Abstract
Geothermal waste from PT. Geo Dipa Energy Dieng was transformed to Na-P zeolite (CFA-ZP) experimentally at a low temperature (100 °C) via conventional hydrothermal. This study was conducted to determine the effect of the hydrothermal process holding time on the characterization of Na-P zeolite. Synthesis process via hydrothermal method was performed with different holding times of 10, 15 and 20 h at a constant temperature of 100 °C. The synthesized material were characterized using XRD and SEM techniques. The results showed conventional hydrothermal process successfully converts geothermal waste into Na-P zeolite, zeolite A and sodalite. Increasing the holding time in conventional hydrothermal method has affected the material characterization of the synthesized zeolite.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Indonesia is geologically located at the confluence of three major tectonic plates; the Eurasian Plate, the Indo-Australian Plate and the Pacific Plate and this strategic geological condition makes a real contribution to the availability of geothermal energy in Indonesia. However, in terms of geothermal energy development, Indonesia is still behind the United States and the Philippines which produces 3093 and 1904 MW of geothermal energy respectively, in comparison with Indonesia which produces a total of 1341 MW [1]. Geothermal energy is a renewable energy resource producing considerable amount of wastes in the form of geothermal brine and geothermal sludge. The geothermal sludge typically contains solids which precipitate out in the waste water treatment plant during the power generation process and the sludge can be highly concentrated in a variety of heavy metal salts namely iron, titanium, manganese, zinc, arsenic, boron, cadmium, lead, nickel and copper [2]. It also contains a large proportion of silica [2, 3]. Due to these, the geothermal waste cannot be directly disposed into a landfill without any prior treatments [3, 4].
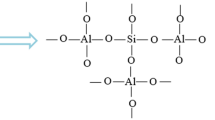
Disposal of hazardous solid wastes at a geothermal power plant is a major concern where large amounts of geothermal sludge volumes are generated. Recent studies have shown that many countries were able to further refine the silica contained in the geothermal sludge into a saleable product. There is a variety of markets and possible uses for amorphous silica of which, depending on the form and purity, can be worth up to 15 US$/kg [5]. Silica is a chemical compound, also known as Silicon Dioxide and is most commonly found in nature as Quartz. It is used for a variety of applications such as an additive for structural materials, silicon for microelectronics, components in the food industry and pharmaceutical industry as well as a mineral for human intake, amongst others. Silica is a valuable product whose costs vary, depending on its properties. The desired properties are often the high surface area, large pore volume and a uniform pore size distribution. Some studies reported that the composition has less significance than the corresponding texture for most applications [6]. Zeolites are microporous crystalline aluminosilicates, composed of TO tetrahedra (T = Si, Al) with O atoms connecting neighboring tetrahedral [7]. There are two kinds of zeolite; natural zeolite and synthesized zeolite. The synthetic zeolites have been used commercially than natural zeolites due to its purity of crystalline products and the uniformity of its particle size. The main advantages of synthetic zeolites in comparison to naturally-occurring zeolites are that they can be engineered with a wide variety of chemical properties and different pore sizes and that they have greater thermal stability. The zeolite synthesis involves the hydrothermal crystallization of aluminosilicate gels [8].
The major drawback of zeolite synthesis is the availability and the cost of the raw material, specifically the silica source. The usage of silica contained in the geothermal waste as a starting material for zeolite synthesis does not only help to reduce its cost production but also contributes to the mitigation of environmental problems [9]. Many studies have used geothermal wastes as a silica source in zeolite synthesis. Zeolite A and hydroxysodalite (HS) were successfully synthesized from a geothermal sludge [3, 10]. Zeolite Na-P was also synthesized from coal fly ash, kaolin waste, clinoptilolite, paper sludge ash and coal bottom ash [11,12,13,14,15,16]. Zeolite A and zeolite X were successfully synthesized from silica in rice husk [17, 18]. The types and characteristics of zeolites produced from the synthesis processes are influenced by reaction time, temperature, pressure and synthesis conditions [3].
Previous experiments reported that geothermal waste contains silica and alumina: 81.26 and 0.14% (calcinated at a temperature of 850 °C for 3 h). The novelty of this study is to synthesize Na-P zeolite from geothermal wastes via hydrothermal reaction. Na-P zeolite is one of the most interesting synthesized zeolites because of its high cation exchange and adsorption capacity. This synthetic zeolite can be used in different applications such as gas separation application; removal of heavy metal, ammonium, toxic and radioactive waste species; extraction of potassium from sea water; water-softening; and as a builder and water-softening agent for eco-friendly detergent [19].
2 Experimental Procedures
The geothermal waste was obtained from Geo Dipa geothermal power plant, Dieng, Indonesia. The geothermal waste was dried under sunlight for a period of time until it was completely dried and screened with a mesh 100 to obtain the geothermal powder. The geothermal powder was calcined at 850 °C for 3 h. This process aimed to drive off volatile expunges, moisture, water of hydrates, organic matter and increase the yield of SiO2 from the geothermal powder. The chemical compositions of geothermal powder before and after calcinations were characterized by atomic absorption spectroscopy (AAS). Calcined geothermal powder was used as a silica source in the synthesis of zeolite and was prepared through conventional hydrothermal method.
The precursor for zeolite synthesis process was carried out by reacting 30 mL of sodium silicate solution and 30 mL of sodium aluminate solution using a magnetic stirrer with 200 rpm for 2 h at room temperature. This process produced natrium aluminosilicate. Sodium silicate was made from 3 g of geothermal powder mixed with 30 mL of NaOH 3M, and then stirred at a temperature of 1000 °C for 20 min. Sodium aluminate was made from 8.5 g of Al(OH)3 mixed with 100 mL of NaOH 5M [20]. The precursor was poured into a Teflon autoclave and hydrothermal reaction was conducted on a conventional hydrothermal reactor at a temperature of 100 °C for 10, 15, and 20 h. The synthesized products were washed with distilled water and filtered with a Whatman 42 paper to separate filtrate from solid. The solid was dried in the oven at a temperature of 80 °C for 4 h to obtain the final product. The final products were examined by X-ray powder diffraction (XRD) for amorphicity and crystalline analysis. Crystalline phases present in the samples were identified with the help of Joint Committee on Powder Diffraction Standards (JCPDS). The morphological structures of the synthesized products were obtained and analysed using Scanning Electron Micrograph (SEM).
3 Results and Discussions
In this study, Atomic Absorption Spectrophotometer (AAS) is used to characterize the chemical constituents of geothermal powder. The potential application of atomic absorption principle is necessary to determine metallic constituents of silicate materials. The analysis of raw geothermal powder is presented in Table 5.1.
XRD plays an important role in the qualitative and quantitative characterization of synthesized products. Data obtained in the form of the intensity and angle 2θ were then matched with the XRD pattern JCPDS in order to identify the crystal phases present in the sample [10, 20]. An effective method to determine the phase of the grown samples is by comparing the XRD data with standard database of JCPDS. As presented in Fig. 5.1, some similarities were observed with respect to the diffractogram from JCPDS data number 11-0401 (hydroxy-sodalite), 40-1464 (zeolite Na-P) and 31-1269 (Zeolite A ). This result shows that the synthesized products contain crystals of Zeolite A , zeolite Na-P and hydroxy-sodalite. It is also observed that the XRD peaks at 24.7663, 24.1773 and 43.2287 indicates that the synthesized product contains sodalite . The presence of zeolite A is indicated with the XRD peaks at 13.8076, 13.4088 and 13.9422 while the presence of zeolite Na-P is observed with the XRD peaks at 28.2511, 28.0813 and 28.3809.
The crystal size was determined using the Scherer formula based on the XRD characterization of synthesized products [10, 21]. The crystal size of zeolite synthesized from geothermal waste at variations of hydrothermal holding time are shown in Fig. 5.2.
The respective crystal size of zeolite Na-P at holding time variations of 10, 15 and 20 h were 28.5, 21.4 and 29.2 nm while the crystal size of zeolite A at holding time variations of 10 h, 15 h and 20 h were 83.6 nm, 104.4 nm and 17.1 nm, respectively. The crystal size of sodalite at holding time variations of 10 h, 15 h and 20 h were 59.7 nm, 18.9 nm and 30.4 nm, respectively. It was also observed that the hydrothermal holding time affects the crystal size of zeolite A , zeolite Na-P and sodalite formed. By increasing the hydrothermal holding time from 10 to 15 h increases the crystal size of zeolite A . The same phenomenon was discovered in the crystal size of zeolite Na-P and sodalite by increasing the hydrothermal holding time from 15 to 20 h. The results from this study indicate that the crystal size of zeolite Na-P, zeolite A and sodalite increases significantly with increasing hydrothermal holding time. Similar results were reported by Muhammad et al. and Zou et al. that increasing the reaction time resulted in an increase in the average particle size [22, 23]. As reported by Liang et al. the duration of the hydrothermal reaction affected the size and morphology of nanoapatite crystals due to larger crystal growth with prolonged reaction times. Prolonging the hydrothermal treatment time promotes solvent evaporation and maintains the solution in a supersaturation state, thereby assisting crystal growth [24].
On the other hand, increasing the hydrothermal holding time beyond 15 h resulted in a decrease in the crystal size of zeolite A. The same phenomenon was discovered in the crystal size of zeolite Na-P and sodalite where it decreased with an increase of holding time from 10 to 15 h. Increasing the crystallization time results in dissolved synthesized zeolites in the alkaline solution and this causes a decrease in its crystallinity and crystal size [21]. This observation agrees with the work of Hanipa et al. which reported the longer the crystallization time the slower the crystal growth rate and nucleation rate and thus producing a small crystal size [25].
The crystallization time beyond 15 h for zeolite Na-P could decrease the intensity for zeolite Na-P and increase the intensity for sodalite and zeolite A , which is consistent with the XRD results as shown Fig. 5.1. Figure 5.1 illustrates the crystallinity which increased with extending times at 100 °C. It started to decrease beyond 20 h due to collapsed of zeolite framework. As can be seen in Fig. 5.2, the optimum crystallinity of zeolite Na-P in 15 h hydrothermal holding time was characterized by the smallest crystal size [26, 27]. Table 5.2 summarizes the effects of crystallization time on XRD patterns of the prepared zeolite samples. It is observed that the synthesized zeolite tends to have higher crystallinity with flat baseline and no amorphous was formed in comparison to the provided JCPDS data base.
The result of this study is also in accordance with the result reported by Alrubaye and Zou et al. [23, 26]. In general, Ostwald’s law of successive reactions is followed in zeolite synthesis, i.e., a metastable phase appears first and then successively more stable phases are then replaced. For instance, with prolonged crystallization time, zeolite-A (LTA) is dissolved to form zeolite-sodalite (SOD), when synthesized in an alkaline aluminosilicate-gel [21].
The photographs show the morphology of the zeolites formed where Fig. 5.3a shows SEM micrographs of zeolite Na-P (scale bar, 2 μm) while Fig. 5.3b shows SEM micrographs of zeolite A (scale bar, 8 μm). Figure 5.3c shows SEM micrographs of sodalite (scale bar, 2 μm). As evident from Fig. 5.3a–c, zeolite Na-P is identified by pseudo-spherical forms constituted by small plates, which formed polycrystals, zeolite A particles formed cubic shapes and sodalite particles presented spherical shapes with an obvious array of long fibers [10, 20, 23].
4 Conclusions
Zeolite Na-P, zeolite A and sodalite have been successfully synthesized from geothermal wastes using silica as the source via the conventional hydrothermal method with holding time variations of 10, 15 and 20 h. The effects of hydrothermal holding time (hydrothermal duration) in the synthesis of possible zeolites on the crystalline end products were investigated. The results of this study showed that increasing the hydrothermal holding time from 10 to 15 h increases the crystal size of zeolite A and reduces the crystal size of zeolite Na-P and sodalite . However, increasing the hydrothermal holding time from 15 to 20 h reduces the size of zeolite A crystals but increases the size of Na-P and sodalite zeolite crystals.
References
P. Suharmanto, A.N. Fitria, S. Ghaliyah, in Indonesian geothermal energy potential as source of alternative energy power plant. Renewable Energy and Energy Conversion Conference and Exhibition (2015), pp. 119–124
D. Gallup, F. Sugiaman, V. Capunoc, A. Manceaud, Laboratory investigation of silica removal from geothermal brines to control silica scaling and produce usable silicates. Appl. Geochem. 18, 1597–1612 (2003)
D.F. Sulardjaka, Fitriyana, A.P. Adi, Synthesis of zeolite from geothermal waste. Appl. Mech. Mater. 660, 157–161 (2014)
A.J. Karabelas, N. Andritsos, A. Mouza, M. Mitrakas, F. Vrouzi, K. Christanis, Characteristics of scales from the Milos geothermal plant. Geothermics 18, 169–174 (1989)
J.O. Were, in Aspects of Waste Management and Pollution Control. Olkaria Geothermal Field, Kenya, Geothermal Training Programme (1998), pp. 16–54
A. Hauksdóttir, Geothermal Energy: Silica Precipitation and Utilization. Thesis of the School of Science and Engineering at Reykjavík University, Iceland, 2016
P. Payra, P.K. Dutta, Zeolite: a primer, The Ohio State University, Columbus Ohio. U.S.A., in Handbook of Zeolite Science And Technology, ed. by Auerbach, M.C. Scott, A. Kathleen, P.K. Dutta (The Ohio State University Columbus Ohio, U.S.A., 2003)
D. Georgiev, B. Bogdanov, K. Angelova, I. Markovska, Y. Hristov, Synthetic zeolites—structure, classification, current trends in zeolite synthesis, (Review). Int. Sci. Conf., Stara Zagora 7, 1–5 (2003)
I. Petrov, T. Michalev, Synthesis of Zeolite A: A Review (Scientific Work of The Russian University, 2012), pp. 30–35
A.P.A. Sulardjaka, S. Nugroho, in Microwave-Hydrothermal Versus Conventional-Hydrothermal Synthesis of Zeolite A from Geothermal Waste. 1st International Joint Conference on Advanced Engineering and Technology proceeding (2012). ISBN: 978-602-097-299-2
W. Chansiriwat, D. Tanangteerapong, K. Wantala, Synthesis of zeolite from coal fly ash by hydrothermal method without adding alumina and silica sources: effect of aging temperature and time. Sains Malays. 45(11), 1723–1773 (2016)
M. Chigondo, U. Guyo, M. Shumba, F. Chigondo, B. Nyamunda, M. Moyo, T. Nharingo, Synthesis and characterisation of zeolites from coal fly ash (CFA). Synthesis 3(4) (2013)
E.A. Hildebrando, C.G.B. Andrade, C.A.F.D. Rocha Junior, R.S. Angélica, F.R. Valenzuela-Diaz, R.D.F. Neves, Synthesis and characterization of zeolite NaP using kaolin waste as a source of silicon and aluminum. Mater. Res. 17, 174–179 (2014)
J. Behin, H. Kazemian, S. Rohani, Sonochemical synthesis of zeolite NaP from clinoptilolite. Ultrason. Sonochem. 28, 400–408 (2016)
P.W.D. Plessis, T.V. Ojumu, L.F. Petrik, Waste minimization protocols for the process of synthesizing zeolites from South African coal fly ash. Materials 6(5), 1688–1703 (2013)
T. Wajima, M. Haga, K. Kuzawa, H. Ishimoto, O. Tamada, K. Ito, T. Nishiyama, R.T. Downs, J.F. Rakovan, Zeolite synthesis from paper sludge ash at low temperature (90 °C) with addition of diatomite. J. Hazard Mater. 132(2), 244–252 (2006)
C. Bhavornthanayo, P. Rungrojchaipon, Synthesis of zeolite A membrane from rice husk ash. J. Met. Mater. Miner. 19(2), 79–83 (2009)
D. Prasetyoko, Z. Ramli, S. Endud, H. Hamdan, B. Sulikowski, Conversion of rice husk ash to zeolite beta. Waste Manag. 26, 1173–1179 (2006)
Z. Huo, X. Xu, Z. Lü, J. Song, M. He, Z. Li et al., Synthesis of zeolite Na-P with controllable morphologies. Microporous Mesoporous Mater. 158, 137–140 (2012)
Sulardjaka, D.F. Fitriyana, The effect of concentration of NaOH and holding time on characteristic of zeolite sinthesized from geothermal waste. Reactor 17(1), 17–24 (2017)
M. Mirfendereski, T. Mohammadi, in Effects of Synthesis Parameters on the Characteristics of NaA Type Zeolite Nanoparticles. Proceedings of the World Congress on Recent Advances in Nanotechnology (2016), pp. 1–8
S. Muhammad, I.M. Sofyana, A. Munandar, T.E. Agustina, E. Saputra, S. Wang, M.O. Tade, in Hydrothermal Synthesis of Nanocrystalline Zeolite using Clear Solution. Proceedings of The 5th Sriwijaya International Seminar on Energy and Environmental Science and Technology (2014), pp. 80–86
J. Zou, C. Guo, C. Wei, F. Li, Y. Jiang, Synthesis of pure Na-X and Na-P zeolite from acid-extracting residues of CFB fly ash by a single-step hydrothermal method. Mater. Trans. 57(5), 726–731 (2016)
W. Liang, Y. Niu, S. Ge, S. Song, J. Su, Z. Luo, Effects of hydrothermal treatment on the properties of nanoapatite crystals. Int. J. Nanomedicine. 7, 5151–5158 (2012)
P.H. Pardoyo, T. Arnelli, Y. Astuti, Effect of hydrothermal time variation on synthesis and characterization of zeolite A nanocrystal from rice husk ash. J. Sci. Appl. Chem. (2), 79–83 (2017)
R.T.A. Alrubaye, in Influence Factors on Zeolite Y Crystal growth. 1st International Conference on Recent Trends of Engineering Science and Sustainability (2017)
M. Farshid, H. Rouein, A. Sima, The synthesis of nano-sized ZSM-5 zeolite by dry gel conversion method and investigating the effects of experimental parameters by Taguchi experimental design. J. Exp. Nanosci. 13(1), 160–173 (2018)
Acknowledgements
The first Author gratefully acknowledge the Faculty of Engineering Diponegoro University for funding this research which made this work possible (strategic research scheme, 2017).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Fitriyana, D.F., Suhaimi, H., Sulardjaka, Noferi, R., Caesarendra, W. (2020). Synthesis of Na-P Zeolite from Geothermal Sludge. In: Murakami, RI., Koinkar, P., Fujii, T., Kim, TG., Abdullah, H. (eds) NAC 2019. Springer Proceedings in Physics, vol 242. Springer, Singapore. https://doi.org/10.1007/978-981-15-2294-9_5
Download citation
DOI: https://doi.org/10.1007/978-981-15-2294-9_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2293-2
Online ISBN: 978-981-15-2294-9
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)