Abstract
Photocatalysis as a technology does satisfy the criteria as a clean technology in application and is suitable for the degradation of petrochemical pollutants. An ideal photocatalyst is expected to conform to properties of photoactivity, biological and chemical inertness, stability toward photo-corrosion, suitable for visible or near UV light energy harnessing, be low cost and be nontoxic in nature. The high stability of TiO2 allows diverse applications such as in electro-ceramics, glass and in photocatalytic degradation of chemicals in water and air. The oxide particles can be used in the form of suspensions in slurry reactors as well as thin film coating agents. The suspended photocatalyst has been demonstrated to be very efficient degrading different classes of organic compounds. The major concern of the suspended photocatalyst system is the inability to reclaim the semiconductor catalyst in suspended slurry-type applications. This drawback has been addressed in various ways through innovative developments, which are specifically aimed at addressing this issue.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
In modern society, one of the biggest commercial global industries is the petroleum industry, also commonly referred to as the oil and gas industry. The petroleum industry constitutes of many commercial products, including gasoline, natural gas, naphtha, fuel and lubricating oils, asphalt, paraffin wax, kerosene, and many more chemicals that are produced from petroleum. These chemicals are applied in the manufacture of a large number of consumer products from different sectors, such as the pharmaceuticals, electronics, motor vehicle, health care, household, and home-based manufacturers among others. Petrochemicals are so versatile that they are found in many of the facets of the human environment, these include medicine (where they are used in the production of resins, films, and plastics) (Hess et al. 2011; Frumkin et al. 2007), food (where they are used in the manufacture of food preservatives) (Neff et al. 2011), agriculture (where they are used in plastic sheeting, in pesticides, and fertilizers), household products (where they are used in many ways in the forms of films, rubbers, fibers, and plastics).
It is apparent from the above information that the petrochemical industry performs an important function in the betterment of human society through activities applied in diverse sectors. The major concern proceeding the utilization of the petrochemical functions and the end-user products is their removal from the environment once the many consumer cycles have run their course. Petrochemical industrial activities and the waste associated with it result in significant amounts of harmful substances being transferred to the environment through liquid waste discharges, solid waste and through the atmosphere (Mechhoud et al. 2016; Chen et al. 2014). The harmful petrochemical pollutant substances can pose serious risks to human health and wildlife through different exposure (Álvarez et al. 2016; Haghollahi et al. 2016; Zolfaghari et al. 2016; Hu et al. 2013; Zhong and Zhu 2013). There have been many documentations of exposure to petroleum product’s harmful substances leading to severe health conditions such as organ and tissue damage in the form of liver, nerve, birth defects, cancer, asthma, brain and hormonal disorders among many more (Bustillo-Lecompte et al. 2018; Axelsson et al. 2010; Belli et al. 2004; Luginaah et al. 2002; Lin et al. 2001a, b).
Environmental contamination by petrochemical pollutants from industrial chemical plant activities most predominantly occurs through effluent discharges in the form of wastewater. This effluent is formally referred to as industrial discharge and constitutes of the waste liquid matrices produced in the chemical plant’s processes, which contains industrial production materials, intermediate compounds products, and the toxic substances produced as part of the production process. The petrochemical industry boasts a diverse repertoire of products that are produced using many different reaction schemes. For this reason, industrial processes discharge wastewater that is made up of a huge range and variety of chemical components, some of which may be resistant to natural degradation processes (Liang et al. 2019; Varjani et al. 2017; Xiaoqiang et al. 2019).
8.2 Petrochemical Pollutants
Petrochemical pollutants are chemical contaminants that are typically present in effluent matrices discharged from industrial activities associated with fossil fuels refinery processes by chemical plants. Various types of chemical plants processes are utilized in efforts to process natural materials toward reusable chemical products. Some of the natural materials converted to chemical products for commercial applications include minerals, metals, natural gases, air, water, and fossil fuel oil. Petrochemical pollutant chemical compounds specifically result from the conversion of natural gas liquids through cracking and distillation processes and through the extraction processes of crude oil. In essence, petrochemicals can be classified as compounds sourced from the hydrocarbons of natural gases and petroleum fractions.
Typical extraction and conversion process from the natural forms of these hydrocarbons toward the desired end products are complex and require many chemical reaction steps. Each of these steps results in the production of multiple and various types of chemical by-products that represent many different chemical groups. Commonly, petrochemicals can be classified into two groups. These classification groups are known as the olefins and the aromatics. Olefins, also termed alkenes, are hydrocarbon chemical species that only contain carbon–carbon double bonds, whereas the alkanes constitute of single bonds (Bruice 2004). Petrochemical olefins include chemical compounds such as propylene, butadiene and ethylene. Aromatic compounds are associated with benzene as the base compound, which has a six-carbon π system in a cyclic form (Bruice 2004). Petrochemical aromatics include chemical compounds such as benzene, xylene and its isomers, ethylbenzene, and toluene.
The above described petrochemical compounds are also referred to as primary petrochemicals; these chemical hydrocarbon molecules are further processed to manufacture petrochemical intermediates (Souza et al. 2014). The manufactured petrochemical intermediates and reaction steps process chemical derivatives produced are products of the primary petrochemical starting products and are referred to as secondary petrochemicals. The secondary petrochemical are also further processed to form daughter products; these are collectively referred to downstream petrochemicals. The term downstream simply means that the particular petrochemical compound is reactively formed later in the sequence of chemical reaction events.
8.3 Diversity of Petrochemical Pollutants
Petrochemical pollutants are sourced and can be sourced from many different hydrocarbon starting materials (primary petrochemicals) of the natural environment. The following brief description will detail some of the sources of petrochemicals that are processed toward usable end products. A subsequent acknowledgement is that due to the diversity of the petrochemical groups that are converted from the different primary petrochemicals, the refining processes generate large quantities of petrochemical wastewater, which upon discharge contaminate natural water bodies, and when discharged toward treatment facilities, the sheer complex nature of the chemical intricacies overwhelms most conventional treatment processes, and/or require large amounts of resources to treat and process to acceptable standards. This is one of the primary drivers with regard to seeking cleaner and less resource-intensive technologies in dealing with petrochemical industrial waste.
8.3.1 Petrochemicals from Downstream Products
Industrial wastewater matrices can be immensely complicated due to the high number of different chemical groups derived from the primary petrochemicals, secondary petrochemicals, and the diverse products formed from the reactions of compounds. The following discussion segment will elucidate the nature of this phenomenon by accounting for some of the products formed from downstream petrochemicals such as methane, ethylene, and benzene. The complex nature of treated petrochemical influent poses a real challenge for most conventional treatment installations, and where advanced treatment technologies are in place, the amount of resources required for the discharged product to meet standard regulation stipulated in the various legislations around the world can be costly. The vast number and the various chemical products derived from downstream reaction processes will be evaluated in an attempt to garner appreciation for the technological challenge of treating petrochemical wastewater.
8.3.1.1 Downstream Petrochemical Products from Methane
Methane is derived from petroleum refining processes in large quantities. Many petrochemical compounds from related groups can subsequently be reactively formed. Some of these products include but not limited to:
-
Methyl alcohol—methane is converted to methanol (methyl alcohol) under an oxidative catalyst in the presence of oxygen molecules. Methanol is used in many industrial and commercial products as a versatile solvent. Methanol can also be further oxidized to produce formaldehyde, which is also used as a raw agent for various products.
-
Unsaturated hydrocarbons—these are compound complexes that comprise of double and triple bonds. Through pyrolysis protocols and in the presence of suitable catalysts, methane is converted to unsaturated products such as acetylene ethylene and propylene. These unsaturated hydrocarbons are also the starting compounds for many other downstream products.
-
Chlorinated products—Chlorinated solvents are formed from the substitution of hydrogen molecules from the methane compound. All four carbon–hydrogen bonds can be replaced to form carbon tetrachloride.
-
Hydrogen—the pyrolysis of methane produces hydrogen, the primary application of this form of hydrogen is in the manufacture of ammonia gas. Ammonia is a primary starting compound in the production of many useful products, which themselves serve in the formation of different end-user products
-
Carbon black—this substance is used in the pigment industry in the form of ink and in the rubber industry. This is achieved by heat stripping the hydrogen molecules from methane.
8.3.1.2 Downstream Petrochemical Products from Ethylene
Ethylene is the simplest of the olefins group, derived from the pyrolysis of naphtha. It has a carbon–carbon double bond, deeming it very reactive and easily converted to lower-end petrochemical products. It is predominantly applied in the synthesis of products such as paints and cosmetics among many more (Medianu et al. 2012). The more common petrochemical products derived from ethylene include:
-
Ethyl benzene—this compound is a reaction of benzene and ethylene, the reaction takes place in the presence of a catalyst. The conversion of ethylbenzene to styrene allows the manufacture of polystyrene.
-
Ethyl alcohol—this compound is the product of the hydration of ethylene. It is the solvent used in the manufacture of a large number of compounds, including acetic acid and ethyl acetate.
-
Polyethylene—this compound is polymerized to form plastic materials.
-
Ethylene oxide—ethylene is reactively oxidized to form ethylene oxide, which is used in the production of ethylene glycol.
-
Vinyl chloride—this compound is formed directly from ethylene
-
Ethylene glycol—this compound is used in the manufacture of polyester materials.
-
Dichloromethane—a reaction of ethylene and chloride produces this compound.
8.3.1.3 Down-Stream Petrochemical Products from Benzene
Benzene is primary produced from naphtha through a process of aromatization, where the aliphatic hydrocarbons from the naphtha are used to produce aromatic hydrocarbons. The more common petrochemicals manufactured from benzene include:
-
Chlorobenzene
-
Nitrobenzene
-
Ethyl benzene
-
Cyclohexane
-
Alkyl benzenes (linear and branched)
8.3.1.4 Down-Stream Petrochemical Products from Other Primary Compounds
Butadiene, propylene, and acetylene are the other primary chemical compounds that account for numerous downstream petrochemical compounds. The unsaturated hydrocarbons compounds propylene and acetylene are derived from natural gas, while the two carbon–carbon double-bonded butadiene is derived from naphtha. The more common petrochemical compounds that are produced from these compounds include:
-
Butadiene monomers
-
Acetaldehyde
-
Vinyl acetate
-
Acrylonitrile
-
Glycerol
-
Isopropyl benzene
-
Polypropylene
-
Isopropyl alcohol
The compounds listed above Sect. 8.3.1 are just some of the many prominent petrochemicals that form part of most chemical plant wastewater constituents. Most of the petrochemicals listed are in a lot of cases the starting products for many simple and complex chemical compounds that are classified as pollutants.
8.4 Treatment of Wastewater and Petrochemical Pollutants
The chains of application of petrochemical compounds utilized in industrial operations are seldom confined to the chemical plants. The manufactured products take on many forms and have variety of application intended for the end users. The eventual end users include both the general public and the privatized firms. Privatized firms typically consume and/or convert the raw acquired product into secondary (downstream) products by further reprocessing these in various ways and forms, which in turn lead to the goods being accessed by the final end users, who are the general public and service provider entities. It should therefore be understood that petrochemical pollution emanating from the manufactured and consumed products exists in the natural environment, meaning that the issue of petrochemical waste treatment directly affects the whole water management cycle. Though it is imperative to stress the importance of petrochemical treatment from the primary sources, these being the oil refinery chemical plants and related facilities, the petrochemical pollutants are also found in secondary sources.
Secondary sources would be those that contribute to wastewater that is generally treated from public households and the commercial sectors. Therefore, different levels of dealing with petrochemical pollution should be recognized. Downstream-level manufactured products that are derived from primary and secondary petrochemicals are conventionally classified separately, and usually form part of larger classification group of these pollutants, one such group for example is the persistent organic pollutants (POPs). POPs are organic compounds with properties that are resistant to environmental degradation using conventional methods. To stay aligned to the subject focus of this chapter, the treatment of downstream-level manufactured products will be limited to comparative referencing with regard to treatment process analysis. The area of interest is the prospect of treating petrochemical industrial effluent using cleaner technologies.
It however should be noted that in most parts of the world, especially in the developing economies, conventional and traditional wastewater are still employed in treating petrochemical waste, without special consideration of the process required. Industrial discharged waste is approached similarly to common waste from the households and commercial environments (Naderi et al. 2017; Stasik et al. 2015; Wu et al. 2015; Viguri et al. 2002; Zheng and Richardson 1999). The analysis of traditional, conventional, and advanced wastewater treatment methodologies should form part of the narrative that promotes the application of cleaner technologies in the degradation of petrochemicals.
8.4.1 Treatment of Wastewater Pollutants
The treatment objective of the wastewater is to remove any and all contaminants that may deem the water not fit for human consumption. There are predominantly two types of wastewater treatment methods, namely, unit operations and unit processes. Methods of treatment in which physical forces predominate are called unit operations, while methods that remove pollutants by chemical or biological reactions are called unit processes (Metcalf and Eddy, Inc. 2003). Typical treatment plants combine physical, chemical, and biological methods in treating wastewater. There are different levels of wastewater treatment; these are described in Table 8.1.
Traditional water and wastewater treatment technologies are designed to deal with particulates, biological removal of pollutants and chemical contaminants dissolved in water. These methods include:
-
Filtration, the removal of suspended solids from the water by passing water through a porous medium; this process is completely ineffective in removing chemical pollutants.
-
Sedimentation, a process that allows the flocculated or coagulated particles time to settle by gravity in a sedimentation tank. Unless the dissolved compounds are precipitated and agglomerated into the flocculants or coagulated particles, this process in mostly ineffective, and in cases where it might be effective for a single class of compounds, it would be impractical to develop a method for each of the multitude of emerging pollutants and known species.
-
Flocculation, the agglomeration of particles in water or wastewater to promote settling by using high-molecular-weight materials. Most organic micro-pollutants are unamenable to flocculation.
-
Coagulation, this is the process of using physiochemical methods to promote particulate settling by reducing net electrical repulsion forces between particles. This is strictly a solute-based method.
-
Activated carbon absorption, this is a process used to remove low concentrations of contaminants from water that are difficult to remove by other means. Given that a suitable material is used, both adsorption and absorption are possible as a means of using surface charges and adhesion to garner particulate and chemical species of relevant properties. Activated carbon can be made extremely porous, thereby creating a very large surface area available for adsorption of contaminants
Tradition chemical processes for water treatment include disinfection as a means of removing living biological specimen that are present in natural waters and the treatment of infrastructure damaging chemical components. Disinfection in conducted in forms of
-
Chlorination, hypochlorites and other forms of chloride compound complexes are used to kill harmful bacteria, parasites, and other organisms. Disinfection reactions can lead to the formation of complex intermediates that may need further treatment.
-
Ozonation, is a treatment process that kills harmful bacteria and other microorganisms through an infusion of ozone. Ozone (O3) is a gas created when oxygen molecules are subjected to high electrical voltage.
-
Ultraviolet radiation, or UV application, is a disinfection process for water and wastewater treatment that involves passing ultraviolet light through water or wastewater. UV light kills microorganisms present in water. This process in advanced oxidation is called photolysis, though the intended end result in application to advanced oxidation process is different to that of traditional disinfection, the application may be the same, and the UV intensity properties may be a point of interest when applied in advanced oxidation.
-
Ion exchange softening, is a process of using either natural or synthetic ion exchange resins to remove hardness from water. The resins exchange non-hardness-causing sodium ions for hardness-causing calcium and magnesium ions.
The primary physical treatment methods, such as screening and centrifugation, and secondary treatment methods, such as clarification, trickling filtration, are not intended for advanced chemical removal and do little to rid water of dissolved pollutants. Traditional physical and chemical water treatment technologies do not affect most organic micro-pollutants, and in some cases through by-product reactions, it exacerbates the toxicity of post-treatment water quality.
8.4.2 Treatment of Petrochemical Pollutants
Discussed earlier are the conventional treatment protocols for general wastewater constituents, and included is the treatment of petrochemical pollutant compounds from secondary source waste. The pertinent challenge under scrutiny is in the treatment and degradation of petrochemicals from primary sources such as the oil refinery and related industries. To obtain a better understanding of the type of waste produced from refining industry processes, a brief description of the core operation processes will be outlined. Refinery chemical plants apply a wide range of physical and chemical processes in their operational processes (Wong 2000). Refinery flow process configurations are therefore guided and determined by the composition of the source feedstock and the desired final petroleum product. Operational processing and auxiliary units in a generic refinery plant follow the treatment process steps as presented below, sourced from the EPA (1977):
-
Step 1: The separation process ((1) atmospheric distillation; (2) vacuum distillation; (3) recovery, gas processing).
-
Step 2: The petroleum conversion process ((1) cracking (thermal and catalytic); (2) reforming; (3) alkylation; (4) polymerization; (5) isomerization; (6) cooking; (7) visbreaking).
-
Step 3: The petroleum treatment process ((1) hydrodesulfurization; (2) hydrotreating; (3) chemical sweetening; (4) acid gas removal; (5) deasphalting).
-
Step 4: Feedstock and product handling ((1) storage; (2) blending; (3) loading; (4) unloading).
-
Step 5: Auxiliary facilities ((1) boilers; (2) wastewater treatment; (3) hydrogen production; (4) sulfur recovery).
The above listed steps should give indication to the complex nature of the oil refinery process, and also provide comprehension of the petrochemical pollutants that are produced. Various petrochemical wastewater treatment strategies have been developed in efforts to enhance conventional treatment processes in dealing with these pollutants, some of these strategies include bioelectro-Fenton reactions, biological aerated filters, and anaerobic–aerobic processes (Zhang et al. 2018; Li et al. 2018; Mattiusi et al. 2015; Yang et al. 2015; Mirzaei et al. 2013. The biological treatment process was advocated to be the more environmentally friendlier in the treatment of petrochemicals and boasted advantages such as low operational costs, small occupation, resistance to impact load, and improved efficiency (Jemli et al. 2017; Wang et al. 2016; Lettinga et al. 2001).
8.5 Advance Methods for the Treatment of Petrochemical Pollutants
The term clean technology mostly describes the processing of any material in a manner that reduces waste, minimizes environmental contamination, and requires the utilization of little to no nonrenewable resources. In chemistry terms, clean technology is mostly referred to as green technology or green chemistry, in general. Green chemistry is defined as the “design of chemical products and processes to reduce or eliminate the use and generation of hazardous substances” (Horvath and Anastas 2007). These terms will be used interchangeably in this chapter and will essentially hold the same meaning. Green technology will be used as the expression with regard to clean technology of petrochemical pollution treatment.
There are several new technologies that have been developed aimed at treatment of petrochemical pollutants; however, most of these do not necessarily qualify for the green technology bracket. Below are some of these technologies, adapted from Balasubramani and Sivarajaseka (2018);
-
Treatment by the use of wetlands (Knight et al. 1999)
-
Sulfide degradation by autotrophic denitrification (Vaiopoulou et al. 2005)
-
Anaerobic treatment (Macarie 2005)
-
Observer-based time-optimal control of an aerobic SBR (Vargas et al. 2000)
-
Enhanced biodegradation using ozonation and BAC advanced treatment systems (Lin et al. 2001a, b)
Dimoglo et al. (2004) published one of the novel works that delved into the treatment of petrochemical wastewater using the concept of clean technologies. An electrochemical reaction protocol was applied toward the removal of hydrocarbons and grease from petrochemical wastewater. The electrolytic process applied demonstrated the successful removal of oil and grease from the chemical plant discharge without the inclusion of any chemical reagents. Advances have since been made in the development of green technology that is primarily focused on the treatment of pollutants resistant to conventional treatment methods. One potential technological area that has proven efficient in achieving this feat is through the application of advanced oxidation processes (AOP) methods. These techniques utilize chemical oxidation processes to treat organic pollutants resulting in noble constituent products, achieving complete mineralization of pollutants.
8.5.1 Advanced Oxidation Processes
There are a range of AOPs reported in literature dealing with treatment of water pollutants. AOPs technologies are generally accepted to be environmental clean-up methods that are both efficient and inexpensive (Tisa et al. 2014; Vaferi et al. 2014). They include catalysis, electrochemical, Fenton’s reagent, ferrate ionizing radiation, microwave, photo Fenton’s reagent, and photocatalysis. Some of the processes are commercially applied to full scale while others are being tested at pilot scale and at laboratory bench levels (Khuzwayo and Chirwa 2015; Parsons 2004; Andreozzi et al. 1999; Hoffmann et al. 1995). Several other advanced oxidation technologies such as ozonation, ozonation combined with H2O2, and certain types of UV irradiation are currently used for disinfection purposes in the water treatment industry (Lazar et al. 2012; Lathasree et al. 2004).
Among the mentioned AOPs, heterogeneous photocatalysis has yielded the most promising efficiencies in degrading a wide range of organic pollutants in the environment. Photocatalysis is so far the only chemical method than has been demonstrated to completely mineralize organic compounds to water and carbon dioxide under ambient operational conditions. In this process, a chemically inert semiconductor metal oxide is used as a catalyst to generate oxidative oxygen species such as OH° and O° radicals. Heterogeneous photocatalysis has been uniquely useful in wastewater and water application as a result of some important features, such as (1) low operational cost and ease of operation of the method; (2) standard operating temperatures and pressure; and (3) complete mineralization achieved without by-products formed.
There are various commercially available semiconductors; titanium dioxide (TiO2) is the most commercially used, as it offers unique principles, including (Parsons 2004):
-
high activity;
-
significant stability to light illumination;
-
low cost;
-
nontoxic and remains stable after repeated catalytic cycles
Titanium dioxide absorbs light in the visible or low-energy range (300–370 nm) of the ultraviolet regions of the spectrum. The mechanism of photocatalysis and the band gap produced has been well documented by Vinod and Anirudhan (2002), Peral et al. (1997) Hoffmann et al. (1995), and Fox and Dulay (1993).
8.5.2 Photocatalytic Degradation of Organic Pollutants
Heterogeneous photocatalysis has grown as a discipline in the last couple of decades. Its relevance to areas related to energy conservation and environmental pollutant remediation has prompted diverse developments in its applications. One of the most significant applications of semiconductor photocatalysis is the purification of water containing low concentrations of chemical contaminants (Ibhadon and Fitzpatrick 2013). The range of applicability of the heterogeneous photocatalytic concept includes surface material physics, surface sciences, photo reaction chemical and physical chemistry, chemical and environmental engineering, and material sciences (Teoh et al. 2012). Such diverse applications are mostly possible because the semiconductor element provides an environment to directly influence the chemical reactivity of a wide range of adsorbates and a means to initiate photo-induced reduction and oxidation reactions (Fox and Dulay 1993).
In the chemistry discipline, photocatalysis is defined as the acceleration of photoreactions in the presence of a catalyst. The natures of reactions that are amenable to the photocatalytic principle include oxidation, dehydrogenation, hydrogen transfer, metal deposition, water detoxification, gaseous pollutant removal, and more. The photocatalysis discipline at present is focused on the development and optimization of the technology in the removal of total organics from aqueous and gas medium systems of; chemical industry waste, water and wastewater, and environmental pollution.
An analytical review by Serpone and Emeline reported that despite many studies conducted on plenty compatible materials, no semiconductor metal oxide besides titanium dioxide (TiO2) has been discovered that can act as a viable photoanode, with properties of the conduction and valence band edges that straddle the redox potentials of water and where rapid charge-transfer events are the rule and not the exception.
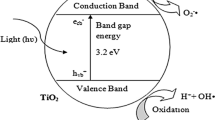
The nucleus of the photocatalytic concepts is embedded on the generation of energy bandgaps upon photon excitation of semiconductor materials. Each semiconductor material has wavelength regions at which directed photons can initiate their most active energy states. When light photons of a sufficient quantum and corresponding wavelength comes into contact with electrons on material surfaces such as semiconductors, the energy carried by the photons is absorbed, which results in the electrons moving to a higher energy state. With additional energy in spin that can overcome the energy difference, the electrons jump to the next higher orbital spin where they re-establish and settle in phase and in wavelength. The additional absorbed energy can also be relinquished by the electrons and dissipated in the form of photon energy, this results in electron descent to lower orbitals where they settle at characteristic ground energy states.
The underlining overall mechanism of the semiconductor photocatalytic process is relatively well understood and documented in scientific literature, though many intricacies about its applications remain unanswered. The champion materials to photocatalytic principle in its current format are semiconductors, those that are amenable to excitation and bandgap formation in the region of the spectrum that can be taken advantage of. These materials have requisitions of reduction potential that are less positive than their oxidation potentials. At the interface between the photocatalyst particle and the present molecules, a double layer of charge exists in metal-liquid medium junctions. This layer can be formed either by adsorption of ions or molecules or by the formation of surface bonds between the solid and the species in solution.
The photocatalytic mechanism of organic substances centers on the generation and formation of hydroxyl (°OH) radicals. Free hydroxyl radicals are one of the most active nonselective initiators of photocatalytic oxidation; they are efficient hydrogen atom abstractors and attack electron-rich moieties such as complex hydrocarbon compounds (Teoh et al. 2012). Energy from UV radiation of solar sources or artificial sources is used to excite the electrons from the valence band to the conduction band; this produces electron-hole pairs. Figure 8.1 shows some of the reaction pathway platforms that are plausible in heterogeneous photocatalysis. The predominant reaction pathways for the oxidation process take place at the valence band edge of the photocatalysts surface via a positive hole formation mechanism.
Schematic depicting of the redox steps in the photocatalytic process. (1) Formation of charged carriers (h+ e−) electron–hole pair. (2) Charge transport and trapping. (3) Charge transfer. (4) Chemical adsorption. (5) Redox pathway mechanisms. (6) Scavenger species and reaction promoters. (7) The semiconductor particle surface
The oxidation process as a whole and the efficiency of the reaction process is driven by the prevention of the electron-positive hole recombination process, which is a spontaneous and a very rapid step. The prevention of the recombination step is achieved through the reductive removal of the ejected electron at the valence band edge. This process is known as scavenging, where a scavenging molecule complex is used to occupy the loose electrons through reductive pathways generation. Oxygen molecules are typically used as the electron accepting agents, where trioxygen (O3) molecules are formed. The trioxygen molecule is also called ozone, and this molecule is formed through the electric discharges and ultraviolet (UV) light actions. Ozone is a good scavenging molecule, due to a much higher oxidation potential than oxygen, and is used in many industrial processes as an oxidation agent.
Gerischer and Heller (1991) reported that the electron transfer to oxygen and subsequent formation of H2O2 might be a rate limiting step; this was also substantiated by a follow-up study conducted by Schwitzgebel et al. (1995) on the role of oxygen in photocatalysis. Hydrogen peroxide has been reported to assist in the oxidation of inorganic and organic compound by acting as a direct electron acceptor or as a direct source of hydroxyl radicals. Hydrogen peroxide can be formed via both the conduction band and valence band pathways in oxygenated matrices (Sahel et al. 2016; Lousada et al. 2015; Yamazaki et al. 2008; Hoffmann et al. 1995; Linsebigler et al. 1995). Hydroxyl radicals are formed on the surface of the titanium dioxide particle; the reaction is initiated by the valence band surface holes with titanol groups in the forms of TiOH, adsorbed water and hydroxide molecules.
Secondary radical formations from the oxidation of organic substrates increase the complexity of the photocatalytic reaction mechanism (Teoh et al. 2012; Schwarz and Dodson 1989). The secondary formed radicals can contribute to the current-doubling effect; this is one of the photocatalytic reaction possibilities indicated in Fig. 8.1. In the current-doubling effect, a single photon of light has the capacity to generate two photoelectrons, one from the excitation, ejection, and promotion at the valence band level and another that is sourced from hydroxyalkoxyl radicals in the presence of suitable organic substrates. The photocatalytic mechanism in application is not a simple and straightforward one, as it has been established that surface hydroxyl groups or adsorbed water molecules may contribute to the chemical oxidation process. This section briefly explains some of the underlining principles of the heterogeneous photocatalytic oxidation process for organic molecules in general.
Photocatalysis applications though economical and do present aspects of environmental friendliness, these aspects may qualify it in terms of green chemistry, but do not necessarily deem it a green technology without additional developments. Green technology defined earlier encompasses both the clean technology and green chemistry principles, where clean technologies mostly describe the processing of any material in a manner that reduces waste, minimizes environmental contamination, and requires the utilization of little to no nonrenewable resources. Conventional application of the photocatalytic principle requires the activation of the semiconductor photocatalyst using energy in the UV region of the spectrum. The UV sources are predominantly artificial in most research and pilot scale applications, though a fair amount of research by the scientific community is being conducted on the utilization of solar sources (Wang et al. 2018; Pelaez et al. 2012; Chong et al. 2010).
8.5.2.1 Photocatalysis as a Clean Technology
When applied accordingly with the latest technological developments, photocatalysis in its current state qualifies as a clean technology. This is not to be confused with green technology – a working context has been defined for the use of these terms earlier. From a chemical reaction and technological design perspective, the ideal photocatalysts are expected to conform to properties of photoactivity, biological and chemical inertness, stability toward photo-corrosion, suitable for visible or near UV light energy harnessing, be low cost and be nontoxic (Hassan et al. 2016; Aghighi and Haghighat 2015; Ibhadon and Fitzpatrick 2013; Mills and Hunte 1997).
The criterion and feats outlined above have been somewhat achieved through technological developments of titanium dioxide as a most viable semiconductor. The high stability of TiO2 allows for diverse applications such as in electro-ceramics, glass, and in photocatalytic degradation of chemicals in water and air. The oxide particles can be applied in the form of suspensions in slurry reactors and in the form of thin film coating. The suspended photocatalyst principle has been extensively researched over the years and has been demonstrated to be very efficient degrading different classes of organic compounds. The major concern of the suspended photocatalyst system is the inability to reclaim the semiconductor catalyst in suspended slurry-type applications. This drawback has been addressed in various ways through innovative developments which are specifically aimed at addressing this issue.
In light of the justification above, photocatalysis as a technology does satisfy the criteria as a clean technology in application and is suitable to the degradation of petrochemical pollutants. The only other area of concern with regard to heterogeneous photocatalysis is the energy-intense irradiation in the ultraviolet region of the spectrum required to excite electrons from the semiconductor surface sites to facilitate reduction and oxidation pathways in nonoptimal transparent mediums. This factor is not necessarily a prerequisite for clean technology classification, but would be a requirement for a green technology status classification.
Several studies have investigated the degradation of petroleum waste using heterogeneous photocatalysis and reported success with regard to the removal in the form of COD and TOC (Al-Sabahi et al. 2017; Aljuboury et al. 2015; Tony et al. 2009). Petrochemical organic pollutants are unlike conventional organic chemical contaminants with regard to the treatment reaction protocols used in the degradation process. There is a huge body of scientific knowledge that supports the treatment of organic pollutants using photocatalysis as the successful method of choice. The use of photocatalysis as a clean technology has been demonstrated to be a viable process in the treatment of most organic pollutants.
8.5.2.2 Photocatalysis as a Green Technology
It was alluded that though photocatalysis is in the clean technology bracket, this does not necessarily deem it a green technology, in accordance with the definition context provided. To achieve the green technology status, photocatalysis in application would require the utilization of renewable resources. The major reaction step and element of heterogeneous photocatalysis that has potential in the form of renewable energy is the photo-excitation step. In this step, photon energy is used to create the electron-hole pair that facilitates the oxidation-reduction pathways on the surface of semiconductor photocatalyst. The photon energy required to activate this step falls within the UV region of the light energy spectrum. The UV radiation range of the light spectrum exists at approximately 300 nm (Oppenlander 2003) and has a flux range of 20–30 W/m2 (De la Cruz et al. 2013).
UV light can naturally be sourced from solar energy, that is, sunlight. Solar radiation constitutes of visible light (43%), infrared light (53%), and UV light (4%). The UV light portion of solar energy can be harnessed and directed toward photocatalytic applications. Various papers have been published on titanium dioxide facilitated oxidation through the application of direct sunlight as the electron energy excitation and promotion step (Blanco-Galvez et al. 2007). Though solar energy is subjected to variations in terms of the geographic latitude, period of the day, cloud cover, atmospheric properties, and seasons (Sirtori et al. 2010), this source of energy qualifies as a green sustainable source.
Advancement of photocatalysis technology toward the utilization of solar energy is the current goal in this sub-discipline. The major obstacle being the amount of energy required to elevate electrons from the valence band edge to the conduction band edge of a semiconductor photocatalyst, such as titanium dioxide. TiO2, though the most useful and common semiconductor in application, has a relatively large band gap, requiring significant amount of energy in the UV region in order to activate and facilitate the redox reaction schemes. The photon package within the UV domain of solar energy represents a small fraction of this energy requirement, and thus limits the mass transfer efficiency. To fully realize the potential of photocatalysis as a green technology, modifications are required to minimize the energy band gap in order to facilitate visible light absorption. Various scientific literatures have been dedicated to this process with some promising results (Fujishima et al. 2008).
8.6 Challenges in Treatment of Petrochemical Pollutants
The steps involved in petroleum treatment are resource intense. One of the major resource required in the treatment process is water, large amounts of water are used in the refinery process. The principal processes that are responsible for the consumption of most water are hydro-treatment, desalting, distillation, and cooling. In turn, large amount of wastewater effluent are produced from these processes. The wastewater produced from different processes contains constituents indicative to each process and therefore, classified accordingly. In better developed refinery systems, the wastewater from the different petroleum treatment steps is separated and the effluent diverted to different reservoirs. This can allow for treatment of wastewater matrices in accordance to the process of pollutant types. The pollutant loads and the source of the pollutants can determine the level of treatment in the form of the following three treatment step and the preferred methods:
-
Primary treatment, the methods that can be applied in this step include buffer tanks, sour water stripper, separators (API/CPI?PPI).
-
Secondary treatment, biological treatment and coagulation flocculation-flotation methods are options.
-
Tertiary treatment, these include membrane filtration, chemical oxidation, and sand filtration.
Other advantages of such setups are that the loading capacity of wastewater into the treatment plants are reduced thus increasing the efficiency of treatment within unit and allows for a greater variety of reuse options.
Oil and water distribution ratios are another challenge to the treatment of petroleum waste. Treatment of petroleum waste requires insight with regard to the nature of the wastewater in terms of the oil droplet size, distribution, as this has an impact on the efficiency of treatment (Romano 1990). Treatment methods can be configured and adapted in line with the nature of the wastewater from each of the processing units. Several other wastewater chemical treatment methods have been used, such as Fenton process, precipitation, and electrochemical techniques in the treatment of petrochemical waste. These methods have succeeded at producing low quality sludge; the downside of these chemical methods is that they also require large quantities of chemicals, is very costly in terms of operation and maintenance, and requires highly skilled labor (Singh et al. 2016; Hu et al. 2013; El-Naas et al. 2009).
8.7 Conclusion
Photocatalysis as a technology satisfies the criteria of an efficient clean technology, but do not necessarily deem it a green technology without additional developments. As an ideal, photocatalysts are expected to conform to properties of photoactivity, biological, and chemical inertness, stability toward photo-corrosion, suitable for visible or near UV light energy harnessing, be low cost and be nontoxic in nature. The major concern of the suspended photocatalyst system is the inability to reclaim the semiconductor catalyst in suspended slurry-type applications. Several chemical wastewater treatment methods have been used such as Fenton process, precipitation, and electrochemical techniques in treatment of petrochemical pollutants. These methods have succeeded at producing low quality sludge, but the downside of these chemical methods is that these also require large quantities of chemicals, is very costly in terms of operation and maintenance, and highly skilled labors are required.
References
Aghighi A, Haghighat F (2015) Evaluation of nano-titanium dioxide (TiO2) catalysts for ultraviolet photocatalytic oxidation air cleaning devices. J Environ Chem Eng 3:1622–1629
Aljuboury DA, Palaniandy P, Aziz HBA, Feroz S (2015) Treatment of petroleum wastewater using combination of solar photo-two catalyst TiO2 and photo-Fenton process. J Environ Chem Eng 3(2):1117–1124
Al-Sabahi J, Bora T, Al-Abri M, Dutta J (2017) Efficient visible light photocatalysis of benzene, toluene, ethylbenzene and xylene (BTEX) in aqueous solutions using supported zinc oxide nanorods. PLoS One 12(12). https://doi.org/10.1371/journal.pone.0189276
Álvarez AM, Carral P, Hernández Z, Almendros G (2016) Hydrocarbon pollution from domestic oil recycling industries in peri-urban soils. Lipid molecular assemblages. J Environ Chem Eng 4(1):695–703
Andreozzi R, Caprio V, Insola A, Marotta F (1999) Advanced Oxidation Processes (AOP) for water purification and recovery. Catal Today 53:51–59
Axelsson G, Barregard L, Sallsten G, Holmberg E (2010) Cancer incidence in a petrochemical industry area in Sweden. Sci Total Environ 408(20):4482–4487
Balasubramani K, Sivarajaseka N (2018) A short account on petrochemical industry effluent treatment. Int J Petrochem Sci Eng 3(1):12–13
Belli S, Benedetti M, Comba P, Lagravinese D, Martucci V et al (2004) Case-control study on cancer risk associated to residence in the neighbourhood of a petrochemical plant. Eur J Epidemiol 19(1):49–54
Blanco-Galvez J, Fernández-Ibánez P, Malato-Rodríguez S (2007) Solar photocatalytic detoxification and disinfection of water: recent overview. J Sol Energy Eng 129:4–15
Bruice PY (2004) Organic Chemistry. Upper Saddle River, Pearson/Prentice Hall
Bustillo-Lecompte CF, Kakar D, Mehrvar M (2018) Photochemical treatment of benzene, toluene, ethylbenzene, and xylenes (BTEX) in aqueous solutions using advanced oxidation processes: Towards a cleaner production in the petroleum refining and petrochemical industries. J Clean Prod 186:609–617
Chen YM, Lin WY, Chan CC (2014) The impact of petrochemical industrialisation on life expectancy and per capita income in Taiwan: an 11-year longitudinal study. BMC Public Health 14:247–248
Chong MN, Jin B, Chow CWK, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44:2997–3027
De la Cruz N, Dantas RF, Gimenez J, Esplugas S (2013) Photolysis and TiO2 photocatalysis of the pharmaceutical propranolol: solar and artificial light. Appl Catal Environ 130:249–256
Dimoglo A, Akbulut HY, Cihan F, Karpuzcu M (2004) Petrochemical wastewater treatment by means of clean electrochemical technology. Clean Technol Environ Policy 6(4):288–295
El-Naas MH, Al-Zuhair S, Al-Lobaney A, Makhlouf S (2009) Assessment of electrocoagulation for the treatment of petroleum refinery wastewater. J Environ Manage 91:180–185
Environmental Protection Agency (EPA) (1977) Revision of emission factors for petroleum refining, EPA-450/3-77-030. U. S. Environmental Protection Agency, Research Triangle Park
Fox AM, Dulay MT (1993) Heterogeneous photocatalysis. Chem Rev 93:341–357
Frumkin H, Hess J, Vindigni S (2007) Peak petroleum and public health. JAMA 298(14):1688–1690
Fujishima A, Zhang X, Tryk DA (2008) Surf Sci Rep 63:515–582
Gerischer H, Heller A (1991) The role of oxygen in photooxidation of organic molecules on semiconductor particles. J Phys Chem 95:5261–5267
Haghollahi A, Fazaelipoor MH, Schaffie M (2016) The effect of soil type on the bioremediation of petroleum contaminated soils. J Environ Manage 180:197–201
Hassan M, Zhao Y, Xie B (2016) Employing TiO2 photocatalysis to deal with landfill leachate: Current status and development. Chem Eng J 285:264–275
Hess J, Bednarz D, Bae J, Jessica P (2011) Petroleum and health care: evaluating and managing health care’s vulnerability to petroleum supply shifts. Am J Public Health 101(9):1568–1579
Hoffmann RM, Martin ST, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:65–96
Horvath I, Anastas PT (2007) Introduction: green chemistry. Chem Rev 107:2167–2168
Hu G, Li J, Zeng G (2013) Recent development in the treatment of oily sludge from petroleum industry: a review. J Hazard Mater 261:470–490
Ibhadon AO, Fitzpatrick P (2013) Heterogeneous photocatalysis: recent advances and applications. Catalysts. 3:1–29
Jemli M, Zaghden H, Rezgi F, Kchaou S, Aloui F, Sayadi S (2017) Biotreatment of petrochemical wastewater: a case study from northern Tunisia. Water Environ Res 89:228–237
Khuzwayo Z, Chirwa EMN (2015) Modelling and simulation of photocatalytic oxidation mechanism of chlorohalogenated substituted phenols in batch systems: Langmuir-Hinshelwood approach. J Hazard Mater 300:459–466
Knight RL, Kadlec RH, Ohlendorf HM (1999) The use of treatment wetlands for petroleum industry effluents. Environ Sci Tech 33(7):973–980
Lathasree S, Rao AN, SivaSankar B, Sadasivam V, Rengaraj K (2004) Heterogeneous photocatalytic mineralisation of phenols in aqueous solutions. J Mol Catal A Chem 223:101–105
Lazar MA, Varghese S, Nair SS (2012) Photocatalytic water treatment by titanium dioxide: recent updates. Catalysts 2:572–601
Lettinga G, Rebac S, Zeeman G (2001) Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol 19:363–370
Li XH, Chen S, Angelidaki I, Zhang YF (2018) Bio-electro-Fenton processes for wastewater treatment: advances and prospects. Chem Eng J 354:492–506
Liang J, Mai W, Tang J, Wei Y (2019) Highly effective treatment of petrochemical wastewater by a super-sized industrial scale plant with expanded granular sludge bed bioreactor and aerobic activated sludge. Chem Eng J 360:15–23
Lin CK, Tsai TY, Liu JC, Chen MC (2001a) Enhanced biodegradation of petrochemical wastewater using ozonation and BAC advanced treatment system. Water Res 35(3):699–704
Lin MC, Yu HS, Tsai SS, Cheng BH, Hsu TY et al (2001b) Adverse pregnancy outcome in a petrochemical polluted area in Taiwan. J Toxicol Environ Health A 63(8):565–574
Linsebigler AL, Lu G, Yates JT Jr (1995) Photocatalysis on TiO2 surfaces - principles, mechanisms, and selected results. Chem Rev 95:735–758
Lousada CM, Brinck T, Jonsson M (2015) Application of reactivity descriptors to the catalytic decomposition of hydrogen peroxide at oxide surfaces. Comput Theoret Chem 1070:108–116
Luginaah IN, Taylor SM, Elliott SJ, Eyles JD (2002) Community reappraisal of the perceived health effects of a petroleum industry. Soc Sci Med 55(1):47–61
Macarie H (2005) Overview of the application of anaerobic treatment to chemical and petrochemical wastewaters. Water Sci Technol 42(5–6):201–214
Mattiusi EM, Kaminari NMS, Ponte MJJS, Ponte HA (2015) Behavior analysis of a porous bed electrochemical reactor the treatment of petrochemical industry wastewater contaminated by hydrogen sulfide (H2S). Chem Eng J 275:305–314
Mechhoud E-A, Rouainia M, Rodriguez M (2016) A new tool for risk analysis and assessment in petrochemical plants. Alex Eng J 55(3):2919–2931
Medianu S, Mircioiu M, Popescu D (2012) Supervisory control for ethylene production in petrochemical installations. IFAC Proc 45(6):230–235
Metcalf and Eddy, Inc. (2003) Wastewater engineering: treatment and reuse. McGraw-Hill, Boston
Mills A, Le Hunte S (1997) An overview of semiconductor photocatalysis. J Photochem Photobiol A 108:1–35
Mirzaei A, Ebadi A, Khajavi P (2013) Kinetic and equilibrium modeling of single and binary adsorption of methyl tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) onto nano-perfluorooctyl alumina. Chem Eng J 231:550–560
Naderi KV, Bustillo-Lecompte CF, Mehrvar M, Abdekhodaie MJ (2017) Combined UV-C/H2O2-VUV processes for the treatment of an actual slaughterhouse wastewater. J Environ Sci Health B 52(5):314–325
Neff RA, Parker CL, Kirschenmann FL, Tinch J, Lawrence RS (2011) Peak oil. Food Syst Public Health 101(9):1587–1597
Oppenlander T (2003) Photochemical purification of water and air-advanced oxidation processes (AOPs): principles, reaction mechanisms, reactor concepts. Wiley-VCH, New York
Parsons S (2004) Advanced oxidation processes for water and wastewater treatment. IWA Publishing, London
Pelaez M, Nolan NT, Pillai SC, Seery MK, Falaras P, Kontos AG, Dunlop PSM, Hamilton JWJ, Byrne JA, O’Shea K, Entezari MH, Dionysiou DD (2012) A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal Environ 125:331–349
Peral J, Domènech X, Ollis DF (1997) Heterogeneous photocatalysis for purification, decontamination and deodorization of air. J Chem Technol Biotechnol 70(2):117–140
Romano F (1990) Oil and water don’t mix: the application of oil-water separation technologies in storm water quality management. Metro, Seattle
Sahel K, Elsellami L, Mirali I, Dappozze F, Bouhent M, Guillard C (2016) Hydrogen peroxide and photocatalysis. Appl Catal Environ 188:106–112
Schwarz HA, Dodson RW (1989) Reduction potentials of CO2- and the alcohol radicals. J Phys Chem 93:409–414
Schwitzgebel J, Ekerdt JG, Gerischer H, Heller A (1995) Role of oxygen molecule and of the photogenerated electron in TiO2. Photocatalysed air oxidation reactions. J Phys Chem 99:5633–5638
Singh P, Ojha A, Borthakur A, Singh R, Lahiry D, Tiwary D, Kumar-Mishra P (2016) Emerging trends in photodegradation of petrochemical wastes: a review. Environ Sci Pollut Res 23(22):22340–22364
Sirtori C, Agüera A, Gernjak W, Malato S (2010) Effect of water-matrix composition on trimethoprim solar photodegradation kinetics and pathways. Water Res 44:2735–2744
Souza EC, Vessoni-Penna TC, de Souza Oliveira RP (2014) Biosurfactant-enhanced hydrocarbon bioremediation: an overview. Int Biodeter Biodegr 89:88–94
Stasik S, Wick LY, Wendt-Potthoff K (2015) Anaerobic BTEX degradation in oil sands tailings ponds: impact of labile organic carbon and sulfate-reducing bacteria. Chemosphere 138:133–139
Teoh WY, Amal R, Scott J (2012) Progress in heterogeneous photocatalysis: from classical radical chemistry to engineering nanomaterials and solar reactors. J Phys Chem Lett 3:629–639
Tisa F, Abdul-Raman AA, Wan-Daud WMA (2014) Applicability of fluidized bed reactor in recalcitrant compound degradation through advanced oxidation processes: a review. J Environ Manage 146:260–275
Tony MA, Zhao YQ, Purcell PJ, El-Sherbiny MF (2009) Evaluating the photo-catalytic application of Fenton’s reagent augmented with TiO2 and ZnO for the mineralization of an oil-water emulsion. J Environ Sci Health A 44(5):488–493
Vaferi B, Bahmani M, Keshavarz P, Mowla D (2014) Experimental and theoretical analysis of the UV/H2O2 advanced oxidation processes treating aromatic hydrocarbons and MTBE from contaminated synthetic wastewaters. J Environ Chem Eng 2(3):1252–1260
Vaiopoulou E, Melidis P, Aivasidis A (2005) Sulphide removal in wastewater from petrochemical industries by autotrophic denitrification. Water Res 39(17):4101–4109
Vargas A, Soto G, Moreno J, Buitron G (2000) Observer-based time-optimal control of an aerobic SBR for chemical and petrochemical wastewater treatment. Water Sci Technol 42(5-6):163–170
Varjani SJ, Gnansounou E, Pandey A (2017) Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere 188:280–291
Viguri J, Verde J, Irabien A (2002) Environmental assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Santander Bay, Northern Spain. Chemosphere 48:157–165
Vinod VP, Anirudhan TS (2002) Photocatalytic degradation for environmental applications - a review. J Chem Technol Biotechnol 77(1):102–116
Wang Y, Wang Q, Li M, Yang Y, He W, Yan G, Guo S (2016) An alternative anaerobic treatment process for treatment of heavy oil refinery wastewater containing polar organics. Biochem Eng J 105:44–51
Wang M, Peng Z, Qian J, Li H, Zhao Z, Fu X (2018) Highly efficient solar-driven photocatalytic degradation on environmental pollutants over a novel C fibers@MoSe2 nanoplates core-shell composite. J Hazard Mater 347:403–411
Wong JM (2000) Petrochemicals. Water Environ Res 72:1–21
Wu C, Gao Z, Zhou Y, Liu M, Song J, Yua Y (2015) Treatment of secondary effluent from a petrochemical wastewater treatment plant by ozonation-biological aerated filter. J Chem Technol Biotechnol 90:543–549
Xiaoqiang J, Dayao J, Chen L, Wenyu L (2019) Characterization and analysis of petrochemical wastewater through particle size distribution, biodegradability, and chemical composition. Chin J Chem Eng 27(2):444–451
Yamazaki S, Siroma Z, Senoh H, Ioroi T, Fujiwara N, Yasuda K (2008) A fuel cell with selective electrocatalysts using hydrogen peroxide as both an electron acceptor and a fuel. J Power Sources 178:20–25
Yang Q, Xiong PP, Ding PY, Chu LB, Wang JL (2015) Treatment of petrochemical wastewater by microaerobic hydrolysis and anoxic/oxic processes and analysis of bacterial diversity. Bioresour Technol 196:169–175
Zhang SY, Wu CY, Zhou YX, Wang YN, He XW (2018) Effect of wastewater particles on catalytic ozonation in the advanced treatment of petrochemical secondary effluent. Chem Eng J 345:280–289
Zheng G, Richardson B (1999) Petroleum hydrocarbons and polycyclic aromatic hydrocarbons (PAHs) in Hong Kong marine sediments. Chemosphere 38:2625–2632
Zhong Y, Zhu L (2013) Distribution input pathway and soil–air exchange of polycyclic aromatic hydrocarbons in Banshan Industry Park, China. Sci Total Environ 444:177–182
Zolfaghari R, Fakhru’l-Razi A, Abdullah LC, Elnashaie SSEH, Pendashteh A (2016) Demulsification techniques of water-in-oil and oil-in-water emulsions in petroleum industry. Sep Purif Technol 170:337–407
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Khuzwayo, Z.(., Chirwa, E.M.N. (2020). Photocatalysis as a Clean Technology for the Degradation of Petrochemical Pollutants. In: Bharagava, R. (eds) Emerging Eco-friendly Green Technologies for Wastewater Treatment. Microorganisms for Sustainability, vol 18. Springer, Singapore. https://doi.org/10.1007/978-981-15-1390-9_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-1390-9_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1389-3
Online ISBN: 978-981-15-1390-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)