Abstract
Heart failure is the end result of a variety of cardiovascular disease states. Heart failure remains a challenge to treat, and the incidence continues to rise with an aging population, and increasing rates of diabetes and obesity. Non-coding RNAs, once considered as “junk DNA”, have emerged as powerful transcriptional regulators and potential therapeutic targets for the treatment of heart failure. Different classes of non-coding RNAs exist, including small non-coding RNAs, referred to as microRNAs, and long non-coding RNAs. Both microRNAs and long non-coding RNAs play a role in cardiac development as well as in the pathogenesis of cardiovascular disease, prompting many studies to investigate their role as potential therapeutic targets. Most studies manipulate miRNAs and lncRNAs of interest via antisense oligonucleotides; however, several challenges remain limiting their potential clinical value. As such, viral and non-viral delivery methods are being developed to achieve targeted delivery in vivo.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Non-coding RNAs
- Long non-coding RNAs
- microRNAs
- Cardiovascular disease
- Heart failure
- Antisense oligonucleotides
1 Background
Heart failure (HF) is the end result of a variety of disease states, including coronary artery disease and hypertension. It is a devastating disorder characterized by chamber remodeling, hypertrophy, fibrosis and poor heart function. It is a significant global health problem which is increasing in prevalence as the population ages [1, 2]. Despite improvements in cardiovascular therapies, medical management and prevention, mortality rates remain high, with almost 50% patients with HF dying within 5 years of diagnosis [3]. As a multifactorial clinical syndrome, HF represents an epidemic threat; highlighting the need to better understand disease mechanisms. The increasing burden of HF on health systems has prompted a number of investigations to identify and develop new therapies for the prevention and treatment of HF [4].
Advances in genome-wide profiling has found that over 90% of the genome encodes a vast range of non-coding RNAs (ncRNAs) instead of protein-coding messenger RNA. NcRNAs can differ in length, from small ncRNAs of approximately 18–25 nucleotides (i.e. microRNAs [miRNAs]), to larger ncRNAs of over 200 nucleotides called long non-coding RNAs (lncRNAs). There are many different types of ncRNAs and they are generally classified into groups based on their length and mechanism of gene regulation (see review [5]). These types include small interfering (siRNAs), miRNAs, piwi-associated RNAs, circular RNAs, small nucleolar RNAs, small nuclear RNAs and lncRNAs [6]. Of these, miRNAs have been extensively studied in the heart, where they have a role in cardiac biology and can influence cardiac remodeling in cardiovascular disease (see reviews [7,8,9,10,11]). Evidence from preclinical studies points to potential applications of miRNAs for diagnostic and therapeutic purposes (see reviews [9, 10, 12,13,14,15]). In contrast, less is known about lncRNAs, but they have a demonstrated role in cardiac development and prognostic potential in the clinic [16, 17].
Earlier chapters in this series have discussed in detail the biology of ncRNAs, as well as the research progress of ncRNAs and heart failure. This particular chapter will focus on miRNAs and lncRNAs and aims to provide readers with an updated summary on those miRNAs and lncRNAs with translational potential for cardiovascular disease, with an emphasis on translational hurdles and new technologies that are being developed to deliver ncRNAs to the heart.
2 Therapeutic Applications of ncRNAs
2.1 Targeting miRNAs in the Heart
Among all the ncRNAs, miRNAs have the capacity to target several genes simultaneously within a similar signaling network or pathway; therefore, they may serve as preferable therapeutic targets compared with other ncRNAs. Two main strategies are used to manipulate the expression of miRNAs: chemically modified inhibitors and miRNA mimics. These strategies aim to normalize miRNA expression in the tissue by either silencing over-expressed miRNAs (using inhibitors) or restoring miRNAs (using miRNA mimics) that have a deficit in expression under pathological conditions (for review see [9]). As miRNAs have been shown to control pathophysiological changes of the heart, including cardiomyocyte cell death, autophagy, contractility, fibrosis and hypertrophy, researchers have investigated the therapeutic potential of miRNAs intensively for the treatment of cardiovascular disease. These studies have been reviewed elsewhere, and have mainly focused on inhibiting miRNAs [9, 10, 13, 18, 19]. Here we present an example of a miRNA that may have a dual therapeutic effect, and the need to consider miRNAs in respect to sex and severity of disease.
miR-208a is a potential therapeutic candidate demonstrating the synergistic effect of miRNAs. Not only did pharmacologic inhibition of miR-208a prevent pathological cardiac remodeling, improve cardiac function and survival in a rat hypertensive model [20] (Fig. 21.1), it was also found to control whole-body metabolism, by protecting mice against high fat diet-induced obesity, despite being a cardiac specific miRNA [21]. These protective actions of miR-208a are due to upregulation of its target gene, thyroid hormone-associated protein 1 (THRAP1, also known as MED13) in cardiac tissue. Thus, pharmacological inhibition of miR-208a potentially has a dual effect not only to improve cardiac function in patients following a cardiac insult, but also in those patients with co-morbidities such a diabetes or a metabolic syndrome to improve whole-body metabolism.
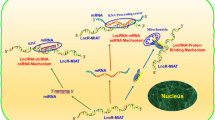
Non-coding RNA therapies for the failing heart. A schematic showing examples of microRNAs and long non-coding RNAs that are dysregulated in the failing heart and have been successfully targeted in preclinical models of cardiac disease. For microRNA therapeutics, disease severity, disease etiology, sex and type of inhibitor can influence therapeutic outcome. miR-208a has a dual-therapeutic affect having also been shown to protect against high fat diet induced obesity in mice
Recently, it has become apparent that the pharmacological effect of miRNA inhibitors is dependent on type and severity of disease, and sex, which may determine therapeutic outcome [22,23,24,25]. In our own studies using inhibitors against the miR-34 family and miR-34a in heart failure mouse models, we found that inhibiting the miR-34 family was therapeutically more effective at protecting the heart against myocardial infarction than inhibiting miR-34a alone [23] (Fig. 21.1). Further, inhibiting miR-34a alone was able to attenuate cardiac pathology in a moderate mouse model of hypertrophic cardiomyopathy, but was ineffective in a more severe model [22] suggesting that therapies that inhibit miR-34a alone may have limited potential in settings of established cardiac pathology. Our follow up studies on miR-34a further confirmed that treatment with miR-34a inhibitors showed little benefit in a setting of severe dilated cardiomyopathy associated with atrial fibrillation, when compared to a setting of moderate dilated cardiomyopathy [24]. In the same study, we showed that males and females respond differently to a miRNA-34a based drug, and identified sex- & treatment-dependent regulation of miRNAs in the diseased heart [24]. Several other studies have reported sexual dimorphism in the miRNA transcriptome. Whole genome wide studies have reported differentially-expressed miRNAs between males and females across four human tissues including brain, colorectal mucosa, peripheral blood, and cord blood [26], as well as in human and murine normal and diseased heart [27], indicating that gender specific treatment strategies may need to be considered. More recently, sex-specific regulation of miRNAs targeting proteins involved in mitochondrial metabolism in the heart were identified [28].
Further, Eding and colleagues [25] demonstrated that the pharmacological effect of antimiR-208a was (i) stronger under disease conditions (compared to basal) in both small and large animal models of cardiac stress, (ii) target regulation can be dependent on the type of stress, and (iii) that both the type and severity of disease determine the therapeutic outcome [25].
Collectively, these studies indicate that disease etiology and sex influences the therapeutic outcome of miRNA-based drug therapies. These factors will be important to consider when assessing the therapeutic dose and predicting therapeutic outcome in the clinic.
2.2 Targeting lncRNAs in the Heart
LncRNAs have been identified to play a role in cardiovascular health and disease and have recently been extensively reviewed [5, 6, 29,30,31,32,33]. Here, we will focus on those lncRNAs that show promise for therapeutic application in cardiovascular disease (Fig. 21.1). One of the most widely used antisense oligonucleotides (ASOs) to inhibit lncRNAs are GapmeRs. GapmeRs are highly potent, single-stranded ASOs that function by RNase H-dependent degradation of complementary RNA targets [34]. GapmeRs are designed to have 2–5 locked nucleic acid (LNA) moieties at each terminus which flank a central “gap” of 5–10 single stranded DNA nucleotides [35]. The LNA:DNA nucleotide combination increases binding affinity, half-life and improved stability of the GapmeR, as well as facilitating unassisted cellular uptake [35]. Only recently have GapmeRs been used to inhibit lncRNAs in preclinical models of heart failure [36, 37]. Three lncRNAs with translation potential into clinical scenarios (due to identification of a human homolog) are cardiac hypertrophy-associated transcript (Chast) [37], Wisp2 super-enhancer-associated RNA (Wisper) [36] and maternally expressed gene 3 (Meg3) [38] (Fig. 21.1).
The lncRNA Chast was identified from whole-genome lncRNA profiling, and upregulated in hypertrophic mouse hearts [37]. Of translational relevance for humans, a human homolog of CHAST was identified and found to be conserved in sequence and structure. Further highlighting the potential translational relevance, CHAST expression was upregulated in the hearts from patients with aortic stenosis (which causes hypertrophy of the heart) compared to healthy hearts [37]. The therapeutic potential of Chast inhibition using GapmeRs was tested in a preclinical mouse model of established cardiac disease (induced by transverse aortic constriction, TAC). Chast inhibition in TAC mice resulted in attenuation of cardiac hypertrophy, smaller cardiomyocyte size and improved cardiac function compared to TAC animals treated with the control-GapmeR [37] (Fig. 21.1). Importantly, there were no signs of toxicological side effects from GapmeR treatment [37].
Wisper is a heart enriched lncRNA that is highly expressed in cardiac fibroblasts and up-regulated in the fibrotic myocardial tissue following myocardial infarction [36]. As Wisper was found to correlate with cardiac fibrosis in both a mouse model of myocardial infarction, and in heart tissue from patients with aortic stenosis [36], it represents a potential anti-fibrotic therapy. To determine whether Wisper could be a potential therapy to combat cardiac fibrosis, Wisper was inhibited in a mouse model of myocardial infarction two and nine days after injury using GapmeRs. At both seven and 28 days post-myocardial infarction, Wisper-depleted myocardial infarction mice had (i) improved cardiac function, (ii) decreased expression of the fibrotic gene program, (iii) reduction of infarct size, and (iv) attenuation of cardiac fibrosis compared to control treated myocardial infarction mice [36] (Fig. 21.1). These findings in a preclinical mouse model, coupled with the observation that human WISPER expression correlates with fibrosis in patients with aortic stenosis, identifies Wisper as a potential therapeutic target to treat cardiac fibrosis and prevent pathological remodeling in the diseased heart [36].
Another lncRNA with potential to be an anti-fibrotic therapy is Meg3. Meg3 was identified from global profiling of lncRNAs in cardiac fibroblasts from hearts of mice that had undergone 13 weeks of pressure overload (induced by TAC) [38]. Meg3 is a fibroblast-enriched lncRNA which was downregulated following TAC. GapmeR-mediated silencing of Meg3 one week after TAC (i) prevented the development of cardiac fibrosis, (ii) attenuated cardiomyocyte hypertrophy, (iii) decreased the expression of the cardiac stress genes atrial natriuretic peptide and B-type natriuretic peptide, (iv) inhibited matrix metalloproteinase 2; and (v) improved diastolic function of the heart [38] (Fig. 21.1). Meg3 is highly conserved across species, and a human homolog of MEG3 has been identified [39, 40], demonstrating the translational potential of Meg3 as a target for the prevention of extracellular matrix remodeling in the heart.
Together, these studies support translation into the clinic, although careful consideration into GapmeR design and delivery will need to be considered (discussed further in Sect. 3.2).
2.3 Circulating Non-coding RNAs as Potential Biomarkers
The detection and stability of circulating miRNAs (ci-miRNAs) in plasma, and with emerging techniques that can detect ci-miRNAs in a quantitative manner (e.g. quantitative PCR, droplet digital PCR, RNA sequencing) suggests that ci-miRNAs can be used as clinical biomarkers for cardiovascular disease. Indeed, several groups have characterized the levels of miRNAs in the circulation of patients with cardiovascular disease ([41,42,43,44]; also see reviews [45, 46]). Studies suggest that in some conditions measurement of a panel of ci-miRNAs may be an alternative way to conventional markers for early detection in acute myocardial infarction [47, 48]. Whilst ci-miRs may be useful for diagnostics and monitoring approaches, further studies are still required before ci-miRNAs can be eventually used as biomarkers for cardiovascular pathologies.
Although not as extensively studied compared to ci-miRNAs, there have been several studies reporting lncRNAs in circulation as useful predictors of disease prognosis ([32, 49, 50]). The lncRNA long intergenic noncoding RNA predicting cardiac remodeling (LIPCAR) is an example of a potential biomarker as increased plasma levels were associated with left ventricular remodeling post myocardial infarction, and increased risk of cardiovascular death in heart failure patients [16]. In addition, numerous other lncRNAs have been identified as potential biomarkers. The lncRNA GAS5 was found to be downregulated in the plasma of patients with coronary artery disease, which might be a promising biomarker for the diagnosis of coronary artery disease [51]. Myocardial infarction-associated transcript (MIAT) and smooth muscle and endothelial cell-enriched migration/differentiation-associated long noncoding RNA (SENCR) were associated with left ventricular remodeling in patients with diabetic cardiomyopathy [52]. Vausort and colleagues [50] identified a number of lncRNAs to be dysregulated in peripheral blood cells of patients with acute myocardial infarction which may help predict outcome. Other potential lncRNAs as biomarkers for acute myocardial infarction include Zinc finger antisense 1 (ZFAS1), Cdr1 antisense (CDR1AS), urothelial carcinoma-associated 1 (UCA1), HOX antisense intergenic RNA (HOTAIR) [53,54,55], and for heart failure are non-coding repressor of NFAT (NRON) and myosin heavy-chain-associated RNA transcripts (MHRT) [56]. Collectively, these findings encourage future studies to determine the value of lncRNAs as novel cardiac biomarkers.
2.4 miRNAs in Clinical Trials
Despite convincing preclinical studies demonstrating therapeutic effect of miRNA inhibitors there are currently no miRNA targeted clinical trials for heart disease. However, there are positive progresses of RNA-based treatments (siRNAs and miRNAs) in other fields of disease [57]. Translational efficacy and safety of miRNA-based therapeutics to patients (an inhibitor against miR-122, miravisen) has been shown in phase IIa clinical trials for the treatment of hepatitis C virus, where results indicate that the treatment was well tolerated [58]. In July 2018, miRAgen Therapeutics Inc. announced they would initiate a Phase 2 clinical trial to evaluate MRG-201 (a synthetic mimic of miRNA-29) in patients with a predisposition for keloid formation (keloids are raised overgrowths of scar tissue). This follows successful testing of MRG-201 in Phase 1 clinical trials which demonstrated MRG-201 could reduce fibrogenesis in patients after skin trauma (http://www.miragen.com/pipeline/). miR-29 targets proteins involved in fibrosis including collagens, fibrillins and elastin, thus representing a potential therapeutic target for tissue fibrosis in other pathological conditions. Despite the well-documented role of miR-29 in cardiac remodelling and fibrosis [59, 60], there have been confounding studies that may influence the development of miR-29 as a therapy for the treatment of heart failure [59,60,61].
MiRagen, in collaboration with Servier, are also developing a synthetic miRNA inhibitor of miRNA-92a (MRG-110) to promote the revascularization process for treatment of ischemic heart failure. Other clinical trials using novel oligonucleotides to inhibit miR-17 (RGLS4326) for the treatment of autosomal dominant polycystic kidney disease are being studied in Phase 1 trials.
No clinical trials targeting lncRNAs for cardiovascular disease have been reported so far.
3 Translational Hurdles of ncRNAs
3.1 miRNAs
Most of preclinical and clinical studies use antisense oligonucleotides (ASOs) to inhibit the miRNA of interest. They have been chemically modified to improve stability, binding affinity and nuclease resistance, most commonly with 2′ sugar modifications such as 2′-O-methyl (2′-OMe), 2′-O-Methyoxyethyl (2′-Moe), 2′-fluoro (2′-F) or LNA, incorporation of phosphodiester and phosphorothioate linkages or conjugation to cholesterol (see reviews [5, 8, 9]). Often referred to as “antagomiRs” [cholesterol conjugated] or “antimiRs” [LNA based], these inhibitors are non-tissue specific and impact on several organs (such as liver and kidney) upon systemic administration. Given the ubiquitous expression of some miRNAs, and the different functions of miRNAs in various tissues and/or oncogenic efficacies, this may be problematic. For example, inhibition of miR-34 in the heart is protective [22, 23], but miR-34 is also recognized as a master regulator of tumor suppression and miR-34 replacement therapy is being investigated as a cancer treatment [62]. Under these conditions, a targeted-tissue specific method may be preferable. Furthermore, miRNA inhibitors have the potential to affect RNA species beyond their intended targets [63, 64], which may make clinical intervention complex.
Over the years, there have been significant progresses in next generation sequencing and miRNA systems biology. Recent studies have demonstrated that not only do miRNAs regulate their target mRNAs, but miRNAs in the heart can also regulate the expression of other secondary miRNAs in the heart [63, 65]. Thus, a better understanding of miRNA-miRNA crosstalk and complex signaling networks in a normal and diseased state will be important for the successful design of miRNA-based therapies for cardiovascular disease.
Further complicating the use of ASOs are inconsistent results when investigators have used antagomiRs or antimiRs targeting the same miRNA in preclinical mouse models of cardiac disease [59, 60, 66, 67]. Whilst one group reported that inhibition of miR-21 (using antagomiRs) prevented cardiac hypertrophy and fibrosis in a mouse model of pressure overload [67], another group was unable to replicate these findings using a LNA-antimiR-21 approach [66]. Similarly, mice subjected to pressure overload were less susceptible to cardiac fibrosis and hypertrophy following inhibition of miR-29 using LNA-antimiRs [59], whereas earlier reports suggested inhibition of miR-29b with antagomiRs promoted the fibrotic response [60]. The disparity in these studies may be partially explained by different oligonucleotide chemistries, specific targeting of an individual miRNA vs. a miRNA family, experimental protocols utilized, or different effects of miRNAs in different cells types (e.g. cardiomyocytes vs fibroblasts). These inconsistencies have yet to be resolved, demonstrating that further studies are required before these miRNAs can enter clinical trials as a therapeutic for cardiac fibrosis and hypertrophy.
3.2 lncRNAs
Several challenges need to be resolved before a lncRNA based therapy enters the clinic for cardiovascular disease (see reviews [29, 30, 68, 69]). The most challenging issue is target specificity. A single lncRNA has pleiotropic actions, where some act through more than one mechanism, can regulate multiple signaling pathways and have a number of functions within an organism. For example, the lncRNA gene, antisense non-coding RNA in the INK4 locus (ANRIL) is associated with an increased risk of atherosclerotic cardiovascular disease [70], but also has a role in cancer cell proliferation [71], thus making it a difficult therapeutic target.
LncRNAs are not well conserved across species which may limit the use of animal models for preclinical studies. This is illustrated by lncRNAs, such as Braveheart (Bvht; regulates cardiac cell fate), Mirt1 and Mirt2 (thought to have a protective role on cardiac function and left ventricular remodeling post myocardial infarction), in which a human homolog has not been identified [72, 73]. Further, for those potentially important lncRNAs that have no rodent homolog, experimental analysis is restricted to human cells and tissues, making translation from bench to bedside more difficult. The low homology between species makes characterization and clinical testing of human lncRNAs more difficult. However, it is thought that the secondary structure of lncRNAs is conserved rather than the primary sequence, and that structure may be more important to function than sequence [29]. Thus, lncRNAs may have structural homologs in other species, which may allow the use of animal models for preclinical testing. However, the relationship between lncRNA structure and function is not well understood and needs to be studied further.
Another hurdle facing the development of lncRNAs as therapeutic targets is cellular location. LncRNAs have widely varying subcellular distributions. Some lncRNAs reside in the cytoplasm, nucleus, mitochondria and other extracellular locations, and can even shuttle to various subcellular locations [74]. The same lncRNA can reside in multiple cellular compartments and have a functional effect in each, which may make therapeutic intervention complex. Antisense and RNAi-based gene-knockdown methods vary in efficacy between different cellular compartments [74], thus the subcellular distribution of lncRNAs need to be determined in order to employ the best potential therapeutic approach [75].
ASOs (such as GapmeRs) are commonly used to inhibit lncRNAs, however, potency, toxicity, route of delivery, dose, duration of treatment, off-target effects, stability and specificity need to be considered when developing and testing ASOs as pharmacological agents. With the use of bioinformatics tools and gene sequence databases, the sequence of GapmeRs, specifically of the DNA gap and flanking LNAs, need to be carefully designed before they can be used in vivo. A carefully designed GapmeR should have favourable therapeutic properties including high target affinity, specificity, stability and favourable pharmacokinetic and tissue-penetrating properties.
Overexpression of lncRNA is also possible with the use of viral vectors, nanoparticles and RNA mimics, although these approaches also have their limitations. Challenges facing viral delivery of lncRNAs include (i) efficiency of lncRNA upregulation, (ii) low packaging limit of adeno-associated virus (AAV) vectors and these cannot be used for packaging lncRNA >3–4 kb; and (iii) ability to overexpress the lncRNA in the subcellular localization in which it resides [32]. Despite these challenges, two studies have used viral-mediated overexpression of lncRNAs in a mouse model of myocardial infarction demonstrating feasibility of this approach [76, 77]. The elevated risk of toxicity needs to be considered when using nanoparticles, and RNA mimics are prone to degradation and can have difficulties entering the cell [78].
4 Emerging Approaches to Deliver ncRNAs to the Heart
There are intense efforts to identify agents that are capable of targeted delivery of oligonucleotides to tissues and cells (see reviews [5, 9, 79, 80]). One common method to achieve targeted delivery is using viral approaches. AAV is the preferred method, and allows for greater flexibility as there are a number of AAV serotypes, promoters and reporter genes to choose from to enhance tissue specificity [81]. AAVs have been shown to be effective in delivering protein coding genes in preclinical models [82], and no side-effects were reported from clinical trials in patients with heart failure [83], demonstrating translational potential. AAVs are commonly employed to deliver miRNA “sponges” or “tough decoys” to inhibit miRNAs in the tissue of interest [84,85,86,87,88], although developing a cardiac-specific technology may be more difficult [89].
Other non-viral methods have recently been demonstrated to have the potential to deliver miRNA therapeutics to the heart. Ultrasound microbubbles coupled with a specific single-chain antibody has allowed targeted delivery of miRNA-126 mimics in abdominal aortic aneurysm [90], although antibodies specifically targeting cardiomyocytes need to be developed. Light-induced antimiR activation (a technique which facilitates local delivery) against miR-92a to improve angiogenesis has been demonstrated in human cells [91]. Coronary angiogenesis is reduced in hearts as they undergo pathological remodeling and this contributes towards the transition to heart failure [92]. This particular method of local delivery could potentially be applied at open-heart surgery in the clinic. An unlockable core-shell nanocomplex (Hep@PGEA) was used to deliver miR-499 to the hearts of mice following myocardial infarction [93]. This approach suppressed cardiomyocyte apoptosis and promoted cardiac repair, without showing any obvious toxic effects in other tissues [93]. Hydrogels are cross-linked polymers which can carry and release therapeutics after injection in tissues, are safe in large animal models [94], and successfully used to deliver miR-302 mimics to the heart to promote cardiomyocyte proliferation and regeneration following myocardial infarction [95]. These hydrogels could be delivered to the heart by catheter in a clinical setting. Negatively-charged calcium phosphate nanoparticles (CaP-NPs) for the delivery of miRNAs to cardiac cells in vitro and in vivo have been developed and used successfully [96], with a follow-up study showing an inhalation approach was effective for the delivery of therapeutics via CaP-NPs to the diseased heart [97]. Finally, CRISPR/cas9 technology is a powerful ncRNAs editing tool and has been shown to be an efficient and stable technology for inhibiting miRNA in vitro and in vivo [98].
5 Conclusion
NcRNAs have emerged as critical regulators of gene expression and function. Studies conducted over the last two decades clearly demonstrate that miRNAs and lncRNAs play an pivotal role in cardiovascular health and disease. Functional studies targeting these classes of ncRNAs demonstrate their therapeutic potential in treating pathology associated with cardiovascular disease such as hypertrophy, fibrosis and cardiac dysfunction. As a result of these favorable outcomes in preclinical models, approaches to deliver ncRNAs to the heart are being continually developed. However, further studies are necessary to clarify the role and regulation of ncRNAs in the heart to develop effective treatments for cardiovascular disease.
References
Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385(9970):812–24.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322.
Liu L, Eisen HJ. Epidemiology of heart failure and scope of the problem. Cardiol Clin. 2014;32(1):1–8.
Bernardo BC, Blaxall BC. From bench to bedside: new approaches to therapeutic discovery for heart failure. Heart Lung Circ. 2016;25(5):525–34.
Das A, Samidurai A, Salloum FN. Deciphering non-coding RNAs in cardiovascular health and disease. Front Cardiovasc Med. 2018;5:73.
Wadley GD, Lamon S, Alexander SE, McMullen JR, Bernardo BC. Non-coding RNAs regulating cardiac muscle mass. J Appl Physiol (Bethesda, Md: 1985). 2018.
Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease. J Am Coll Cardiol. 2016;68(23):2577–84.
Bernardo BC, Charchar FJ, Lin RCY, McMullen JR. A MicroRNA guide for clinicians and basic scientists: background and experimental techniques. Heart Lung Circ. 2012;21(3):131–42.
Bernardo BC, Ooi JYY, Lin RCY, McMullen JR. miRNA therapeutics: a new class of drugs with potential therapeutic applications in the heart.Future. Med Chem. 2015;7(13):1771–92.
Hata A. Functions of MicroRNAs in cardiovascular biology and disease. Annu Rev Physiol. 2013;75(1):69–93.
Xiao J, Chen Y-H. MicroRNAs: novel regulators of the heart. J Thorac Dis. 2010;2(1):43–7.
Gidlöf O, Erlinge D. MicroRNAs in the failing heart – novel therapeutic targets? Scand Cardiovasc J. 2014;48(6):328–34.
Ooi JYY, Bernardo BC, McMullen JR. The therapeutic potential of microRNAs regulated in settings of physiological cardiac hypertrophy. Future Med Chem. 2014;6(2):205–22.
Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol. 2015;213(1):60–83.
Zhou SS, Jin JP, Wang JQ, Zhang ZG, Freedman JH, Zheng Y, Cai L. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018;39(7):1073–84.
Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J, Holzmann A, Lemesle G, de Groote P, Pinet F, Thum T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res. 2014;114(10):1569–75.
Kumarswamy R, Thum T. Non-coding RNAs in cardiac remodeling and heart failure. Circ Res. 2013;113(6):676–89.
Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469(7330):336–42.
Lv D, Liu J, Zhao C, Sun Q, Zhou Q, Xu J, Xiao J. Targeting microRNAs in cardiac hypertrophy and heart failure. Mini Rev Med Chem. 2015
Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure/clinical perspective. Circulation. 2011;124(14):1537–47.
Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, Gautron L, Elmquist JK, Bassel-Duby R, Olson EN. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149(3):671–83.
Bernardo BC, Gao XM, Tham YK, Kiriazis H, Winbanks CE, Ooi JY, Boey EJ, Obad S, Kauppinen S, Gregorevic P, Du XJ, Lin RC, McMullen JR. Silencing of miR-34a attenuates cardiac dysfunction in a setting of moderate, but not severe, hypertrophic cardiomyopathy. PLoS One. 2014;9(2):e90337.
Bernardo BC, Gao XM, Winbanks CE, Boey EJ, Tham YK, Kiriazis H, Gregorevic P, Obad S, Kauppinen S, Du XJ, Lin RC, McMullen JR. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci U S A. 2012;109(43):17615–20.
Bernardo BC, Ooi JYY, Matsumoto A, Tham YK, Singla S, Kiriazis H, Patterson NL, Sadoshima J, Obad S, Lin RCY, McMullen JR. Sex differences in response to miRNA-34a therapy in mouse models of cardiac disease: identification of sex-, disease- and treatment-regulated miRNAs. J Physiol. 2016;594(20):5959–74.
Eding JE, Demkes CJ, Lynch JM, Seto AG, Montgomery RL, Semus HM, Jackson AL, Isabelle M, Chimenti S, van Rooij E. The efficacy of cardiac anti-miR-208a therapy is stress dependent. Mol Ther. 2017;25(3):694–704.
Cui C, Yang W, Shi J, Zhou Y, Yang J, Cui Q, Zhou Y. Identification and analysis of human sex-biased MicroRNAs. Genomics Proteomics Bioinformatics. 2018;16(3):200–11.
Tsuji M, Kawasaki T, Matsuda T, Arai T, Gojo S, Takeuchi JK. Sexual dimorphisms of mRNA and miRNA in human/murine heart disease. PLoS One. 2017;12(7):e0177988.
Sanchez-Ruderisch H, Queiros AM, Fliegner D, Eschen C, Kararigas G, Regitz-Zagrosek V. Sex-specific regulation of cardiac microRNAs targeting mitochondrial proteins in pressure overload. Biol Sex Differ. 2019;10(1):8.
Gomes CPC, Spencer H, Ford KL, Michel LYM, Baker AH, Emanueli C, Balligand JL, Devaux Y, Cardiolinc network. The function and therapeutic potential of Long non-coding RNAs in cardiovascular development and disease. Mol Ther Nucleic Acids. 2017;8:494–507.
Greco S, Salgado Somoza A, Devaux Y, Martelli F. Long noncoding RNAs and cardiac disease. Antioxid Redox Signal. 2018;29(9):880–901.
Hermans-Beijnsberger S, van Bilsen M, Schroen B. Long non-coding RNAs in the failing heart and vasculature. Non-coding RNA Res. 2018;3(3):118–30.
Hobuss L, Bar C, Thum T. Long non-coding RNAs: at the heart of cardiac dysfunction? Front Physiol. 2019;10:30.
Shen S, Jiang H, Bei Y, Xiao J, Li X. Long non-coding RNAs in cardiac remodeling. Cell Physiol Biochem. 2017;41(5):1830–7.
Castanotto D, Lin M, Kowolik C, Wang L, Ren XQ, Soifer HS, Koch T, Hansen BR, Oerum H, Armstrong B, Wang Z, Bauer P, Rossi J, Stein CA. A cytoplasmic pathway for gapmer antisense oligonucleotide-mediated gene silencing in mammalian cells. Nucleic Acids Res. 2015;43(19):9350–61.
Frieden M, Christensen SM, Mikkelsen ND, Rosenbohm C, Thrue CA, Westergaard M, Hansen HF, Orum H, Koch T. Expanding the design horizon of antisense oligonucleotides with alpha-L-LNA. Nucleic Acids Res. 2003;31(21):6365–72.
Micheletti R, Plaisance I, Abraham BJ, Sarre A, Ting CC, Alexanian M, Maric D, Maison D, Nemir M, Young RA, Schroen B, Gonzalez A, Ounzain S, Pedrazzini T. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci Transl Med. 2017;9(395)
Viereck J, Kumarswamy R, Foinquinos A, Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, Remke J, Just A, Fendrich J, Scherf K, Bolesani E, Schambach A, Weidemann F, Zweigerdt R, de Windt LJ, Engelhardt S, Dandekar T, Batkai S, Thum T. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med. 2016;8(326):326ra322.
Piccoli MT, Gupta SK, Viereck J, Foinquinos A, Samolovac S, Kramer FL, Garg A, Remke J, Zimmer K, Batkai S, Thum T. Inhibition of the cardiac fibroblast-enriched lncRNA Meg3 prevents cardiac fibrosis and diastolic dysfunction. Circ Res. 2017;121(5):575–83.
Miyoshi N, Wagatsuma H, Wakana S, Shiroishi T, Nomura M, Aisaka K, Kohda T, Surani MA, Kaneko-Ishino T, Ishino F. Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes Cells. 2000;5(3):211–20.
Zhang X, Rice K, Wang Y, Chen W, Zhong Y, Nakayama Y, Zhou Y, Klibanski A. Maternally expressed gene 3 (MEG3) noncoding ribonucleic acid: isoform structure, expression, and functions. Endocrinology. 2010;151(3):939–47.
Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, Pinto YM. MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010;106(6):1035–9.
Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail. 2012;14(2):147–54.
Ovchinnikova ES, Schmitter D, Vegter EL, Ter Maaten JM, Valente MA, Liu LC, van der Harst P, Pinto YM, de Boer RA, Meyer S, Teerlink JR, O’Connor CM, Metra M, Davison BA, Bloomfield DM, Cotter G, Cleland JG, Mebazaa A, Laribi S, Givertz MM, Ponikowski P, van der Meer P, van Veldhuisen DJ, Voors AA, Berezikov E. Signature of circulating microRNAs in patients with acute heart failure. Eur J Heart Fail. 2016;18(4):414–23.
Vegter EL, Schmitter D, Hagemeijer Y, Ovchinnikova ES, van der Harst P, Teerlink JR, O’Connor CM, Metra M, Davison BA, Bloomfield D, Cotter G, Cleland JG, Givertz MM, Ponikowski P, van Veldhuisen DJ, van der Meer P, Berezikov E, Voors AA, Khan MA. Use of biomarkers to establish potential role and function of circulating microRNAs in acute heart failure. Int J Cardiol. 2016;224:231–9.
Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease?Circulation. Research. 2012;110(3):483–95.
Xu J, Zhao J, Evan G, Xiao C, Cheng Y, Xiao J. Circulating microRNAs: novel biomarkers for cardiovascular diseases. J Mol Med (Berl). 2012;90(8):865–75.
Wang G-K, Zhu J-Q, Zhang J-T, Li Q, Li Y, He J, Qin Y-W, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31(6):659–66.
Wang R, Li N, Zhang Y, Ran Y, Pu J. Circulating MicroRNAs are promising novel biomarkers of acute myocardial infarction. Intern Med. 2011;50(17):1789–95.
Viereck J, Thum T. Circulating noncoding RNAs as biomarkers of cardiovascular disease and injury. Circ Res. 2017;120(2):381–99.
Vausort M, Wagner DR, Devaux Y. Long noncoding RNAs in patients with acute myocardial infarction. Circ Res. 2014;115(7):668–77.
Yin Q, Wu A, Liu M. Plasma Long non-coding RNA (lncRNA) GAS5 is a new biomarker for coronary artery disease. Med Sci Monit. 2017;23:6042–8.
de Gonzalo-Calvo D, Kenneweg F, Bang C, Toro R, van der Meer RW, Rijzewijk LJ, Smit JW, Lamb HJ, Llorente-Cortes V, Thum T. Circulating long-non coding RNAs as biomarkers of left ventricular diastolic function and remodelling in patients with well-controlled type 2 diabetes. Sci Rep. 2016;6:37354.
Gao L, Liu Y, Guo S, Yao R, Wu L, Xiao L, Wang Z, Liu Y, Zhang Y. Circulating long noncoding RNA HOTAIR is an essential mediator of acute myocardial infarction. Cell Physiol Biochem. 2017;44(4):1497–508.
Zhang Y, Sun L, Xuan L, Pan Z, Li K, Liu S, Huang Y, Zhao X, Huang L, Wang Z, Hou Y, Li J, Tian Y, Yu J, Han H, Liu Y, Gao F, Zhang Y, Wang S, Du Z, Lu Y, Yang B. Reciprocal changes of circulating Long non-coding RNAs ZFAS1 and CDR1AS predict acute myocardial infarction. Sci Rep. 2016;6:22384.
Yan Y, Zhang B, Liu N, Qi C, Xiao Y, Tian X, Li T, Liu B. Circulating long noncoding RNA UCA1 as a novel biomarker of acute myocardial infarction. Biomed Res Int. 2016;2016:8079372.
Xuan L, Sun L, Zhang Y, Huang Y, Hou Y, Li Q, Guo Y, Feng B, Cui L, Wang X, Wang Z, Tian Y, Yu B, Wang S, Xu C, Zhang M, Du Z, Lu Y, Yang BF. Circulating long non-coding RNAs NRON and MHRT as novel predictive biomarkers of heart failure. J Cell Mol Med. 2017;21(9):1803–14.
Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee S-S. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids. 2017;8:132–43.
Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–94.
Sassi Y, Avramopoulos P, Ramanujam D, Gruter L, Werfel S, Giosele S, Brunner AD, Esfandyari D, Papadopoulou AS, De Strooper B, Hubner N, Kumarswamy R, Thum T, Yin X, Mayr M, Laggerbauer B, Engelhardt S. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat Commun. 2017;8(1):1614.
van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13027–32.
McMullen JR, Bernardo BC. Inhibition of miR-29 protects against cardiac hypertrophy and fibrosis: new insight for the role of miR-29 in the heart. Non-coding RNA Investig. 2018;2:3.
Slabakova E, Culig Z, Remsik J, Soucek K. Alternative mechanisms of miR-34a regulation in cancer. Cell Death Dis. 2017;8(10):e3100.
Ooi JYY, Bernardo BC, Singla S, Patterson NL, Lin RCY, McMullen JR. Identification of miR-34 regulatory networks in settings of disease and antimiR-therapy: implications for treating cardiac pathology and other diseases. RNA Biol. 2017;14(5):500–13.
Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S. Inhibition of microRNA function by antimiR oligonucleotides. Silence. 2012;3(1):1.
Matkovich SJ, Hu Y, Dorn GW. Regulation of cardiac microRNAs by cardiac microRNAs. Circ Res. 2013;113(1):62–71.
Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, van Rooij E, Olson EN. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120(11):3912–6.
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–4.
McMullen JR, Drew BG. Long non-coding RNAs (lncRNAs) in skeletal and cardiac muscle: potential therapeutic and diagnostic targets? Clin Sci. 2016;130(24):2245–56.
Lucas T, Dimmeler S. RNA therapeutics for treatment of cardiovascular diseases: promises and challenges. Circ Res. 2016;119(7):794–7.
Holdt LM, Beutner F, Scholz M, Gielen S, Gabel G, Bergert H, Schuler G, Thiery J, Teupser D. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol. 2010;30(3):620–7.
Zhao B, Lu YL, Yang Y, Hu LB, Bai Y, Li RQ, Zhang GY, Li J, Bi CW, Yang LB, Hu C, Lei YH, Wang QL, Liu ZM. Overexpression of lncRNA ANRIL promoted the proliferation and migration of prostate cancer cells via regulating let-7a/TGF-beta1/Smad signaling pathway. Cancer Biomark. 2018;21(3):613–20.
Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152(3):570–83.
Zangrando J, Zhang L, Vausort M, Maskali F, Marie PY, Wagner DR, Devaux Y. Identification of candidate long non-coding RNAs in response to myocardial infarction. BMC Genomics. 2014;15:460.
Lennox KA, Behlke MA. Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res. 2016;44(2):863–77.
Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–307.
Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY, Fan YY, Li PF. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun. 2014;5:3596.
Wang K, Sun T, Li N, Wang Y, Wang JX, Zhou LY, Long B, Liu CY, Liu F, Li PF. MDRL lncRNA regulates the processing of miR-484 primary transcript by targeting miR-361. PLoS Genet. 2014;10(7):e1004467.
Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9(1):60.
Biglino G, Caputo M, Rajakaruna C, Angelini G, van Rooij E, Emanueli C. Modulating microRNAs in cardiac surgery patients: novel therapeutic opportunities? Pharmacol Ther. 2017;170:192–204.
Kwekkeboom RF, Lei Z, Doevendans PA, Musters RJ, Sluijter JP. Targeted delivery of miRNA therapeutics for cardiovascular diseases: opportunities and challenges. Clin Sci. 2014;127(6):351–65.
Bass-Stringer S, Bernardo BC, May CN, Thomas CJ, Weeks KL, McMullen JR. Adeno-associated virus gene therapy: translational Progress and future prospects in the treatment of heart failure. Heart Lung Circ. 2018;27(11):1285–300.
Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15(23):1550–7.
Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M, Hajjar RJ. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res. 2014;114(1):101–8.
Jeong D, Yoo J, Lee P, Kepreotis SV, Lee A, Wahlquist C, Brown BD, Kho C, Mercola M, Hajjar RJ. miR-25 tough decoy enhances cardiac function in heart failure. Mol Ther. 2018;26(3):718–29.
Krol J, Busskamp V, Markiewicz I, Stadler MB, Ribi S, Richter J, Duebel J, Bicker S, Fehling HJ, Schubeler D, Oertner TG, Schratt G, Bibel M, Roska B, Filipowicz W. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141(4):618–31.
Winbanks CE, Beyer C, Hagg A, Qian H, Sepulveda PV, Gregorevic P. miR-206 represses hypertrophy of myogenic cells but not muscle Fibers via inhibition of HDAC4. PLoS ONE. 2013;8(9):e73589.
Xie J, Ameres SL, Friedline R, Hung JH, Zhang Y, Xie Q, Zhong L, Su Q, He R, Li M, Li H, Mu X, Zhang H, Broderick JA, Kim JK, Weng Z, Flotte TR, Zamore PD, Gao G. Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nat Methods. 2012;9(4):403–9.
Wang F, Fang Q, Chen C, Zhou L, Li H, Yin Z, Wang Y, Zhao CX, Xiao X, Wang DW. Recombinant Adeno-associated virus-mediated delivery of MicroRNA-21-3p lowers hypertension. Mol Ther Nucleic Acids. 2018;11:354–66.
Bernardo BC, Gregorevic P, Ritchie RH, McMullen JR. Generation of microRNA-34 sponges and tough decoys for the heart: developments and challenges. Front Pharmacol Transl Pharmacol. 2018;9:1090.
Wang X, Searle AK, Hohmann JD, Liu AL, Abraham M-K, Palasubramaniam J, Lim B, Yao Y, Wallert M, Yu E, Chen Y-C, Peter K. Dual-targeted theranostic delivery of miRs arrests abdominal aortic aneurysm development. Mol Ther. 2018;26(4):1056–65.
Schafer F, Wagner J, Knau A, Dimmeler S, Heckel A. Regulating angiogenesis with light-inducible AntimiRs. Angew Chem Int Ed Engl. 2013;52(51):13558–61.
Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Investig. 2005;115(8):2108–18.
Nie JJ, Qiao B, Duan S, Xu C, Chen B, Hao W, Yu B, Li Y, Du J, Xu FJ. Unlockable nanocomplexes with self-accelerating nucleic acid release for effective staged gene therapy of cardiovascular diseases. Adv Mater. 2018;30(31):e1801570.
Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. J Transl Med. 2013;5(173):173ra125.
Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, Cavanaugh CA, Zhou S, Kanade R, Atluri P, Morrisey EE, Burdick JA. Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischaemic injury. Nat Biomed Eng. 2017;1(12):983–92.
Di Mauro V, Iafisco M, Salvarani N, Vacchiano M, Carullo P, Ramirez-Rodriguez GB, Patricio T, Tampieri A, Miragoli M, Catalucci D. Bioinspired negatively charged calcium phosphate nanocarriers for cardiac delivery of MicroRNAs. Nanomedicine (Lond). 2016;11(8):891–906.
Miragoli M, Ceriotti P, Iafisco M, Vacchiano M, Salvarani N, Alogna A, Carullo P, Ramirez-Rodríguez GB, Patrício T, Esposti LD, Rossi F, Ravanetti F, Pinelli S, Alinovi R, Erreni M, Rossi S, Condorelli G, Post H, Tampieri A, Catalucci D. Inhalation of peptide-loaded nanoparticles improves heart failure. Sci Transl Med. 2018;10(424)
Chang H, Yi B, Ma R, Zhang X, Zhao H, Xi Y. CRISPR/cas9, a novel genomic tool to knock down microRNA in vitro and in vivo. Sci Rep. 2016;6:22312.
Acknowledgments
The authors would like to thank Julie R. McMullen at Baker Heart & Diabetes Institute for proofreading the manuscript. The authors acknowledge funding support from the Sir Edward Dunlop Medical Research Foundation (to J.Y.Y.O and B.C.B). B.C.B is supported by an Alice Baker and Eleanor Shaw Fellowship (The Baker Foundation, Melbourne, Australia).
Competing Financial Interests
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ooi, J.Y.Y., Bernardo, B.C. (2020). Translational Potential of Non-coding RNAs for Cardiovascular Disease. In: Xiao, J. (eds) Non-coding RNAs in Cardiovascular Diseases. Advances in Experimental Medicine and Biology, vol 1229. Springer, Singapore. https://doi.org/10.1007/978-981-15-1671-9_21
Download citation
DOI: https://doi.org/10.1007/978-981-15-1671-9_21
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1670-2
Online ISBN: 978-981-15-1671-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)