenesis in various organs of mice and rats, including the breast, urinary bladder, liver, lung, colon, and stomach. Likewise, sulforaphane of cruciferous plants is found effective as antioxidant, anticancer, and chemopreventive agent. These compounds safeguard cells from DNA damage, induce apoptosis, nullify carcinogens, and inhibit angiogenesis and migration of tumor cells. In vitro and in vivo experiments have disclosed various potential pathways through which these compounds prevent cancer. This chapter aims to highlight the anticancer and chemopreventive effects of various phytochemicals isolated from cruciferous plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Chemoprevention is defined as “the use of non-cytotoxic nutrients or pharmacological agents to enhance physiological mechanisms that protect the organism against mutant clones of malignant cells” (Morse and Stoner 1993). Cancer chemoprevention is achieved by using naturally or synthetically derived molecules to inhibit, interrupt, or reverse carcinogenesis before it leads to malignancy. Various preclinical and epidemiological studies have led to the identification of a varied range of naturally occurring compounds and dietary substances with significant chemopreventive effects. In vitro and in vivo experiments endorse that phytochemicals may modulate various signaling pathways associated with cell multiplication and apoptosis in cancerous cells, boosting cellular immunity, and sensitizing cancer cells to facilitate the action of antiproliferative agents (Kotecha et al. 2016). A relationship between health and diet has attracted attention for centuries, but a link between diet and cancer has attracted attention only in recent years. Consumption of cruciferous vegetables has been linked with prevention of risk from various cancers, especially cancers of the lung, gastrointestinal tract, and prostate (Razis and Noor 2013). These vegetables contain diverse health promoting natural compounds including ascorbic acids, folic acids, phenolics, carotenoids, glucosinolates (GSs), and brassinosteroids (BRs) which offer effective broad-spectrum protection against the cancer-stimulating agents encountered in our everyday life. Unlike vegetables from other plants, cruciferous veggies have considerable quantities of sulfur-comprising GSs, which, on hydrolysis, by the enzymatic action of myrosinase or enzymes of few intestinal microbes, are transformed into bioactive molecules, such as isothiocyanates (ITCs) and indoles (Shapiro et al. 2006; Becker and Juvik 2016). These phytochemicals are thought to be responsible for the chemopreventive effects offered by higher consumption of vegetables and fruits from the cruciferous plants. Plant secondary metabolites are one of the most promising bioactive molecules for cancer prevention. This chapter aims to highlight the anti-carcinogenic and chemopreventive effects of various phytochemicals isolated from cruciferous plants.
17.2 Important Cruciferous Vegetables
Vegetables obtained from the plants, belonging to the family of Brassicaceae are commonly recognized as cruciferous vegetables. These vegetables include arugula or rocket (Eruca sativa), bok choy (Brassica rapa subsp. chinensis), broccoli (Brassica oleracea var. gemmifera and B. oleracea var. Italic), cabbage (B. oleracea var. capitata), cauliflower (B. oleracea var. botrytis), collard greens, horseradish (Armoracia rusticana), radishes (Raphanus raphanistrum subsp. sativus), kale (B. oleracea var. sabellica), Rutabaga (B. napobrassica), turnips (B. rapa subsp. Rapa), watercress (Nasturtium officinale), and wasabi (Eutrema japonicum) (Fig. 17.1).
17.3 Glucosinolates (GSs or GLs)
Glucosinolates (GSs or GLSs) are a group of sulfur-rich, amino acid derived phyto-metabolites occurring in the plants of Brassicaceae family. Various GSs like sinigrin, gluconapin, glucobrassicanapin, progoitrin, epiprogoitrin, napoleiferin, glucoiberin, glucoraphanin, glucoalysin, gluconasturtiin, glucobrassicin, 4-OH glucobrassicin, 4-OMe glucobrassicin, and neoglucobrassicin have been evaluated in edible parts of B. oleracea (Brussels sprouts, broccoli, kale, cabbage, and cauliflower) (Fig. 17.2). Broccoli is an important source of glucoraphanin, while cabbage possesses higher levels of sinigrin. Likewise, watercress (Nasturtium officinale) is rich in gluconasturtiin content, which is a type of Gas, and most likely imparts pest-inhibiting property to growing crucifers. There are more than one hundred fifty known GLs, and all of them share a general sulfur-allied β-D-glucopyranose skeleton; however they vary in the nature of the substituent or side chain R. Side chains of these GLs are from various amino acids during their biosynthesis in cruciferous plants. These GLs are classified into various subgroups on the basis of the molecular arrangement of the side chains (R). For example, the alkylthioalkyl chain of glucoraphanin has a sulfur containing functionality (sulfinyl group), while the aryl or aromatic side chain of gluconasturtiin is a phenethyl substituent (Fig. 17.2) (Navarro et al. 2011).
17.4 Hydrolysis Products of Glucosinolate and Their Chemopreventive Effect
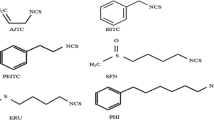
Crucifers contain many biologically active components; glucosinolates (GLSs) are one such important components which have been extensively investigated. These GLSs undergo enzyme-catalyzed hydrolysis and yield different hydrolytic products like ITCs and various others products like epithionitriles, indoles, thiocyanates, nitriles, and oxazolidine-2-thione. Out of various hydrolytic products of GLSs, ITCs are strongly linked with cancer chemoprevention of various tissues or organs in human beings (Singh and Singh 2012). Many numbers of in vitro and in vivo experiments propose that ITCs exert their biological effects through diverse interconnected signaling pathways, including detoxification, apoptosis, and cell cycle regulation, which are essential for inhibition of carcinogenesis. The effects of ITCs on human health have been broadly evaluated, and these hydrolytic products are involved in the anti-carcinogenic effects of these cruciferous vegetables (Verhoeven et al. 1997). Glucoraphanin of broccoli is hydrolyzed to sulforaphane (Kushad et al. 1999) (Fig. 17.3). Sulforaphane has been recognized as an important anticancer ITC in humans, which induced apoptosis in tumor cells (Gamet-Payrastre et al. 2000) and inhibited the rate of tumor growth in various model organisms (Zhang et al. 1994; Chung et al. 2000).
The biological activities of hydrolysis products of glucosinolates (GSHPs) linked to cancer chemoprevention in human beings have been supported by various researches. The crucial mechanism of chemoprevention provided by GSHPs is by modulating phase I/phase II enzymes and inducing various antioxidant enzymes, such as heme oxygenase 1, NAD(P)H quinone reductase, and glutathione S transferases via the Keap1-Nrf2-ARE signaling. The stimulation of this molecular pathway is commonly linked with aliphatic ITCs, while few indole-based GSHPs have also been linked with the stimulation of antioxidant enzymes (Becker and Juvik 2016) (Fig. 17.4).
Mechanism of chemoprevention by glucosinolate hydrolysis products, mainly ITCs. PII enzymes: UDP-glucuronosyltransferases (UGTs), sulfotransferases (SULTs), glutathione S-transferases (GSTs), N-acetyltransferases (NATs), and S-and O-methyltransferases (MTs). PI enzymes: many cytochrome P450s (CYPs). Antioxidant (AO) enzymes: catalases (CAT), superoxide dismutases (SOD), glutathione reductases (GSR), glutathione peroxidases (GPX), glutaredoxins (GLRX), thioredoxins (TXN), thioredoxinreductases (TXNRD), heme oxygenase 1 (HO-1), and NAD(P)H:quinoneoxidoreductase 1 (NQO1)
17.5 Sulforaphane (SFN) and Its Chemopreventive Effect
Common cruciferous ITCs, such as sulforaphane (SFN) and phenethyl isothiocyanate (PEITC), have been confirmed to inhibit carcinogenesis via inducing cancerous cell growth apprehension and modulating apoptosis in various cancers, including skin (Xu et al. 2006), bladder (Munday et al. 2008), colon (Gamet-Payrastre et al. 2000), breast (Li et al. 2010), ovary (Chuang et al. 2007), blood (Suppipat et al. 2012), and prostate cells (Singh et al. 2004). The mechanism by which ITCs achieve this assignment is not well explained and perhaps not widely accepted, but a few of the well-known effects of ITC-treated cells include alternative gene splicing and modulation of gene expression (Traka et al. 2010). Perhaps more significantly, many ITCs have revealed to improve the functioning of Nrf2 (the nuclear factor erythroid 2-related factor 2) (Saw et al. 2011). After activation, Nrf2 encourages the rate of transcription antioxidant enzymes and phase II genes that eventually help cells to overcome cancer development (Surh 2003; Zhang and Gordon 2004). It appears that most ITCs from crucifer vegetables induce phase II enzymes; however there might be disparity amongst aromatic ITCs and aliphatic ITCs in their modulatory activities of phase I enzymes (Leibelt et al. 2003; Jeffery and Araya 2009; La Marca et al. 2012).
Sulforaphane (SFN) exhibit its anti-carcinogenic effects via modulation of key genes and signaling pathways involved in cell cycle blocking and induction of apoptosis in different cancer cells. Various researches on the molecular mechanisms of the antitumor effects of sulforaphane have revealed that SFN might reverse epigenetic changes in different cancer cells by affecting various enzymes like histone deacetyltransferases, DNA methyltransferases, and noncoding RNAs (Su et al. 2018). In basal environment, Keap1 gene binds to Nrf2 gene, which promotes degradation of proteasomes by ubiquitination. Due to oxidative stresses, Nrf2 gene detaches from Keap1 gene, and later it gets translocated into the nucleus of the cells, where it interacts with the promoter sites of the target genes. This signaling carries out the expression of various cytoprotective genes, such as heme oxygenase and superoxide dismutase. In TRAMP C1 (prostate cancer) cells, sulforaphane can check the expression and action of various enzymes, such as DNA methyltransferases and histone deacetylases. A significant inhibition of these enzymes is also being identified in tetradecanoylphorbol acetate-stimulated mouse epidermal skin (JB6 P+) cells treated by sulforaphane. The compound alleviated the CpG methylation and increased histone acetylation of the Nrf2 gene. Eventually, this epigenetic modulation by sulforaphane promoted the transcription, nuclear translocation, and activation of Nrf2 gene (Su et al. 2018) (Fig. 17.5).
Anticancer effects of sulforaphane (SFN) against various cancers (based on Su et al. 2018)
17.6 Indole-3-Carbinol (I3C) and Its Chemopreventive Effect
Indole-3-carbinol (I3C) is a derivative of glucobrassicin hydrolysis by the enzymes, which is found exclusively in various cruciferous veggies, including radish, cabbage, broccoli, daikon, Brussels sprouts, and cauliflower (Broadbent and Broadbent 1998). The hydrolysis of glucobrassicin by the enzyme (myrosinase) at pH 7 produce 3-indomethyl ITC, which further converts to thiocyanate ion and indole-3-carbinol. During cooking of cruciferous vegetables, myrosinase is denatured and thus the hydrolysis of glucosinolates is checked. Non-hydrolyzed glucosinolates then transport to the colon and are metabolized by human intestinal bacteria. The formation of I3C from glucobrassicin may still occur to a smaller degree in the large intestine due to the enzymatic action of myrosinase of colonic bacteria (Barba et al. 2016).
I3C has been revealed to suppress the multiplication of several types of human cancer cells, including breast, colon (Howells et al. 2002; Frydoonfar et al. 2002; Rahman et al. 2003; Hudson et al. 2003), and prostate cancer cells (Frydoonfar et al. 2003; Nachshon-Kedmi et al. 2003). I3C has a remarkable potential of preventing cancers, and it exerts its effects via various modes of actions. Interestingly, it offers cytoprotective activity for the normal cells; however it functions as a unique apoptotic agent for cancerous cells. This valuable potential of indole-3-carbinol should be further investigated in combination cancer therapy with various chemotherapeutic agents (Fig. 17.6).
17.7 Brassinosteroids (BRs) and Its Chemopreventive Effect
Brassinosteroids (BRs), a unique group of plant-based steroidal hormones that are necessary for plants growth and development, provide resistance and tolerance against disease stress and regulation of senescence (Bishop and Koncz 2002). Brassinosteroids affect vegetal development via various physiological responses. Studies have revealed their activities against stress, viruses, human cancers, and genotoxic effects. The most common naturally occurring brassinosteroids, 24-epibrassinolide and 28-homocastasterone (Fig. 17.7), were investigated by different research groups to evaluate their anticancerous activity. BRs were investigated against various cancer cells including CEM, multiple myeloma RPMI 8226, T-lymphoblastic leukemia, cervical carcinoma, A-549, lung carcinoma, HeLa, osteosarcoma HOS cell lines (Malikova et al. 2008), breast cancer, and prostate cancer cells. The outcome of their studies has revealed that BRs can induce apoptosis by interacting with the cell cycle (Malikova et al. 2008; Steigerova et al. 2010, 2012). BRs may targets ER (estrogen receptor), EGFR (epidermal growth factor receptor), and HER-2 (human EGFR-2) proteins, which are essential for the treatment of breast cancer as they are abundant in breast cancer cells, such as MCF-7, T47D, MDA-MB-468, and MDA-MB-231 (Pledgie-Tracy et al. 2007; Steigerova et al. 2010, 2012). Treatment of 28-homocastasterone and 24-epibrassinolide with breast cancer cells showed reduction in cyclin proteins which are involved in G1 phase of the cell cycle. The treatment of prostate cancer cells with these BRs induces programmed cell death by increasing levels of the pro-apoptotic protein (Bax) and reduction of anti-apoptotic protein (Bcl-2) (Steigerova et al. 2012).
17.8 Conclusions
Cruciferous vegetables are important sources of various phytochemicals that have remarkable inhibitory effects on pathways of carcinogenesis for different cancers, due to their antiproliferative and chemopreventive properties. In vitro and in vivo experiments have disclosed various potential pathways through which these phyto compounds prevent cancer. The consumption of these vegetables is advantageous in the sense that they are sources of glucosinolates which on enzymatic action give rise to isothiocyanates like sulforaphane and indole-3-carbinol. In addition, significant inhibition of uncontrolled cell growth as well as induction of apoptosis has been reported with the integration of indole-3-carbinol and indole-3-carbinols. However, clinical studies, carried out till now on the role of cruciferous vegetables in preventing cancers in humans have shown mixed results, and further research is needed to conclusively establish the use of cruciferous veggies for cancer prevention in humans.
References
Barba FJ, Nikmaram N, Roohinejad S, Khelfa A, Zhu Z, Koubaa M (2016) Bioavailability of glucosinolates and their breakdown products: impact of processing. Front Nutr 3:24. https://doi.org/10.3389/fnut.2016.00024
Becker T, Juvik J (2016) The role of glucosinolate hydrolysis products from brassica vegetable consumption in inducing antioxidant activity and reducing cancer incidence. Diseases 4(2):2. https://doi.org/10.3390/diseases4020022
Bishop GJ, Koncz C (2002) Brassionsteroids and plant steroid hormone signaling. Plant Cell 14:97–110
Broadbent TA, Broadbent HS (1998) The chemistry and pharmacology of indole-3-carbinol (indole-3-methanol) and 3-(methoxymethyl) indole. [part II]. Curr Med Chem 5:469–491
Chuang LT, Moqattash ST, Gretz HF, Nezhat F, Rahaman J, Chiao JW (2007) Sulforaphane induces growth arrest and apoptosis in human ovarian cancer cells. Acta Obstet Gynecol Scand 86:1263–1268
Chung FL, Conaway CC, Rao CV, Reddy BS (2000) Chemoprevention of colonic aberrant crypt foci in fischer rats by sulforaphane and phenethylisothiocyanate. Carcinogenesis 21:2287–2291
Frydoonfar HR, McGrath DR, Spigelman AD (2002) Inhibition of proliferation of a colon cancer cell line by indole-3-carbinol. Colorectal Dis 4:205–207
Frydoonfar HR, McGrath DR, Spigelman AD (2003) The effect of indole-3-carbinol and sulforaphane on a prostate cancer cell line. ANZ J Surg 73:154–156
Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F (2000) Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res 60:1426–1433
Howells LM, Gallacher-Horley B, Houghton CE, Manson MM, Hudson EA (2002) Indole-3-carbinol inhibits protein kinase B/Akt and induces apoptosis in the human breast tumor cell line MDA MB468 but not in the nontumorigenic HBL100 line. Mol Cancer Ther 1:1161–1172
Hudson EA, Howells LM, Gallacher-Horley B, Fox LH, Gescher A, Manson MM (2003) Growth-inhibitory effects of the chemopreventive agent indole-3-carbinol are increased in combination with the polyamine putrescine in the SW480 colon tumour cell line. BMC Cancer 3(2)
Jeffery EH, Araya M (2009) Physiological effects of broccoli consumption. Phytochem Rev 8:283–298
Kotecha R, Takami A, Espinoza JL (2016) Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget 7:52517–52529
Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH (1999) Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem 47:1541–1548
La Marca M, Beffy P, Della Croce C, Gervasi PG, Iori R, Puccinelli E, Longo V (2012) Structural influence of isothiocyanates on expression of cytochrome P450, phase II enzymes, and activation of Nrf2 in primary rat hepatocytes. Food Chem Toxicol 50:2822–2830
Leibelt DA, Hedstrom OR, Fischer KA, Pereira CB, Williams DE (2003) Evaluation of chronic dietary exposure to Indole-3-Carbinol and absorption-enhanced 3,3′-Diindolylmethane in Sprague-Dawleyrats. Toxicol Sci 74:10–21
Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D (2010) Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res 16:2580–2590
Malikova J, Swaczynova J, Kolar Z, Strnad M (2008) Anticancer and antiproliferative activity of natural brasinosteroids. Phtyochemistry 69:418–426
Morse MA, Stoner GD (1993) Cancer chemoprevention: principles and prospects. Carcinogenesis 14:1737–1746
Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, Tang L, Munday JS, Lister C, Wilson P, Fahey JW, Davis W, Zhang Y (2008) Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res 68:1593–1600
Nachshon-Kedmi M, Yannai S, Haj A, Fares FA (2003) Indole-3-carbinol and 3,3′-diindolylmethaneinduce apoptosis in human prostate cancer cells. Food Chem Toxicol 41:745–752
Navarro SL, Li F, Lampe JW (2011) Mechanisms of action of isothiocyanates in cancer chemoprevention: an update. Food Funct 2:579–587
Pledgie-Tracy A, Sobolewski MD, Davidson NE (2007) Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther 6(3):1013–1021
Rahman KM, Aranha O, Sarkar FH (2003) Indole-3-carbinol (I3C) induces apoptosis in tumorigenic but not in nontumorigenic breast epithelial cells. Nutr Cancer 45:101–112
Razis AFA, Noor NM (2013) Cruciferous vegetables: dietary phytochemicals for cancer prevention. Asian Pac J Cancer Prev 14:1565–1570
Saw CL, Huang MT, Liu Y, Khor TO, Conney AH, Kong AN (2011) Impact of Nrf2 on UVB-induced skininflammation/photoprotection and photoprotective effect of sulforaphane. Mol Carcinog 50:479–486
Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P (2006) Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer 55:53–62
Singh SV, Singh K (2012) Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis 33:1833–1842
Singh AV, Xiao D, Lew KL, Dhir R, Singh SV (2004) Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis 25:83–90
Steigerova J, Oklestkova J, Levkova L, Kolar Z, Strnad M (2010) Brassinosteroids cause cell cycle arrest and apoptosis of human breast cancer cells. Chem Biol Interact 188:487–496
Steigerova J, Rarova L, Oklestkova J, Krizova K, Levkova M, Svachova M, Kolar Z, Strnad M (2012) Mechanisms of natural brassinosteroid-induced apoptosis of prostate cancer cells. Food Chem Toxicol 50:4068–4076
Su X, Jiang X, Meng L, Dong X, Shen Y, Xin Y (2018) Anticancer activity of sulforaphane: the epigenetic mechanisms and the Nrf2 signaling pathway. Oxidative Med Cell Longev 2018:5438179. https://doi.org/10.1155/2018/5438179
Suppipat K, Park CS, Shen Y, Zhu X, Lacorazza HD (2012) Sulforaphaneinduces cell cycle arrest and apoptosis in acute lymphoblastic leukemia cells. PLoS One 7(12):e51251. https://doi.org/10.1371/journal.pone.0051251
Surh YJ (2003) Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3:768–780
Traka MH, Spinks CA, Doleman JF, Melchini A, Ball RY, Mills RD, Mithen RF (2010) The dietary isothiocyanatesulforaphane modulates gene expression and alternative gene splicing in a PTEN null preclinical murine model of prostate cancer. Mol Cancer 9:189. https://doi.org/10.1186/1476-4598-9-189
Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G (1997) A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem Biol Interact 103:79–129
Xu C, Huang MT, Shen G, Yuan X, Lin W, Khor TO, Conney AH, Kong ANT (2006) Inhibition of 7,12-dimethylbenz(a) anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res 66:8293–8296
Zhang Y, Gordon GB (2004) A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol Cancer Ther 3:885–893
Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P (1994) Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornylisothiocyanates. Proc Natl Acad Sci U S A 91:3147–3150
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Idrees, N., Saquib, M., Azmi, S., Ahmad, I., Hussain, M.K. (2020). Anticancer and Chemopreventive Phytochemicals from Cruciferous Plants. In: Swamy, M. (eds) Plant-derived Bioactives. Springer, Singapore. https://doi.org/10.1007/978-981-15-2361-8_17
Download citation
DOI: https://doi.org/10.1007/978-981-15-2361-8_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2360-1
Online ISBN: 978-981-15-2361-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)