Abstract
Recently, Enterobacter spp. are encountered as significant clinical pathogens. Most of them are naturally resistant to older and few newer antimicrobial agents. They have the inherent ability to develop antibiotic resistance to novel antibiotics. This genus is prevalent in nosocomial infections. Among the Enterobacteriaceae family, two well-known clinically important opportunistic pathogens emerged recently are E. aerogenes and E. cloacae. These are versatile pathogens known to cause nosocomial infections in intensive care unit patients. Recently, Enterobacter spp. are emerged in community other than the hospital settings causing life-threatening infections. This bacteria possesses intrinsic resistance to broad-spectrum beta-lactam drugs owing to the presence of beta lactamases. Majority of the Enterobacter resistance is due to the production of chromosomal AmpC beta-lactamases. AmpC is responsible for the resistance profiles of Enterobacter spp. toward first-, second-, and third-generation cephalosporins. Due to the acquisition of plasmids harboring genes that encode extended spectrum beta-lactamases and cephalosporinases, they often exhibit multiple drug resistance and are common in hospitalized patients. Emergence of plasmid-mediated quinolone resistance, aminoglycoside resistance, and carbapenem resistance appears to be rare cases. However, these resistant strains are troublesome during the antibiotic therapy as these drugs known as last line of treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
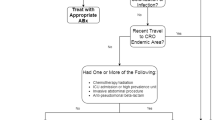
Recently, Enterobacter spp. are rapidly establishing as deadly nosocomial pathogens. Hospital-acquired infections are one of the worse outcome associated with the multidrug-resistant Enterobacter spp. Recently, third-generation antibiotic, cephalosporin resistance among Enterobacter is important nosocomial pathogen associated with high mortality and infection morbidity (Ye et al. 2006). Enterobacter spp. causes several nosocomial infections such as bloodstream infections (BSIs), urinary tract infections (UTIs), ophthalmic infections, central nervous system infections, and skin and soft tissue infections. Among all, BSIs are the most invasive nosocomial infection caused by Enterobacter spp. These bacteria are ranked as the seventh most common infectious agent associated with nosocomial pneumonia and ninth most common pathogen causing nosocomial BSIs. The severity of this pathogen in hospital settings is high, where bloodstream infections are the second most invasive disease due to these bacteria (Kus 2014) (Fig. 11.1).
The genus Enterobacter was first suggested by Hormaeche and Edwards in 1960 (Grimont and Grimont 2006). Enterobacter spp. are having similar phenotypic and biochemical features of the genus Klebsiella but differ in their motility. Colonies of this genus are slightly mucoid in nature and less fermentative than Klebsiella. Clinically important and highly pathogenic one is known as E. cloacae. An anaerobic and yellow pigmented, E. agglomerans formerly called as Erwinia herbicola is encountered occasionally in clinical settings (Greenwood 2012). This genus of bacteria is facultative anaerobes and straight Gram-negative bacilli having an approximate size of 0.6–1 mm × 1.2–3 mm. They are motile by peritrichous flagella and are nonspore formers. Bacteria are having capsules for protection from unfavorable conditions. Enterobacter colonies are pigmented or nonpigmented. Biochemical features of bacteria include mannitol fermenter, Voges-Proskauer test positive, methyl red test negative, ornithine positive, and citrate positive. They can grow on Moller’s potassium cyanide medium at 30 °C and are lysine decarboxylase negative, gelatin test positive, indole negative, and oxidase test negative. Some of the species exhibit different biochemical properties. E. agglomerans do not deaminate phenylalanine and are ornithine decarboxylase negative. Also, they cannot produce hydrogen sulfide in triple sugar iron agar. Gelatin liquefaction test, indole test, oxidase test, and lysine decarboxylase test are some of the different tests for E. aerogenes and E. gergoviae (Kus 2014).
Enterobacter spp. belongs to the family Enterobacteriaceae. These are ubiquitous in the environment and are able to survive on dry and skin surfaces. This genus was recognized as pathogens after a nationwide septicemia outbreak at 25 hospitals in 1976 from unsterile intravenous solutions. These bacteria can cause sporadic outbreak owing to their ability to divide in the glucose-containing parental fluids (Maki et al. 1976). Several outbreaks of enterobacterial infections have been reported due to the use of unsterile humidifiers, respiratory therapy equipment, hydrotherapy water in burn unit, and enteral feedings. This genus causes a wide variety of nosocomial infections (infections of lung, urinary tract, abdominal cavity, and intravascular devices). E. sakazakii is one of the bacteria that causes neonatal sepsis along with meningitis (Nazarowec-White and Farber 1997; Bar-Oz et al. 2007). Like other enteric Gram-negative bacilli, this genera is gifted with several factors such as siderophores, endotoxins, and adhesions capable of initiating pathogenesis. This genus can be readily separated from other members of Enterobacteriaceae family due to the ease in the isolation from the clinical specimens (Patel and Patel 2016).
11.2 Enterobacter Infections
These bacteria inhabit as commensal bacteria in the human gastrointestinal tract and in the environment including soil, water, sewage, and plants. Historically, this genus was categorized as mild pathogens causing low threat to humans. Now, Enterobacter spp. acquired attention as opportunistic pathogens causing infections in immunocompromised patients within the clinics. E. cloacae are responsible for most of the nosocomial infections in human beings and known as the most medically significant species (Davin-Regli and Pagès 2015). Enterobacter species are associated with a wide variety of clinical infections such as lower respiratory tract infections, urinary tract infections, bacteremia, skin and soft tissue infections, endocarditis, central nervous system infections, bone and joint infections, and gastrointestinal tract infections. In case of animals, these species are rarely linked with pneumonia, peritonitis, intravenous catheter infections, wound infections, dermatitis, otitis media infections, and urinary tract infections (Weese 2008). The arrival of antibiotic resistance profiles among this genus is of great threat in global health care system, which affects both humans and companion animals. The failure of antibiotic therapy emerges with new risks in humans and animals. This may further end up with several significant public health consequences. Hence, alternatives strategies for the elimination of antimicrobial-resistant strains among Enterobacter spp. are important from the view of veterinary medicine and public health care system (Harada et al. 2017; Weese 2008).
This genus has been associated with several clinical syndromes and occasionally some of the syndromes mimics with the disease patterns of easily treatable pathogens such as Staphylococcus aureus and group A Streptococci. In recent times, high rates of coinfections with other pathogens are detected in the liver and lung transplant areas with Enterobacter infections. This includes the growing dominance in a variety of clinical syndromes and their etiologic part in cotton fever (Sanders and Sanders 1997). Overall, infections by Enterobacter spp. are mostly similar to those by other facultative Gram-negative bacilli. A broad range of infections of this genus include bacteremia, infections of urinary tract, lower respiratory tract, central nervous system, skin, soft tissue, bone, gastrointestinal tract, and other organs (Fig. 11.2). Recently, an Enterobacter spp., E. bugandensis is isolated from neonates and immunocompromised patients with sepsis. This is known as one of the newly isolated and highly pathogenic species of Enterobacter (Pati et al. 2018).
11.2.1 Enterobacter bacteremia
Often Enterobacter infections are complicated by their resistance to antibiotics of choice such as cephalosporins. In a study, 36% Enterobacter infections in intensive care units (ICUs) showed resistance to broad-spectrum cephalosporins. During the course of antibiotic therapy, these bacteria are adapted to the drugs through the increased production of beta-lactamase. Generally, cephalosporin-resistant Enterobacter bacteremia is significantly higher than those associated with the susceptible bacteremia. Species associated with Enterobacter bacteremia are E. aerogenes, E. agglomerans, E. cloacae, E. asburiae, E. sakazakii, E. amnigenus, E. gergoviae, and E. hormaechei (Kang et al. 2004). In all cases, bacteremia occurs in debilitated patients like those who had recently hospitalized, or received corticosteroid therapy, or previously received antibiotics or admitted to ICU. The mortality rate associated with Enterobacter bacteremia was 20 and 24% at 14 and 28 days after the diagnosis of bacteremia (Blot et al. 2003). Combination therapy is suggested for preventing the emergence of Enterobacter resistant strains. Combination therapy includes the administration of beta-lactam drugs other than cephalosporins and aminoglycosides. Combination therapy is more effective than monotherapy as the organism will become resistant to only one of the two drugs given and remain susceptible to other drugs. Even, more sensible use of third-generation cephalosporins may reduce the prevalence of nosocomial multidrug-resistant bacteremia associated with Enterobacter spp. (Chow et al. 1991).
There are several risk factors involved in bacteremia such as malignancies, prematurity, gastrointestinal disease, life-threatening infections, use of a ventriculoperitoneal shunt catheter, parenteral nutrition, immunosuppressive therapy, use of ventriculostomy, and prolonged antibiotic therapy (Andresen et al. 1994). A survey in the USA reported that 3.9% of all nosocomial bloodstream infections are caused by E. cloacae (Wisplinghoff et al. 2004). Around 5–6% of bacteremia is contributed by Enterobacter bacteremia and also known for 8.7% of neonatal sepsis. This bacteremia is severe in children younger than 18 months. The predisposing factors of deaths associated with neonatal bacteremia are prematurity, respiratory problems, and leukocytosis (Chen et al. 2014). Generally, 56–100% of bacteremia is developed institutionally. The most common species associated with bacteremia in decreasing order are E. cloacae (46–91% of isolates), E. aerogenes (9–43%), E. agglomerans, E. sakazakii, and others. Some of the infections are contributed by Enterobacter spp. that are polymicrobial (14–53%) (Sanders and Sanders 1997).
11.2.2 Lower Respiratory Tract Infections
Like Enterobacter spp. are common cause of bacteremia, most of these species are implicated in lower respiratory tract infections. They involved in lung abscess, pneumonia, emphysema, asymptomatic colonization in respiratory secretions, and purulent bronchitis (John et al. 1982). These species surpassed Klebsiella spp. and known as the third most cause of nosocomial respiratory tract infections in the USA. It is recognized as a cause of community-acquired pneumonia (Pareja et al. 1992). In last decades, prevalence of lower respiratory tract infections by Enterobacter spp. increased continuously. Statistics suggested that only 2–9% cases of respiratory tract infections were reported in 1970s. This rate was increased from 9.5% in the 1980s to 11% in 1990 (Jarvis and Martone 1992). There was a prevalence of Enterobacter infections in lung transplant recipients. It is reported that around 40% of lung transplant patients developed acute bacterial pneumonia exactly following the transplantation. Manifestations of clinical pneumonia caused by other Gram-negative bacilli differ from those caused by Enterobacter spp. (Sanders and Sanders 1997).
11.2.3 Endocarditis
Infective endocarditis is usually caused by Gram-positive bacteria and accounts for high risk of mortality in renal failure patients. Occasionally Gram-negative bacteria are encountered as one of the etiologic agents. Enterobacter is known to be a rare etiology of endocarditis. In a meta analyis of 2761 patients, two were confirmed with Enterobacter endocarditis (Karasahin et al. 2018). Another case series reviewed 37.7% mortality rate with Enterobacter. In a study, Enterobacter endocarditis was treated through monotherapy using carbapenem (Moon et al. 2012). Generally vancomycin along with meropenem is administered for critically ill patients and has risk factors for ESBL producing Enterobacter spp. (Gould et al. 2012). A case report showed that multidrug-resistant E. cloacae can be another possible pathogen of infective endocarditis and treated by continuous administration of beta-lactam drug and aminoglycoside (Yoshino et al. 2015).
11.2.4 Urinary Tract Infections
E. cloacae are one of the chief pathogens involved in urinary tract infection. Extended-spectrum beta-lactamase (ESBL) producing Enterobacter spp. are major in hospital-acquired urinary tract pathogens. One of the effective antibiotics against ESBL producing strains were carbapenems but the expression of carbapenemase provided resistance to all beta-lactam drugs (Xu and He 2019). In a case report, New Delhi metallo-β-lactamase 1 (NDM-1) producing E. aerogenes was reported to cause UTI after the insertion of JJ ureteric stents in areas of Croatia and Europe. Urinary pathology and even urosepsis are the most common complications developed after JJ stent insertion due to the antibiotic resistance determinants of bacteria (Franolić et al. 2019). Pathogens enter the lower urinary tract, urethra, and then spread to upper urinary tract. Sometimes, nosocomial UTI is acquired through bloodstream infections. Incidence of UTI is 50 times higher in women than men (Lipsky et al. 1980). Another resistant UTI causing species that express AmpC beta-lactamase is E. cloacae. They confer resistance to a wide range of antibiotics such as cephalosporins, penicillins, clavulanic acid, and quinolones (Pallett and Hand 2010). The known risk factors associated with ESBL producing UTI are recent hospitalization, presence of comorbidities, bladder catheterization, and prolonged stay in health care facility (Vardi et al. 2012).
11.3 Versatile Enterobacter spp. Challenging Antibiotic Treatment
Vast majority of Enterobacter infections in humans are caused by four important versatile species such as E. cloacae, E. aerogenes, E. agglomerans (P. agglomerans), and E. sakazakii. These bacteria are lactose fermenters, motile, and form mucoid colonies. These strains arise from endogenous intestinal flora of hospitalized patients and occur as cause of common source outbreaks or transmit from patient to patient. Especially those patients who had undergone antibiotic therapy and those admitted in intensive care units encounters more infections. These species cause variety of nosocomial infections (UTIs, pneumonia, wound, and burn infections, infections of intravascular devices, meningitis, and prosthetic devices) (Sanders and Sanders 1997). Among these, meningitis is primarily observed in neonates associated with contaminated powdered milk products (Bowen and Braden 2006).
E. aerogenes is isolated from clinical samples of urinary, blood, respiratory, or gastrointestinal tract. Epidemiology of these species was common in nosocomial infection outbreaks in Western Europe since 1993. E. aerogenes was regarded as a significant multidrug-resistant pathogen in intensive care units till 2003. International spread and transmission of extended-spectrum beta-lactam carrying epidemic plasmid in Europe resulted in Enterobacter infections in European hospitals and health care facilities. Also, antibiotic therapy using extended-spectrum cephalosporins and carbapenems caused an increase in infections caused by them in clinical wards. As a consequence of this therapy, pandrug-resistant E. aerogenes strains resistant to last line of drugs (colistin and carbapenems) are emerged. Identification of efflux pump mechanism in drug-resistant E. aerogenes highlighted the new methods in adaptive evolution of bacteria (Davin-Regli and Pagès 2015) (Fig. 11.3).
11.3.1 Enterobacter cloacae
E. cloacae are Gram-negative facultative bacteria widely found as saprophytes (sewage and soil) in nature. They are also found as commensals in human gastrointestinal tract. These are known as one of the important nosocomial pathogens causing UTIs, wound infections, sepsis in ICUs, and pneumonia with clinical significance (Wang et al. 2018). It is a known clinically significant species in Enterobacter genus owing to the presence of several antibiotic-resistant genes (Liu et al. 2013). This situation has become worse as there is a need for novel therapeutic drug discovery for broad-spectrum antibiotic-resistant E. cloacae (resistant to quinolones, carbapenems, and aztreonam). There are reports of combating E. cloacae ceftazidime-resistant and cefotaxime-susceptible strains with a triple combination of colistin with amikacin and cefepime (Lima et al. 2017). This bacterium has also been isolated from rice, meat, vegetables, and food processing plants. They lead to food spoilage and food safety problems due to the production of putrescine and cadaverine (Liu et al. 2018).
Various virulence-associated genes are present in the pathogenic islands and codes for type IV or III secretion system that is acquired by horizontal gene transfer. Different antagonistic mechanisms present in E. cloacae allow them to endure in diverse environments (Liu et al. 2013). A case study reported these bacteria as unusual cause of necrosis of nasal mucosa. These species are intrinsically resistant to amoxicillin, ampicillin, and cephalosporins. Many studies revealed that the disproportionate use of broad-spectrum drugs (e.g., cephalosporins) developed Enterobacter sp. as significant nosocomial pathogens (Binar et al. 2015). Monotherapy or combination therapy using aminoglycosides was suggested for treatment of carbapenem or beta-lactam drug-resistant E. cloacae. Later, draft genome analysis showed that bacteria acquired high-level aminoglycoside resistance through rmtD-2 gene (Martins et al. 2017). In an outbreak of bacteremia, E. cloacae were found to be the causative agent for 4.5% of all cases and remain endemic at medical center for 5 years (John et al. 1982). Infections caused by E. cloacae accounts for 5% hospital-acquired sepsis, 4% nosocomial pneumonia, 10% postsurgical peritonitis, and 4% nosocomial urinary tract pneumonia. Pathogenic mechanism in disease development includes the production of hemolysin, enterotoxins, and thiol activated pore-forming cytotoxins. Antibiotic-resistant biofilms are produced with the help of curli fimbriae. In general, these are widely found in nature but can also act as pathogens (Mezzatesta et al. 2012).
11.3.2 Enterobacter aerogenes
There is a greater concern associated with the nosocomial infections in immunocompromised individuals caused by Enterobacter sp. Among them, E. aerogenes and E. cloacae are the most important opportunistic pathogens, especially in patients on ventilation. E. aerogenes possess multiple resistance and virulence genes that contribute to increased pathogenesis. They produce extended-spectrum lactamases such as AmpC lactamase and have acquired resistance to cefoxitin, ampicillin, first-generation cephalosporins and amoxicillin (Azevedo et al. 2018). These bacteria were responsible for several outbreaks of nosocomial infections in Europe. Various redundant regulatory cascades present in bacteria efficiently allow the bacterial dissemination through control of membrane permeability during bacterial protection and expresses several detoxifying enzymes that codes for antibiotic resistance. Different factors involved in conferring antibiotic resistance include activation of OmpX porin membrane protein and other drug transporters such as AcrAB-TolC system, MacA, MdfA, OqxAB, Mar, Ram, Sox, and EmrE (Davin-Regli and Pagès 2015). The improved resistance toward broad-spectrum antibiotics was linked with the alterations in outer membrane that resulted in porin decrease and modification in the lipopolysaccharide components. To circumvent the emergence of beta-lactam-resistant strains, combination therapy of imipenem and colistin are suggested (Thiolas et al. 2005). Another mechanism involved in multiple drug resistance (MDR) is the overexpression of efflux pumps. These efflux pumps exclude the antibiotics before they reach to their target. Two efflux pumps, such as ABC transporter- and proton motive force-dependent types, were reported as active in MDR strains of E. aerogenes (Martins et al. 2010). E. aerogenes is known as the fourth most regularly isolated bacteria from hospital. This is due to the emergence of extended-spectrum cephalosporins and carbapenems. After that, there was an emergence of pan drug-resistant E. aerogenes isolates, which are resistant to last line of drugs. It has been studied that around 40% of the MDR strains possess active efflux pump systems (McCusker et al. 2019).
11.3.3 Enterobacter sakazakii
E. sakazakii is a known food-borne pathogen causing necrotizing enterocolitis, bacteremia, and meningitis in preterm and full-term immunocompromised infants (Hu et al. 2013). Now, E. sakazakii is called as Cronobacter sakazakii and in 2002, International Commission on Microbiological Specification for Foods classified this species as a severe threat for restricted populations. It is associated with life-threatening food-borne disease in infants where powdered infant formula acts as source of infection (Akineden et al. 2017). This organism was first characterized as yellow-pigmented coliform causing septicemia in infants. Antibiotics effective against this pathogen are acyl ureidopenicillins, carbapenems, aminoglycosides, aztreonam, antifolates, cephalosporins, chloramphenicol, quinolones, nitrofurantoin, and tetracyclines. They show resistance to benzylpenicillin oxacillin, some macrolides, and clindamycin (Abdesselam and Pagotto 2014). Fatal rate of E. sakazakii infection in infants can range up to 80% (Shukla et al. 2018).
Severe reported outcomes of this bacterial infection are brain abscess, seizures, hydrocephalus, developmental delay, and death in 40–80% cases. People at greater risk rate are premature infants rather than mature infants, children, and adults (Bowen and Braden 2006). According to Center for Disease Control and Prevention in the USA, four to six infection cases related to these bacteria are reported per year. Furthermore, immunocompromised elderly patients have also been reported with infections (Lou et al. 2014). Virulence traits of bacteria were found through complete genome sequencing. These virulence factors associated with pathogenesis include hemolysin, plasminogen activator (cpa), and siderophore interacting protein. Other proteins found in bacteria such as outer membrane protein (Omp) A and X plays vital part in the adhesion and internalization to the cells. They are resistant to antibiotic treatment as E. sakazakii form biofilms (Holý et al. 2019).
11.3.4 Enterobacter agglomerans
E. agglomerans are now classified into a new taxon of Enterobacteriaceae family; Pantoea based numerical phonotypical analysis. These are isolated widely from humans (wounds, internal organs, urine, and blood), animals, plant parts, water, and seeds. Some of them cause stalk and leaf necrosis on onions and some develop galls on Wisteria japonica, Gypsophila paniculata, etc. (Gavini et al. 1989). P. agglomerans are known as obligate infectious agents of plants and opportunistic human pathogens. In humans, mostly, infection by P. agglomerans is acquired through wound infection by plant material or by nosocomial infection in immunocompromised individuals. Possible clinical outcomes of P. agglomerans infection include synovitis, septic arthritis, peritonitis, osteomyelitis, endophthalmitis, and endocarditis. Epidemics of nosocomial septicemia caused by P. agglomerans have been reported in both adult and pediatric patients. Generally, nosocomial infections were mild and proper antibiotic therapy led to complete recovery. Reports of infections by P. agglomerans in vertebrate animals are few compared to humans (Dutkiewicz et al. 2016). The pathogenic determinants of P. agglomerans are described as pathogenicity island containing plasmid (150 kb pPATH) (Barash and Manulis-Sasson 2009), large Pantoea Plasmid (De Maayer et al. 2012) and the type III secretion system (T3SS) (Nissan et al. 2006). These bacteria cause soft tissue or bone joint infections followed by the penetration of trauma by vegetation (Völksch et al. 2009).
E. agglomerans causes a variety of nosocomial infections such as UTIs, septic arthritis, intra-abdominal infections, ophthalmic infections, central nervous system (CNS) infections, bacteremia, endocarditis, lower respiratory tract infections, skin infections, and osteomyelitis in individuals associated with intravenous lines and immunocompromised patients. This bacterium is an unusual cause of spondylodiscitis, which is one of the manifestations of osteomyelitis. Only 31 suspected cases of E. agglomerans spondylodiscitis reported previously (Jayaweera et al. 2016). A report showed P. agglomerans as an infrequent cause of peritonitis in peritoneal dialysis patients (Sastre et al. 2017). It is also known to be an unknown cause of infections in children. Often, these bacteria in association with other conventional pathogens cause bacteremia with indwelling central access in children. P. agglomerans is suspected as an etiologic agent of penetrating trauma through vegetation or by soil coated objects that persist to be resistant to the conventional therapy (Cruz et al. 2007). The relative contribution of most common infections of P. agglomerans includes UTIs (21.4%), wound infections (35.7%), and pneumonia (21.4%). Infections by these bacteria may cause serious morbidity and mortality, especially in children with pneumonia. The drug-resistant patterns of community and hospital-acquired strains may vary with their different pathogenic and clinical features. Even though, 21.4% of the P. agglomerans isolates obtained from hospital setting showed resistance to carbapenem and caused infection (Büyükcam et al. 2018). One report showed nosocomial outbreaks of P. agglomerans in a tertiary care center associated with in-house prepared anticoagulant dextrose solution which was used for the priming of the plasmapheresis machine and for hemodialysis in acute care. Nosocomial outbreaks reported due to the contaminated parenteral nutrition, transference tubes (used for intravenous purposes), blood and blood products (Boszczowski et al. 2012).
11.4 Antimicrobial Resistance Mechanisms and Associated Factors
Enterobacter spp. among Enterobacteriaceae family are documented as a foremost pathogen in hospital-acquired pathogen and continuously involved in several nosocomial outbreaks worldwide. The emergence of MDR strains is found to be responsible for the life threatening and more expensive outbreaks, which constitute a greater threat to the infection control teams. Increase in the number of carbapenemase and extended-spectrum beta-lactamase (ESBL) producing Enterobacter spp. remain as a concern for the physicians and scientists (Noël et al. 2019).
11.4.1 Antibiotic Resistance and Mechanism
Major mechanisms involved in Enterobacter spp. antibiotic resistance are alteration in the target of drug, production of an inactivating enzyme and modulating the ability of the drug to enter the cells. Enterobacter spp. acquires resistance to beta-lactam antibiotics and aminoglycosides through the production of an inactivating enzyme, whereas resistance to quinolones and trimethoprim is acquired by altering drug targets and their ability to accumulate in the cell. Studies have shown that all species of Enterobacter possess chromosomally encoded Bush group type 1 beta-lactamases (Bush et al. 1995; Cohen et al. 1993; Conus and Francioli 1992). In certain strains of E. gergoviae, E. aerogenes, and E. sakazakii, lactamases are produced in very low and noninducible concentrations. These strains have greater sensitivity toward ampicillin, cefoxitin, and older cephalosporins. Mostly, uniform resistance pattern is observed in wild-type strains of E. sakazakii, E. cloacae, E. taylorae, E. asburiae, and E. aerogenes owing to the presence of Bush type 1 beta-lactamases (Pitout et al. 1997).
Resistance among wild-type strains arises mainly from great specificity of drug to this enzyme or from the drug that acting as inducer of enzyme. There is an ampD gene in Enterobacter spp. which on mutation causes the emergence of resistance to extended-spectrum cephalosporins, aztreonam, and broad-spectrum penicillins. Usually, this gene prevents high level expression of beta-lactamases. Hence, such a mutation to this gene has been referred as stable depression mutation. These stable depressant mutants are also resistant to beta-lactam inhibitor beta-lactam drug combinations. Among beta-lactam drugs, only carbapenems and newer expanded spectrum cephalosporins (cefepime) maintain their activity toward Enterobacter infections (Schaberg et al. 1991; Ehrhardt and Sanders 1993; Bonten et al. 1994). Wild-type strains may become resistant to broad-spectrum penicillins like piperacillin through the achievement of plasmids encoding Bush group 2 TEM1, TEM2 or SHV lactamases (Huovinen et al. 1989; Liu et al. 1992). Recently scientists reported chromosomally encoded carbapenemases in E. cloacae which exhibited carbapenem resistance. Like Bush group 1 lactamases, this enzyme can also be induced by cefoxitin and carbapenems. Enterobacter spp. with aminoglycoside resistance are thought to produce one or multiple aminoglycoside inactivating enzymes. Acetylating enzymes such as AAC (6′), AAC (3) II, AAC (3) I, AAC (3) V and AAC (3) III, and nucleotidylating enzymes such as ANT (2″) have been investigated in Enterobacter spp. which exhibits aminoglycoside resistance (Huovinen et al. 1989; Maes and Vanhoof 1992) (Table 11.1).
11.4.2 Extended-Spectrum Beta-Lactamases (ESBL) and AmpC Beta-Lactamases
Beta-lactamases are bacterial enzymes produced to cleave beta-lactam antibiotics which results in the generation of inactive molecules. ESBLs have the ability to inactivate oxyimino aminothiazolyl cephalosporins such as monobactam, aztreonam, ceftazidime, cefepime, and cefotaxime (Pitout and Laupland 2008). As they are resistant to extended-spectrum cephalosporins, ESBL producers are difficult to detect through zone diameters or MICs. Also, detection of ESBL producers is difficult due to the presence of inducible AmpC chromosomal enzymes. It has been shown that clavulanate induces AmpC beta-lactamases and hydrolyze the cephalosporins. Enterobacter spp. are resistant to third-generation (cefepime) and fourth-generation cephalosporins that act as poor substrates for beta-lactamases (Crowley and Ratcliffe 2003). Mostly, resistance to beta-lactam drugs is mediated by the hyperproduction of chromosomal AmpC beta-lactamase which resulted by induction or by selection of depressed mutant strains (Barnaud 2001). It has been reported a plasmid-mediated ESBL production among resistant Enterobacter spp. Most common beta-lactamases found in Enterobacter spp. belong to SHV-, CTX-M-, and TEM-derived lactamases. There are several other lactamases reported in different geographical areas. IBC-1 is one of the recently reported enzymes in E. cloacae in Greece. Another VEB-1 was reported in clinical samples containing E. cloacae and E. sakazakii in Bangkok and Thailand. SFO-1 reported in Japan and associated with E. cloacae (Schlesinger et al. 2005).
There are different classes of beta-lactamases based on their substrate and inhibitor specificity. Group 1 describes cephalosporinases which are not inhibited well by clavulanate. Group 2 describes enzymes such as penicillinase, broad-spectrum beta-lactamase, and cephalosporinase activity inhibited by beta-lactamase. Group 3 are metallo beta-lactamase hydrolyzing penicillins, carbapenems, and cephalosporins poorly inhibited by most of the beta-lactamase inhibitors (Bush 2013). Ambler class C (Bush-Jacoby group 1) enzymes do not belong to the ESBL-type enzymes but hydrolyze third-generation cephalosporins. Several Gram-negative bacteria possess chromosomally located genes coding AmpC and have been recognized in 1960s. In some species AmpC gene is chromosomally located and is intrinsic, especially in E. aerogenes and E. cloacae. This is due to the inducible expression of AmpC gene controlled by transcription factors in those species (Corvec et al. 2007; Macdougall 2011; Harris and Ferguson 2012). Some species are found with AmpC gene located on plasmids which can transfer between species. Plasmid-mediated AmpC gene is found in clinical species associated with community onset, nosocomial and health care-related infections. There are ESBL producing strains with plasmid-mediated AmpC gene that are frequently resistant to quinolones or trimethoprim sulfamethoxazole. These plasmid-mediated AmpC genes are not inducible in nature but there are reports of plasmid-mediated inducible AmpC gene transmitting into new hosts (Alvarez et al. 2004).
Further, there are no reports describing the superior action of any antimicrobial agent over carbapenems to combat or treat infections by ESBL or AmpC producers. Overuse of carbapenem has raised a new alarming threat of carbapenem-resistant strains that developing on a global scale. Beta-lactam/beta-lactamase inhibitors (BLBIs) such as piperacillin and tazobactam can be suggested for treatment of ESBL producers. But theoretical studies limited the use of piperacillin against the AmpC producers. For urinary infections caused by ESBL producers, amoxicillin–clavulanate is the choice of treatment. Some uncommon antibiotics such as temocillin, pivmecillinam, and fosfomycin are used to treat less critical infections (Harris 2015). A study reported intrinsic chromosomal resistance of E. cloacae to first-generation cephalosporins, penicillins, cephamycins, and beta-lactam or lactamase inhibitors due to the AmpC gene. One of the reasons for the resistance to cephalosporins by Enterobacter spp. is due to the overexpression of beta-lactamases. This class I beta-lactamases are encoded by chromosomal AmpC gene (Uzunović et al. 2018).
11.5 Conclusion and Future Perspectives
Recently, Enterobacter spp. are considered as significant clinical pathogens causing nosocomial infections. Most of the species associated with Enterobacter infections are innately resistant to older antibiotics. They have the ability to develop resistance to newer antibiotics also. Multiple drug-resistant strains are emerged in hospitals using beta-lactam drugs and cephalosporins. They have high prevalence of nosocomial infections in neonates and immunocompromised individuals. Among all isolates, E. aerogenes and E. cloacae are the most versatile opportunistic pathogens associated with nosocomial outbreaks. The saga of Enterobacter infections is associated with a logarithmic increase in the expression of beta-lactamases and extended-spectrum cephalosporinases. There are many fundamental questions to be answered for understanding the pathogenic mechanisms relevant to clinical infections caused by Enterobacter spp. that are different from other Gram-negative enteric bacilli. Strategies should be developed to suppress the expression of multiple drug-resistant strains and to minimize new emergence of resistance.
References
Abdesselam K, Pagotto F (2014) Bacteria: Cronobacter (Enterobacter) sakazakii and other Cronobacter spp. In: Encyclopedia of food safety. Elsevier, Cambridge, pp 424–432
Akineden Ö, Heinrich V, Gross M, Usleber E (2017) Reassessment of Cronobacter spp. originally isolated as Enterobacter sakazakii from infant food. Food Microbiol 65:44–50. https://doi.org/10.1016/j.fm.2017.01.021
Alvarez M, Tran JH, Chow N, Jacoby GA (2004) Epidemiology of conjugative plasmid-mediated AmpC β-lactamases in the United States. Antimicrob Agents Chemother 48:533–537. https://doi.org/10.1128/AAC.48.2.533-537.2004
Andresen J, Asmar BI, Dajani AS (1994) Increasing Enterobacter bacteremia in pediatric patients. Pediatr Infect Dis J 13:787–791. https://doi.org/10.1097/00006454-199409000-00007
Annavajhala MK, Gomez-Simmonds A, Uhlemann A-C (2019) Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front Microbiol 10:1–8. https://doi.org/10.3389/fmicb.2019.00044
Azevedo PAA, Furlan JPR, Oliveira-Silva M, Nakamura-Silva R, Gomes CN, Costa KRC, Stehling EG, Pitondo-Silva A (2018) Detection of virulence and β-lactamase encoding genes in Enterobacter aerogenes and Enterobacter cloacae clinical isolates from Brazil. Brazilian. J Microbiol 49:224–228. https://doi.org/10.1016/j.bjm.2018.04.009
Barash I, Manulis-Sasson S (2009) Recent evolution of bacterial pathogens: the gall-forming Pantoea agglomerans case. Annu Rev Phytopathol 47:133–152. https://doi.org/10.1146/annurev-phyto-080508-081803
Barnaud G (2001) Extension of resistance to cefepime and cefpirome associated to a six amino acid deletion in the H-10 helix of the cephalosporinase of an Enterobacter cloacae clinical isolate. FEMS Microbiol Lett 195:185–190. https://doi.org/10.1016/S0378-1097(01)00010-6
Bar-Oz B, Preminger A, Peleg O, Block C, Arad I (2007) Enterobacter sakazakii infection in the newborn. Acta Paediatr 90:356–358. https://doi.org/10.1111/j.1651-2227.2001.tb00319.x
Binar M, Arslan F, Tasli H, Karakoc O, Kilic A, Aydin U (2015) An unusual cause of necrosis and nasal septum perforation after septoplasty: Enterobacter cloacae. New Microbes New Infect 8:150–153. https://doi.org/10.1016/j.nmni.2015.07.002
Blot SI, Vandewoude KH, Colardyn FA (2003) Evaluation of outcome in critically ill patients with nosocomial Enterobacter bacteremia: results of a matched cohort study. Chest 123:1208–1213. https://doi.org/10.1378/chest.123.4.1208
Bonten MJ, Gaillard CA, van Tiel FH, Smeets HG, van der Geest S, Stobberingh EE (1994) The stomach is not a source for colonization of the upper respiratory tract and pneumonia in ICU patients. Chest 105:878–884. https://doi.org/10.1378/chest.105.3.878
Boszczowski I, Nóbrega de Almeida Júnior J, Peixoto de Miranda EJ, Pinheiro Freire M, Guimarães T, Chaves CE, Cais DP, Strabelli TM, Risek CF, Soares RE, Rossi F, Costa SF, Levin AS (2012) Nosocomial outbreak of Pantoea agglomerans bacteremia associated with contaminated anticoagulant citrate dextrose solution: new name, old bug? J Hosp Infect 80:255–258. https://doi.org/10.1016/j.jhin.2011.12.006
Bowen AB, Braden CR (2006) Invasive Enterobacter sakazakii disease in infants. Emerg Infect Dis 12:1185–1189. https://doi.org/10.3201/eid1208.051509
Bush K (2013) Proliferation and significance of clinically relevant β-lactamases. Ann N Y Acad Sci 1277:84–90. https://doi.org/10.1111/nyas.12023
Bush K, Jacoby GA, Medeiros AA (1995) A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother 39:1211–1233. https://doi.org/10.1128/AAC.39.6.1211
Büyükcam A, Tuncer Ö, Gür D, Sancak B, Ceyhan M, Cengiz AB, Kara A (2018) Clinical and microbiological characteristics of Pantoea agglomerans infection in children. J Infect Public Health 11:304–309. https://doi.org/10.1016/j.jiph.2017.07.020
Chen HL, Lu JH, Wang HH, Chen SJ, Chen CJ, Wu KG, Tang RB (2014) Clinical analysis of Enterobacter bacteremia in pediatric patients: a 10-year study. J Microbiol Immunol Infect 47:381–386. https://doi.org/10.1016/j.jmii.2013.03.016
Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, Ramphal R, Wagener MM, Miyashiro DK, Yu VL (1991) Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med 115:585–590
Cohen SP, Yan W, Levy SB (1993) A multidrug resistance regulatory chromosomal locus is widespread among enteric bacteria. J Infect Dis 168:484–488. https://doi.org/10.1093/infdis/168.2.484
Conus P, Francioli P (1992) Relationship between ceftriaxone use and resistance of Enterobacter species. J Clin Pharm Ther 17:303–305. https://doi.org/10.1111/j.1365-2710.1992.tb01308.x
Corvec S, Prodhomme A, Giraudeau C, Dauvergne S, Reynaud A, Caroff N (2007) Most Escherichia coli strains overproducing chromosomal AmpC beta-lactamase belong to phylogenetic group A. J Antimicrob Chemother 60:872–876. https://doi.org/10.1093/jac/dkm284
Cosgrove SE, Kaye KS, Eliopoulous GM, Carmeli Y (2002) Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch Intern Med 162:185–190
Crowley B, Ratcliffe G (2003) Extended-spectrum β-lactamases in Enterobacter cloacae: underestimated but clincially significant. J Antimicrob Chemother 51:1316–1317
Cruz AT, Cazacu AC, Allen CH (2007) Pantoea agglomerans, a plant pathogen causing human disease. J Clin Microbiol 45:1989–1992. https://doi.org/10.1128/JCM.00632-07
Davin-Regli A, Pagès JM (2015) Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol 6:1–10. https://doi.org/10.3389/fmicb.2015.00392
De Maayer P, Chan WY, Blom J, Venter SN, Duffy B, Smits TH, Coutinho TA (2012) The large universal Pantoea plasmid LPP-1 plays a major role in biological and ecological diversification. BMC Genomics 13:625. https://doi.org/10.1186/1471-2164-13-625
Dutkiewicz J, Mackiewicz B, Kinga Lemieszek M, Golec M, Milanowski J (2016) Pantoea agglomerans: a mysterious bacterium of evil and good. Part III. Deleterious effects: infections of humans, animals and plants. Ann Agric Environ Med 23:197–205. https://doi.org/10.5604/12321966.1203878
Ehrhardt AF, Sanders CC (1993) Lactam resistance amongst Enterobacter species. J Antimicrob Chemother 32:1–11. https://doi.org/10.1093/jac/32.suppl_B.1
Franolić I, Bedenić B, Beader N, Lukić-Grlić A, Mihaljević S, Bielen L, Zarfel G, Meštrović T (2019) NDM-1-producing Enterobacter aerogenes isolated from a patient with a JJ ureteric stent in situ. CEN Case Rep 8:38–41. https://doi.org/10.1007/s13730-018-0360-z
Gavini F, Mergaert J, Beji A, Mielcarek C, Izard D, Kersters K, De Ley J (1989) Transfer of Enterobacter agglomerans (Beijerinck 1888) ewing and fife 1972 to Pantoea gen. nov. as Pantoea agglomerans comb. nov. and description of Pantoea dispersa sp. nov. Int J Syst Bacteriol 39:337–345. https://doi.org/10.1099/00207713-39-3-337
Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW (2012) Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 67:269–289. https://doi.org/10.1093/jac/dkr450
Greenwood D (2012) Medical microbiology, a guide to microbial infections: pathogenesis, immunity, laboratory investigation and control. Churchill Livingstone, London, p 795
Grimont F, Grimont PAD (2006) The genus Enterobacter. In: The prokaryotes. Springer, New York, pp 197–214
Harada K, Shimizu T, Mukai Y, Kuwajima K, Sato T, Kajino A, Usui M, Tamura Y, Kimura Y, Miyamoto T, Tsuyuki Y, Ohki A, Kataoka Y (2017) Phenotypic and molecular characterization of antimicrobial resistance in Enterobacter spp. isolates from companion animals in Japan. PLoS One 12:e0174178. https://doi.org/10.1371/journal.pone.0174178
Harris P (2015) Clinical management of infections caused by Enterobacteriaceae that express extended-spectrum β-lactamase and AmpC enzymes. Semin Respir Crit Care Med 36:056–073. https://doi.org/10.1055/s-0034-1398387
Harris PNA, Ferguson JK (2012) Antibiotic therapy for inducible AmpC β-lactamase-producing gram-negative bacilli: what are the alternatives to carbapenems, quinolones and aminoglycosides? Int J Antimicrob Agents 40:297–305. https://doi.org/10.1016/j.ijantimicag.2012.06.004
Holý O, Cruz-Córdova A, Xicohtencatl-Cortes J, Hochel I, Parra-Flores J, Petrželová J, Fačevicová K, Forsythe S, Alsonosi A (2019) Occurrence of virulence factors in Cronobacter sakazakii and Cronobacter malonaticus originated from clinical samples. Microb Pathog 127:250–256. https://doi.org/10.1016/j.micpath.2018.12.011
Hu X, Dou W, Fu L, Zhao G (2013) A disposable immunosensor for Enterobacter sakazakii based on an electrochemically reduced graphene oxide-modified electrode. Anal Biochem 434:218–220. https://doi.org/10.1016/j.ab.2012.11.013
Huovinen S, Klossner M, Katila M, Huovinen P (1989) Plasmid-mediated beta-lactamases among aminoglycoside resistant gram-negative bacilli. Scand J Infect Dis 21:303–309. https://doi.org/10.3109/00365548909035700
Jarvis WR, Martone WJ (1992) Predominant pathogens in hospital infections. J Antimicrob Chemother 29:19–24. https://doi.org/10.1093/jac/29.suppl_A.19
Jayaweera JAAS, Kothalawala M, Devakanthan B, Arunan S, Galgamuwa D, Rathnayake M (2016) Spondylodiscitis caused by Enterobacter agglomerans. Case Rep Infect Dis 2016:1–4. https://doi.org/10.1155/2016/8491571
John JF, Sharbaugh RJ, Bannister ER (1982) Enterobacter cloacae: Bacteremia, epidemiology, and antibiotic resistance. Rev Infect Dis 4:13–28. https://doi.org/10.1093/clinids/4.1.13
Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Oh MD, Kim EC, Choe KW (2004) Bloodstream infections caused by Enterobacter species: predictors of 30-day mortality rate and impact of broad-Spectrum cephalosporin resistance on outcome. Clin Infect Dis 39:812–818. https://doi.org/10.1086/423382
Karasahin O, Yildiz Z, Unal O, Arslan U (2018) A rare cause of healthcare-associated infective endocarditis: Enterobacter cloacae. IDCases 12:18–20. https://doi.org/10.1016/j.idcr.2018.03.004
Khajuria A, Praharaj AK, Kumar M, Grover N (2014) Carbapenem resistance among Enterobacter species in a tertiary care hospital in Central India. Chemother Res Pract 2014:1–6. https://doi.org/10.1155/2014/972646
Kus JV (2014) Infections due to Citrobacter and Enterobacter. In: Reference module in biomedical sciences. Elsevier, Amsterdam, pp 1–12
Leverstein-van Hall MA, Blok HEM, Donders R et al (2003) Multidrug resistance among Enterobacteriaceae is strongly associated with the presence of integrons and is independent of species or isolate origin. J Infect Dis 187:251–259. https://doi.org/10.1086/345880
Lima TB, Silva ON, de Almeida KC, Ribeiro SM, Motta DO, Maria-Neto S, Lara MB, Filho CR, Ombredane AS, de Faria Junior C, Parachin NS, Magalhães BS, Franco OL (2017) Antibiotic combinations for controlling colistin-resistant Enterobacter cloacae. J Antibiot (Tokyo) 70:122–129. https://doi.org/10.1038/ja.2016.77
Lipsky BA, Hook EW, Smith AA, Plorde JJ (1980) Citrobacter infections in humans: experience at the Seattle veterans administration medical center and a review of the literature. Clin Infect Dis 2:746–760. https://doi.org/10.1093/clinids/2.5.746
Liu F, Wang F, Du L, Zhao T, Doyle MP, Wang D, Zhang W, Suna Z, Xu W (2018) Antibacterial and antibiofilm activity of phenyllactic acid against Enterobacter cloacae. Food Control 84:442–448. https://doi.org/10.1016/j.foodcont.2017.09.004
Liu PYF, Gur D, Hall LMC, Livermore DM (1992) Survey of the prevalence of βlactamases amongst 1000 gram-negative bacilli isolated consecutively at the Royal London Hospital. J Antimicrob Chemother 30:429–447. https://doi.org/10.1093/jac/30.4.429
Liu WY, Wong CF, Chung KM, Jiang JW, Leung FC (2013) Comparative genome analysis of Enterobacter cloacae. PLoS One 8:e74487. https://doi.org/10.1371/journal.pone.0074487
Lou X, Si G, Yu H, Qi J, LiuT FZ (2014) Possible reservoir and routes of transmission of Cronobacter (Enterobacter sakazakii) via wheat flour. Food Control 43:258–262. https://doi.org/10.1016/j.foodcont.2014.03.029
Macdougall C (2011) Beyond susceptible and resistant, part I: treatment of infections due to gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther 16:23–30
Maes P, Vanhoof R (1992) A 56-month prospective surveillance study on the epidemiology of aminoglycoside resistance in a belgian general hospital. Scand J Infect Dis 24:495–501. https://doi.org/10.3109/00365549209052636
Maki DG, Rhame FS, Mackel DC, Bennett JV (1976) Nationwide epidemic of septicemia caused by contaminated intravenous products. Am J Med 60:471–485. https://doi.org/10.1016/0002-9343(76)90713-0
Marchaim D, Navon-Venezia S, Schwaber MJ, Carmeli Y (2008) Isolation of imipenem-resistant Enterobacter species: emergence of KPC-2 carbapenemase, molecular characterization, epidemiology, and outcomes. Antimicrob Agents Chemother 52:1413–1418. https://doi.org/10.1128/AAC.01103-07
Martins A, Spengler G, Martins M, Rodrigues L, Viveiros M, Davin-Regli A, Chevalier J, Couto I, Pagès JM, Amaral L (2010) Physiological characterisation of the efflux pump system of antibiotic-susceptible and multidrug-resistant Enterobacter aerogenes. Int J Antimicrob Agents 36:313–318. https://doi.org/10.1016/j.ijantimicag.2010.06.036
Martins ER, Casella T, Bueno MFC, Francisco GR, Tolentino FM, de Freitas ACT, Cerdeira L, Costa MN, Cevada C, Lincopan N, Garcia DO, Nogueira MCL (2017) Draft genome sequence of an aminoglycoside-resistant RmtD2-producing Enterobacter cloacae subsp. cloacae ST395 in Brazil. J Glob Antimicrob Resist 10:308–309. https://doi.org/10.1016/j.jgar.2017.07.012
McCusker MP, Alves Ferreira D, Cooney D, Martins Alves B, Fanning S, Pagès JM, Martins M, Davin-Regli A (2019) Modulation of antimicrobial resistance in clinical isolates of Enterobacter aerogenes: a strategy combining antibiotics and chemosensitisers. J Glob Antimicrob Resist 16:187–198. https://doi.org/10.1016/j.jgar.2018.10.009
Mezzatesta ML, Gona F, Stefani S (2012) Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future Microbiol 7:887–902. https://doi.org/10.2217/fmb.12.61
Millar M, Philpott A, Wilks M, Whiley A, Warwick S, Hennessy E, Coen P, Kempley S, Stacey F, Costeloe K (2008) Colonization and persistence of antibiotic-resistant Enterobacteriaceae strains in infants nursed in two neonatal intensive care units in East London, United Kingdom. J Clin Microbiol 46:560–567. https://doi.org/10.1128/JCM.00832-07
Moon J, Smith T, Sahud AG, Bhanot N (2012) An unusual etiology of infective endocarditis: Enterobacter cloacae. J Infect Chemother 18:925–930. https://doi.org/10.1007/s10156-012-0376-9
Nazarowec-White M, Farber JM (1997) Enterobacter sakazakii: a review. Int J Food Microbiol 34:103–113. https://doi.org/10.1016/S0168-1605(96)01172-5
Nissan G, Manulis-Sasson S, Weinthal D, Mor H, Sessa G, Barash I (2006) The type III effectors HsvG and HsvB of gall-forming Pantoea agglomerans determine host specificity and function as transcriptional activators. Mol Microbiol 61:1118–1131. https://doi.org/10.1111/j.1365-2958.2006.05301.x
Noël A, Vastrade C, Dupont S, de Barsy M, Huang TD, Van Maerken T, Leroux-Roels I, Delaere B, Melly L, Rondelet B, Dransart C, Dincq AS, Michaux I, Bogaerts P, Glupczynski Y (2019) Nosocomial outbreak of extended-spectrum β-lactamase-producing Enterobacter cloacae among cardiothoracic surgical patients: causes and consequences. J Hosp Infect 102:54–60. https://doi.org/10.1016/j.jhin.2019.01.001
Pallett A, Hand K (2010) Complicated urinary tract infections: practical solutions for the treatment of multiresistant gram-negative bacteria. J Antimicrob Chemother 65:iii25–iii33. https://doi.org/10.1093/jac/dkq298
Pareja A, Bernal C, Leyva A, Piedrola G, Maroto MC (1992) Etiologic study of patients with community-acquired pneumonia. Chest 101:1207–1210. https://doi.org/10.1378/chest.101.5.1207
Patel KK, Patel S (2016) Enterobacter spp.: an emerging nosocomial infection. Conf Proc 2:532–538
Pati NB, Doijad SP, Schultze T, Mannala GK, Yao Y, Jaiswal S, Ryan D, Suar M, Gwozdzinski K, Bunk B, Mraheil MA, Marahiel MA, Hegemann JD, Spröer C, Goesmann A, Falgenhauer L, Hain T, Imirzalioglu C, Mshana SE, Overmann J, Chakraborty T (2018) Enterobacter bugandensis: a novel enterobacterial species associated with severe clinical infection. Sci Rep 8:5392. https://doi.org/10.1038/s41598-018-23069-z
Pitout JD, Laupland KB (2008) Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8:159–166
Pitout JD, Moland ES, Sanders CC, Thomson KS, Fitzsimmons SR (1997) Beta-lactamases and detection of beta-lactam resistance in Enterobacter spp. Antimicrob Agents Chemother 41:35–39
Sanders WE, Sanders CC (1997) Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin Microbiol Rev 10:220–241. https://doi.org/10.1128/CMR.10.2.220
Sastre A, González-Arregoces JE, Romainoik I, Mariño S, Lucas C, Monfá E, Stefan G, de León B, Prieto M (2017) Peritonitis causada por Pantoea agglomerans en diálisis peritoneal. Nefrologia 37:108–109. https://doi.org/10.1016/j.nefro.2016.09.006
Schaberg DR, Culver DH, Gaynes RP (1991) Major trends in the microbial etiology of nosocomial infection. Am J Med 91:S72–S75. https://doi.org/10.1016/0002-9343(91)90346-Y
Schlesinger J, Navon-Venezia S, Chmelnitsky I, Hammer-Münz O, Leavitt A, Gold HS, Schwaber MJ, Carmeli Y (2005) Extended-spectrum beta-lactamases among Enterobacter isolates obtained in Tel Aviv, Israel. Antimicrob Agents Chemother 49:1150–1156. https://doi.org/10.1128/AAC.49.3.1150-1156.2005
Shukla S, Haldorai Y, Bajpai VK, Rengaraj A, Hwang SK, Song X, Kim M, Huh YS, Han YK (2018) Electrochemical coupled immunosensing platform based on graphene oxide/gold nanocomposite for sensitive detection of Cronobacter sakazakii in powdered infant formula. Biosens Bioelectron 109:139–149. https://doi.org/10.1016/j.bios.2018.03.010
Singh NK, Bezdan D, Checinska Sielaff A, Wheeler K, Mason CE, Venkateswaran K (2018) Multi-drug resistant Enterobacter bugandensis species isolated from the international Space Station and comparative genomic analyses with human pathogenic strains. BMC Microbiol 18:175. https://doi.org/10.1186/s12866-018-1325-2
Thiolas A, Bollet C, La Scola B, Raoult D, Pagès JM (2005) Successive emergence of Enterobacter aerogenes strains resistant to imipenem and colistin in a patient. Antimicrob Agents Chemother 49:1354–1358. https://doi.org/10.1128/AAC.49.4.1354-1358.2005
Uzunović S, Ibrahimagić A, Bedenić B (2018) Antibiotic resistance in Enterobacter cloacae strains with derepressed/partly derepressed/inducible AmpC and extended-spectrum beta-lactamases in Zenica-Doboj Canton, Bosnia and Herzegovina. Med Glas 15:37–45. https://doi.org/10.17392/925-18
Vardi M, Kochavi T, Denekamp Y, Bitterman H (2012) Risk factors for urinary tract infection caused by enterobacteriaceae with extended-spectrum beta-lactamase resistance in patients admitted to internal medicine departments. Isr Med Assoc J 14:115–118
Völksch B, Thon S, Jacobsen ID, Gube M (2009) Polyphasic study of plant- and clinic-associated Pantoea agglomerans strains reveals indistinguishable virulence potential. Infect Genet Evol 9:1381–1391. https://doi.org/10.1016/j.meegid.2009.09.016
Wang YQ, Xiao D, Li J, Zhang HF, Fu BQ, Wang XL, Ai XM, Xiong YW, Zhang JZ, Ye CY (2018) Rapid identification and subtyping of Enterobacter cloacae clinical isolates using peptide mass fingerprinting. Biomed Environ Sci 31:48–56. https://doi.org/10.3967/bes2018.005
Weese JS (2008) Investigation of Enterobacter cloacae infections at a small animal veterinary teaching hospital. Vet Microbiol 130:426–428. https://doi.org/10.1016/j.vetmic.2008.02.009
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. https://doi.org/10.1086/421946
Xu J, He F (2019) Genomic analysis of two bacterial strains co-isolated from a urinary tract infection: NDM-1-producing Enterobacter cloacae accompanied by extended-spectrum β-lactamase-producing Escherichia coli. J Glob Antimicrob Resist 17:198–200. https://doi.org/10.1016/j.jgar.2019.04.007
Ye Y, Li JB, Ye DQ, Jiang ZJ (2006) Enterobacter bacteremia: clinical features, risk factors for multiresistance and mortality in a Chinese University Hospital. Infection 34:252–257. https://doi.org/10.1007/s15010-006-5038-3
Yoshino Y, Okugawa S, Kimura S, Makita E, Seo K, Koga I, Matsunaga N, Kitazawa T, Ota Y (2015) Infective endocarditis due to Enterobacter cloacae resistant to third- and fourth-generation cephalosporins. J Microbiol Immunol Infect 48:226–228. https://doi.org/10.1016/j.jmii.2012.07.015
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Anju, V.T., Siddhardha, B., Dyavaiah, M. (2020). Enterobacter Infections and Antimicrobial Drug Resistance. In: Siddhardha, B., Dyavaiah, M., Syed, A. (eds) Model Organisms for Microbial Pathogenesis, Biofilm Formation and Antimicrobial Drug Discovery. Springer, Singapore. https://doi.org/10.1007/978-981-15-1695-5_11
Download citation
DOI: https://doi.org/10.1007/978-981-15-1695-5_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1694-8
Online ISBN: 978-981-15-1695-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)