Abstract
Freshwater in the environment is getting contaminated day by day due to constant pollution of freshwater bodies through different anthropogenic sources which leads to scarcity of freshwater for the people usage. Green synthesis of nanoparticle is a very efficient method of nanoparticle synthesis. Nowadays these nanoparticles are used to treat wastewater system. The overview of recent advancement in nanotechnology for water and wastewater treatment mechanisms is provided, including nanobased materials, such as nanoadsorbents, nanometals, nanomembranes, and photocatalysts. Various advantageous roles governed of these materials as well as technical barriers when compared with conservative processes are being demonstrated. The commercial value of these materials is presented and viewpoint on further research value is given for each type of nanobased material and process. This chapter includes recent trends in green synthesis of nanoparticles using different plants and their parts like leaves, stem, etc., and their application in treatment of wastewater.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
The freshwater is essentially significant for humans, animals, and plants, not just for drinking, but also for irrigation, domestic use, and other vital activities. It is expected to become increasingly inadequate in the near future, and this is partly because of environmental change (Pretty 2007). Due to the rapid increase in world population as well as global warming, water reservoirs are decreasing at a very fast rate. In this approach, discreet use of water resources and reuse of treated wastewater for various purposes have been perceived as the best method for monitoring the restricted resource of freshwater (Shittu and Ihebunna 2017). The occurrence of microorganisms and trace metals is the principle indication of water contamination. The trace metal contamination of water is a public health concern, with several health risks associated with it (Bharagava et al. 2018; Kishor et al. 2019; Rahman and Singh 2018). The prokaryotic organisms, for example, Escherichia coli, Shigella spp., Salmonella spp., Vibrio spp., and Cryptosporidium, are known to be transmitted by water and cause ill-health when this water defiled by microorganisms is consumed (Ashbolt 2004).
Nowadays, metal nanoparticles especially gold and silver NPs are of particular interest to researchers due to their specific properties and wide applications in the field of medical sciences. Biosynthesis approach of NPs is considered as a better alternative than physical and chemical method (Ahmad et al. 2003; Song et al. 2010; Gautam et al. 2018) as it is eco-friendly, cost effective, and less time requiring. Different materials are categorized as biological for nanoparticle synthesis; these include plant extracts, microscopic organisms, growth chemicals, and actinomycetes (Bharagava and Chandra 2010; Chandra et al. 2012; Agarwal et al. 2017). But plant extracts have been the major focus due to their abundant nature and the phytochemical composition (Bharagava et al. 2008).
There is constrained chance of an expansion in the supply of freshwater due to ever-increasing demand by growing population throughout the world; additionally, water-related issues are anticipated to increase further because of atmospheric changes and because of population growth throughout the following two decades (Vörösmarty et al. 2000). It is evaluated that overall population will increment by about 2.9 billion as of now till 2050 (as indicated by UN’s normal projections) (Rockström 2003). Nanotechnology has been considered as a useful method for identifying and solving problems related to the water quality (Bottero et al. 2006). Nanomaterials are adding to the improvement of progressively effective treatment forms among the new water system (Obare and Meyer 2004).
There are numerous parts of nanotechnology that address the different issues of water quality so as to make sure the ecological stability is maintained. This information gives a unique point of view on fundamental research of nanotechnology for water/wastewater treatment and reuse by concentrating on difficulties of future research. Advancement in the field of nanoscience technology and designing propose that huge numbers of the flow issues including water quality could be resolved or greatly diminished by utilizing nanoadsorbents, nanocatalysts, bioactive nanoparticles, nanostructured synergist layers, nanotubes, attractive nanoparticles, granules, chip, high surface region metal molecule supra subatomic assemblies with trademark length sizes of 9–10 nm including clusters, small-scale particles, nanoparticles, and colloids which significantly affect water quality in natural environment (Diallo and Savage 2005).
13.2 Green Synthesis of Nanoparticles
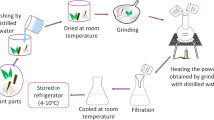
Nanoparticles can be prepared by various methods which are usually categorized in to two major synthetic routes which are the top-down and the bottom-up approaches (Fig. 13.1) (Rahman and Padavettan 2012). In the top-down (physical) approach, nanoparticles are obtained from their bulk materials (macroscopic) using different methods and techniques like mask, ball milling thermal decomposition, irradiation, laser ablation, arc discharge, etc. (Mijatovic et al. 2005). In bottom-up (chemical and biological) approach, nanoparticles are obtained from their atomic level building blocks which react to generate nanoparticles of the desired shape and size. These methodologies utilize larger of nanostructures (Barth et al. 2010). During self-gathering the physical forces working at the nanoscale are utilized to join fundamental units into bigger stable structures. Physical and chemical methods are being utilized broadly for generation of metal and metal oxide nanoparticles as shown in Fig. 13.1 (Ma et al. 2017). However, these methods of production require the use of highly reactive and toxic reducing agents such as sodium borohydride and hydrazine hydrate, which account for undesired detrimental impacts on the environment, plant, and animal life (Saif et al. 2016). Researchers are putting continue efforts to develop facile, effective, and reliable green chemistry processes for the production of nanomaterials.
Different organisms behave as clean, eco-friendly, and sustainable precursors in order to produce stable and well-functionalized nanoparticles. These may incorporate bacteria, actinomycetes, fungi, yeast, viruses, etc. Therefore, it is critically important to develop a more reliable and sustainable process for the synthesis of nanomaterials. The idea of economic viability, ecological sustainability, and social adaptability as well as the availability of local resources should be kept in mind during the production of nanomaterials (Saif et al. 2016). In order to keep the cost of the final completed nanotechnology-based products affordable to consumers, industries must maintain a delicate balance between environmentally sound green processes and their sustainability. The green nanotechnology-based production processes operate under such conditions where the involvement of toxic chemicals is negligible. Metal NPs have been extensively studied because of their specific characteristics such as catalytical, optical, electronic, antimicrobial, and magnetic properties.
Among noble metals, silver is the metal of preference in the field of biological systems, living organisms, and medicine (Bootharaju et al. 2017). The biosynthesis of nanoparticles involves use of biogenic matter including plant extracts, biopolymers, and microbial residue like bacteria, fungi, algae, and yeast for nanomaterial fabrication (Sharma et al. 2015). Development of biocompatible, nontoxic, and eco-friendly methods for the synthesis of nanoparticles is mainly concerned in green chemistry (Narayanan and Sakthivel 2010). Biosynthesis of metal nanoparticles using plants is currently under exploitation. The biological synthesis of metal nanoparticles (especially gold and silver nanoparticles) using plants (inactivated plant tissue, plant extracts, and living plant) has gained more attention as a suitable alternative to chemical procedures and physical methods (Iravani 2011). Synthesis of metal nanoparticles using plant extracts is quite cost effective and therefore can be used as an economic and useful alternative for the large-scale production of metal nanoparticles. Extracts from plants may act as both reducing and capping agents in nanoparticle synthesis. The bioreduction of metal nanoparticles by combinations of biomolecules found in plant extracts (e.g., enzymes, proteins, amino acids, vitamins, polysaccharides, and organic acids such as citrates) is environmentally beneficial, yet chemically complex (Sharma et al. 2009).
Because of the important and critical role of plants in biosynthesis of metal nanoparticle production, the green synthesis of metal nanoparticles using plants has been discussed in this part. Current research in biosynthesis of nanometals using plant extracts has provided a new opportunity in fast and nontoxic methods for production of nanoparticles. Many researchers have reported the biosynthesis of metal nanoparticles by plant leaf extracts and their subsequent potential applications (Ghosh et al. 2012). MubarakAli et al. (2011) have studied the bioreduction of gold and silver ions by leaf broth of Pelargonium graveolens and Azadirachta indica (Thakkar et al. 2010). Moreover, they have explored the formation mechanism of triangular gold nanoprisms by Cymbopogon flexuosus (lemongrass) extracts where the nanotriangles seemed to grow by a process involving rapid bioreduction, assembly, and room temperature sintering of “liquid-like” spherical gold nanoparticles (Brumbaugh et al. 2014).
Also rapid synthesis of stable gold nanotriangles can be achieved which involves the use of Tamarindus indica (tamarind) leaf extract as a reducing agent (Kumar and Yadav 2009). The shape of metal nanoparticles subsequently changed their optical and electronic properties (Storhoff et al. 2000). They have also demonstrated the synthesis of gold and silver nanoparticles having variety of shapes (spherical and triangular) and sizes using Aloe vera plant extracts (Bar et al. 2009). It was explained that only biomolecules having molecular weight less than 3 kDa caused reduction of chloroaurate ions, leading to the formation of gold nanotriangles. Nevertheless, the bioreduction of silver ions proceeded barely in the presence of ammonia. The aqueous solution of gold ions when exposed to Coriandrum sativum leaf extract was reduced and resulted in the extracellular biosynthesis of gold nanoparticles with spherical, triangle, truncated triangle and decahedral morphologies ranging from 6.75 to 57.91 nm. These nanoparticles were stable in solution over a period of 1 month at room temperature (Korbekandi et al. 2009).
Njagi et al. (2010) used aqueous Sorghum sp. (hybrid sorghum) bran extract for nZVI synthesis; eucalyptus globulus leaf extract was used as a bioreducing agent to synthesize nZVI. P. Wang synthesized iron nanoparticles using Eucalyptus leaf extract by adding 0.1 M FeCl3 solution in a ratio of 1:2; Wang et al. synthesized polydispersed iron nanoparticles employing Eucalyptus leaf extract obtained from its leaf litter. nZVI, Fe3O4, and Fe2O3 were the different forms of nanoparticles synthesized during the process; FeO/Fe3O4 nanoparticles were successfully synthesized using pomegranate leaf extract by Rao et al. (2013); these nanoparticles were coated on two strains (NCIM 3589 and NCIM 3590) of heat-killed yeast cells Yarrowia lipolytica, which is considered a good biosorbent itself; Venkateswarlu et al. (2014) used plantain peel extract as a low-cost bioreducing agent for synthesizing magnetite nanoparticles.
Iron salt solution was hydrolyzed which led to the formation of ferric hydroxide, and then it was subsequently reduced by various biomolecules to form Fe3O4 nanoparticles, banana peel ash extract was used to synthesize iron oxide nanoparticles, and aqueous extract of Colocasia esculenta leaves was used to reduce graphene oxide by Thakur and Karak (2014). Daniel et al. (2013) used leaf extract of the evergreen shrub Dodonaea viscosa to synthesize iron nanoparticles where the effect of leaf extract concentration on nanoparticle synthesis was studied. Senthil and Ramesh (2012) reported the green synthesis of Fe3O4 nanoparticles at room temperature using leaf extract of Tridax procumbens, and Narayanan et al. (2011) synthesized superparamagnetic magnetite/gold (Fe3O4/Au) hybrid nanoparticles at room temperature using grape seed proanthocyanidin (GSP) for the first time. Machado et al. (2014) screened leaf extracts of 26 plants for the production of nZVI. During synthesis variables like leaf extraction, temperature, time, and leaf mass to solvent volume ratio were checked. Eighty degree Celsius was identified as the optimum temperature, whereas the other variables like extraction, time, and leaf mass to solvent volume ratio varied as per leaf type.
In another study, Machado et al. (2014) synthesized nZVI using grape marc, black tea, and vine leaf extract. Kumar et al. (2013) synthesized stable iron oxide (Wustite) using aqueous extract of Terminalia chebula dry fruit pericarp. Leaves of three plants native to Australia, namely, Eucalyptus tereticornis (A), Melaleuca nesophila (B), and Rosmarinus officinalis (C), were explored by Wang et al. (2014). Phumying et al. (2013) synthesized Fe3O4 nanoparticles by the hydrothermal method using Aloe vera plant extract. Ahmmad et al. (2013) successfully synthesized highly pure hematite (𝛼-Fe2O3) nanoparticles by the hydrothermal method using green tea (Camellia sinensis) leaf extract. Nanoparticle synthesis was carried out by exposing pretreated and milled powder of Medicago sativa (Alfalfa) to the salt solution of ferrous ammonium sulfate.
13.3 Use of Various Green Synthesized Nanoparticles in Wastewater Treatments
Treating polluted water has become a major challenge as various heavy metals and pathogenic organisms cause human health problems and can present a major threat to the environment (Ayangbenro and Babalola 2017). The use of magnetite nanoparticles as a sorbent has shown promise as an emerging treatment of polluted water. In this study, a common weed plant (Lantana camara) was used for synthesis of magnetite nanoparticles (MNPs). Synthesized MNPs were characterized for physical properties and batch absorption studies were undertaken to assess the potential for removal of heavy metals, namely, lead, under varying conditions (Sun et al. 2011). Nanotechnology can enable a distributed water reuse and treatment paradigm and offer leapfrogging opportunities to obviate concerns of water quality degradation within distribution networks, alleviate dependence on major system infrastructure, exploit alternative water sources (e.g., recycled new water) for potable and agricultural use, and abate energy consumption.
This vision may be particularly appropriate for developing countries that face rapid degradation of water quality with increasing pressure for cleaner water to meet more stringent environmental, public health, and food safety standards. This scenario underscores the need for a new class of “high-performance” water treatment technology. Future urban systems in developing countries will likely increasingly rely on nanotechnology-enabled water monitoring, treatment, and reuse systems that target a wide variety of water pollutants and are affordable and easy to operate. This will also contribute toward a zero discharge paradigm, which is an ultimate goal of sustainable urban water management. Examples of engineered nanomaterials (ENMs) that can enable this vision are summarized in this chapter. Such novel technologies for water treatment at both point of use and community scale are of great value for increasing the effectiveness and robustness of water distribution networks, allowing access to clean water to users that are not connected to a central network, and for emergency response following catastrophic events.
Various industries such as paper and pulp, textile, tanneries, cosmetic, coffee pulping, pharmaceutical food processing, electroplating, and dye manufacturing units discharge colored and toxic effluents to water (Erdem et al. 2005; Hameed 2009; Chandra et al. 2011; Zainith et al. 2019; Kishor et al. 2019). The untreated wastewater containing colored compounds which have complex structures is difficult to biodegrade. Some dyes used in the textile industries are toxic and carcinogenic, which present eco-toxic hazard and introduce the potential danger of bioaccumulation and also affect humans through the food chain (Babu et al. 2007; Sujata and Bharagava 2016; Kishor et al. 2019). Green synthesized NPs are highly proficient for recycling and removal of heavy metal from wastewaters without loss of their stability and degradation of a variety of organic pollutants from wastewaters and, thus, purify the wastewaters for reuse and recycling and could solve various water quality issues worldwide.
Nickel oxide is considered as good adsorbent due to its chemical and magnetic properties (Nateghi et al. 2012). Rafique et al. (2012) synthesized nano NiO using coprecipitation method and the obtained particles were probed for their adsorption nature toward sulphate and nitrate. It was noted that the adsorbent was effective at pH 7 for the removal of both anions from waters. The synthesized silver nanoparticle also shows heavy metal removal activity in laboratory simulated wastewater. The safety toxicity studies show no significant difference between the orally administered silver nanoparticle-treated water group and control group, while the histopathological studies show well-preserved hepatic architecture for the orally administered silver nanoparticle-treated wastewater group when compared with the control group.
Therefore, it can be concluded that the biosynthesized silver nanoparticles have efficient ability in heavy metal removal without subchronic adverse effects in experimental rats (Shittu and Ihebunna 2017). As water becomes scarcer and more contaminated, the food industry stands to gain much from advanced technology that would provide cleaner and more plentiful water supplies. Nanotechnology has the potential to meet these water-related needs in an inexpensive, efficient, yet flexible way that is crucial for developing nations. Wang et al. (2014) utilized the leaf extracts of green tea and eucalyptus separately for the formation of iron nanoparticles (Fe NPs) and employed for the efficient removal of nitrate from wastewater. Synthesis of spheroidal iron nanoparticles (Fe NPs) was confirmed by employing characterization techniques. A comparison study was conducted between plant-synthesized and chemically synthesized iron materials. Green tea and eucalyptus-mediated Fe NPs were able to remove 59.7% and 41.4% of nitrate from wastewater, respectively, compared to 87.6% and 11.7% removal of nitrate by nZVI and Fe3O4 nanoparticles, respectively.
Despite the higher removal efficiency of nZVI, the green synthesized Fe NPs were found to be more stable in nature. Reactivity of aged nZVI, green tea, and eucalyptus synthesized Fe NPs was compared after being completely exposed to air for 2 months. Green tea and eucalyptus synthesized Fe NPs retained the same efficiency of 51.7% and 40.7%, respectively, whereas the efficacy of nZVI significantly dropped about 2.1-fold (45.4%). New study, a green one-pot synthesis of l-serine (L-Ser) capped magnetite nanoparticles (Fe3O4 NPs) and its potential application for adsorption of RhB dye from aqueous solution (Belachew et al. 2017).
13.4 Conclusion and Future Prospective
This chapter highlights the recent advancement in deciphering iron NP synthesis by plant extracts and their applications in water and wastewater treatment. Use of physical and chemical methods for NP synthesis is common but recently several new eco-friendly and economically feasible synthesis techniques are being explored. Green synthesized NPs are being effectively exploited in the area of medical and environmental remediation. Green synthesized NPs and several characterization techniques are currently being utilized in water and wastewater treatment because of their high proficiency and biocompatible nature. Green synthesized NPs are greatly proficient in recycling and eradication of heavy metal contaminants from wastewaters without disrupting their stability, consequently, for purification of wastewaters for reuse and recycling could solve numerous water quality problems globally. Moreover, experiments and problems correlated with the use of green synthesized Fe NPs in water and wastewater treatment are also discussed. However, the enormity of future research scope in this field cannot be accentuated enough.
References
Agarwal HS, Kumar V, Kumar SR (2017) A review on green synthesis of zinc oxide nanoparticles-an eco-friendly approach. Resour Efficient Technol 3(4):406–413
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B Biointerfaces 28(4):313–318
Ahmmad B, Leonard K, Islam MS, Kurawaki J, Muruganandham M, Ohkubo T, Kuroda Y (2013) Green synthesis of mesoporous hematite (α-Fe2O3) nanoparticles and their photocatalytic activity. Adv Powder Technol 24(1):160–167
Ashbolt NJ (2004) Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 198(1–3):229–238
Ayangbenro A, Babalola O (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14(1):94
Babu BR, Parande AK, Raghu S, Kumar TP (2007) Cotton textile processing, waste generation and effluent treatment. J Cotton Sci 11:141–153
Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A (2009) Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A Physicochem Eng Asp 339(1–3):134–139
Barth JV, Costantini G, Kern K (2010) Engineering atomic and molecular nanostructures at surfaces. In: Nanoscience and technology: a collection of reviews from Nature journals. World Scientific Publishing, Singapore, pp 67–75
Belachew N, Rama Devi D, Basavaiah K (2017) Green synthesis and characterization of L-Serine capped magnetite nanoparticles for removal of Rhodamine B from contaminated water. J Exp Nanosci 12(1):114–128
Bharagava RN, Chandra R (2010) Effect of bacteria treated and untreated post-methanated distillery effluent (PMDE) on seed germination, seedling growth and amylase activity in Phaseolus mungo L. J Hazard Mater 180:730–734
Bharagava RN, Chandra R, Rai V (2008) Phytoextraction of trace elements and physiological changes in Indian mustard plants (Brassica nigra L.) grow in post methanated distillery effluent (PMDE) irrigated soil. Bioresour Technol 99(17):8316–8324
Bharagava RN, Saxena G, Mulla SI, Patel DK (2018) Characterization and identification of recalcitrant organic pollutants (ROPs) in tannery wastewater and its phytotoxicity evaluation for environmental safety. Arch Environ Contam Toxicol 75(2):259–272
Bootharaju MS, Kozlov SM, Cao Z, Harb M, Maity N, Shkurenko A, Parida MR, Hedhili MN, Eddaoudi M, Mohammed OF, Bakr OM (2017) Doping-induced anisotropic self-assembly of silver icosahedra in [Pt2Ag23Cl7 (PPh3) 10] nanoclusters. J Am Chem Soc 139(3):1053–1056
Bottero JY, Rose J, Wiesner MR (2006) Nanotechnologies: tools for sustainability in a new wave of water treatment processes. Integr Environ Assess Manage 2(4):391–395
Brumbaugh AD, Cohen KA, St. Angelo SK (2014) Ultrasmall copper nanoparticles synthesized with a plant tea reducing agent. ACS Sustain Chem Eng 2(8):1933–1939
Chandra R, Bharagava RN, Kapley A, Purohit HJ (2011) Bacterial diversity, organic pollutants and their metabolites in two aeration lagoons of common effluent treatment plant during the degradation and detoxification of tannery wastewater. Bioresour Technol 102:2333–2341
Chandra R, Bharagava RN, Kapley A, Purohit HJ (2012) Characterization of Phargmites cummunis rhizosphere bacterial communities and metabolic products during the two stage sequential treatment of post methanated distillery effluent by bacteria and wetland plants. Bioresour Technol 103:78–86
Diallo MS, Savage N (2005) Nanoparticles and water quality. J Nanopart Res 7(4):325–330
Erdem E, Çölgeçen G, Donat R (2005) The removal of textile dyes by diatomite earth. J Colloid Interface Sci 282(2):314–319
Gautam SP, Saxena G, Singh V, Yadav AK, Bharagava RN, Thapa KB (2018) Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem Eng J 336:386–396
Ghosh S, Patil S, Ahire M, Kitture R, Kale S, Pardesi K, Cameotra SS, Bellare J, Dhavale DD, Jabgunde A, Chopade BA (2012) Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int J Nanomedicine 7:483
Hameed BH (2009) Spent tea leaves: a new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. J Hazard Mater 161(2–3):753–759
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13(10):2638–2650
Jebali A, Ramezani F, Kazemi B (2011) Biosynthesis of silver nanoparticles by Geotricum sp. J Cluster Sci 22(2):225–232
Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K (2011) Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc 79(3):594–598
Kisa F (2015) New approach to selective stem cell sorting: separation of undifferentiated stem cells from differentiated stem cells by using iron oxide core shell nanoparticles. Doctoral dissertation, The University of Wisconsin-Madison
Kishor R, Bharagava RN, Saxena G (2019) Industrial wastewaters: the major sources of dye contamination in the environment, ecotoxicological effects, and bioremediation approaches. In: Bharagava RN (ed) Recent advances in environmental management. CRC Press Taylor & Francis, Boca Raton, p 13. ISBN: 978-0-8153-8314-7
Korbekandi H, Iravani S, Abbasi S (2009) Production of nanoparticles using organisms. Crit Rev Biotechnol 29(4):279–306
Kumar V, Yadav SK (2009) Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 84(2):151–157
Kumar KM, Mandal BK, Kumar KS, Reddy PS, Sreedhar B (2013) Biobased green method to synthesise palladium and iron nanoparticles using Terminalia chebula aqueous extract. Spectrochim Acta A Mol Biomol Spectrosc 102:128–133
Ma L, Fan H, Fu K, Lei S, Hu Q, Huang H, He G (2017) Protonation of graphitic carbon nitride (g-C3N4) for an electrostatically self-assembling carbon@ g-C3N4 core–shell nanostructure toward high hydrogen evolution. ACS Sustain Chem Eng 5(8):7093–7103
Machado S, Grosso JP, Nouws HP, Albergaria JT, Delerue-Matos C (2014) Utilization of food industry wastes for the production of zero-valent iron nanoparticles. Sci Total Environ 496:233–240
Mijatovic D, Eijkel JC, van den Berg A (2005) Technologies for nanofluidic systems: top-down vs. bottom-up—a review. Lab Chip 5(5):492–500
MubarakAli D, Thajuddin N, Jeganathan K, Gunasekaran M (2011) Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B Biointerfaces 85(2):360–365
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156(1–2):1–3
Narayanan S, Sathy BN, Mony U, Koyakutty M, Nair SV, Menon D (2011) Biocompatible magnetite/gold nanohybrid contrast agents via green chemistry for MRI and CT bioimaging. ACS Appl Mater Interfaces 4(1):251–260
Nateghi R, Bonyadinejad GR, Amin MM, Mohammadi H (2012) Decolorization of synthetic wastewaters by nickel oxide nanoparticle. Int J Environ Health Eng 1(1):25
Njagi EC, Huang H, Stafford L, Genuino H, Galindo HM, Collins JB, Hoag GE, Suib SL (2010) Biosynthesis of iron and silver nanoparticles at room temperature using aqueous Sorghum bran extracts. Langmuir 27(1):264–271
Obare SO, Meyer GJ (2004) Nanostructured materials for environmental remediation of organic contaminants in water. J Environ Sci Health 39(10):2549–2582
Pretty J (2007) Agricultural sustainability: concepts, principles and evidence. Philos Trans R Soc B Biol Sci 363(1491):447–465
Qu F, Liang H, He J, Ma J, Wang Z, Yu H, Li G (2012) Characterization of dissolved extracellular organic matter (dEOM) and bound extracellular organic matter (bEOM) of Microcystis aeruginosa and their impacts on UF membrane fouling. Water Res 46(9): 2881–2890
Rafique U, Imtiaz A, Khan AK (2012) Synthesis, characterization and application of nanomaterials for the removal of emerging pollutants from industrial waste water, kinetics and equilibrium model. J Water Sustain 4:233–244
Rahman IA, Padavettan V (2012) Synthesis of silica nanoparticles by sol-gel: size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—a review. J Nanomater 2012:8
Rahman Z, Singh VP (2018) Assessment of heavy metal contamination and Hg-resistant bacteria in surface water from different regions of Delhi, India. Saudi J Biol Sci 25(8):1687–1695
Rao A, Bankar A, Kumar AR, Gosavi S, Zinjarde S (2013) Removal of hexavalent chromium ions by Yarrowia lipolytica cells modified with phyto-inspired Fe0/Fe3O4 nanoparticles. J Contam Hydrol 146:63–73
Rockström J (2003) Water for food and nature in drought–prone tropics: vapour shift in rain–fed agriculture. Philos Trans R Soc Lond Ser B Biol Sci 358(1440):1997–2009
Saif S, Tahir A, Chen Y (2016) Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 6(11):209
Senthil M, Ramesh C (2012) Biogenic synthesis of Fe3O4 nanoparticles using Tridex procumbens leaf extract and its antibacterial activity on Pseudomonas aeruginosa. Dig J Nanomater Biostruct (DJNB) 7(4):1655–1661
Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci 145(1–2):83–96
Sharma D, Kanchi S, Bisetty K (2015) Biogenic synthesis of nanoparticles: a review. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.11.002
Shittu KO, Ihebunna O (2017) Purification of simulated waste water using green synthesized silver nanoparticles of Piliostigma thonningii aqueous leave extract. Adv Nat Sci Nanosci Nanotechnol 8(4):045003
Song JY, Kwon EY, Kim BS (2010) Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst Eng 33(1):159
Storhoff JJ, Lazarides AA, Mucic RC, Mirkin CA, Letsinger RL, Schatz GC (2000) What controls the optical properties of DNA-linked gold nanoparticle assemblies? J Am Chem Soc 122(19):4640–4650
Sujata, Bharagava RN (2016) Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. Rev Environ Contam Toxicol 237:71–104
Sun H, Cao L, Lu L (2011) Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Research 4(6):550–562
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomed Nanotechnol Biol Med 6(2):257–262
Thakur S, Karak N (2014) One-step approach to prepare magnetic iron oxide/reduced graphene oxide nanohybrid for efficient organic and inorganic pollutants removal. Mater Chem Phys 144(3):425–432
Togola A, Diallo D, Dembélé S, Barsett H, Paulsen BS (2005) Ethnopharmacological survey of different uses of seven medicinal plants from Mali,(West Africa) in the regions Doila, Kolokani and Siby. J Ethnobiol Ethnomed 1(1):7
Venkateswarlu S, Kumar BN, Prathima B, SubbaRao Y, Jyothi NV (2014) A novel green synthesis of Fe3O4 magnetic nanorods using Punica Granatum rind extract and its application for removal of Pb (II) from aqueous environment. Arab J Chem 12:589–596
Vörösmarty CJ, Green P, Salisbury J, Lammers RB (2000) Global water resources: vulnerability from climate change and population growth. Science 289(5477):284–288
Wang T, Jin X, Chen Z, Megharaj M, Naidu R (2014) Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci Total Environ 466:210–213
Zainith S, Purchase D, Saratale GD, Ferreira LFR, Bilal M, Bharagava RN (2019) Isolation and characterization of lignin-degrading bacterium Bacillus aryabhattai from pulp and paper mill wastewater and evaluation of its lignin-degrading potential. 3 Biotech 9(3):92
Acknowledgments
Amit K. Singh acknowledges Council of Scientific and Industrial Research, New Delhi, for the financial support in the form of Senior Research Fellowship. Amit K. Singh and Abhay K. Pandey also acknowledge the financial support given by UGC/DST India to the Department of Biochemistry, University of Allahabad, Allahabad India.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yadav, R.K. et al. (2020). Green Synthesized Nanoparticle-Mediated Wastewater Treatment. In: Bharagava, R. (eds) Emerging Eco-friendly Green Technologies for Wastewater Treatment. Microorganisms for Sustainability, vol 18. Springer, Singapore. https://doi.org/10.1007/978-981-15-1390-9_13
Download citation
DOI: https://doi.org/10.1007/978-981-15-1390-9_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1389-3
Online ISBN: 978-981-15-1390-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)