Abstract
Cardiovascular disease (CVD) is a common disease which poses a serious threat to human health and it is characterized by high prevalence, high disability and high mortality. Myocardial hypertrophy (MH) is a common pathological process of various cardiovascular diseases and is considered as an independent risk factor for increased cardiovascular morbidity and mortality. Therefore, it is particularly important to understand its pathological mechanism and treatment. In recent years, it has been found that many non-coding RNAs (ncRNAs) play key regulatory roles in humans’ various pathophysiological processes. Abnormal expression of ncRNAs in different types of cardiac cells is associated with pathological cardiac hypertrophy. Understanding the relationship between various ncRNAs and intercellular communication through extracellular vesicles (EV) can identify the key ncRNAs which are the accurate targets of precise therapy in this network of action, it also can potentially be a marker for clinical disease diagnosis, which will reflect the progress of the disease earlier and more accurately. There are many factors that regulate the occurrence and development of cardiac hypertrophy, ncRNAs are only a part of them. There are also mutual promotion or inhibition between ncRNAs and other molecules. It will be helpful for us to comprehend the mechanism of cardiac hypertrophy better and provide a sufficient theoretical basis for clinical diagnosis and treatment by defining these relationships.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Background
Normal myocardium consists of cardiomyocytes and non-cardiomyocytes. The cardiomyocytes account for only one-third in the number, but they assume the two-thirds of the function of the heart; the non-cardiac cells include cardiac fibroblasts, smooth muscle cells, macrophages and so on. Cardiac hypertrophy is classified into physiological hypertrophy and pathological hypertrophy. Physiological hypertrophy is a protective response. In order to adapt to the increase of work force under the action of various physiological factors, it increases the contraction to increase the myocardial reserve capacity, which is mainly shown as increased myocardial weight and myocardial cells hypertrophy along the horizontal axis of the cell, but it isn’t accompanied by fibrosis and is usually reversible [1]. Pathological cardiac hypertrophy refers to an injurious reaction that occurs when the heart is overloaded, including long-term mechanical stimulation [2, 3] or endocrine factors change or metabolic disorders [4,5,6], which mainly due to an increase of myocardial cell volume, protein synthesis and sarcomere, and also due to the re-expression of embryonic genes and the proliferation of mesenchymal cells, the proliferation of collagen and other connective tissues. It eventually leads to myocardial structure disorder, reduced contractility, insufficient blood supply, myocardial contraction and diastole dysfunction, which prone to heart failure, arrhythmia, and even sudden death [7]. At present, pathological cardiac hypertrophy is considered to be one of the independent risk factors for increased cardiovascular morbidity and mortality [8]. Cardiac hypertrophy is common in clinical practice, and there is a high risk in the progression of the disease. Therefore, it is particularly important to understand its pathological mechanism and treatment. However, the pathogenesis of cardiac hypertrophy is complicated, and there is still no thorough research. The main mechanisms of cardiomyocyte involvement in cardiac hypertrophy are as follows: calcium regulation mechanism, metabolism-related regulation, gene expression regulation, and cell death process (such as apoptosis process and autophagy process) [9]. Pathological cardiac hypertrophy is usually accompanied by cardiomyocyte death and myocardial fibrosis, resulting in functional deficits in contraction and relaxation, which further progress to heart failure. Neurohormonal regulation, such as the adrenaline and renin-angiotensin system, is widely activated, with early protective effects, and later decompensation will result in irreversible cardiac dysfunction. Studies have confirmed that it can be achieved by activating NFAT, CaMKII, cGMP/PKG, MAPK, PI3/Akt and other pathways [10]. In recent years, studies have found that non-coding RNA plays an important role in the occurrence and development of cardiac hypertrophy.
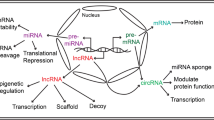
Recent data show that less than 2% of the human genome encodes proteins, and most sequences can be transcribed but not encoded. These gene sequence transcripts cannot encode proteins are called as non-coding RNAs (ncRNAs). Until now, we have discovered many of the ncRNAs play roles in DNA replication, chromatin processing, transcription and post-transcriptional gene expression of other RNAs, genomic integrity, and the controlling stability of mRNA [11]. Different ncRNAs have been found to play a key role in regulating pathophysiological process. In different types and tissues of cardiomyocyte, abnormal expression of miRNAs and lncRNAs is associated with many cardiovascular diseases. Circular RNA (circRNA) is another RNA which is classic, diversity, endogenous and lack of research, it also regulate eukaryotic gene expression leading to cardiovascular disease. Below is a summary of the relationship between non-coding RNA and cardiac hypertrophy (Fig. 13.1).
Part of non-coding RNAs which is significantly associated with cardiac hypertrophy
Different types of non-coding RNAs are essential regulators for cellular function. The chronic stress of cardiomyocytes can induce hypertrophic growth, microRNAs, long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) interact with their respective targets to regulate specific cellular functions and induce pathological cell hypertrophic growth, which will eventually develop into heart failure
2 MicroRNAs in Cardiac Hypertrophy
So far, there are about 2000 microRNAs (miRNAs) found in humans, and new microRNAs are constantly being discovered [12, 13]. MicroRNAs are endogenous small molecule non-coding RNAs, whose length is from 21 to 25 nucleotides. The sequence and hairpin structure of mature miRNAs are highly evolutionarily conserved among different species; gene clustering and space-time specificity [14, 15]; mature miRNAs expression is tissue-specific; the same miRNA can regulate multiple messenger RNAs at the same time, and a messenger RNA can also be regulated simultaneously by multiple miRNAs. These features are the functional basis for miRNAs to play important regulatory roles in the development of different organs at different stages of growth and development of organisms. There are two ways for microRNAs to silence the target mRNA expression: ① the single-stranded microRNA is complete complementary pairing to the specific sequence base of the 3′-non-coding region (3′-UTR) of the mRNA of the target protein, cleaving the messenger RNA;②The single-stranded microRNA is incomplete complementary pairing to the 3′-UTR specific sequence base of the mRNA of the target protein, restraining the translation of target mRNA without affecting the stability of messenger RNA. MicroRNAs participate in the regulation of multiple physiological and pathological activities by the above two methods. Studies have reported that the changes in microRNAs are closely related to the occurrence development of cardiac hypertrophy. The network of microRNAs regulating cardiac hypertrophy is very complicated. The role of MicroRNAs in cardiac hypertrophy ventricular remodeling is described below.
2.1 MicroRNAs That Inhibit Cardiac Hypertrophy
miR-1 is highly expressed in heart tissue and its absence can lead to cardiac malformations. Tracking the changes of miRNA expression levels timely, during the 14-day stress overload period, miR-1 was the only miRNA that was first discovered to be down-regulated. The specific overexpression of miR-1 in myocardium leads to inhibition of target genes such as Ras GTPase activating protein (RasGAP), cyclin-dependent kinase 9 (Cdk9), fibronectin and Ras homolog (Rheb), which means that the miR- 1 by acting on multiple target genes associated with cardiac hypertrophy can reduce cardiac hypertrophy, reduce fibrosis, reduce myocardial apoptosis and improve calcium signaling [16]. miR-1 reduces the expression of CaM and MEF2a at the level of post-transcriptional modification by binding to calmodulin (CaM) and 3″-UTR of MEF2a. In cardiomyocytes, NFATs and MEF2a can act together on GATA4, activating the transcription of hypertrophic genes. GATA4 mRNA 3′-UTR lacks a sequence which directly binds to miR-1, so it is speculated that miR-1 reduces the expression of GATA4 protein by direct action of MEF2a to act indirectly on GATA4, without altering the level of GATA4 mRNA [17]. In addition, the direct target of miR-1 acting on cardiomyocytes including insulin-like growth factor (IGF1) and IGF1-receptor (IGF1-R). IGF1 usually binds to IGF1-R to activate different pathways, such as PI3K-AKT and hence inhibitory FOXO3A. In turn, these factors in cardiomyocytes also directly affect the level of miR-1 expression [18]. In summary, the interaction of miR-1 with IGF1 plays a role in many processes of regulating cardiac function.
miR-133 is abundant in cardiomyocytes and markedly decreased on serious pathological hypertrophy. MiR-133 regulates extracellular matrix deposition via acting as a repressor of connective tissue growth factor (CTGF). During pathological cardiac remodeling CTGF is secreted by cardiomyocytes as well, although it is mostly expressed in fibroblasts [19]. In addition, miR-133 regulates cell proliferation, cytoskeletal formation and rearrangement of muscle fibers mainly by inhibiting the expression of its target gene RHOA (a GTP-GDP exchange protein gene), CDC42 (a signal transduction kinase gene) and NELF-A/WHSC2 (a nuclear factor gene involved in cardiac development) [20]. The nuclear factor-activated T (NFAT)-mediated hypertrophic signaling pathway plays an important role in cardiac hypertrophy caused by various stimuli. It has been confirmed that NFAT is a target gene of miRNA-133, and miRNA-133 can also participate in the regulation of cardiac hypertrophy by affecting the expression of NFAT. miR-133a inhibits the expression of NFAT3. NFAT3 has two regions in the 3-UTR that can bind to miR-133a. With overexpression of miR-133a, the expression of NFAT3 mRNA and protein were both reduced, and the cardiomyocyte hypertrophy was alleviated. It is suggested that miR-133a can protect the heart by inhibiting the CaN-NFATs signaling pathway [21]. Specific transgenic mice with miR-133 heart can maintain cardiac performance and decrease myocardial apoptosis and collagen deposition under overload stress, which is related to inhibition of, a target gene of miR-133,β1-adrenergic receptor kinase [22].
miR-378 is mainly expressed in cardiomyocytes. MiR-378 overexpression in neonatal rat cardiomyocytes can inhibit PE-stimulated cardiomyocyte hypertrophy; under the same conditions, the number of hypertrophic cardiomyocytes increased significantly after myocardial cells were transfected with anti-miR-378. In the myocardium, miR-378 can inhibit the expression of the following proteins: MAPK, insulin-like growth factor receptor 1 (IGF1R), growth factor receptor-bound protein 2(GRB2), kinase suppressor of ras l (KSP1). miR-378 inhibit cardiac hypertrophy by binding with the 3′-UTR of mRNA and regulating the expression of these four proteins at the post-transcriptional level [23]; in addition, miR-378 overexpression in primary cardiomyocytes inhibits PE-stimulated Ras activity, thus suppressing the activation of two major cell growth signaling pathways, PI3K-AKT and Raf1-MEK1-ERK 1/ 2, which act in the downstream of Ras signaling [24].
miR-9 reduces the expression of myocardin. Myocardin is a transcriptional cofactor, and various hypertrophic stimulating factors can up-regulate myocardin, which mediates cardiac hypertrophy signals. Overexpression of myocardin can induce cardiac hypertrophy. Myocardin is a member of the CaN-NFAT4 signaling pathway. Knockout of the myocardin gene can attenuate the amplification of surface area in NFAT4-induced myocardial cell. miR-9 can binds directly to the 3 UTR of myocardin mRNA, affecting the translation of myocardin protein to reduce cardiac hypertrophy [25].
miR-98/let-7i reduces cyclin D2 expression. AngII significantly up-regulates the expression of cyclin D2, which promotes AngII-induced cardiac hypertrophy. Up-regulation of miR-98/let-7i can significantly reduce the basal expression of cyclin D2. It partially inhibits AngII-induced cardiac hypertrophy by inhibiting the expression of cyclin D2 induced by AngII. The overexpression of miR-98/let-7i can significantly reduced the expression of atrial natriuretic peptide (ANP) mRNA and cardiomyocyte hypertrophy induced by AngII, suggesting that miR-98/let-7i can inhibit AngII-induced cardiac hypertrophy [26].
miR-26b reduces the expression of GATA4. GATA4 has a zinc finger structure that binds to a specific DNA. GATA4 regulates the expression of some genes in the myocardium by interacting with other transcription factors such as myocyte enhancer factor 2a (MEF2a), NFATs, and plasma response factor (SRF). miR-26b can regulate the occurrence of cardiac hypertrophy by acting on the 3′-UTR of GATA4 mRNA. Down-regulation of miR-26b can up-regulate GATA4 expression and cause cardiac hypertrophy induced by pressure overload; overexpression of miR-26b can inhibit cardiac hypertrophy [27].
In a mouse model with cardiac hypertrophy conducted by TAC and AngI1, miR-21-3p was found to effectively inhibit cardiac enlargement, inhibit cardiomyocyte hypertrophy and reduce the expression of cardiac hypertrophy marker protein. It was also detected that it can directly target the 3′-UTR of histone deacetylase 8(HDAC8) mRNA, and can also inhibit the expression of HDAC8. HDAC8 belongs to the class II of HDACs and is a group of proteases that promote cardiac hypertrophy [28].
Thioredoxin 1 (Trx1) produced by miR-98 can inhibit cardiac hypertrophy. Therefore, in a rodent model with cardiac hypertrophy induced by angiotensin II (AngII), the down-regulation of miR-98 accelerates the cardiac growth, which most likely due to the increased expression levels of its target gene cyclin D2. Trx1 acts as a negative feedback regulator of cardiac hypertrophy induced by Ang II [26].
The miR-30 family is significantly down-regulated in hypertrophic heart of mouse and cardiac biopsies from patients with left ventricular hypertrophy (LVH). MiR-30c regulates connective tissue growth factor (CTGF), and plays a role in myocardial matrix remodeling and participates in cardiac remodeling [19]. By culturing rat primary cardiomyocytes in vitro, the level of miR-30a in myocardial cells of cardiomyocyte hypertrophy induced by AngII were down-regulated. The overexpression of miR-30a in cardiomyocytes can attenuate myocardial autophagy and myocardial cell morphological hypertrophy induced by AngII; inhibiting the activity of miR-30a in myocardial cell can aggravate myocardial autophagy and morphological hypertrophy of cardiomyocytes induced by AngII [29].
The expression of miR-92b-3p was significantly decreased in the hypertrophic myocardium of rat induced by Ang-II perfusion and also decreased in the myocardium of patients with cardiac hypertrophy. miR-92b-3p can inhibit the expression of MEF2D at the post-transcriptional level. Enhancing the expression of miR-92b-3p or decreasing the level of MEF2D can consistently inhibit the cardiomyocytes hypertrophic phenotype in milk mouse induced by AngII. miR-92b-3p can inhibit cardiomyocyte hypertrophy [30].
2.2 MicroRNA That Promotes Cardiac Hypertrophy
miR-350 plays an important role in regulating the pathological process of cardiac hypertrophy, especially in the late stage of cardiac hypertrophy. The expression of miR-350 is increased in rats with myocardial hypertrophy induced by pressure overload. miR-350 inhibits protein synthesis of P38 and JNK at the post-transcriptional level, leading to dephosphorylation of NFAT4, promoting NFAT4 entry into the nucleus, and increasing transcription of ANP, brain natriuretic peptide (BNP) and α-actinin. Transfection of H9c2 cells with anti-miR-350 can reduce the level of intracellular miR-350 and inhibit the silencing effect of miR-350 on its target gene and reduce cardiac hypertrophy [31].
miR-206 increases in cardiac hypertrophy. Adenovirus transfectes mouse ventricular myocytes leading to the overexpression of miR-206, and 48 hours later, cardiomyocyte hypertrophy happens; mice with cardiac-specific overexpression of miR-206 is prone to catch cardiac hypertrophy; inhibition of miR-206 can reduce cardiac hypertrophy induced by stress. miR-206 inhibits the expression of forkhead box protein P1 (FOXP1). FOXP1 is an anti-cardiac-hypertrophy protein. Down-regulation of FOXP1 can significantly enlarge cardiomyocytes. Overexpression of FOXP1 attenuates cardiac hypertrophy induced by miR-206 [32].
MiR-195 was one of the first miRNAs which demonstrated to be up-regulated in pathological cardiac remodelling. Increased expression of miR-195 leads to cardiomyocytes growth disorganization followed by development of severe hypertrophy already at 6 weeks of age in mice [33]. The AMPK pathway is also involved in the regulation of cardiac hypertrophy. The MO25/Ste20 Related Adaptor (STRAD)/liver kinase B1 (LKB1) complexus is an important molecule of the AMPK pathway. miR-195 can target mouse protein-25 (MO25) to promote cardiac hypertrophy [34].
The overexpression of miR-208 induces cardiac hypertrophy by inhibiting the nuclear transfer factor SOX6 (Y-box 6 protein, SOX6). It shows that miR-208 is increased and SOX6 is decreased in hypertrophic cardiomyocytes induced by PE. After knocking out miR-208 in cardiomyocytes, SOX6 expression is increased, accompanied by decreased expression of ANP and α-actinin, and cardiomyocyte hypertrophy was inhibited [35].
miR-19a/b inhibits the expression of atroginl and Murfl. Atroginl and muscle ring finger protein (Murf1) are the two common E3 ligases in ubiquitination. Atroginl inhibits cardiac hypertrophy by inhibiting the expression of calcineurin and alpha-actinin. The overexpression of miR-19a/b in neonatal mouse cardiomyocytes can significantly induce cardiomyocyte hypertrophy. The miR-19a/b family directly inhibits the expression of atroginl and Murfl, increases the expression of CaN, and then activates the CaN-NFATs signaling pathway to promote cardiac hypertrophy [36].
miR-328 inhibits the expression of Serca2a. And the expression of ANP, BNP and β-myosin heavy chain (β-MHC) is significantly increased in mice with overexpressing miR-328 induced by pressure overload. Sarco/endoplasmic reticulum Ca2 + -ATPase 2a(ATP2a2 or Serca2a) is responsible for maintaining intracellular Ca2+ balance. The expression of miR-328 increased during cardiac hypertrophy. It can directly act on Serea2a to reduce its expression, and increase the intracellular Ca2+ concentration to activate CaN-NFATs signaling pathway, then promote cardiac hypertrophy [37].
miR-199a inhibits the expression of GSK313. Overexpression of miR-199a in neonatal rat can increase the size of cardiomyocytes; knocking out miR-199a reduces cardiomyocyte hypertrophy induced by isoproterenol. In mice with overexpressing miR-199a, miR-199a inhibits the expression of GSK3B by binding to the 3′-UTR terminus of GSK3B, which activates the PI3K-AKT-mTOR signaling pathway to attenuate autophagy and promote cardiac hypertrophy [38].
We also discover the up-regulation of MiR-499 in human and mouse hypertrophic hearts. Under cardiac stress overload, the expression of miR-499 is increased, leading to cardiac maladaptation and accelerates the transition to heart failure, via Akt and MAPK targeting the cardiac kinase and phosphatase pathways [39].
The up-regulated expression of the miR-212/132 family results in cardiac hypertrophy, heart failure, and death through regulation of their target gene FOXO3 and its subsequent alteration of calcineurin-NFAT signaling. Thus, in a genetic animal model or animals treated with antagomir, the reduction of miR-212/132 inhibits cardiac hypertrophic growth [40].
2.3 Controversial microRNA in Cardiac Hypertrophy
The role of miR-21 in cardiac hypertrophy is still controversial. Studies have shown that the expression of miRNA-21 in myocardial tissue under pressure load continues to increase [41]. In cardiomyocytes whose miRNA-21 gene is knocked out, cell proliferation and embryonic gene expression induced by factors that promote cardiac hypertrophy were both inhibited. MiRNA-21 may promote cardiomyocyte proliferation by regulating the expression of SPRY2 protein as the inhibitor of the mitogen-activated protein kinase MAPK [42]. However, in neonatal rat cardiomyocytes, inhibiting the expression of miR-21 can prevent cardiomyocyte hypertrophy caused by adrenal and angiotensin 2 [43]. Some studies show that, in the regulation of cardiac hypertrophy, miR-21 does not directly regulate the target but regulate it by an indirect mechanism [44].
3 Long Non-coding RNAs in Cardiac Hypertrophy
Long non-coding RNA (lncRNA) is a class of pseudogenes (about 200 nucleotides in length) that lose the function of protein coding. It belongs to the non-coding RNA family and its diversity and complexity in function is determined by its high heterogeneity in sequence structure [45]. By targeting promoters, enhancers and insulators as a cis- or trans- functional regulatory element, lncRNA is the central component to regulate and modify epigenetics, regulate alleles (genomic imprinting) and regulate transcription/transcriptional genes [46]. Studies have found that changes in lncRNA structure or expression levels can cause many diseases by affecting gene expressions and the regulatory of signaling pathways. More and more scholars have begun to study long-chain non-coding RNAs that affect myocardial function and value their pathophysiological effects in the heart [47].
3.1 Myosin Heavy Chain Associated RNA Transcripts (Mhrt)
Long non-coding RNA Mhrt is an antisense transcript of myosin heavy chain 7(Myh7) [48]. In mouse myocardium with Pre-overexpression of Mhrt and pathological stimulation, we find the progression of cardiac hypertrophy becomes slow, suggesting that Mhrt has a protective effect on the heart. During this process, Mhrt achieves it by inhibiting cardiac stress-activated chromatin remodeling factor (Brg1). First, Mhrt recognizes the target gene of Brg1 and represses its abnormal gene expression under pathological stimulation (inhibit the pathological conversion of α-MHC to β-MHC). At the same time, Mhrt inhibits chromatin remodeling by competitively inhibiting the combination of chromatinized NDA and Brg1, thereby inhibiting cardiac hypertrophy.
3.2 Chaer (Cardiac-Hypertrophy-Associated Epigenetic Regulator)
Chaer affects the function of the PRC2 sequence, rendering PRC2 unable to target its genomic locus, thereby inhibiting the methylation of histone H3 lysine 27 on the promoter region of genes associated with cardiac hypertrophy. Studies have shown that, by inhibiting the expression of Chaer in the heart, it can significantly reduce cardiac hypertrophy and myocardial dysfunction caused by stress stimulation. Chaer and PRC2 can be transiently induced to interact with each other under the stimulation of hormones, which is one of the prerequisites for epigenetic reprogramming and related pathological gene expression in the occurrence of cardiac hypertrophy [49].
3.3 Chast (Cardiac-Hypertrophy-Associated Transcript)
In a model of cardiac hypertrophy in the mouse with thoracic aortic coarctation, the expression of Chast in cardiomyocytes is specifically up-regulated. The expression of this lncRNA is rising in cardiac tissue derived from human aortic stenosis and cardiomyocytes derived from human embryonic stem cell under hypertrophic irritation. The overexpression of Chast in cell and animal models with cardiac hypertrophy is sufficient to induce cardiomyocyte hypertrophy, while the silence of Chast can prevent and reverse pathological cardiac remodeling induced by pressure overload. The mechanism is to activate Chast by NFST which is the factor of promoting hypertrophic transcrition, and up-regulate the expression of the Plekhm1 protein (Plekhm1, also known as platelet-leukocyte C kinase substrate) of the autophagy regulator protein family M member 1, then block the myocardium cell autophagy [50].
3.4 Cardiac Hypertrophy Related Factor (CHRF)
The expression of cardiac hypertrophy related factor (CHRF) is up-regulated in the mouse heart with transverse aortic coarctation and the human samples with heart failure, it is also extensively expressed in cardiovascular cells and has peculiar functions in cardiomyocytes. CHRF induces cardiomyocyte hypertrophy and apoptosis by acting as a sponge of miRNA-489. By chelating with miR-489, CHFR up-regulates its target gene, myeloid differentiation primary response gene (Myd88), and induces cardiac hypertrophy through NFkB pathway [51]. In addition, studies have found that CHRF inhibits the expression of miR-93 by direct interaction, and the inhibition of miR-93 attenuates the anti-hypertrophic response mediated by si-CHRF in Iso-treated cardiomyocytes. miR-93 blocks the hypertrophy induced by Iso, which can be reversed by exogenous overexpression of Akt3 [52]. Study finds that the persistent overexpression of Akt3 triggers systolic dysfunction and enhances the sensitivity to injury for heart, ultimately making adaptive hypertrophy evolve into maladaptive hypertrophy [53]. In summary, at least, CHRF promotes cardiac hypertrophy by partially modulating the miR-93 / Akt3 axis in Iso-induced cardiomyocytes. These studies connect the effects of long non-coding RNA, microRNA and its downstream target genes, inflammatory signaling pathways and so on, by the specific combination role of long non-coding RNA, which is the latest discovery of the mechanism of cardiac hypertrophy.
3.5 Long Non-coding RNA H19
It is up-regulated in the cardiac hypertrophy model of mouse with thoracic aortic coarctation, and the silence of H19 or microRNA-675 in primary cardiomyocytes of mouse can both lead to cardiomyocyte hypertrophy. The overexpression of microRNA-675 can reverse the cell hypertrophy induced by the knockdown of H19, but the overexpression of H19 and knockdown of microRNA-675 can not inhibit cardiomyocyte hypertrophy. Thus, it is confirmed that long non-coding RNA H19 can inhibit cardiac hypertrophy by regulating microRNA-675. And it is determined that Ca/calmodulin-dependent protein kinase IIδ (CaMKIIδ) is the direct target of microRNA-675, and partially mediating the effect of H19 on cardiomyocyte hypertrophy. It reveals a new function of H19-microRNA-675 axis targeting CaMKIIδ as a negative regulator of cardiac hypertrophy, which shows its potential therapeutic effects in heart disease [54].
3.6 Long Non-coding RNA ROR (Reprogramming Regulator)
Long non-coding RNA ROR, which is up-regulated during cardiac hypertrophy, is also involved in the occurrence and development of cardiac hypertrophy. ROR has been verified to regulate reprogramming and inhibit the damage from P53 to DNA. Its function is to promote the occurrence of cardiac hypertrophy by adsorbing microRNA-133 [55].
3.7 Long Non-coding RNA TINCR (Terminal Differentiation Inducing Non-coding RNA)
TINCR is down-regulated in a mouse model with aortic coarctation. While up-regulating TINCR can reduce cardiac hypertrophy. It was also found that primary cardiomyocyte hypertrophy caused by angiotensin II (Ang II) in blood culture was associated with the decreased expression of TINCR. TINCR can directly combine with EZH2 in cardiomyocytes, and EZH2 can directly combine with the promoter region of CaMKII, which mediates the modification of h3k27me3. So the knock-down of TINCR can reduce its combination with EZH2 and decrease the combination between CaMKII promoter and h3k27me3 in cardiomyocytes. Furthermore, the enhanced expression of TINCR can reduce the expression of CaMKII and attenuated cardiomyocyte hypertrophy induced by Ang II. TINCR can alleviate cardiac hypertrophy by epigenetic silencing of CaMKII, which may provide a new therapeutic strategy for cardiac hypertrophy [56].
3.8 Long Non-coding RNA HOX Transcript Antisense RNA (HOTAIR)
HOTAIR facilitates the pathogenic mechanism of cardiac hypertrophy mainly by functioning as a miRNA sponge to derepress the miRNA target mRNAs in the ceRNA regulatory network. It may serve as a ceRNA for miR-19 to modulate the dis-inhibition of its endogenous target phosphatase and tensin homolog (PTEN) and attenuate cardiac hypertrophy progress [57].
3.9 Long Non-coding RNA MIAT
LncRNA myocardial infarction-associated transcript (Miat), MIAT was upregulated while miR-93 was downregulated in cardiac hypertrophy induced by Ang-II, and the expressions of the hypertrophic markers including ANF and β-MHC were increased. Knockdown of MIAT inhibited AngII-induced cardiac hypertrophy by decreasing cell surface area and lowering the expressions of ANF and β-MHC. It has been varified that TLR4 as a target of miR-93, and MIAT acted as a ceRNA to up-regulate TLR4 expression by sponging miR-93 in cardiac hypertrophy. The over-expression of TLR4 facilitated AngII-induced cardiac hypertrophy through PI3K/Akt/mTOR pathway. Knockdown of MIAT inhibited AngII-induced cardiac hypertrophy by regulating miR-93/TLR4 axis. It clarifies a potential therapy target for cardiac hypertrophy [58]. In addition, it was identified that MIAT which was increased in AngII-induced cardiac hypertrophy was contributed to the pathological process of cardiac hypertrophy by sponging miR-150 [59].
4 Circular RNAs in Cardiac Hypertrophy
Circular RNAs (circRNAs) is a kind of special noncoding RNAs (ncRNAs). Unlike linear RNAs, circRNAs have a covalently closed circular structure and, lacking of both 5’ and 3’polarity and a poli-A tail [60]. The current study suggests that circRNAs have stable structure and high conservation, and have tissue-specific and developmental stage-specific expression [61]. Studies have found that circRNAs have the following functions: the sponge of microRNAs [62, 63]; regulating cleavage or transcription [64, 65]; regulating gene expression by interacting with RNA binding proteins (RBPs) [66, 67]. Recently, they have been received many attentions in in many processes, including ageing, cancer, cardiovascular diseases and tissue development [60].
4.1 Heart-Related circRNA (HRCR)
As an endogenous sponge of miR-223, HRCR can inhibit cardiac hypertrophy by adsorbing miR-223 and inhibiting the action of miR-223. miR-223 can induce cardiac hypertrophy by modulating the apoptotic repressor with CARD domain (ARC). ARC can inhibit cardiac hypertrophy, which is high-expressive in myocardium and skeletal muscle cells. ARC is a downstream target of miR-223. And HRCR inhibits the activity of miR-223 by adsorbing miR-223, resulting in the increased expression of cytoskeleton-associated protein ARC which targeting gene activity in downstream increased, it is associated with mitigating cardiac hypertrophy induced by stress overload [62], a novel regulatory pathway consisting of HRCR, miR-223 and ARC. Regulating their levels provides promising therapeutic targets for the treatment of cardiac hypertrophy.
4.2 Circular RNA ciRS-7/CDR1as
ciRS-7/CDR1as has the binding sites of miR-7 up to 70 and can adsorb miR-7 to inhibit its biological function [62]. miR-7a inhibits cardiomyocyte apoptosis by inhibiting the expression of PARP (poly ADP-ribose polymerase) and transcription factor SP1; ciRS-7 inhibits the action of miR-7a by adsorbing it. The up-regulated PARP and SP1, which aggravates the apoptosis of myocardial cells after myocardial infarction, can be reversed by the overexpression of miR-7a and reduce cardiac hypertrophy [68].
4.3 Circular RNA circ-Foxo3
In the mouse model of cardiac hypertrophy stimulated by doxorubicin, the extremely high expression of circ-Foxo3 can aggravate myocardial lesions induced by doxorubicin, and the inhibition of circ-Foxo3 expression can inhibit aging of mouse embryonic fibroblasts, while the abnormally high expression of circ-Foxo3 can promote the aging of mouse embryonic fibroblasts. Circ-Foxo3, which is mainly distributed in the cytoplasm, takes effect by combining with the aging-related proteins ID-1, E2F1 and stress-related proteins FAK and HIF1α. The expression of ID-1, E2F1, FAK, and HIF1αis inhibited by the up-regulation of circ-Foxo3. The decreased expression of these anti-aging proteins can accelerate myocardial cell aging. However the down-regulation of circ-Foxo3 can inhibit cardiomyocyte and apoptosis again, and can reduce cardiac hypertrophy [69].
5 Other Non-coding RNAs in Cardiac Hypertrophy
A new class of small RNAs, tRFs (tRNA-derived fragments), are produced by stress-released ribonuclease cleaves mature tRNA into fragments. Study has been reported that lots of stress conditions can specifically induce tRNA cleavage [70]. Besides, tRFs could function as a paternal epigenetic factor in sperm, and mediate the intergenerational inheritance of paternal disease [71]. Other studies also revealed that tRFs could serve as small interfering RNA that modulated diverse biological processes [72]. In a model of typical cardiac hypertrophy induced by isoproterenol, the tRFs were extremely enriched (84%) in the hypertrophic heart. tRFs1 and tRFs2 overexpression would both increase cardiomyocytes area and elevation the expression of hypertrophic markers (ANF, BNP, and β-MHC) through target 3’UTR of Timp3. Besides, tRFs1, tRFs2, tRFs3, and tRFs4 were highly expressed in Hyp F0 sperm and in Hyp F1 offspring hearts. Compared to Con F1 offspring, Hyp F1 offspring had elevated expression levels of β-MHC and ANP genes, as well as increased cardiac fibrosis and apoptosis. These data revealed that tRFs are involved in regulating the response of myocardial hypertrophy. Also, tRFs might serve as novel epigenetic factors that contribute to the intergenerational inheritance of cardiac hypertrophy [73].
6 Intercellular Delivery of Non-coding RNA in Cardiac Hypertrophy
Different Cell types cross-talk with each other and create specific microenvironments to share resources that are essential to maintain homeostasis and respond to external stimuli. To gain deep views into the molecular mechanisms underlying pathological cardiac hypertrophy, the contribution of cell to cell communication in the heart related to this process must be taken into consideration. More researches have been focused on extracellular vesicles (EVs) that allow long-range cellular communication. EVs are secreted by cells and act as transport vehicles for a lot of small molecules like mRNA, miRNAs, lncRNAs, small amounts of DNA, as well as low molecular weight lipids and proteins [74, 75]. EVs can be classified in three different subgroups: microvesicles (MVs) (0.1–1 μm), exosomes (20–100 nm) and apoptotic bodies (ABs) (0.5–2 μm). All major cardiac cell types, including cardiomyocytes, endothelial cells and fibroblasts, can release exosomes to modulate recipient cellular functions under physiological and pathological conditions, and might hence be involved in the process of cardiomyocyte hypertrophy.
6.1 Cardiomyocytes and Endothelial Cells
In the heart, cardiomyocytes-derived exosomes can lead to different metabolic functions when taken in by different cells [76]. In the glucose deprivation conditions, both the number and the contents of cardiomyocytes secreted exosomes are markedly differing from the normal glucose conditions [77]. Cardiomyocytes can exchange the exosomes’ content with the recipient cells and therefore affecting their angiogenesis. Besides, in starved conditions, on one side, a class of miRNAs which have pro-angiogenic effects are enriched in exosomes derived from starved conditions in ECs. On the other side, cardiomyocytes could also absorbed ECs secreted exosomes and subsequently affect their physiological functions and responsive mechanisms to stress. As in women suffered from peripartum cardiomyopathy (PPCM), the anti-angiogenic 16-kDa N-terminal prolactin fragment (16 K PRL) acts on ECs, inducing the release of miR-146a-enriched exosomes. These released exosomes could be absorbed by cardiomyocytes, and consequently, increased miR-146a will affect the physiological metabolism of cardiomyocytes, leading to the development of hypertrophy [78].
6.2 Cardiac Fibroblasts and Cardiomyocytes
Fibroblasts would secrete exosomes to induce the expression of angiotensin and its receptor (AT1R and AT2R) in cardiomyocytes while stimulated with angiotensin II. These could finally cause hypertrophic cell growth. Therefore, AT1R and AT2R antagonists or with exosome inhibitors could both attenuate this exosome-induced effect. Besides, fibroblasts can cross-talk with cardiomyocytes via paracrine effects. Specifically, release the exosomes which contain the passenger strand of miR-21 (miR-21∗) and absorbed by cardiomyocytes. In cardiomyocytes, miR-21∗ induces cardiac hypertrophy by down-regulating sorbin and SH3 domain containing 2 (SORBS2) or PDZ and LIM domain 5 (PDLIM5), both involved in regulation of cardiac muscle structure and function [79].
6.3 Immune Cells and Cardiomyocytes
Mir-155 has been demonstrated to modulate pathological cardiac hypertrophy, while miR-155 knockout mice could prevent mice hearts from this pathological process. It has been reported that this benefit effect are due to the decreased miR-155 level in macrophages rather than in cardiomyocytes [80]. miR-155-deficient macrophages could prevent this hypertrophic phenotype through a paracrine effect.
7 Perspective
Cardiovascular disease (CVD) is a common disease which poses a serious threat to human health and it is characterized by high prevalence, high disability and high mortality. It imposes a heavy burden on society and family. Myocardial hypertrophy (MH) is a common pathological process of various cardiovascular diseases and is considered to be an independent risk factor for increased cardiovascular morbidity and mortality. Therefore, understanding its pathological mechanism and treatment is particularly important. However, the pathogenesis of cardiac hypertrophy is complicated, and there is still no thorough research.
In recent years, ncRNAs have been reported to take important parts in pathophysiological processes, and abnormal expression of ncRNAs in different cardiac cell types being associated with many cardiovascular abnormalities. The types of ncRNAs involved in different kind of cardiovascular disease are not unique. Different cardiovascular diseases may also be regulated by the same ncRNAs. Extracellular vesicles (EV) secreted by cells play an important role in cell-to-cell communication. A more detailed understanding of the relationship between ncRNAs can help us find the key ncRNAs of this network of action, provide a target for precise treatment, and may also become a marker for clinical disease diagnosis, reflecting the progress of the disease earlier and more accurately. NcRNAs have promising therapeutic potential. Using antisense oligonucleotides to inhibit the ncRNAs may bring hope to the treatment of the disease, but currently the technical means are still immature, and there are still some problems left to be solved. There are many factors regulating the development of cardiac hypertrophy, and NcRNAs are only a part of them. There are also mutual promotions or inhibition between ncRNAs with other molecules. Defining these relationships will inspire us to understand the mechanism of cardiac hypertrophy better, and it also will be beneficial to provide a sufficient theoretical basis for clinical diagnosis and treatment.
References
Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114(3):565–71.
Mohamed BA, Asif AR, Schnelle M, Qasim M, Khadjeh S, Lbik D, Schott P, Hasenfuss G, Toischer K. Proteomic analysis of short-term preload-induced eccentric cardiac hypertrophy. J Transl Med. 2016;14(1):149.
Maack C. The cardiac re-AKT-ion to chronic volume overload. Eur J Heart Fail. 2016;18(4):372–4.
Ferrario CM. Cardiac remodelling and RAS inhibition. Ther Adv Cardiovasc Dis. 2016;10(3):162–71.
Pires A, Martins P, Pereira AM, Silva PV, Marinho J, Marques M, Castela E, Sena C, Seica R. Insulin resistance, dyslipidemia and cardiovascular changes in a group of obese children. Arq Bras Cardiol. 2015;104(4):266–73.
Houstek J, Vrbacky M, Hejzlarova K, Zidek V, Landa V, Silhavy J, Simakova M, Mlejnek P, Kazdova L, Miksik I, Neckar J, Papousek F, Kolar F, Kurtz TW, Pravenec M. Effects of mtDNA in SHR-mtF344 versus SHR conplastic strains on reduced OXPHOS enzyme levels, insulin resistance, cardiac hypertrophy, and systolic dysfunction. Physiol Genomics. 2014;46(18):671–8.
Hou J, Kang YJ. Regression of pathological cardiac hypertrophy: signaling pathways and therapeutic targets. Aliment Pharmacol Ther. 2012;135(3):337–54.
Zakharov P, Dewarrat F, Caduff A, Talary MS. The effect of blood content on the optical and dielectric skin properties. Physiol Meas. 2011;32(1):131–49.
Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol. 2015;89(9):1401–38.
Lyon RC, Zanella F, Omens JH, Sheikh F. Mechanotransduction in cardiac hypertrophy and failure. Circ Res. 2015;116(8):1462–76.
Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10(2):94–108.
Condorelli G, Latronico MV, Cavarretta E. microRNAs in cardiovascular diseases: current knowledge and the road ahead. J Am Coll Cardiol. 2014;63(21):2177–87.
Braunwald E. The war against heart failure: the lancet lecture. Lancet. 2015;385(9970):812–24.
Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100(8):1164–73.
Wang N, Zhou Z, Liao X, Zhang T. Role of microRNAs in cardiac hypertrophy and heart failure. IUBMB Life. 2009;61(6):566–71.
Karakikes I, Chaanine AH, Kang S, Mukete BN, Jeong D, Zhang S, Hajjar RJ, Lebeche D. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2013;2(2):e000078.
Ikeda S, He A, Kong SW, Lu J, Bejar R, Bodyak N, Lee KH, Ma Q, Kang PM, Golub TR, Pu WT. MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol Cell Biol. 2009;29(8):2193–204.
Elia L, Contu R, Quintavalle M, Varrone F, Chimenti C, Russo MA, Cimino V, De Marinis L, Frustaci A, Catalucci D, Condorelli G. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation. 2009;120(23):2377–85.
Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104(2):170–8.. 176p following 178
Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13(5):613–8.
Li Q, Lin X, Yang X, Chang J. NFATc4 is negatively regulated in miR-133a-mediated cardiomyocyte hypertrophic repression. Am J Phys Heart Circ Phys. 2010;298(5):H1340–7.
Castaldi A, Zaglia T, Di Mauro V, Carullo P, Viggiani G, Borile G, Di Stefano B, Schiattarella GG, Gualazzi MG, Elia L, Stirparo GG, Colorito ML, Pironti G, Kunderfranco P, Esposito G, Bang ML, Mongillo M, Condorelli G, Catalucci D. MicroRNA-133 modulates the beta1-adrenergic receptor transduction cascade. Circ Res. 2014;115(2):273–83.
Ganesan J, Ramanujam D, Sassi Y, Ahles A, Jentzsch C, Werfel S, Leierseder S, Loyer X, Giacca M, Zentilin L, Thum T, Laggerbauer B, Engelhardt S. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation. 2013;127(21):2097–106.
Knezevic I, Patel A, Sundaresan NR, Gupta MP, Solaro RJ, Nagalingam RS, Gupta M. A novel cardiomyocyte-enriched microRNA, miR-378, targets insulin-like growth factor 1 receptor: implications in postnatal cardiac remodeling and cell survival. J Biol Chem. 2012;287(16):12913–26.
Wang K, Long B, Zhou J, Li PF. miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. J Biol Chem. 2010;285(16):11903–12.
Yang Y, Ago T, Zhai P, Abdellatif M, Sadoshima J. Thioredoxin 1 negatively regulates angiotensin II-induced cardiac hypertrophy through upregulation of miR-98/let-7. Circ Res. 2011;108(3):305–13.
Han M, Yang Z, Sayed D, He M, Gao S, Lin L, Yoon S, Abdellatif M. GATA4 expression is primarily regulated via a miR-26b-dependent post-transcriptional mechanism during cardiac hypertrophy. Cardiovasc Res. 2012;93(4):645–54.
Kee HJ, Kook H. Roles and targets of class I and IIa histone deacetylases in cardiac hypertrophy. J Biomed Biotechnol. 2011;2011:928326.
Li P, Hao Y, Pan FH, Zhang M, Ma JQ, Zhu DL. SGK1 inhibitor reverses hyperglycemia partly through decreasing glucose absorption. J Mol Endocrinol. 2016;56(4):301–9.
Hu ZQ, Luo JF, Yu XJ, Zhu JN, Huang L, Yang J, Fu YH, Li T, Xue YM, Feng YQ, Shan ZX. Targeting myocyte-specific enhancer factor 2D contributes to the suppression of cardiac hypertrophic growth by miR-92b-3p in mice. Oncotarget. 2017;8(54):92079–89.
Ge Y, Pan S, Guan D, Yin H, Fan Y, Liu J, Zhang S, Zhang H, Feng L, Wang Y, Xu R, Yin JQ. MicroRNA-350 induces pathological heart hypertrophy by repressing both p38 and JNK pathways. Biochim Biophys Acta, Mol Cell Res. 2013;1832(1):1–10.
Yang Y, Del Re DP, Nakano N, Sciarretta S, Zhai P, Park J, Sayed D, Shirakabe A, Matsushima S, Park Y, Tian B, Abdellatif M, Sadoshima J. miR-206 mediates YAP-induced cardiac hypertrophy and survival. Circ Res. 2015;117(10):891–904.
van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103(48):18255–60.
Chen H, Untiveros GM, McKee LA, Perez J, Li J, Antin PB, Konhilas JP. Micro-RNA-195 and -451 regulate the LKB1/AMPK signaling axis by targeting MO25. PLoS One. 2012;7(7):e41574.
Huang X, Li Z, Bai B, Li X, Li Z. High expression of microRNA-208 is associated with cardiac hypertrophy via the negative regulation of the sex-determining region Y-box 6 protein. Exp Ther Med. 2015;10(3):921–6.
Song DW, Ryu JY, Kim JO, Kwon EJ, Kim DH. The miR-19a/b family positively regulates cardiomyocyte hypertrophy by targeting atrogin-1 and MuRF-1. Biochem J. 2014;457(1):151–62.
Li C, Li X, Gao X, Zhang R, Zhang Y, Liang H, Xu C, Du W, Zhang Y, Liu X, Ma N, Xu Z, Wang L, Chen X, Lu Y, Ju J, Yang B, Shan H. MicroRNA-328 as a regulator of cardiac hypertrophy. Int J Cardiol. 2014;173(2):268–76.
Xydous M, Prombona A, Sourlingas TG. Corrigendum to “the role of h3k4me3 and H3K9/14ac in the induction by dexamethasone of Per1 and Sgk1, two glucocorticoid early response genes that mediate the effects of acute stress in mammals” [Biochim Biophys Acta 1839 (2014) 866–872]. Biochimica et Biophysica Acta Gene Regul Mech. 2017;1860(3):392.
Matkovich SJ, Hu Y, Eschenbacher WH, Dorn LE, Dorn GW 2nd. Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy. Circ Res. 2012;111(5):521–31.
Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun. 2012;3:1078.
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–4.
Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170(6):1831–40.
Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, Rojas M, Hammond SM, Wang DZ. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42(6):1137–41.
Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3(3):251–5.
Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation. 2014;129(10):1139–51.
Nakagawa S. Lessons from reverse-genetic studies of lncRNAs. BBA-Biomembranes. 2016;1859(1):177–83.
Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152(3):570–83.
Han P, Li W, Lin CH, Yang J, Shang C, Nuernberg ST, Jin KK, Xu W, Lin CY, Lin CJ, Xiong Y, Chien H, Zhou B, Ashley E, Bernstein D, Chen PS, Chen HV, Quertermous T, Chang CP. A long noncoding RNA protects the heart from pathological hypertrophy. Nature. 2014;514(7520):102–6.
Wang Z, Zhang XJ, Ji YX, Zhang P, Deng KQ, Gong J, Ren S, Wang X, Chen I, Wang H, Gao C, Yokota T, Ang YS, Li S, Cass A, Vondriska TM, Li G, Deb A, Srivastava D, Yang HT, Xiao X, Li H, Wang Y. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat Med. 2016;22(10):1131–9.
Viereck J, Kumarswamy R, Foinquinos A, Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, Remke J, Just A, Fendrich J, Scherf K, Bolesani E, Schambach A, Weidemann F, Zweigerdt R, de Windt LJ, Engelhardt S, Dandekar T, Batkai S, Thum T. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med. 2016;8(326):326ra322.
Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, Li PF. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res. 2014;114(9):1377–88.
Wo Y, Guo J, Li P, Yang H, Wo J. Long non-coding RNA CHRF facilitates cardiac hypertrophy through regulating Akt3 via miR-93. Cardiovasc Pathol. 2018;35:29–36.
Taniyama Y, Ito M, Sato K, Kuester C, Veit K, Tremp G, Liao R, Colucci WS, Ivashchenko Y, Walsh K, Shiojima I. Akt3 overexpression in the heart results in progression from adaptive to maladaptive hypertrophy. J Mol Cell Cardiol. 2005;38(2):375–85.
Liu L, An X, Li Z, Song Y, Li L, Zuo S, Liu N, Yang G, Wang H, Cheng X, Zhang Y, Yang X, Wang J. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc Res. 2016;111(1):56–65.
Jiang F, Zhou X, Huang J. Long non-coding RNA-ROR mediates the reprogramming in cardiac hypertrophy. PLoS One. 2016;11(4):e0152767.
Shao M, Chen G, Lv F, Liu Y, Tian H, Tao R, Jiang R, Zhang W, Zhuo C. LncRNA TINCR attenuates cardiac hypertrophy by epigenetically silencing CaMKII. Oncotarget. 2017;8(29):47565–73.
Lai Y, He S, Ma L, Lin H, Ren B, Ma J, Zhu X, Zhuang S. HOTAIR functions as a competing endogenous RNA to regulate PTEN expression by inhibiting miR-19 in cardiac hypertrophy. Mol Cell Biochem. 2017;432(1–2):179–87.
Li Y, Wang J, Sun L, Zhu S. LncRNA myocardial infarction-associated transcript (MIAT) contributed to cardiac hypertrophy by regulating TLR4 via miR-93. Eur J Pharmacol. 2018;818:508–17.
Zhu XH, Yuan YX, Rao SL, Wang P. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur J Pharmacol. 2016;20(17):3653–60.
Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365(2):141–8.
Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160(6):1125–34.
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–8.
Li F, Zhang L, Li W, Deng J, Zheng J, An M, Lu J, Zhou Y. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/beta-catenin pathway. Oncotarget. 2015;6(8):6001–13.
Yang W, Du WW, Li X, Yee AJ, Yang BB. Foxo3 activity promoted by non-coding effects of circular RNA and Foxo3 pseudogene in the inhibition of tumor growth and angiogenesis. Oncogene. 2016;35(30):3919–31.
Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, Zhou LY, Sun T, Wang M, Yu T, Gong Y, Liu J, Dong YH, Li N, Li PF. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016;37(33):2602–11.
Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, Zhu P, Chang Z, Wu Q, Zhao Y, Jia Y, Xu P, Liu H, Shan G. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22(3):256–64.
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44(6):2846–58.
Geng HH, Li R, Su YM, Xiao J, Pan M, Cai XX, Ji XP. The circular RNA Cdr1as promotes myocardial infarction by mediating the regulation of miR-7a on its target genes expression. PLoS One. 2016;11(3):e0151753.
Du WW, Yang W, Chen Y, Wu ZK, Foster FS, Yang Z, Li X, Yang BB. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J. 2017;38(18):1402–12.
Selitsky SR, Baran-Gale J, Honda M, Yamane D, Masaki T, Fannin EE, Guerra B, Shirasaki T, Shimakami T, Kaneko S, Lanford RE, Lemon SM, Sethupathy P. Small tRNA-derived RNAs are increased and more abundant than microRNAs in chronic hepatitis B and C. Sci Rep. 2015;5:7675.
Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, Feng GH, Peng H, Zhang X, Zhang Y, Qian J, Duan E, Zhai Q, Zhou Q. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351(6271):397–400.
Sobala A, Hutvagner G. Small RNAs derived from the 5′ end of tRNA can inhibit protein translation in human cells. RNA Biol. 2013;10(4):553–63.
Shen L, Gan M, Tan Z, Jiang D, Jiang Y, Li M, Wang J, Li X, Zhang S, Zhu L. A novel class of tRNA-derived small non-coding RNAs respond to myocardial hypertrophy and contribute to intergenerational inheritance. Biomolecules. 2018;8(3):54.
S ELA, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–57.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Waldenstrom A, Genneback N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012;7(4):e34653.
Garcia NA, Ontoria-Oviedo I, Gonzalez-King H, Diez-Juan A, Sepulveda P. Glucose starvation in Cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One. 2015;10(9):e0138849.
Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Investig. 2013;123(5):2143–54.
Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Investig. 2014;124(5):2136–46.
Heymans S, Corsten MF, Verhesen W, Carai P, van Leeuwen RE, Custers K, Peters T, Hazebroek M, Stoger L, Wijnands E, Janssen BJ, Creemers EE, Pinto YM, Grimm D, Schurmann N, Vigorito E, Thum T, Stassen F, Yin X, Mayr M, de Windt LJ, Lutgens E, Wouters K, de Winther MP, Zacchigna S, Giacca M, van Bilsen M, Papageorgiou AP, Schroen B. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation. 2013;128(13):1420–32.
Competing Financial Interests
The authors declare no competing finicial interests
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
He, J., Luo, Y., Song, J., Tan, T., Zhu, H. (2020). Non-coding RNAs and Pathological Cardiac Hypertrophy. In: Xiao, J. (eds) Non-coding RNAs in Cardiovascular Diseases. Advances in Experimental Medicine and Biology, vol 1229. Springer, Singapore. https://doi.org/10.1007/978-981-15-1671-9_13
Download citation
DOI: https://doi.org/10.1007/978-981-15-1671-9_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1670-2
Online ISBN: 978-981-15-1671-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)