Abstract
During the years, numerous evidence reported the extraordinary power of nutrition in both prevention and management of several chronic diseases, including metabolic disorders, cardiovascular diseases and cancer. Indeed, epidemiological studies established the existence of strong relationships between the regular consumption of such foods and the risk of disease development, with both positive and negative meanings. In this sense, a particular interest has been focused on the role of nutrition on cancer risk and prevention. Besides the well-established negative impact of high consumption of red meat- or processed meat-based products, several evidence highlighted the preventive effect of fruits and vegetables, or more generally fibre-rich foods, on various types of cancer, mainly colorectal cancer. Among these kinds of foods, cruciferous vegetables gained great interest by the scientific research. More specifically, bioactive compounds contained in cruciferous vegetables, including indole-3-carbinol and isothiocyanates, have been demonstrated to exert a marked anticancer potential. Several studies, indeed, reported that these phytochemicals show a chemo-preventive activity influencing cancer development since the initial phases, acting through various mechanisms. The present chapter aims to summarise the available literature providing the evidence for the use of bioactive compounds from cruciferous vegetables as chemo-protective agents, focusing the attention on the main representative phytochemicals belonging to this group, such as indole-3-carbinol and isothiocyanates. Also, the main putative mechanisms of action and signalling pathways will be presented and discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
“Let food be your medicine and medicine be your food”. With this statement, almost 2500 years ago Hippocrates offered to human population a beautiful recommendation dressed up like a medical prescription that still appears contemporary in our present era. Without having to disturb the first physicians and scientists in the history which, however, understood the medicinal properties of foods (Fenwick and Heaney 1983), it should be noted that, in the last decades, the scientific research paid particular attention on the impact of diet, in general, or such foods, specifically, on both several pathological conditions or maintenance of the wellness. In this sense, we could mention the first studies on the Mediterranean diet and the prevention of cardiometabolic diseases, as well as the previous studies on the bioactive compounds contained in red wine that led to the well-known French paradox. All this is to say that, besides the extraordinary researches in the pharmaceutical field, a relevant piece of evidence supports the potential benefits of natural products, mainly food-deriving bioactive compounds, in both prevention and management of various pathological conditions.
Notably, in the 1989 Professor Stephen DeFelice finalized the concept of the strong interplay between diet and health coining the term “nutraceutical”, as portmanteau of the words “nutrition” and “pharmaceutical”, identifying the pharmacological potential of food-derived compounds (Santini and Novellino 2014). It should be stressed that this is a science studying the effects of bioactive compounds from animal or vegetal foods on human health, following a pharmacological approach. It means that nutraceutical should not be confused with further alternative or non-conventional practices, since the single components of foods are investigated with a meticulous scientific rigour, properly designing in vitro and in vivo studies and providing strong evidence. Interestingly, nutraceuticals are considered as supplements containing food-derived extracts, concentrated and administered in a suitable pharmaceutical form, highlighting once more the pharmaceutical meaning of nutrition. Overall, thus, the careful analysis of the available literature in this field produced in the last 10–15 years, allows considering the nutraceutical as a useful tool to contrast the development of several diseases, acting “Beyond the diet, before the drugs” as stated by Professor Ettore Novellino in 2012 (Santini and Novellino 2014).
This current shift of the scientific interest from the mere study of the drugs to the evaluation of further and eventually natural therapeutic approaches should not be interpreted as a new direction operated by the researchers, but rather as a novel route that run alongside. And it should not scare off, since this just represents the natural progress of the science. We would like to give an example that might sounds like as an off-topic, but may help to understand the importance to keep an open mind when approaching the scientific research.
Until not many decades ago it was thought that Universe consisted solely of ordinary matter, which constitutes stars, planets, gasses and cosmic dust. During ‘70s, several studies and researches provided a novel interpretation of the Universe, proving the existence of both dark matter and dark energy. Interestingly, it was proved that dark matter and dark energy constitute the majority of the Universe, whereas ordinary matter only represent a limited part. These findings opened the way to new fields of application in theoretical physic and a novel comprehension of the complexity of the Universe. This fascinating travel into the galaxy perfectly suggests that static certainties are not admitted in sciences, and that research is a dynamic process that continually remodels the previous discoveries, providing novel interpretations. In this context, medical science is not exempt from this continuous progress.
Coming back to the focus of this chapter, also the research in the tumour field deeply investigated the protective role of such diets or foods in prevention of various cancers (Verhoeven et al. 1996), specifically focusing on vegetable consumption. Among these, cruciferous gained a particular interest since their regular consumption has been linked to reduced development of different kinds of cancers, including colorectal, breast, kidney, and upper digestive tract cancers (Bosetti et al. 2012). Although the exact mechanisms by which these vegetables are able to counteract the processes of carcinogenesis are still unclear, many studies clarified the possible signalling pathways in which the bioactive compounds of cruciferous vegetables may be involved.
In the following sections of the present chapter we will offer you an initial overview on the cruciferous vegetables, in terms of botanical and chemical features, with a focus on the human metabolism of their bioactive compounds. Subsequently, we will discuss the relationship occurring among these vegetables and cancer, reporting the main putative anticancer mechanisms of action and signalling pathways.
2 Cruciferous Vegetables
In this section we will explore the Cruciferous vegetable world, describing in detail the botanical features and their bioactive compounds, in terms of chemical features and human metabolism after ingestion.
2.1 Botanical Features
Cruciferous are vegetables belonging to the botanical family Brassicaceae , order Capparales, in which sixteen families are included (National Resource Conservation Services 2020). The Brassicaceae family is very large, containing about 3000 species in 350 genera, including a large number of edible plants, such as B. oleracea (i.e. cabbage, cauliflower, broccoli, Brussels sprouts), B. rapa and B. napus (including Chinese cabbage and rape), and other genera including radish and cress. The main cruciferous vegetables used in the human diet are listed in Table 7.1.
Generally, the terms Cruciferae or Cruciferaceae refer to the so-called “cabbage family” or “mustard family”, as the latin term “Brassica” means cabbage. The petals of the Brassicaceae family present a peculiar cruciform arrangement, from which it derives its name. These plants can be annuals, biennals or perennials. Due to their good adaption to mild temperatures ranging 16–18 °C, these plants can grow in temperate areas, during the cool season. In particular, Brassicaceae are cultivated in the Mediterranean region, Europe, North America and Southwest and Central Asia (National Resource Conservation Services 2020).
2.2 Chemistry of Bioactive Compounds
Similar to other vegetables, Cruciferous vegetables are a good nutrients and bioactive compounds source, including fibre, folate, carotenoids and chlorophyll, which chemopreventive properties have been demonstrated. Among these, glucosinolates deserve to be discussed more in detail.
2.2.1 Glucosinolates
Glucosinolates are the most abundant phytochemicals contained in Cruciferous vegetables, and they are responsible for their pungent aroma and spicy taste (Drewnowski and Gomez-Carneros 2000). In plants, they derive from valine, alanine, leucine, phenylalanine, isoleucine, methionine, tryptophan and tyrosin (IARC 2004) with a complex biosynthetic process involving the formation of aldoximes as intermediate, the oxidation of the N-hydroxy-amino acids and the subsequent decarboxylation and glucosidation reactions of the thiol function. The so-produced glucosinolates may be involved in further transamination, condensation, isomerisation and decarboxylation reactions, resulting in the synthesis of different structures (Popolo et al. 2017). The chemical structures of the main cruciferous vegetables are represented in Fig. 7.1.
Glucosinolates are also subject to hydrolytic processes operated by such enzymes, mainly myrosinases, leading to the formation of further bioactive compounds, including indoles and isothiocyanates (Holst and Williamson 2004). In particular, myrosinase is a β-thioglucosidase catalysing the hydrolysis of the S-glucoside giving an aglycone and the D-glucose; the aglycone, in turn, rearranges giving sufate and other compounds, such as nitriles, isothiocyanates, thiocyanates and epithionitriles (Hayes et al. 2008). Among these, one of the most widely distributed glucosinolates is the indole-3-ylmethylglucosinolate, starting from which both indole-3-acetonitrile and indole-3-carbinol (I3C) are produced. Although these reactions occur spontaneously, glucosinolates and myrosinases are naturally stored in different compartments of the plant cell, and they can meet only when the cell structure is ruptured. This physical separation is an evolutionary adaptation aimed to preserve the plant integrity protecting from cell damages, since the glucosinolate breakdown is toxic to both herbivores and pathogens (Katz et al. 2018).
Interestingly, the glucosinolate profile may be severely affected by such cooking procedures (Palermo et al. 2014), including boiling that inactivates the myrosinases (Campbell and Slominski 1990). Moreover, since most glucosinolates are thermally stable and the myrosinases can be inactivated by high temperature, the formation of isothiocyanates and indoles might be avoided by such cooking procedures; however, both chopping and chewing cause a cell rupture leading glucosinolates and myrosinases come in contact (Higdon et al. 2007). In this context, it should be taken into account that glucosinolates are water-soluble, thus they may be lost into the cooking water. 9–15 min-boiling process of cruciferous vegetables, indeed, causes a total glucosinolates content decrease around the 18–59% (McNaughton and Marks 2003); in this sense, the use of such cooking methods including steaming or microwaving should be encouraged in order to reduce the glucosinolate losses (Higdon et al. 2007).
More than a hundred glucosinolates have been identified in plants, leading to unique hydrolysis products. As examples, broccoli is a rich source of glucoraphanin and glucobrassicin, which are precursors of sulforaphane and I3C, respectively. On the other hand, watercress is a source of gluconasturtiin, the precursor of phenethyl isothiocyanate (Higdon et al. 2007; Popolo et al. 2017).
2.3 Metabolism of Bioactive Compounds
Once ingested throughout the diet, cruciferous vegetable-derived bioactive compounds follow the physiological gastrointestinal digestion, thus they undergo several physic-chemical and biochemical conditions naturally occurring during these processes, which may severely affect both their chemical features and absorption rate. Overall, the metabolic fate of these compounds can be summarised as following described at different levels:
-
Stomach : at the gastric acid pH, glucosinolate-derived products can combine with each other forming complexes of bioactive compounds, also known as acid condensation products (Shertzer and Senft 2000), including dimer 3,3′-diindolylmethane and a cyclic trimer, which biological activities differ from those of glucosinolate-derived products (Bjeldanes et al. 1991; Anderton et al. 2004a)
-
Upper intestine: at the intestinal neural pH, the major glucosinolate hydrolysis products are stable isothiocyanates; in contrast, β-hydroxy-isothiocyanates are unstable and undergo spontaneous processes leading to the formation of further compounds, including oxazolidine-2-thiones or alcohols (Higdon et al. 2007)
-
Lower intestine: the enzymatic activities of the human microbiota may contribute to further metabolise glucosinolates, forming hydrolysis products (Shapiro et al. 1998).
Additionally, the main pharmacokinetic features may be summarised with the so-called ADMET scheme (abbreviation for absorption, distribution, metabolism, excretion and toxicity).
-
A – absorption: glucosinolate-derived products are quickly absorbed; isothiocyanates can be absorbed by both upper and lower intestine (Popolo et al. 2017)
-
D – distribution: after the absorption, glucosinolate-derived products are distributed in high-vascularised tissues, such as heart, kidney, lung, liver and brain (Anderton et al. 2004b)
-
M – metabolism: after the intestinal absorption, glucosinolate-derived products undergo complex metabolic processes. Firstly, isothiocyanates are conjugated to glutathione, forming complexes that are further metabolized to mercapturic acid through the activities of various enzymes, including γ-glutamyltranspeptidase, cysteinylglycinase and N-acetyltransferase (Higdon et al. 2007)
-
E – excretion: glucosinolates and their hydrolysis products have a low half-life, due to the quick renal clearance, the rapid inactivation, the low molecular weight and the low water solubility. However, glucosinolate-derived metabolites have been detected in urine (Popolo et al. 2017)
-
T – toxicity: at high dose may be laxative. As a major concern, prolonged exposure to elevated amounts of glucosinolates may cause cytotoxicity and genotoxicity, resulting in dysfunctions at renal, thyroid gland, liver and pancreas level (Stoewsand 1995; Kassie et al. 1999; Holst and Williamson 2004). These toxic doses, however, are not reachable with the dietary consumption.
3 Cruciferous Vegetables and Cancer
Over the years, cruciferous vegetables gained great interest by the scientific research due to their potential chemopreventive effect. In this sense, one of the main challenges in investigating the relationship existing between the consumption of cruciferous vegetable and the risk of cancer in humans is related to the need to separate the benefits deriving by a general vegetable rich diet from those deriving directly by those rich in cruciferous vegetables (McNaughton and Marks 2003). However, evidence reports that the regular cruciferous vegetable consumption is linked to reduced rates of incidence of cancer (IARC 2004). In particular, epidemiologic studies published in the last century evidenced an inverse association between the consumption of some type of cruciferous vegetables and the risk of developing such types of cancer (Verhoeven et al. 1996), including lung, digestive tract, breast and prostate cancers (Higdon et al. 2007; Popolo et al. 2017). In general, these chemopreventive effects are attributable to the phytochemicals contained in this class of vegetables, acting via different mechanism of action, as will be discussed in the next section. In Table 7.2 the main evidences reporting the association between the consumption of cruciferous vegetables and cancer risk are summarised.
In addition to these studies investigating the effect of cruciferous consumption on the cancer risk, interesting evidence reported the existence of such human genetic differences that may play a role in modulating the chemopreventing potential of these vegetables (Lampe and Peterson 2002). In particular, genetic polymorphisms affecting the enzymatic activities of glutathione S-transferases (GST ; a family of enzymes metabolizing various compounds, including isothiocyanates, resulting in enhancing their excretion) may severely affect the metabolism of glucosinolate-deriving products. More specifically, homozygous individuals for the null variants of the GSTM1 and GSTT1 genes are not able to produce the GSTs (Coles and Kadlubar 2003), resulting in reduced elimination and increased exposure to isothiocyanates following the consumption of cruciferous vegetables (Seow et al. 1998). In this context, studies reported that in GSTM1-null and/or GSTT1-null individuals the inverse association between cruciferous vegetable-derived isothiocyanate intake and the risk of lung (Lewis et al. 2001; Spitz et al. 2000; London et al. 2000; Zhao et al. 2001) or colorectal cancer (Slattery et al. 2000; Seow et al. 2002; Turner et al. 2004) was more pronounced, suggesting that these genetic polymorphisms may enhance the chemoprotective effects of cruciferous vegetables via slowing the elimination rate of the bioactive compounds.
4 Main Putative Anticancer Mechanisms of Action
As aforementioned, there is evidence suggesting the chemompreventive potential of cruciferous vegetable-deriving bioactive compounds. In the following section we will discuss the main putative mechanisms of action related to this effect. In general, the anticancer activity of this class of phytochemicals is exerted through various signalling pathways involving hormone regulation, DNA repairing, cell division and growth, angiogenesis, apoptosis and inflammation (Wang and Jiang 2012), that are altered in cancer cells (Popolo et al. 2017).
4.1 Cell Cycle Arrest
The cell cycle consists of a complex process in which cells go from a resting phase (G0) to a subsequent proliferation, following distinct phases named G1, S, G2 and M. Cyclins are key proteins regulating the progression toward the single phases of the cell cycle. Cyclins may form complexes with cyclin-dependent kinases (CDKs), that are inhibited by specific enzymes, including p21 and p27. This mechanism that control the cell proliferation is deregulated in cancer cells (Diaz-Moralli et al. 2013). It has been demonstrated that isothiocyanates may modulate the expression levels of both cyclins and CDKs and up-regulate the p21 and p27 expression, resulting in induced cell cycle arrest. This effect has been reported in various studies conducted on different cancer cell lines, including human colon carcinoma, cervical cancer cells, bladder cancer cells, oral carcinoma cells, prostate cancer cells and human pancreatic cancer cells (Watson et al. 2013; Mitsiogianni et al. 2019).
4.2 Apoptosis
Apoptosis is a process inducing a programmed cell death that, in physiological conditions, is finely regulated, contributing to the maintenance of the normal cell number. Differently, in cancer cells the ability to respond to apoptosis signals is lost and cells proliferate rapidly and constantly. The pro-apoptotic effect of cruciferous vegetable-deriving phytochemicals, mainly I3C, was investigated in different cancer cell lines, including prostate, cervical and breast cancer cells, reporting that these compounds act at different levels, including (a) shifting in the expression levels of Bax and Bcl2 toward a ratio promoting the cell death, (b) PARP cleavage, (c) DNA laddering and (d) decreasing the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) activation (Higdon et al. 2007; Watson et al. 2013).
4.3 Signalling Pathways Involving Akt
One of the most frequent targets of sporadic cancers is the phosphatidylinositol 3-kinases (PI3K)/Akt/mammalian target of rapamycin (mTOR) signalling pathway (Ahmad et al. 2013). PI3Ks are lipid kinases that phophorylate various targets, including phosphatidylinositol 4,5-bisphosphate (PIP2), leading to the formation of phosphatidylinositol 3,4,5-trisphosphate (PIP3), a second messenger involved in crucial cell pathways (Zhao et al. 2017). PIP3 binds to further kinases, including Akt that in turn, once activated, regulates various cell processes, such as progression of the cell cycle, promotion of cell survival and cell growth by phosphorylating the mTOR complex 1 (Popolo et al. 2017). One of the crucial targets of Akt is NF-κB, which role in cancer development is well-established. In particular, NF-κB represents a link between inflammatory status and cancer, regulating the expression of genes involved in inhibition of the apoptotic processes and cell cycle progression, resulting in promoting angiogenesis and metastasis (Karin 2006; Hussain and Harris 2007). I3C was reported to be able to block the Akt/NF-κB signalling pathways, resulting in exerting an anticancer effect via inhibition of (a) progression of the cell cycle, (b) survival and (c) metastasis; these effects have been demonstrated in different cell lines, including B-cell precursor acute lymphoblastic leukemia and lung cancer cells (Popolo et al. 2017). In particular, the modulation of the NF-κB activation mediated by I3C results in the inhibition of the expression of both proliferative and anti-apoptotic genes, including c-Myc, IAP1, Bcl-xL, Bcl-2 and XIAP (Safa et al. 2015). Moreover, it has been reported that in Hep-2 cells, I3C affect the expression of key proteins involved in the PIP3/Akt signalling pathway, including PI3K p110α, PI3K p110β, PI3K class III, GSK3-β, p-PDK1, Akt, p-Akt and p-c-Raf (Popolo et al. 2017). Similarly, in breast and prostate cancer cells, the loss in the activity of Akt mediated by I3C resulted in alteration of various growth factors, including FOXO3a (Rahman et al. 2004). Furthermore, I3C was shown to inhibit the prostate cancer cell proliferation down-regulating the IGF1/Akt signalling pathway (Li et al. 2014).
4.4 AHR Signalling Pathway
The aryl hydrocarbon receptor (AHR) is a transcription factor regulating the expression of genes involved in various processes, including inflammation, detoxification and cancer. More specifically, acting as a xenobiotic chemical sensor, AHR binds to xenobiotic responsive elements in the promoters of targeted genes, regulating their expression. It has been demonstrated that indole derivatives are able to modulate the AHR activity (Popolo et al. 2017). In particular, I3C inhibits the binding between AHR and COX-2 promoter, which over-expression plays a crucial role in the development of various cancers, including mammary (Banerjee et al. 2002) and esophangeal cancers (Li et al. 2002). Moreover, the chemopreventive effects mediated by the interaction between I3C and AHR has been also reported in in vitro studies on colorectal cancer (Bonnesen et al. 2001; Frydoonfar et al. 2002; Hudson et al. 2002; Lee et al. 2005; Neave et al. 2005; Díaz-Díaz et al. 2016; Megna et al. 2016). Further evidence reported the capability of I3C to down-regulate the expression of the estrogen receptors (ER)-α (that is involved in promoting the cell proliferation) and activate the ER-β (that is associated with reduced cellular proliferation) (Firestone and Sundar 2009).
4.5 Carcinogen Metabolising Enzymes
Metabolising enzymes, including phase I and phase II enzymes, play a pivotal role in cancer development. Among the phase I enzymes, there is the cytochrome p450 (CYP450), while among the phase II enzymes there are GST, quinone reductase (QR), and NAD(P)H quinone oxidoreductase-1 (NQO1) (Leone et al. 2017). Phase I enzymes increase the hydrophilicity of carcinogenic compounds via various reactions (i.e. oxidation, reduction, hydrolysis), resulting in increasing their reactivity and the consequent DNA damage. On the other hand, phase II enzymes cause the reactive intermediates conjugation, promoting their urinary excretion through the mercapturic acid pathway (Talalay and Fahey 2001; Keum et al. 2009). It has been reported that cruciferous vegetable-deriving bioactive compounds are able to inhibit the activity of phase I enzymes and activate the phase II enzymes; in particular, this activation seems to be exerted through increased transcription of these enzymes (Abbaoui et al., 2018).
4.6 Epigenetic Effects
Besides the aforementioned effects of both indoles and isothiocyanates in signalling pathways directly involved in cancer development, evidence suggests a role of these phytochemicals in epigenetic processes, in particular, altering the epigenetic modulators expression, such as enzymes controlling histone methylation and microRNAs. Human in vivo studies, indeed, demonstrated that the supplementation with idole-deriving products increased the expression of Let family microRNA and decreased the expression of the histone methyltransferase EZH2; moreover, these compounds may restore the expression levels of the silenced miR-34a, that is recognized as a regulator of cancer suppression (Watson et al. 2013).
5 Conclusion
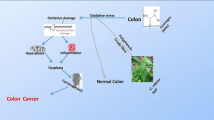
Cruciferous vegetables may be considered as good sources of bioactive compounds which chemopreventive effect has been widely reported and the mechanisms of action well-described, as schematically summarised in Fig. 7.2. Overall, this evidence supports the observational data showing the direct association between reduced cancer risk and regular consumption of this kind of vegetables reported in various studies. Such promising, this evidence may serve to drive the scientific research in the nutraceutical field toward the further investigation of this beneficial potential of Cruciferous vegetable-derived bioactive compounds, formulating proper nutraceuticals aimed to prevent the risk to develop various cancers. This in turn, may be a start point for designing animal-based studies and clinical trials aimed to substantiate these effects in vivo.
Abbreviations
- AHR:
-
Aryl hydrocarbon receptors
- CDKs:
-
Cyclin-dependent kinases
- CYP450:
-
Cytochrome p450
- ER:
-
Estrogen receptors
- GTS:
-
Glutathione S-transferase
- I3C:
-
Indole 3-carbinol
- mTOR:
-
Mammalian target of rapamycin
- NFκB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NQO1:
-
NAD(P)H quinone oxidoreductase-1
- PI3K:
-
Phosphatidylinositol-3-kinase
- PIP2:
-
Phosphatidylinositol 4,5-bisphosphate
- PIP3:
-
Phosphatidylinositol 3,4,5-trisphosphate
- QR:
-
Quinone reductase
References
Abbaoui B, Lucas CR, Riedl KM, Clinton SK, Mortazavi A (2018). Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol Nutr Food Res 62(18):e1800079. https://doi.org/10.1002/mnfr.201800079
Ahmad A, Biersack B, Li Y, Kong D, Bao B, Schobert R et al (2013) Targeted regulation of PI3 K/Akt/mTOR/NF-κB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy. Anticancer Agents Med Chem 13:1002–1013
Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG (2004) Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr 134:1134–1138
Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB et al (2004a) Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res 10:5233–5241
Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML et al (2004b) Physiological modeling of formulated and crystalline 3, 3′- diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos 32:632–638
Banerjee S, Bueso-Ramos C, Aggarwal BB (2002) Suppression of 7,12-dimethylbenz(a) anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappa B, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res 62:4945–4954
Benito E, Obrador A, Stiggelbout A, Bosch FX, Mulet M, Munoz N et al (1990) A population-based casecontrol study of colorectal cancer in Majorca. I. Dietary factors. Int J Cancer 45:69–76
Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA (1991) Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A 88:9543–9547
Bonnesen C, Eggleston IM, Hayes JD (2001) Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res 61:6120–6130
Bosetti C, Filomeno M, Riso P et al (2012) Cruciferous vegetables and cancer risk in a network of case-control studies. Ann Oncol 23(8):2198–2203
Bradlow HL, Telang NT, Sepkovic DW, Osborne MP (1996) 2-hydroxyestrone: the ‘good’ estrogen. J Endocrinol 150(Suppl):S259–S265
Campbell LD, Slominski BA (1990) Extent of thermal decomposition of indole glucosinolates during the processing of canola seed. J Am Oil Chem Soc 67(2):73–75
Cohen JH, Kristal AR, Stanford JL (2000) Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst 92:61–68
Coles BF, Kadlubar FF (2003) Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinants of individual response to chemical carcinogens and chemotherapeutic drugs? Biofactors 17:115–130
Díaz-Díaz CJ, Ronnekleiv-Kelly SM, Nukaya M, Geiger PG, Balbo S, Dator R et al (2016) The aryl hydrocarbon receptor is a repressor of inflammation-associated colorectal tumorigenesis in mouse. Ann Surg 264:429–436
Diaz-Moralli S, Tarrado-Castellarnau M, Miranda A, Cascante M (2013) Targeting cell cycle regulation in cancer therapy. Pharmacol Ther 138:255–271
Drewnowski A, Gomez-Carneros C (2000) Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr 72:1424–1435
Fenwick GR, Heaney RK (1983) Glucosinolates and their breakdown products in cruciferous crops, foods and feedingstuffs. Food Chem 11(4):249–271
Feskanich D, Ziegler RG, Michaud DS, Giovannucci EL, Speizer FE, Willett WC et al (2000) Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J Natl Cancer Inst 92:1812–1823
Firestone GL, Sundar SN (2009) Minireview: modulation of hormone receptor signaling by dietary anti-cancer indoles. Mol Endocrinol 23:1940–1947
Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C et al (2003) Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res 63:3980–3986
Frydoonfar HR, McGrath DR, Spigelman AD (2002) Inhibition of proliferation of a colon cancer cell line by indole-3-carbinol. Color Dis 4:205–207
Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC (2003) A prospective study of cruciferous vegetables and prostate cancer. Cancer Epidemiol Biomark Prev 12:1403–1409
Graham S, Dayal H, Swanson M, Mittelman A, Wilkinson G (1978) Diet in the epidemiology of cancer of the colon and rectum. J Natl Cancer Inst 61:709–714
Hayes JD, Kelleher OM, Eggleston IM (2008) The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr 47:73–88
Higdon JV, Delage B, Williams DE, Dashwood RH (2007) Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 55(3):224–236. https://doi.org/10.1016/j.phrs.2007.01.009
Holst B, Williamson G (2004) A critical review of the bioavailability of glucosinolates and related compounds. Nat Prod Rep 21:425–447
Hudson EA, Howells LM, Gallacher-Horley B, Fox LH, Gescher A, Manson MM (2002) Growth-inhibitory effects of the chemopreventive agent indole-3- carbinol are increased in combination with the polyamine putrescine in the SW480 colon tumour cell line. BMC Cancer 3:2
Hussain SP, Harris CC (2007) Inflammation and cancer: an ancient link with novel potentials. Int J Cancer 121:2373–2380
International Agency for Research on Cancer Workgroup (2004) Cruciferous vegetables, isothiocyanates and indoles. In: Handbooks of cancer prevention, vol 9. IARC Press, Lyon
Jain MG, Hislop GT, Howe GR, Ghadirian P (1999) Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer 34:173–184
Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, Zhang Y et al (2004) Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr Cancer 50:206–213
Karin M (2006) Nuclear factor-kappa B in cancer development and progression. Nature 441(7092):431–436
Kassie F, Pool-Zobel B, Parzefall W, Knasmüller S (1999) Genotoxic effects of benzyl isothiocyanate:a natural chemopreventive agent. Mutagenesis 14:595–604
Katz E, Nisani S, Chamovitz DA (2018) Indole-3-carbinol: a plant hormone combatting cancer. F1000Res 7:F1000 Faculty Rev-689. https://doi.org/10.12688/f1000research.14127.1
Keum YS, Khor TO, Lin W, Shen G, Kwon KH, Barve A, Li W, Kong AN (2009) Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharm Res 26:2324–2331
Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR et al (2000) Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomark Prev 9:795–804
Lampe JW, Peterson S (2002) Brassica, biotransformation and cancer risk: genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr 132:2991–2994
Larsson SC, Hakansson N, Naslund I, Bergkvist L, Wolk A (2006) Fruit and vegetable consumption in relation to pancreatic cancer: a prospective study. Cancer Epidemiol Biomarkers Prev 15:301–305
Lee SH, Kim JS, Yamaguchi K, Eling TE, Baek SJ (2005) Indole-3-carbinol and 3:3′- diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun 328:63–69
Leone A, Diorio G, Sexton W, Schell M, Alexandrow M, Fahey JW, Kumar NB (2017) Sulforaphane for the chemoprevention of bladder cancer: molecular mechanism targeted approach. Oncotarget 8:35412–35424
Lewis S, Brennan P, Nyberg F, Ahrens W, Constantinescu V, Mukeria A et al (2001) Re: Spitz, M. R., Duphorne, C. M., Detry, M. A., Pillow, P. C., Amos, C. I., Lei, L., de Andrade, M., Gu, X., Hong, W. K., and Wu, X. Dietary intake of isothiocyanates: evidence of a joint effect with glutathione Stransferase polymorphisms in lung cancer risk. Cancer Epidemiol. Biomark. Prev. 9: 1017–1020, 2000. Cancer Epidemiol Biomark Prev 10:1105–1106
Li N, Chen X, Liao J, Yang G, Wang S et al (2002) Inhibition of 7,12-dimethylbenz[a] anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 23:1307–1313
Li Y, Ahmad A, Kong D, Bao B, Sarkar FH (2014) Recent progress on nutraceutical research in prostate cancer. Cancer Metastasis Rev 33:629–640
London SJ, Yuan JM, Chung FL, Gao YT, Coetzee GA, Ross RK et al (2000) Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet 356:724–729
McNaughton SA, Marks GC (2003) Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br J Nutr 90:687–697
Megna BW, Carney PR, Nukaya M, Geiger P, Kennedy GD (2016) Indole-3-carbinol induces tumor cell death: function follows form. J Surg Res 204:47–54
Mitsiogianni M, Koutsidis G, Mavroudis N, Trafalis DT, Botaitis S, Franco R, Zoumpourlis V, Amery T, Galanis A, Pappa A, Panayiotidis MI (2019) The role of isothiocyanates as cancer chemo-preventive, chemo-therapeutic and anti-melanoma agents. Antioxidants (Basel, Switzerland) 8(4):106. https://doi.org/10.3390/antiox8040106
Neave AS, Sarup SM, Seidelin M, Duus F, Vang O (2005) Characterization of the Nmethoxyindole-3-carbinol (NI3C)–induced cell cycle arrest in human colon cancer cell lines. Toxicol Sci 83:126–135
Neuhouser ML, Patterson RE, Thornquist MD, Omenn GS, King IB, Goodman GE (2003) Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET). Cancer Epidemiol Biomark Prev 12:350–358
Palermo M, Pellegrini N, Fogliano V (2014) The effect of cooking on the phytochemical content of vegetables. J Sci Food Agric 94(6):1057–1070
Popolo A, Pinto A, Daglia M, Nabavi SF, Farooqi AA, Rastrelli L (2017) Two likely targets for the anti-cancer effect of indole derivatives from cruciferous vegetables: PI3K/Akt/mTOR signalling pathway and the aryl hydrocarbon receptor. Semin Cancer Biol 46:132–137. https://doi.org/10.1016/j.semcancer.2017.06.002
Rahman KM, Li Y, Sarkar FH (2004) Inactivation of akt and NF-κB play important roles during indole-3-carbinol-induced apoptosis in breast cancer cells. Nutr Cancer 48:84–94
Safa M, Tavasoli B, Manafi R, Kiani F, Kashiri M, Ebrahimi S et al (2015) Indole-3- carbinol suppresses NF-κB activity and stimulates the p53 pathway in pre-B acute lymphoblastic leukemia cells. Tumour Biol 36:3919–3930
Santini A, Novellino E (2014) Nutraceuticals: beyond the diet before the drugs. Curr Bioact Compd 10(1):1–12
Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP et al (1998) Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomark Prev 7:775–781
Seow A, Yuan JM, Sun CL, Van Den Berg D, Lee HP, Yu MC (2002) Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese health study. Carcinogenesis 23:2055–2061
Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P (1998) Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomark Prev 7:1091–1100
Shertzer HG, Senft AP (2000) The micronutrient indole-3-carbinol: implications for disease and chemoprevention. Drug Metabol Drug Interact 17:159–188
Slattery ML, Kampman E, Samowitz W, Caan BJ, Potter JD (2000) Interplay between dietary inducers of GST and the GSTM-1 genotype in colon cancer. Int J Cancer 87:728–733
Spitz MR, Duphorne CM, Detry MA, Pillow PC, Amos CI, Lei L et al (2000) Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol Biomark Prev 9:1017–1020
Stoewsand GS (1995) Bioactive organosulfur phytochemicals in Brassica oleracea vegetables. A review. Food Chem Toxicol 33:537–543
Talalay P, Fahey JW (2001) Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr 131:3027S–3033S
Terry P, Wolk A, Persson I, Magnusson C (2001) Brassica vegetables and breast cancer risk. JAMA 285:2975–2977
Turner F, Smith G, Sachse C, Lightfoot T, Garner RC, Wolf CR et al (2004) Vegetable, fruit and meat consumption and potential risk modifying genes in relation to colorectal cancer. Int J Cancer 112:259–264
United States Department of Agriculture (USDA) (2020) National Resources Conservation Service. https://www.nrcs.usda.gov/wps/portal/nrcs/site/national/home/ [last access April 2020]
Verhoeven DT, Goldbohm RA, van Poppel G et al (1996) Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev 5(9):733–748
Voorrips LE, Goldbohm RA, Verhoeven DT, van Poppel GA, Sturmans F, Hermus RJ et al (2000a) Vegetable and fruit consumption and lung cancer risk in the Netherlands cohort study on diet and cancer. Cancer Causes Control 11:101–115
Voorrips LE, Goldbohm RA, van Poppel G, Sturmans F, Hermus RJ, van den Brandt PA (2000b) Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: the Netherlands cohort study on diet and cancer. Am J Epidemiol 152:1081–1092
Walters DG, Young PJ, Agus C, Knize MG, Boobis AR, Gooderham NJ et al (2004) Cruciferous vegetable consumption alters the metabolism of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) in humans. Carcinogenesis 25:1659–1669
Wang J, Jiang YF (2012) Natural compounds as anti-cancer agents: experimental evidence. World J Exp Med 2:45–57
Watson W, Beaver GM, Williams LE, Dashwood DH, Ho RE (2013) Phytochemicals from cruciferous vegetables, epigenetics, and prostate cancer prevention. AAPS J 15(4):951–961. https://doi.org/10.1208/s12248-013-9504-4
West DW, Slattery ML, Robison LM, Schuman KL, Ford MH, Mahoney AW et al (1989) Dietary intake and colon cancer: sex- and anatomic site-specific associations. Am J Epidemiol 130:883–894
Young TB, Wolf DA (1988) Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer 42:167–175
Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng P et al (2001) Dietary isothiocyanates, glutathione Stransferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomark Prev 10:1063–1067
Zhao W, Qiu Y, Kong D (2017) Class I phosphatidylinositol 3-kinase inhibitors for cancer therapy. Acta Pharm Sin B 7:27–37
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Annunziata, G., Arnone, A., Tenore, G.C. (2021). Cruciferous Vegetables (Indole-3-Carbinol, Isothiocyanates) Against Cancer. In: Jafari, S.M., Nabavi, S.M., Silva, A.S. (eds) Nutraceuticals and Cancer Signaling. Food Bioactive Ingredients. Springer, Cham. https://doi.org/10.1007/978-3-030-74035-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-74035-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-74034-4
Online ISBN: 978-3-030-74035-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)