Abstract
The rapid growth of industrial development sources the insufficiency of valuable land. Accordingly, it is the vibrant necessity to encourage the Research and Development works to attain ecological, financial, and societal benefits from colossal exploitation of their waste for worldwide benefits. The present work encourages the impact of pozzolanic waste material, i.e., ground-granulated blast-furnace slag (GGBS) in geotechnical characteristics of jarosite waste (zinc industries residual). The strength tests (unconfined compressive (UCS) and indirect tensile strength) were conducted on GGBS stabilized jarosite mixtures (GGBS, 10–30%) with different curing periods such as 7 days, 28 days, and 90 days. The outcomes illustrate that strength properties, increase by increase in GGBS percentage as well as curing periods. This strength improvement behavior of stabilized jarosite is also detected from the microstructural study (SEM), in which, denser agglomeration of GGBS stabilized jarosite particles, proves strength advancement. The durability studies (freeze–thaw (F-T)) of jarosite–GGBS mixtures were performed and it was observed that the loss in the UCS after five sequential F-T phases improved from 61.8% (raw jarosite waste) to 36.89, 26.60 and 17.12% with 10, 20, and 30% GGBS content, curing at 28 days period, respectively. The leachate study of jarosite indicates that jarosite contains hazardous constitutes, which were immobilized after stabilization with GGBS. From this study, it may be summarized that mixing of pozzolanic admixtures (GGBS) along with raw jarosite waste lead to a substantial enhancement in geotechnical properties with economic, social as well as environmental concern.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Presently, industrialization advancement is a symbol of any developing country that tracks the way to becoming a developed country. However, advancement in the industrialization produces the significant level of waste and derivatives. Usually, the wastes can be categorized as solid, liquid, radioactive, and e-waste and it can also be categorized as hazardous or non-hazardous contingent on the basis of their production source. Globally, the total wastes are generated about 20 billion tons, in which 60% are produced from industrial evolution, 20% generated as municipal solid wastes, and residual 20% produced as organic and inorganic waste [1]. The land disposal technique is one of the broadly accepted traditional approaches for the disposal of such wastes. However, due to huge production of wastes, the necessity of supplementary precious land is raised. Thus the land scarcity arises. Today, this land scarcity problem is easily solved by adopting waste management techniques. The waste management technique mainly consists of, controlled production of waste; reduce, reclaim, reuse, and recycling (4-R) the waste; and reclamation of energy and also sustainable disposal of waste. In the previous few decades, for the enchantment in the waste management/utilization, the various industrial wastes such as stone slurry residuals, coal combustions residues, rice husk ash, phosphogypsum, metals slag, blast-furnace slag, cement kiln dust, bagasse ash, waste steel chips, ceramic dust, brick dust, and many more, have been profitably used as a stabilizing agents, with or without the addition of small percentage of cementing agents such as cement, hydrated lime etc. [2–6].
Jarosite is a waste by-product of zinc smelters and is produced during metal extraction processes. Worldwide, a huge amount of jarosite waste produces annually, in which around 0.25 million tons jarosite is produced in India and about and 0.60 million tons in European Union, respectively [2]. Chemically, it is acidic and contains mobilize toxic elements, thus from safety and environmental concern, disposal of jarosite residues is a universal issue [7]. For enhancing the opportunity to reduce, reclaim, reuse, and recycle (4-R) jarosite waste, numerous works done have been accomplished in the recent past. The works reported by Mymrin et al. [8, 9], concluded the chances for producing a new material from blends of jarosite along with dumped ferrous slag, aluminum surface cleaning waste, and the small addition of lime or Portland cement. These obtained blends produce a solidified material which possesses significant strength and water resistance. Chen and Dutrizac [10] advocated the morphological study of jarofix material (resulting from blending of jarosite along with 1% lime and 10% ordinary Portland cement) and observed that the stability of jarofix was directly depended upon the percentage combination of jarosite and Portland cement. Katsioti et al. [11] reported the opportunity to use jarosite as a substitute for gypsum content used in the production of cement. Asokan et al. [1, 2, 12, 13] advocated numerous studies on jarosite waste blends with different compositions of inorganic pozzolanic wastes such as coal combustion residues, and stone slurry residues and reported that hazardous jarosite could be detoxified by making immobilized products.
Sinha et al. [14] described the relative study of jarofix blends with different compositions of bottom ash and local clay and reported the utilization potential of these blends in the field of civil engineering. Similarly, numerous investigators have reported their work done for enhancing the possibilities of huge utilization of the waste materials in civil engineering applications [15,16,17,18,19,20,21,22,23,24,25,26].
In India, mainly two industries named as Hindustan Zinc Limited (HZL) and Binani Zinc limited have contributed to the production of zinc metal, among which HZL has the largest multi-unit of mining and smelting industry producing about 4 lac metric tons of jarosite waste annually [2, 18, 27]. On concerning environmental factors involves in the safe disposal practice of zinc by-products in HZL, the jarosite waste is mixed with 2% lime and 10% cement and carried to the disposal area as a stabilized and stable material named as jarofix. From the economic factor applied in the production cost of jarofix, the current study attempts to be the focus on the production of more economical, immobilized, and stable material. For this, the jarosite waste is blended with the pozzolanic activator such as ground-granulated blast-furnace slag (GGBS) (by-product of the iron industries) in different proportions to produce a more stabilized, stable, and economical material, which to be used as construction materials in various civil engineering applications.
2 Scope and Objectives
In this work, the author attempts to evaluate the opportunities to reduce, reclaim, reuse, and recycle (4-R) the jarosite waste as well as enrich the opportunity to huge utilization of jarosite waste in the field of civil engineering. In order to find out the feasibility of GGBS stabilized jarosite waste, the various properties were investigated. The main objectives of the study are as follows:
-
Evaluate of index properties of jarosite waste such as consistency indexes, specific gravity, and grain size distribution (hydrometer analysis).
-
Study the effect of various GGBS content on compaction characteristics (maximum dry density (MDD) and optimum moisture content (OMC)) and examine that the GGBS blend which gives maximum MDD is taken as an optimum blend.
-
Study the strength properties (unconfined compressive and split tensile strength) of GGBS-stabilized jarosite waste with the inclusion of the effect of curing times (7, 28, and 90 days).
-
Perform morphological studies (SEM).
-
Evaluate the durability characteristics (freeze–thaw).
-
Evaluate the leachate characteristics.
Thus, the main objectives of the study focused on exploring and evaluating the practical as well as economical ways for safe utilization of hazardous jarosite waste.
3 Materials
3.1 Jarosite
Jarosite is a waste by-product of zinc smelters and produced during zinc metal extraction processes in which, primarily the zinc ore (which contains about 50% zinc) is roasted at temperature 900 °C and afterward send for the leaching process, where jarosite (iron residual) is produced in the form of a waste [8, 9, 28, 29].
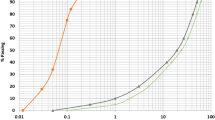
In the present study, jarosite is found from the local zinc industry (Hindustan Zinc Limited, Udaipur, Rajasthan (India)). The jarosite found is yellowish in color (Fig. 1a). The grain size distribution test of jarosite was tested, as mentioned in ASTM D6913-04 (sieve analysis) and ASTM D422-63 (hydrometer analysis) [30, 31], in the laboratory and presented in Fig. 2. As per ASTM D2487-11 [32] (soil classification), jarosite is categorized as silt with high plasticity (MH) fine grained material. The physical characteristics and chemical compositions of jarosite and GGBS are tabulated in Tables 1 and 2, respectively.
3.2 Ground-Granulated Blast-Furnace Slag (GGBS)
GGBS is an iron-manufacturing derivative, generated when iron ore, limestone, and raw coke are blended with each other and then roasted up to 1500 °C in a blast furnace. Afterward, due to the application of heating, the blend is melted and converted into two products such as melted iron and melted slag. The melted slag has a very low specific gravity as compared to the melted iron, thereby floating on the topmost surface, from where it is extracted easily. As the whole process of slag extraction is continued in a blast furnace, it is designated as blast-furnace slag and its granulated residue is designated as GGBS.
Typically the GGBS contains the silicates of Aluminum and calcium [33, 34]. In the present work, the GGBS (specific gravity of 2.74) is found locally from Krishna Udyog, Burdwan, India (Fig. 1b). The particle size distribution of GGBS and its chemical composition is presented in Fig. 2 and Table 2, respectively.
4 Results and Discussions
4.1 Effect of GGBS on Compaction Characteristics
For conducting the compaction test, The Mini Compaction Mold (developed by Sridharan et al. [35] and permitted by ASTM D698-12e1) is used. Primarily, the compaction parameters (optimum moisture content (OMC) and maximum dry density (MDD)) of jarosite blended with varying percentages of GGBS (10, 20, 30, and 40%) were determined and presented in Fig. 3a.
It was examined from the results that the raw jarosite had 1.13 Mg/m3 and 42% of MDD and OMC, respectively. Afterward, the addition of GGBS affects these parameters. With increase in the GGBS content, the MDD of blends was increased up to 30% GGBS; beyond this (i.e., at GGBS content 40%), MDD reduces. This happened mainly due to the rearrangement of particles in the blends. Initially, the void pores between the jarosite particles were filled with the GGBS particles. Thus the structure of particles was varied from a flocculated to dispersed, and henceforth the MDD increases with GGBS (up to 30%). However, beyond the optimum limit of GGBS (i.e., 30%), the blend particles separated in the matrix. Thus the reduction in MDD occurred. Similarly, the OMC was decreased up to 30% of GGBS. It happened due to the less specific surface area and glassy nature of GGBS, requiring a lesser amount of moisture content to attain MDD. Afterward, the OMC was increased at 40% of GGBS, because the separation of particles enhanced the OMC. These variations in compaction parameters are presented in Fig. 3b.
4.2 Effect of GGBS on Strength Characteristics
The unconfined compressive strength (UCS) and split tensile strength test of stabilized jarosite–GGBS blends were conducted in a laboratory by ASTM D2166-06 and ASTM D-3967, respectively [37, 38]. The result of UCS and split tensile strength of jarosite–GGBS blends are presented in Fig. 4a. It was examined from the results that the UCS of jarosite comprising 30% GGBS was increased from 187 kPa (unstabilized jarosite) to 432, 590, and 676 kPa at various curing periods, i.e., 7, 28, and 90 days, respectively. Similarly, in Fig. 4b, it is clear that split tensile strength was increased from 96.32 kPa (unstabilized jarosite) to 83, 108, and 129 at various curing periods, i.e., 7, 28, and 90 days, respectively. After examining the strength test result, it was observed that the increase in both types of strength directly varied increase in the curing periods such as 7, 28, and 90 days. This happened because of the pozzolanic material (GGBS) having time-dependent self-hardening characteristics [34].
It is interesting to note that, after the addition of GGBS content for more than 30%, the segregation of particles happened (MDD reduces), which causes a reduction in both types of strength values. Thus the freeze–thaw study was limited up to 30% jarosite–GGBS blends and GGBS contents up to 30% were considered as a boundary of jarosite–GGBS blends.
4.3 Effect of GGBS on Durability
The durability of stabilized jarosite mixtures was examined by performing a freeze–thaw study by ASTM D560 M-15 [36]. In this study, a sealed-system form of freezing was exposed to the mixtures. Also, throughout the study, moisture is inexistent excluding the present at the start in the voids. After the particular curing cycle, the samples experienced alternate freeze–thaw by placing them in a refrigerator at −18 ± 2 °C and openly at room temperature (23 ± 5 °C) for 1 day, respectively. This temperature slots for complete freeze and thaw were designated by following some preceding studies [39, 40], which conclude that if the temperature in the vessel closed to 0 °C, chances of fractional freeze or perhaps no freeze occurs; thus to achieve comprehensive freezing, −18 ± 2 °C temperature for 1 day was desired. In the same way, to accomplish complete thawing, 23 ± 5 °C temperature for 1 day was maintained. Each jarosite–GGBS blend were endangered to continuously zero, one, three, and five freeze–thaw (F-T) cycles. The numeral of successions was selected as per the existing literature works [41,42,43], which publicized that the lack in the strength typically happens majorly in a few cycles, i.e., up to five cycles.
After the accomplishment of alternative freeze and thaw series, the blends mold were checked for Unconfined Compressive Strength test. The results of the influence of freeze and thaw on the Unconfined Compressive Strength of raw jarosite and treated jarosite–GGBS blends are shown in Fig. 5a, b, correspondingly. It is revealed from Fig. 5 that the blending of GGBS noticeably improves the freeze and thaw resilience resistance as associated with raw jarosite. For example, after five freeze–thaw alternate series, the reduction in UCS values got improved from 62% (natural jarosite waste) to 36.89, 26.60, and 17.12% with 10, 20, and 30% GGBS content, curing at 28 days period respectively.
4.4 Effect of GGBS on Microstructure Study
The fresh samples of the jarosite–GGBS optimum blend (i.e., 30% GGBS) were further studied for their morphological modifications using a scanning electron microscope (SEM). It was examined from the results that by the blending of GGBS, denser packing of particles or agglomeration of particles was achieved as compared to untreated jarosite. This denser packing was achieved due to the pozzolanic reaction of free aluminous and silicious components existing in jarosite by calcium component found in GGBS, which leads to producing cementing gels such as calcium silicate hydrate [C-S-H] and calcium aluminate hydrate [C-A-H]. Similar outcomes have been advocated by many researchers [44,45,46]. The agglomeration or densification in particles is presented in Fig. 6. Figure 6a, b shows a loosely packed (porous) jarosite and GGBS matrix (uncemented) respectively; Fig. 6c, d shows SEM image of optimum jarosite–GGBS blend (i.e., 30% GGBS content) at 28 days curing period, with and without five freeze–thaw cycles. On comparing Fig. 6c, d, it is observed that some closed and dense matrix (some less agglomeration) are still produced after completion of five freeze–thaw cycles as compared to more closed and dense matrix jarosite–GGBS blends (without F-T cycle).
4.5 Effect of GGBS on Toxicity Leachate Potentials
For evaluation of the degree of hazardous nature of raw waste and its stabilized product, the United States Environmental Protection Agency (USEPA) Toxicity Leachate Characteristics Procedure (TCLP) [47] is widely adopted. In this procedure, the powdered sample of stabilized product is blended with a solution of acetic acid and 1 N NaOH as an extraction liquid (pH 4.93), with a liquid to solid weight ratio of 20:1, and then this solution is agitated in a rotary extractor for a period of 18 h at the speed of 30 RPM and 22 °C. After agitation, the solution is loaded into Zero Head Space Extractor (ZHE) and the extractor is airtight closed. To extract leachate fluid, the 50 psi pressure is applied through the extractor, and up to 50 ml of expelled fluid is collected. The collected fluid is screened through a filter paper having 0.6–0.8 µm filtering capacity. This filtrate fluid is distinct as a TCLP extract, and it is analyzed further for the evaluation of hazardous constituents such as Silver (Ag), Chromium (Cr), Cadmium (Cd), Lead (Pb), and Arsenic (As). In the current study, the Inductively coupled plasma (ICP) Spectrophotometer is used for evaluation of heavy metals and toxic elements. The analysis results of leachate samples are tabulated in Table 3.
From the results presented in Table 3; it is revealed that the leachate concentrations of Ag, Cd, Cr, Pb, and As in raw jarosite waste are 27.95, 20.04 ppm, 24.27 ppm, 30.88 ppm, and 2.82 ppm, respectively. All these leachate concentration ranges are higher in raw jarosite waste than that of the limiting in the USPEA-TCLP standard (Table 3). Therefore the jarosite waste is characterized as a hazardous waste.
Similarly, the leachate concentrations of Ag, Cd, Cr, Pb, and As in GGBS stabilized jarosite products are 4.27, 0.16 ppm, 2.70 ppm, 0.58 ppm, and 1.68 ppm, respectively. Comparing the limiting USEPA ranges of heavy metals concentrations with raw jarosite and GGBS stabilized jarosite waste blends, it was observed that stabilized jarosite was found to be within the permissible limits for exploitation in eco-friendly applications of civil engineering.
The reason for the immobilization of heavy metals that occurred in the presence of higher percentage GGBS content is due to the occurrence of more particle agglomeration, which produces the cemented bonds such as calcium silicate hydrate [C-S-H] and calcium aluminate hydrate [C-A-H] bonds (discussed earlier). Thus the heavy metals were trapped and immobilized along with these cemented bonds.
5 Cost Estimation
Traditionally, the jarosite waste is disposed of by its conversion into a more stable form, i.e., jarofix (jarosite–lime–cement blends). In this study, an attempt is made to equate the economic viability of GGBS-stabilized products with that of lime and cement stabilized products (jarofix). Construction rate of 1 cum of embankment is expected as illustrated in the Schedules of Rates of CPWD, Govt. of India [48] regarding the United State currency $. The cost in Indian currency is changed to the United State currency established on the current exchange rate (December 2017).
The above financial estimation (Table 4) advocates that the utilization of jarosite treated with 10, 20, and 30% GGBS can be reduced by up to 74.18, 73.56, and 72.72%, respectively of the rate of making one cum embankment as equalled with lime and cement stabilized jarosite products (jarofix), which is conventionally used by industries of India in order to make jarosite stable.
6 Conclusions
In the universe, nothing is completely waste, whether it is above or below the earth’s surface. For saving the energy, economy, and environment, the potential reduces, reuse, and recycling of all types of wastes becomes a global environmental concern. Thus it is an urgent need to conduct extensive research and development work for optimizing the use of current technology and exploring novel applications for sustainable waste management with social and economic benefits. Stabilization of any waste is a process in which an additive/reagents is employed to reduce the toxic nature by changing its toxic constituents into a more stable form to diminish the contaminant migration rate thus reducing the level of toxicity. In this study, hazardous jarosite waste is treated with GGBS, and the stabilized product is suitable to be used in various applications in an eco-friendly manner. Based on this study, the following conclusions can be drawn:
-
The addition of GGBS affects the compaction characteristics of jarosite waste. As the GGBS percentage increases up to 30%, the OMC of blends goes decreased, and the MDD goes increased. Afterward, further addition of GGBS causes a reduction in MDD and an increase in OMC.
-
Both types of strength, i.e., unconfined compressive and split tensile strength increase considerably with an increase in the GGBS content and curing period.
-
The addition of GGBS increases the durability of jarosite waste. The loss in both types of strength due to alternative F-T cycles decreases with increase in the GGBS content and curing periods. That means the stabilized product is more durable with a higher percentage of GGBS.
-
The comparative financial estimation of treated jarosite revealed that GGBS-treated jarosite could be reduced up to 72.72% of the total construction cost of 1 cum embankment as compared to conventional jarosite stabilization (jarofix). Thus, jarosite–GGBS stabilization is a cheaper alternative to jarofix.
-
Leachate study of jarosite and its stabilized product advocates that this product can be safely used in the various fields of civil engineering and is also eco-friendly.
References
Asokan, P., Saxena, M., Asolekar, S.R.: Waste to wealth-cross sector waste recycling opportunity and challenges. Can. J. Environ. Constr. Civ. Eng. 2(3) (2011)
Asokan, P., Saxena, M., Asolekar, S.R.: Hazardous jarosite use in developing a non-hazardous product for engineering application. J. Hazard. Mater. 137, 1589–1599 (2006)
Havanagi, V.G., Sinha, A.K., Arora, V.K., Mathur, S.: Waste materials for construction of road embankment and pavement layers. Int. J. Environ. Eng. Res. 1(2), 51–59 (2012)
Gupta, C., Prasad, A.: Strength and durability of lime-treated jarosite waste exposed to freeze and thaw. J. Cold Regions Eng. 32(1), 04017025 (2018a). https://doi.org/10.1061/(ASCE)CR.1943-5495.0000154
Gupta, C., Prasad, A.: A parametric strength study of jarosite waste. Proceedings of the Institution of Civil Engineers: Waste and Resource Management, vol. 170, issue 3+4, November 2017, pp. 149–161 (2018b). https://doi.org/10.1680/jwarm.17.00041
Gupta, C., Prasad, A.: Variables controlling strength of lime stabilized jarosite waste. Int. J. Geo-Eng. 9(1) (2018c). https://doi.org/10.1186/s40703-018-0074-2
Kerolli-Mustafa, M., Ćurković, L., Fajković, H., Rončević, S.: Ecological risk assessment of jarosite waste disposal. Croat. Chem. Acta. 88(2), 189–196 (2015)
Mymrin, V., Vaamonde, V.A.: New construction materials from Spanish jarosite processing wastes. Miner. Eng. 12(11) (1999)
Mymrin, V.A., Ponte, H.A., Impinnisi, P.R.: Potential application of acid jarosite wastes as the main component of construction materials. Constr. Build. Mater. 19, 141–146 (2004). https://doi.org/10.1016/j.cemconcomp.2006.12.005
Chen, T.T., Dutrizac, J.E.: A mineralogical study of jarosite products for the stabilization of jarosite residues for disposal. TMS, 917–933 (2000)
Katsioti, M., Boura, P., Tsakiridis, P.E., Agatzini, S., Oustadakis, P.: Use of jarosite/alunite precipitate as a substitute for gypsum in Portland cement. Cem. Concr. Compos. 27, 3–9 (2005)
Asokan, P., Saxena, M., Asolekar, S.R.: Jarosite characteristics and its utilization potentials. J. Total Environ. 359, 232–243 (2005)
Asokan, P., Saxena, M., Asolekar, S.R.: Recycling hazardous jarosite waste using coal combustion residues. Mater. Charact. 61, 1342–1355 (2010)
Sinha, A.K., Havanagi, V.G., Ranjan, A., Mathur, S., Arora V.K.: Feasibility study of Jarofix waste material for road construction. Indian geotechnical conference, Kochi. Vol. 1, pp. 685–688 (2011)
Ding, M., Geusebroek, M., Sloot, H.A.: Self-sealing isolation and immobilization—a geochemical approach to solve the environmental problem of waste acidic jarosite. Appl. Geochem. 17, 93–103 (2002)
Cheilas, A., Katsioti, M.: Impact of hardening conditions on to stabilized/solidified products of cement -sewage sludge -jarosite/alunite. Cem. Concr. Compos. 29, 263–269 (2007)
Vyas, A.K.: Solidification-stabilization technique for metal bearing solid waste from zinc industry—a case study. Proceeding of International Conference on Environmental and Computer Science (IPCBEE). vol. 19, pp. 151–155. BS 5950-3 (2011)
Sinha, A.K., Havanagi, V.G., Arora, V.K., Mathur, S.: Design, construction and evaluation of jarofix embankment and sub grade layers. Int. J. Environ. Eng. Res. 1(3), 97–103 (2012)
Sinha, A.K., Havanagi, V.G., Ranjan, A., Mathur, S.: Geotechnical characterization of jarosite waste material for road construction. Proceedings of Indian Geotechnical Conference, 22–24 (2013)
Arora, V., Sachdeva, S.N., Agrawal, P.: Effect of use of jarosite on workability and early age strength of concrete. Int. J. Comput. Math. Sci. (IJCMS) 4, 136–144 (2015)
Makhatha, M.E., Ndou, M.O. Nheta, W.: Characterisation of jarosite, fly ash and clay for their possible usage in the construction industry. Proceedings of the World Congress on Mechanical, Chemical, and Material Engineering, Barcelona, Spain, 1–9 (2015)
Mehra, P., Chandra, R., Skariah, B.: Assessment of durability characteristics of cement concrete containing jarosite. J. Cleaner Prod. 119, 59–65 (2016a). https://doi.org/10.1016/j.jclepro.2016.01.055
Mehra, P., Chandra, R., Skariah, B.: Properties of concrete containing jarosite as a partial substitute for fine aggregate. J. Cleaner Prod. 120, 241–248 (2016b). https://doi.org/10.1016/j.jclepro.2016.01.015
Yadav, J., Tiwari, S.: Behaviour of cement stabilized treated coir fibre-reinforced clay-pond ash mixtures. J. Building Eng. 8, 131–140 (2016)
Yadav, J., Tiwari, S.: Effect of waste rubber fibres on the geotechnical properties of clay stabilized with cement. Appl. Clay Sci. 149, 97–110 (2017a)
Yadav, J., Tiwari, S.: A study on the potential utilization of crumb rubber in cement treated soft clay. J. Build. Eng. 9, 177–191 (2017b)
Acharya, S., Anand, S., Das, R.P.: Iron rejection through jarosite precipitation during acid pressure leaching of zinc leach residue. Hydrometallurgy 31, 101–110 (1992)
Singh, V.: Technological innovation in the zinc electrolyte purification process of a hydrometallurgical zinc plant through reduction in zinc dust consumption. Hydrometallurgy 40, 247–262 (1996)
Hage, J.L.T., Schuiling, R.D.: Comparative column elution of jarosite waste and its autoclaved product-evidence for the immobilization of deleterious elements in jarosite. Miner Eng. 13(3), 287–296 (2000)
ASTM D6913-04: Standard Test Methods for Particle Size Distribution of Soils. American Society for Testing of Materials, Pennsylvania, PA, USA
ASTM D422-6: Standard Test Methods for Hydro Meter Analysis of Soils. American Society for Testing of Materials, Pennsylvania, PA, USA
ASTM D2487-11: Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). American Society for Testing of Materials, Pennsylvania, PA, USA
Seggiani, M., Vitolo, S.: Recovery of silica gel from blast furnace slag. Resour. Conserv. Recycl. 40, 71–80 (2003)
Sharma, A.K., Sivapullaiah, P.V.: Ground granulated blast furnace slag amended fly ash as an expansive soil stabilizer. Soils Found. 56(2), 205–212 (2016)
Sridharan, A., Sivapullaiah, P.V.: Mini compaction test apparatus for fine grained soils. Geotech. Testing J. 28(3) (2005)
ASTM D560 M-15: Standard Test Methods for Freezing and Thawing Compacted Soil-Cement Mixtures. American Society for Testing of Materials, Pennsylvania, PA, USA
ASTM D2166-06: Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. American Society for Testing of Materials, Pennsylvania, PA, USA
ASTM D-3967: Standard Test Method for Splitting Tensile Strength of Intact Rock Core Specimens. American Society for Testing of Materials, Pennsylvania, PA, USA
Güllü, H., Khudir, A.: Effect of freeze-thaw cycles on unconfined compressive strength of fine-grained soil treated with jute fiber, steel fiber and lime. Cold Reg. Sci. Technol. 106–107, 55–65 (2014). https://doi.org/10.1016/j.coldregions.2014.06.008
Güllü, H.: Unconfined compressive strength and freeze-thaw resistance of fine-grained soil stabilised with bottom ash, lime and superplasticiser. Road Materials and Pavement Design, pp. 608–634 (2015). https://doi.org/10.1080/14680629.2015.1021369
Lee, W., Bohra, N.C., Altschaeffl, A.G., White, T.D.: Resilient modulus of cohesive soils and the effect of freeze-thaw. Can. Geotech. J. 32, 559–568 (1995)
Ghazavi, M., Roustaei M.: The influence of freeze-thaw cycles on the unconfined compressive strength of fiber-reinforced clay. Cold Reg. Sci. Technol. 61, 125–131 (2010)
Roustaei, M., Eslami, A., Ghazavi, M.: Effects of freeze-thaw cycles on a fiber reinforced fine grained soil in relation to geotechnical parameters. Cold Reg. Sci. Technol. 120, 127–137 (2015). https://doi.org/10.1016/j.coldregions.2015.09.011
Eades, J.L., Grim, R.E.: A quick test to determine lime requirements for soil stabilization. Highway Research Record No. 139. Highway Research Board, Washington, DC, 61–72 (1966)
Sherwood, P.T.: Soil Stabilisation with Cement and Lime: State of the Art Review. HMSO Publication, London (1993)
Kalkan, E., Akbulut, S.: The positive effects of silica fume on the permeability, swelling pressure and compressive strength of natural clay liners. Eng. Geol. 73(1–2), 145–156 (2004)
Toxicity Characteristics Leaching Procedure. Method 1311. United States Environmental Protection Agency (USEPA), USA (1992)
Analysis for Rates of Delhi. Central public work department (CPWD), Government of India, New Delhi, Vol. 1 (2016)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Gupta, C., Prasad, A. (2020). The Influence of Ground-Granulated Blast-Furnace Slag on Geotechnical Properties of Jarosite Waste. In: Kalamdhad, A. (eds) Recent Developments in Waste Management . Lecture Notes in Civil Engineering, vol 57. Springer, Singapore. https://doi.org/10.1007/978-981-15-0990-2_13
Download citation
DOI: https://doi.org/10.1007/978-981-15-0990-2_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0989-6
Online ISBN: 978-981-15-0990-2
eBook Packages: EngineeringEngineering (R0)