Abstract
Tooth tissue engineering through advancements in cell biology and bioengineering has proceeded toward regeneration of entire tooth or individual and surrounding components of tooth. Tooth regenerative therapy is a novel therapeutic concept directing toward restoration of physiological function of tooth such as mastication, periodontal ligament function, and response to noxious stimuli. Tooth regeneration is achieved through two distinctive approaches such as cell transplantation and cell homing. Cell-based strategies are a promising potential for regenerating the whole tooth structure in rodents but rendering obstacles in therapeutics. Cell homing is an under-recognized alternative approach to cell delivery-based tooth regeneration. This approach provides tangible pathway toward clinical translation. Scaffold-based or scaffold-free tissue engineering is considered for tooth regeneration. Scaffold-based approach uses scaffolds planted with cells either in vitro or by cell homing. Scaffold-free approach directly induces development of embryonic tooth formation by appropriate signals to produce tooth structure which mimics natural teeth in morphology and size. The combination of biomaterials and human tooth‐associated with stem cell populations isolated from dental pulp and periodontal ligament tissues shows promising approach to regenerate human dental tissues. Scaffolds provide biophysical support for cell recruitment, adhesion, proliferation, differentiation, and metabolism. The designed scaffolds should be biocompatible, non-toxic, and promote regeneration of single or multiple dental tissues. Different types of biomaterials for constructing scaffolds are available that can regenerate tooth components which successfully improves the treatment outcome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Tooth is a biological organ originating from ectomesenchymal cells composed of enamel, dentin, and viable pulp tissue which is altogether called as tooth organ. These tissues usually arise from the interaction of oral epithelium and mesenchyme of cranial neural crest. Ameloblasts of ectodermal origin generally deposit enamel and disappears once hard tissue formation completes. Odontoblast cells of ectomesenchymal origin secrete dentin and persist as dental pulp for the whole life. Tooth loss and dental diseases resultant of caries, trauma, periodontal disease, and genetically inherited disease are considered as a major health issues. The traditional approach of dental treatment uses non-biological substitutes which build up the structure, function, and esthetics component of the tooth but do not promote biological vitality in the tissues. The resultant tooth will be non-vital which is devoid of physiological defense mechanisms and nerves for pain transmission. In the case of immature teeth, the ability to undergo further root formation is hampered. These issues focused toward the development of new biological approach is of concern in vital tissue regeneration. The tissue engineering has focused significantly on the replacement and regeneration of entire tooth or individual components of a tooth which is structurally and functionally sound. The molecular signals associated with odontogenesis and tooth-related phenotypes lead to concept of tooth tissue engineering. Tooth regeneration through advanced and innovative approaches eliminates the pitfall of treatment outcomes linked with traditional approaches. Significant progress toward successful tooth regeneration has been achieved by utilizing a combination of tissue-specific stem cells or progenitor cells, signaling molecules, and biomaterial scaffolds that are the guiding principles in tooth tissue engineering.
2 Strategies for Tooth Regeneration
Forsyth Group in teamwork with Dr. Vacanti in 2002 first achieved tooth regeneration through classical tissue engineering techniques. The dental tissue engineering approaches generate bioengineered replacement teeth and dental tissues mimicking natural tooth by understanding and applying the regulatory mechanism of tooth developmental processes. The formed functional bioengineered tooth has the capability to occlude accurately in dentition with potential interproximal contacts, proprioceptive function, efficient transmission of masticatory forces, and esthetics reestablishment. To regenerate bioengineered teeth with predetermined morphology, it is mandatory for systematic arrangement of epithelial–mesenchymal cell layers onto the scaffold and its interaction with the extracellular matrix. Three variants of dental tissues are essential for tooth regeneration such as dental epithelium, dental pulp, and dental follicle which are associated with enamel, dentin, pulp, and periodontium formation. A range of advanced tooth tissue engineering techniques can be accomplished using these cells through its implantation and interaction with the extracellular matrix. Tooth regeneration techniques involving regulatory processes of odontogenesis are scaffold-based and scaffold-free approaches.
2.1 Scaffold-Based Approach
In this approach, the cells are seeded in an orderly manner on the scaffold in the laboratory. The seeded scaffolds are precultured in vitro for few days and transplanted in vivo into animal tissues such as renal capsule or omentum to obtain adequate blood supply, oxygen, or nutrients (Fig. 1). To promote diffusion of metabolites and its nutrients, the cells can be cultured under perfusion or flow perfusion bioreactors. The desired recipient site can later be grafted with regenerated tissue or organ to obtain an organ of specific shape and size to fulfill the mechanistic requirement at the recipient site. The construct is implanted into the jaw and designed to overcome the mechanical and physiological stresses. This approach results in three-dimensional scaffold that is rich in growth factors and aids in cell adhesion, proliferation, differentiation, and migration thereby imparting mechanical support for extracellular matrix tissue regeneration [1]. Young et al. [2] first successfully bioengineered tooth crowns by seeding dissociated cells on poly (L-lactide-co-glycolide) (PLGA) biodegradable polymer scaffolds. The histological, immunohistochemical, and molecular analyses have shown that the bioengineered tooth contained morphologically similar enamel organ, dentin, predentin, odontoblasts, pulp chamber, and Hertwig’s epithelial root sheath with proper cellular and protein organization as observed in natural tooth. Duailibi et al. [3] suggested that tooth tissue engineering methods can regenerate tooth tissues in both pig and rat by culturing tooth bud cells of postnatal rat and by seeding them on biodegradable scaffold such as PLGA or polyglycolic acid (PGA). It was also demonstrated that during tooth morphogenesis, the tooth cusp number could be controlled by cap stage dental mesenchyme and reaggregation of the mesenchymal and dental epithelium. However, the shortcoming of these studies was that the regenerated tooth did not have morphological size and shape mimicking natural tooth. Honda et al. [4] employed a novel technique wherein epithelial and mesenchymal cells were seeded sequentially such that there is a direct contact between the epithelium and mesenchyme. It was proposed that the cell seeding technique can be beneficial in regulating the tooth morphology. Duailibi et al. [5] cultured cells from tooth bud and seeded them on tooth-like molds composed of PGA/PLGA/poly-L-lactide (PLLA) in the jaw. Subsequently 12 weeks after, an organized tooth crown similar to that of implants grown in the omentum was formed. The resultant tooth displayed physical properties and functions similar to natural teeth. Seeding of dental bud cells on gelatin chondroitin hyaluronan tri-copolymer scaffold regenerated tooth structures with a set of proteins necessary for odontogenesis. Although several studies show that the bioengineered teeth with organized dental tissue in the seeded scaffold are effective, the main disadvantage is that the regenerated tooth is repeatedly very small and occasionally simulates the natural tooth in morphology. The translation of this cell transplantation technique into therapeutics is quite challenging due to the following reasons.
-
Autologous tooth germ cells are inaccessible for humans.
-
Xenogenic tooth germ cells may induce immune rejection and dental abnormalities.
-
Limited availability of autologous postnatal tooth germ cells or dental pulp stem cells and translational barriers.
-
Difficulties in regulatory approval and excessive commercialization cost.
To overcome the limitations of cell-based therapy, an alternative approach without the use of cell delivery system called stem cell homing was first introduced in 2010 to regenerate anatomically shaped tooth-like structures [6]. This approach utilizes the in vivo induction of mesenchymal stem cells with specific target localization which will be circulating in the blood and differentiate into conforming tissues. This therapeutic approach does not involve isolation and ex vivo manipulation of cells in the laboratory, thereby enhancing clinical, commercialization, and regulatory processes. Cell homing bring about tissue regeneration in the injured site by chemotaxis through biomolecular signals. Kim et al. [7] was the first to show that chemotaxis-induced cell homing is suitable for pulp tissue regeneration in an endodontically treated tooth. This method is commonly used in dental pulp and periodontal ligament regeneration and forms a noticeable clinical translational approach. In pulp regeneration, growth factors impregnated in bioactive scaffolds are injected into root canals of the teeth to induce cell migration, proliferation, and differentiation of stem cells [8,9,10]. Growth factors like stromal-derived factor-1 (SDF1) and bone morphogenetic protein (BMP)-7 are delivered into the scaffold to recruit endogenous cells and promote angiogenesis for periodontal ligament regeneration [11].
2.2 Scaffold-Free Approach
This approach involves encouraging embryonic tooth formation processes with appropriate signaling molecules to produce tooth tissue that resembles natural tooth anatomically. In 2002, Ohazama et al. [12] developed artificial embryonic tooth primordia after transplantation into adult jaw using recombinants such as non-dental-derived mesenchymal cell and oral embryonic epithelium. They also showed that non-dental stem cells such as embryonic stem cells, neural stem cells, and bone marrow-derived stem cells can develop tooth structure by the expression of odontogenic genes. They also were the first to develop bioengineered tooth that mimicked natural tooth histologically by the implantation of E14.5 rat tooth primordia into adult mice. Various studies report that epithelial–mesenchymal interactions are important parameters for tooth development [13, 14]. In scaffold-free studies, interaction of epithelial–mesenchymal cells is permitted and tissue is developed through cell aggregation without any external carrier material for cellular attachment [15, 16]. The main limitation associated with this approach is that large quantities of mesenchymal cells are essential to develop morphologically distinct tooth structures. An improved bioengineered three-dimensional organ-germ culture method was developed to generate ectodermal organs such as teeth and whisker follicles by reciprocal interactions of epithelial and mesenchymal cells. After transplantation of bioengineered tooth germ into subrenal capsules in mice, the tooth primordium generated plural incisors composed of enamel, ameloblasts, odontoblasts, dentin, dentinal tubules, pulp tissue, Tomes’ processes, root, periodontal ligaments, alveolar bone, and blood vessels which were organized in similar way to natural tooth on histological observation. The bioengineered tooth germs show an organized arrangement of mineralized tissues, cell types, detectable blood vessels, and nerve fibers in the pulp. This approach produces oral epithelium-like structures or bone but not the whole tooth. Ikeda et al. [17] successfully demonstrated the development of fully functional bioengineered tooth through regenerative therapy using 3D organ culture method. The developed tooth had a similar hardness to the adult natural tooth with normal colony-stimulating factor-1 and parathormone-1 gene expression which could regulate osteoclastogenesis. The nerve fibers such as anti-neurofilament, neuropeptide Y, calcitonin-gene-related peptide, and galanin-immunoreactive neurons effectively re-entered the pulp and periodontal regions which responded to noxious stimuli such as pain and mechanical stress. This study also provided an insight into the tooth eruption mechanisms and masticatory potential of a bioengineered tooth. Oshima et al. [18] developed a bioengineered mature tooth unit possessing physiological tooth functions in the subrenal capsule of the mouse using a size-control device (Fig. 2).
3 Innovative Approaches for Enamel Regeneration
In addition to scaffold-based and scaffold-free approaches, enamel tissue regeneration includes cell pellets culture system, recombination experiments, self-assembly bioactive nanomaterials, and gene-based enamel formation [19, 20].
3.1 Cell Pellet Culture System
Pellet culture system is a beneficial model developed to trigger dental papilla mesenchymal cells (DPMCs) at the advanced bell stage to differentiate dentin–pulp complexes with enamel-shaped cover to promote dentinogenesis on seeding into the renal capsule [21]. Similar study by Iohara et al. [22] showed that cell pellet culture system can provide a three-dimensional growth condition which is essential for cell differentiation and produce a better yield of extracellular matrix which acts as natural scaffolds in cell pellets. Matrigel composed of laminin which forms the principal part of pellet culture system has been used as a substitute to biodegradable scaffolds [23, 24]. Although Matrigel by itself does not promote biomineralization and generate enamel tissue, it can accelerate the expression of adhesion molecules and receptors for ameloblast lineage cells. Human embryonic stem cells along with BMP-4 and retinoic acid, embedded in the Matrigel-coated culture will differentiate into epithelium-like cells, without demonstrable amelogenin. However, ameloblast differentiation demands more complex signals for increased expression of amelogenin, integrin α2, and integrin β7 with the absence of biomineralized enamel [25, 26].
3.2 Recombination Experiments
Recombination experiments were carried out by simulating epithelial–mesenchymal cell interactions during morphogenesis using reciprocal signaling networks. Recombination of epithelial and mesenchymal tissues from different animal species has been attempted to form enamel suggesting that odontogenesis takes place on reactivation of genes by the mesenchyme of different species [27]. Recombination of epithelial cells and mesenchymal tissue results in tooth-like morphology displaying normal cusp number and crown shape [28]. Similarly, epithelium layers obtained from mandible primordia of E10 mice induced the development of dental tissue including enamel with proper organization of a tooth. Recombination of mesenchymal and epithelial cells has been effective in developing ameloblasts that could form well-organized enamel in a bioengineered tooth. Epithelial and mesenchymal cells dissociated from E14.5 mice seeded within a collagen gel drop and then transplanted into subrenal capsules or cultured in vitro led to the formation of ameloblasts which secreted organized enamel in a bioengineered tooth.
3.3 Artificial Bioactive Nanomaterials
Self-assembly peptide amphiphiles (PAs) molecules carrying Arg-Gly-Asp (RGD) peptide were developed for cellular receptor signaling. To induce biological adhesion, the fibronectin RGD epitope is commonly displayed [29, 30]. Huang et al. [31] showed the interaction between ameloblasts or their progenitor cells and PAs through RGD peptide signals. Peptide motif RGD and PAs molecules self-assembled together into nanofiber-induced biomineralization by the ameloblast-like cell line. It showed more mineral formation through increased expression of integrin α5 mRNA and amelogenin upregulation.
3.4 Gene-Manipulated Enamel Regeneration
Borovjagin et al. [32] reported that gene-manipulated treatment was effective in the treatment of amelogenesis imperfecta using vectors and modified delivery system. The localized gene manipulation can re-establish the mineralization procedures by temporally targeting important components in enamel development. It is proven that gene therapy might be a feasible approach for enamel regeneration or repair especially during permanent tooth development. In another study, oral epithelial and odontoblast mesenchymal cells were reprogrammed using Pitx2 and miR-200a-3p into a single type of cell for enamel regeneration. This preprogrammed approach leads to amelogenin expression, Sox2 upregulation, and decreased mesenchymal markers expression, but still regeneration of enamel has not been reported [33]. The study reports that thymosin beta-4 was as an active gene during tooth germ development and TMSB4X-transfected HaCaT cells have mineralizing ability [34, 35]. Several studies have employed gene-manipulated treatment using Ad5-pK7/RGD, Pitx2, miR-200a-3p, TMSB4X as vectors and part of the modified delivery system to regenerate and repair enamel during tooth development. However, successful results could not be achieved as the enamel protein expression was not concordant with that of normal enamel secretion [36].
4 Innovative Approaches for Dentin Regeneration
The inductive molecular signals associated with epithelium are essential for formation of dentin by inducing odontoblasts to produce dentin matrix proteins. Initially, demineralized bone powder was placed on exposed pulp surface to induce mineralization, but was found to be ineffective [37]. BMP present in the dentin is required for stimulation of odontoblasts for formation of reparative dentin. It was found that area of reparative dentin induced is proportionally dependent on the quantity of BMP used, which predetermines the dentin mass formed. Dentin regeneration can be induced using human recombinant proteins with collagen matrices, whereas in case of inflamed pulp, the reparative dentin formation is not effective since active recombinant protein possess short half-life and rapid protein degradation. Growth factor 11 can also induce reparative dentin formation by placing them directly onto the pulp cells. Additionally, bone sialoprotein aids information of reparative dentin through laying down of extracellular matrix by the differentiating dental pulp cells. The reparative dentin laid down by bone sialoprotein exhibits varied morphological features. Isolated stem cells from pulp and periodontal ligament grafted into defects show partial regeneration of dentin and periodontal-like tissue when appropriate signals coexist [38, 39].
Dentin–enamel regeneration using scaffold and cell aggregate methods employs dissociated cells. Shinmura et al. [40] showed that epithelial cell rests of Malassez (ERM) have the capacity to regenerate dental tissues like enamel, stellate reticulum, and ameloblast-like cells by transplanting cultured ERM seeded onto collagen sponge scaffolds into the rat omentum. The complex dental tissue regeneration was examined by reassociation between human dental epithelial stem cells and human dental pulp stem cells from mouse embryos which were cultured in vitro initially and later implanted in vivo.
5 Innovative Approaches for Dentin–Pulp Complex Regeneration
Pulpal regeneration includes three crucial steps: (1) Destructed pulpal tissues must carefully be disinfected, and all microorganisms must be eradicated through antimicrobial therapy; (2) inflammation control at different levels of the tissue injury; (3) successful regeneration of lost pulpal tissue. In the last step, the endodontic regenerative biomaterials should encourage cell migration and proliferation of the different components at the tissue injury [41,42,43]. Pulpal regeneration strategies rely upon the state of the dental pulp, the stage of inflammation, and extent of tissue infected and damaged. In initial lesion with pulp exposure leading to dentin loss and less destruction of pulp, regeneration is performed using pulp capping biomaterials such as calcium hydroxide, mineral trioxide aggregates, and biodentin. These biomaterials encourage stem cells of the pulp to differentiate into odontoblast-like cells or to secrete transforming growth factor (TGF)-b131.
The regenerative strategy can be applied successfully to induce apical closure (apexification) in immature open apex with necrotic pulp tissue by placing biomaterials like calcium hydroxide and mineral trioxide aggregate in the coronal or in the deeper portion of the pulp [44]. The same regenerative biomaterials can be used to encourage physiological root development (apexogenesis) in the case of vital pulp tissue. In the case of a significant lesion with pulpal infection and inflammation, the revascularization method is employed to promote pulpal vitality. In immature teeth with open apex, bleeding is induced that results in filling of the root canal with a blood clot that acts as a scaffold for pulp and promotes root formation. The major pitfall of this approach is that the regenerated pulp tissue is different from the physiologic counterpart. To overcome this drawback, stem cell therapy is advocated for dentin–pulp complex regeneration since dental tissues are abundant with stem cells. To carry out pulp regeneration, different types of stem cells are used such as postnatal dental pulp stem cells (DPSCs), stem cells from exfoliated deciduous teeth (SHED), stem cells from apical papilla (SCAP), periodontal ligament stem cells (PDLSCs), and dental follicle progenitor cells. As DPSCs, SHED, and SCAP are derived from pulp or precursor of pulp, these stem cells are more suitable for dentin–pulp regeneration.
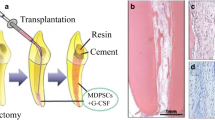
Stem cell-sheet-derived pellet and encapsulated form of stem cells have been tried for dentin–pulp complex regeneration. The commonly tried cell encapsulated materials are enzyme-cleavable, customized self-assembled peptide hydrogels, glycolide, polyethylene gylated fibrin hydrogels, or biodegradable lactide. Gelfoam-encapsulated dental stem cells have been found to be effective in forming dentin–pulp complex in case of non-vital root canals especially in young permanent incisors of beagles. The dentin–pulp complex can be stimulated using Emdogain and platelet-rich plasma combination. Scaffolds encompassed by growth factors such as endothelial growth factor (EGF), basic fibroblast growth factor (bFGF), and TGF-b1 enhance the stem cells function. Bioactive factors can be accumulated or encapsulated and allowed to release from biomaterials in vivo. The adjacent and or systemic cells present next to the root apices of root canal treated teeth migrate into the anatomic part and allow neovascularization and reinnervation. In this case, the root canal of the tooth acts as a scaffold. Single or multiple cytokines, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), or bFGF, alone or in combination with nerve growth factor (NGF), and BMP-7 play a role as critical signaling molecules in pulp regeneration. It is found that the implantation on calvarial site is superior to the dorsum for pulp regeneration using cell homing approach. Injectable scaffolds are excellent candidates for pulp and dentin regeneration since the tooth root canals are small and of irregular shape. Collagen, fibrin, synthetic self-assembled nanofibrous peptide hydrogel (PuraMatrixTM), self-assembling peptide amphiphiles, RGD-modified PAs, and polyethylene glycol maleate citrate hydrogel were used as an injectable scaffold for dentin–pulp complex regeneration. As a first step, a novel anatomically shaped human mandibular first molar scaffold was fabricated with communicating microchannels, 3D layer-by-layer apposition for angiogenesis, and homing of host cells. Suzuki et al. [45] achieved regeneration of dental pulp tissue by recruiting and subsequently differentiating by chemotaxis of selective cytokines such as SDF1, bFGF, or BMP-7. Pan et al. [46] first documented the expression of stem cell factor (SCF) and c-Kit receptor CD117 in dental pulp cells, in the dental follicle, and in periodontal ligament progenitors. It was also observed that application of SCF in regeneration induced chemotaxis, angiogenesis, tissue remodeling, and synthesis of collagen matrix. Takeuchi et al. [47] showed that bFGF is potentially useful in pulp regeneration as cell homing or migration factor is similar to the influence of granulocyte colony-stimulating factor. Yang et al. [48] provided novel in vivo ectopic transplantation model for pulp regeneration and showed that SDF-1a-loaded silk fibroin scaffolds have a promising effect on pulp revascularization by enhancing DPSCs migration, focal adhesion, and stress fiber assembly through autophagy. Zhang et al. [49] observed that cell homing of systemic bone marrow stromal cells to the root canal surface contributed to dental pulp-like tissue regeneration. It was also analyzed that SDF-1 on intracanal application enhanced the efficiency of bone marrow stromal cells homing and angiogenesis. It was also found that stem cell factor in human immature teeth can accelerate cell homing and maturation of the dentin–pulp complex. Li and Wang [50], in an in vivo study, used a combined approach of PDGF-BB, nerve growth factor, and a brain-derived neurotrophic factor for successful dental pulp-like tissue regeneration ectopically by cell homing without using any exogenous cells (Fig. 3).
6 Innovative Approaches for Periodontal Regeneration
In early 1980s to restore the lost periodontal tissues, demineralization of cementum of the tooth was carried out to expose inserted collagen fibers for the integration of new and exposed collagen fibers. The main disadvantage associated with this therapy is ankylosis and root resorption [51]. Hence, utilization of bone grafts into the periodontal defects was adopted, but these materials led to minimal osteoinductive capacity enveloped by dense connective tissue of fibrous nature. Later guided tissue regeneration procedure was employed by draping the biomaterial membrane into the periodontal bony defect extending from the root to the adjacent bone surface. This procedure allows regeneration of the bony defect by migration of cells located in periodontal ligament. Researchers have improved this therapy by incorporating growth factors and stem cells. The common growth factors used for regeneration of periodontal tissues are BMPs, Emdogain, PDGFs, and recombinant amelogenin protein. Further successful demonstration of root periodontal tissue was achieved using pelleted hydroxyapatite or tricalcium phosphate scaffold composed of dental stem cells of the apical papilla. Transplanted cells seeded on multilayered polyglycolic acid sheets when inserted into root surfaces generated new bone, root cementum, and well-organized collagen fibers. In addition to periodontal ligament-derived dental stem cells, other cells such as bone marrow-derived mesenchymal stem cells and adipose-derived stem cells are shown to encourage periodontal regeneration. The combination of transplanted mesenchymal stem cells and exogenous molecular signals is found to be more effective in periodontal tissue regeneration.
7 Challenges in Tooth Regeneration
The three main challenges associated with tooth regeneration are difficult accessibility of clinically applicable cell source, tough to induce odontogenic capacity in these cells, and accelerate total developmental process by gene control. Sufficient quantities of cell sources are essential for tooth regeneration especially DPSCs, SHED, SCAP, and PDLSCs. These stem cells are deficient since dental cells of epithelial origin go through apoptosis subsequently to enamel formation completion and dental epithelial expansion is a difficult task in ex vivo. Hence, non-dental cells were identified for regeneration. It is hard to obtain an applicable embryonic stem (ES) cells source in particular to the patient along with ethical concern. The xenogenically derived ES cells commonly show immune rejection upon clinical use. Autologous-based postnatal stem cells from embryonic tooth germ are difficult to isolate and expand which may not be clinically useful. In addition, these cells lose odontogenic potency after in vitro expansion and may not act as an appropriate source for regeneration of a precise tooth structure. The discovery of induced pluripotential stem cells (iPSc) eliminated ethical concerns associated with ES cells and limited adult stem cells pluripotency. However, the use of iPSC in host tissue has shown cancer-like growth. The molecular signaling networks have been tried for tooth development through epithelial mesenchyme interactions but are minimally understood. Hence, clinically tooth regeneration is not possible via stimulating signaling molecules.
8 Stem Cells in Dental Tissues
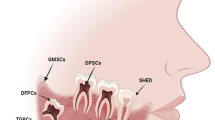
The existence of stem cells within the tooth has been reported for the first time in 2000 [52]. Epithelial stem cell niche in teeth was first isolated from apical end of rodent incisor via organ culture. In humans, the stem cells include adult epithelial and mesenchymal cells, which are essential for regeneration of human tooth. Shi et al. isolated stem cells from adult dental pulp with high self-renewal and multiple cell lineages potential. Dental mesenchymal stem cells and bone marrow-derived mesenchymal stem cells are the two varieties of human adult mesenchymal stem cells used for tooth regeneration. Both these cells have osteo/odontogenic cell lineage capacity analyzed during the process of tooth engineering. Among them, dental mesenchymal cells have been recognized for tooth regeneration because of more odontogenic than osteogenic potential [53]. The dental mesenchymal stem cells in tooth regeneration are stem cells from the DPSCs, SCAP, PDLSCs, SHED, and dental follicle precursor cells (DFPCs). The other non-dental stem cell sources available in oral cavity and used in regenerative dentistry are oral mucosa-derived stem cells, salivary gland-derived stem cells, alveolar bone-derived mesenchymal stem cells, and adipose tissue-derived stem cells with minimal available literature evidence.
8.1 Dental-Derived Stem Cells
8.1.1 Stem Cells from Dental Pulp
Tooth-related DPSCs were isolated first from the pulp of permanent third molars in 2000 by Gronthos et al. [54]. These isolated cells were able to differentiate into various derivates of mesenchymal cells like odontoblasts, adipocytes, chondrocytes, and osteoblasts [55]. DPSCs can be isolated from normal and inflamed pulps with differences in cellular characteristics. DPSCs can also be obtained from aged pulp tissues, and its potency can be maintained using growth factors and nanostructured hydroxylapatite scaffolds [56]. DPSCs exhibit favorable odontogenic potential and generate tissues with morphological and functional features resembling human dental pulp. Literature evidence supports the development of a three-dimensional model representing early odontogenesis through human normal oral epithelial cells and DPSCs [57]. DPSCs are considered to be ideal for tissue reconstruction since it can be obtained with greater efficiency, considerable differentiation capacity, and demonstrable interaction with biomaterials. Scaffold-free three-dimensional cell constructs containing DPSCs reveal a vital part in dental pulp regeneration with increased vascularity.
8.1.2 Apical Papilla-Derived Stem Cells (Stem Cells from Apical Papilla)
Stem cells from the apical papilla (SCAPs) were first reported by Abe et al. in the apical papilla of the roots of developing teeth. The third molars and teeth with open apices are an important source of SCAPs. These cells have high proliferation and multilineage differentiation activity in vitro in comparison to DPSCs. These cells have the ability to differentiate into other cells such as osteoblasts, odontoblasts, and adipocytes. The differentiation of APDCs into dentinogenic cells has been established using animal models. APDCs showed odontoblast-like cells differentiation which secreted dentin in vivo and presented as a primary cell source for root dentin formation [58]. The discovery of SCAP cells led to pulp tissue revascularization concept wherein apical papilla is stimulated and regeneration of damaged or lost pulp tissue through cell homing strategy was executed [59]. The SCAP holds the upper hand in dentin pulpal regeneration compared to DPSCs and SHED. SCAP brings dentin–pulp regeneration by migration of these cells onto the dentin surface and releasing growth factors embedded in dentin which acts as molecular signals for further differentiation of cells into odontoblast-like cells. The cell processes of each differentiated cell spread into the dentinal tubules, and these differentiated cells generate extracellular matrix onto the surface of dentin and into the dentinal tubular spaces. SCAP has been tested with dental bioceramics and showed favorable results toward its differentiation potential [60]. SCAP showed a favorable response to platelet concentrate, i.e., concentrated growth factor and platelet-rich fibrin and showed increased proliferation and differentiation potential [61].
8.1.3 Periodontal Ligament Stem Cells
PDLSCs belong to MSCs subfamily residing in the perivascular space of the periodontium. These cells were first isolated by Seo et al. [62] from human impacted third molars showing differentiation into periodontal ligaments, alveolar bone, cementum, peripheral nerves, and blood vessels. It is found that orthodontic forces can act as a positive modulator and increase the proliferation of PDLSCs and expression of osteogenic and angiogenic factors [63]. It has also been observed that dental bioceramics improve the osteogenic potential of PDLSCs [64]. Chitosan films have shown to promote higher self-renewal, gene expression, osteogenic capacity, and colony-forming units of PDLSCs [65].
8.1.4 Dental Follicle Progenitor Cells (DFPCs)
Dental follicle cells are ectomesenchymal cells surrounding developing tooth germ which can be easily isolated from an impacted third molar. The presence of stem cells in the dental follicle was first reported in 2002 [66]. It is believed that dental follicle tissue contains progenitor cells for cementoblasts, periodontal ligament cells, and osteoblasts. DFPCs have been reported to exhibit better proliferation, colony-forming ability, and differentiation compared to SHED and DPSCs [67].
8.1.5 Stem Cells from Exfoliated Deciduous Teeth (SHED)
Batouli et al. [68] isolated a distinctive population of multipotent stem cells from the pulp remnants of exfoliated deciduous teeth. These cells have the capacity to promote bone formation and secretion of dentin and possess the ability to differentiate into non-dental mesenchymal cells. Immature dental pulp stem cells (IDPSCs) are kind of SHED cells which are isolated from primary teeth. SHED exhibits higher proliferation rates compared to DPSCs and bone marrow-derived MSCs. SHED has different odontogenic and osteogenic differentiation potential than DPSCs. Although SHED cells are incapable of osteoblastic or osteocytic differentiation, they induce osteogenic differentiation thereby demonstrating its osteoinductive potential. Therefore, SHED can be used in vivo in dental pulp tissue engineering, where stem cells replace the infected pulp, resulting in architecturally and cellularly resembling normal dental pulp [69]. SHED shows high expression for genes involved in cell proliferation and extracellular matrix formation like TGF-β and fibroblast growth factor [70]. The exfoliated teeth are one of the sources for SHED cells that can be used in regenerative dentistry by commercial banking of these stem cells. The preserved SHED cells from exfoliated teeth can be used once the child becomes adult for autologous and allogeneic cell replacement.
8.1.6 Tooth Germ Progenitor Cells (TGPCs)
These cells are novel stem cell population that was identified in the dental mesenchyme of the third molar tooth germ during the advanced bell stage. TGPCs have adipogenic, chondrogenic, osteogenic/odontogenic, neurogenic differentiation capacity and able to form tube-like structures, possibly an evidence of vascularization.
9 Selection of Biomaterials for Tooth Regeneration
Biomaterial scaffold is most crucial for tooth regeneration and should satisfy definite general requirements. To achieve significant regeneration of tooth, the biomaterial scaffolds should possess following requirements:
-
Easy to handle, biocompatible, non-toxic, and good physical and mechanical stability.
-
Lesser immunogenicity and should enhance vascularity.
-
Biomaterial scaffold should have acceptable pore size, shape, and volume for cells and/or growth factors diffusion and passage of nutrients and waste products in the cells.
-
Biologically safe biodegradability correlating with the rate of new tissue formation without any noxious by-products.
-
Biomaterial scaffold should exhibit cellular encapsulation or cellular surface adhesion that regenerate single or multiple dental tissues.
-
Permit functionality of at least few multiple cell types like ameloblasts, odontoblasts, fibroblasts, cementoblasts, vascular cells, and neural endings.
-
As tooth organ exhibits diversity in structure and function, multiple polymeric layers may be chosen for regeneration which may be either natural, synthetic or hybrid polymers.
-
Manifest clinical suitability which is readily sterilized and stored in clinical setting with reasonable shelf life.
10 Biomaterials for Tooth Regeneration
The biomaterials are developed to contribute an ideal platform for cell adhesion, proliferation, and differentiation. Biomaterials are basically involved in osteo/odontoconduction, osteo/odontoinduction, and osteo/odontogenesis based on their composition [71, 72]. Biomaterials can be classified into natural and synthetic based on its derivatives. Based on chemical composition, biomaterials can be classified as metals, polymers, ceramics, and composites.
10.1 Natural Biomaterials
10.1.1 Bioceramics
Bioceramics derived from coral or marine sponges have unique interconnected sponges which possess a significant amount of water and helps in the flow of fluid mimicking ideal bone/dentin scaffold. The sponge in the organic component of hard tissues is similar to that of vertebral column and is suitable for tissue regeneration. Biosilica, another mineral component, is able to provide cell proliferation, mineralization, and bone formation [73]. They are hard and brittle with excellent compressive strength, high resistance to wear, and low frictional properties but poor tensile strength. They possess better mechanical properties yet retain their porous structure which benefits cell adhesion, infiltration, and improved vascularization. The naturally derived ceramics have greater biocompatibility compared to synthetic. These materials help in attachment, growth, and differentiation of osteoprogenitor cells.
10.1.2 Natural Polymers
10.1.2.1 Proteins
Natural proteins derived from collagen, gelatin, silk fibroin, and fibrin are the types of polymers used in bone/tooth tissue engineering. Collagen and gelatin are the most commonly used natural proteins in tissue engineering because of their higher biocompatibility, lesser antigenicity, lesser cytotoxicity, and lesser inflammatory reaction. Collagen promotes cellular adhesion, cellular migration, and cell growth. Although collagen does not have high physical strength, it has high tensile strength and hence can be used for pulp regeneration. The drawback with collagen-based scaffold when used in the regeneration of pulp is high degradation rate by collagenase enzyme and noticeable contraction of collagen. To overcome this disadvantage, it is usually coupled with other polymers or cross-linked with other chemicals. Chemical cross-linking of collagen can be done with glutaraldehyde or diphenylphosphoryl azide as it not only enhances the mechanical stiffness but also compromises cell survival and biocompatibility. The mechanical properties and bone conductivity can be enhanced by hybrid scaffolds with β-tricalcium phosphate (TCP)/polyethylene and HA. The pulp with hard tissue in organized manner can be induced by combining DPSCs, collagen scaffold, and dentin matrix protein-1. The osteodent in formation can be induced by combining collagen matrix with BMP-2 or BMP-4. Collagen scaffolds loaded with growth factors such as bFGF, VEGF, PDGF on implantation into endodontically treated root canals after 3 weeks formed re-cellularized and revascularized pulp tissue with neodentin. Clinically, collagen sponge scaffold is effective in tooth production with maximum success. To manage periodontal and peri-implant defects, use of grafting material with collagen membranes is beneficial.
Fibrin has been used in bone/pulp tissue engineering because of biocompatibility, controlled biodegradability, and cells and biomolecules delivering capacity. Injectable 3D-shaped mold fibrin hydrogel is found to be suitable for pulp regeneration since it promotes functional well-organized structures. In vivo transplantation of polyethylene gylated fibrin hydrogel combined with DPSCs or PDLSCs revealed vascularized connective tissue resembling pulp tissue. Platelet-rich fibrin (PRF) with growth factors suggests complete regeneration of tooth with indiscriminate shape with crown, root, enamel, dentin, cementum, odontoblasts, pulp, blood vessels, and periodontal ligament. The PRF and HA graft material and PRF membrane are effective in management of large periapical lesion, whereas HA and PRF are effective in treatment of three-wall intrabony defects, and bone graft composed of PRF and β-tricalcium phosphate can be used to treat periapical cyst and for bone augmentation in periapical defects. The combination of PRF and mineral trioxide is biocompatible and favorable in managing pulpal floor perforation and in apexification. Investigations suggest that PRF on human dental pulpal cells enhances dental pulp cell proliferation, osteoprotegerin expression, and alkaline phosphatase activity. These findings suggest that PRF has a role in reparative dentin formation and in managing pulpitis.
Silk is a natural fibrous protein used in periodontal and maxillofacial therapies because of its biocompatibility, non-toxicity, physical characteristics, cell attachment, and cell proliferation. It also creates three-dimensional soft tissue augmentation. Hexafluoroisopropanol-based silk degrades slowly and supports soft dental pulp formation better than aqueous-based silk. Tooth bud cells when seeded on these scaffolds help in osteodentin formation. Micron-sized silk fibers when added produce high strength and serve as load-bearing bone grafts. They help in both human bone marrow stem cell differentiation and formation of bone-like tissue. Electrospun silkworm silk scaffolds allow gingival fibroblast attachment and proliferation of cells on electrospun fibers, thus promoting gingival tissue regeneration.
10.1.2.2 Polysaccharides
The commonly used natural polysaccharides for tooth regeneration are alginate, hyaluronic acid, agar, dextran, chitosan, and cellulose. Alginate is used as an alternative to proteins, polymers, and glass ceramics for bone/tooth regeneration. It is used in different forms like porous scaffold, injectable hydrogels, and nanofibrous scaffolds. RGD-modified alginate hydrogel helps in adhesion, proliferation, and differentiation of the cells. Alginate hydrogels promote dentin–pulp and periodontal regeneration by delivering TGF–β. Differentiation of odontoblast-like cells along with secretion of tubular dentin matrix is upregulated by TGF-β and acid-treated alginate hydrogels. Fibroblast cells from human periodontal ligament proliferate tremendously on alginate or bioglass scaffolds because nano-active bioglass ceramic has enhanced alkaline phosphatase activity. Hyaluronic acid is biocompatible and has low immunogenic activity so it offers as a preferable scaffold for dental pulp regeneration and blood vessel proliferation. The main disadvantage associated with this hyaluronic acid is in vivo rapid degradation and poor mechanical strength. Hyaluronic acid induces differentiation of mesenchymal cells and reparative dentin formation. Hyaluronic acid sponges and collagen sponges induce dental pulp proliferation with lesser inflammatory response [74]. Agar is a linear polysaccharide agarose which helps dental mesenchymal cells to differentiate into functional odontoblast-like cell, thus promoting tooth tissue formation [1]. Dextran is a complex polysaccharide, which is synthesized by sucrose, and helps in bone formation through the differentiation of bone forming cells. Hydrogel scaffold containing microspheres of dextran/gelatin loaded with BMP enhance periodontal tissue regeneration during periodontal therapy. Chitosan is usually processed into nanofibers, scaffolds, membranes, gels, beads, and sponges. It is a natural biopolymer which is derived from chitin used in dental applications from restorative dentistry to tissue engineered scaffolds for the alveolar bone to periodontal complex healing [75]. It shows high expression for BMP-2 osteoblast gene and increased alkaline phosphatase activity. Chitosan gel combined with demineralized bone matrix and the collagenous membrane has been used for periodontal regeneration, whereas HA chitosan scaffolds find application in bone tissue regeneration. The collagen tetracycline membrane also has beneficial effect on regeneration of bone and cementum in intrabony defects in animal models. Chitosan-based trilayer scaffold and solidified chitosan hydrogel have promising effect in periodontal regeneration by improving the functional ligament length [76]. Chitosan membranes loaded with growth factors like bFGF and PDGF-BB can induce more tissue regeneration and improves guided tissue regeneration. The blending of chitosan with other materials such as HA or silicone and preparation of chitosan nanofibers enhance the mechanical properties and make them appropriate for clinical application in guided tissue regeneration. Cellulose is a polysaccharide found in green plants, with excellent biocompatibility and better mechanical properties. Cellulose scaffolds exhibit angiogenic effect; hence, microfibrous cellulose acetate scaffolds improve the formation of a capillary tube-like structure, thus enhancing pulp revascularization and regeneration.
10.2 Synthetic Biomaterials
10.2.1 Ceramic Scaffold/Bioactive Ceramics
Ceramic scaffolds are calcium/phosphate materials (β-TCP or HA), bioactive glasses, and glass ceramics. They have varied benefits like biocompatibility, lesser immunogenicity, better healing of large bone defects, bone regeneration, and osteoconductivity. β-TCP and HA are two different entities, where β-TCP is biodegradable ceramic, whereas HA is non-biodegradable ceramic. β-TCP and biphasic calcium phosphate scaffold have been used for pulp and dentin tissue regeneration. 3D calcium phosphorus porous granules are used in dental tissue engineering as it provides 3D substrate conditions for the growth and differentiation of human dental pulp stem cell (hDPSCs). Mechanical strength and cellular proliferation can be increased by the addition of silicon dioxide and zinc oxide. Calcium phosphate dissolution products increase the osteoblastic activity of the material. Disadvantages include poor mechanical strength, brittle nature, tough to shape, slower degradation rate, and high density. Glass ceramics-based SiO2–Na2O–CaO–P2O5 helps in osteogenic differentiation of pulp stem cells and provides good crystallization conditions. Ceramic scaffolds can be modified to enhance cellular activity. The mechanical integrity and bioactivity are enhanced during dental tissue regeneration by using Mg-based glass ceramics. Attachment, proliferation, and differentiation of hDPSCs are excellent using niobium-doped fluorapatite glass ceramics. Bioactive glass is considered as a good alternative to HA as they are able to bind to bone and as well as soft tissues. It can replace osseous defects and helps in developing new attachment on tooth surfaces. Dental ceramic coated with bioactive glass accelerates HA formation. Nanobioactive glass ceramic composite scaffold promotes maximum mineral deposition and is considered as a potential candidate for alveolar bone regeneration. They can be used as surface-reactive osteoconductive and osteoinductive biomaterials that promote osteogenesis and pro-angiogenesis and hence used in soft tissue engineering because of its high mechanical strength [77].
10.2.2 Synthetic Polymers
10.2.2.1 Polyesters
PGA, PLGA, PLLA, and polylactic acid (PLA) are commonly used synthetic polyester polymers for dental pulp regeneration. Polycaprolactone (PCL) is less commonly used because of limited bioactivity and extended degradation rate which can alter the molecular weight, crystallinity, and can modify the structure. PGA was first attempted in vitro for pulpal regeneration and revealed cellularity similar to the normal pulp tissue. PGA has better cell proliferation, conduction, increased regeneration of blood vessels, and fibroblast differentiation. PGA and PCL composite scaffolds seeded with genetically modified human cells lead to enhanced periodontal regeneration. PGA and PLA scaffolds were used for seeding SHED, DPSCs, and pulp fibroblasts to result into tissues similar to dentin and pulp by differentiation of stem cells into odontoblast-like cells and endothelial cells. PLLA can also show similar cellularity and pulp tissue formation. Combination of simvastatin and nanofibrous PLLA scaffold promotes the odontogenic potential of dental pulp cells in an inflammatory environment. A PGA/PLLA scaffold has shown to regenerate tooth crown composed of enamel, dentin, and pulp using tooth bud-isolated cells on transplanted rat omentum. The periodontal ligament tissues were also regenerated when similar constructs were translated into rat jaws. Another promising polymer named synthetic open-cell PLA was effective in dental pulp regeneration. Scaffold with SHED was able to adhere to the dentin of the root canal after transplantation into clean and shaped root canals of extracted human teeth.
10.2.2.2 Polyanhydrides
Polyanhydrides are surface-eroding polymers which contain two carbonyl groups linked by ether group. Polysebacic acid, poly1, 3-bisp-carboxyphenoxypropane, and poly1, 6-bis (p-carboxyphenoxy) hexane solely/blending with polymers (e.g., PCL) were explored for bone regeneration and repair. In hard tissue engineering, their use is restricted due to its unsatisfying mechanical properties particularly in load-bearing areas. Hence, to enhance the mechanical properties, the polyanhydrides are combined with polyimides as an alternative approach. In dentistry, polyanhydrides linked with carboxylic acid, amine, thiol, alcohol, or phenol group have gained attention due to its use in oral inflammatory pathologies [78, 79]. Similarly, Hasturk et al. [80] studied the utilization of light/chemically set polymethylmethacrylate, polyhydroxyethylmethacrylate, and calcium hydroxide graft material along with polyanhydride around dental implants and extraction sockets. The microscopic evaluations supported the implant stability with good bone-to-implant contact and well-organized implant–bone interface, effective in crestal augmentation throughout immediate implant placement. A modified perishable polyanhydride into disks, coatings, microspheres, and tubes was used for treatment for periodontal diseases, orthopedic injuries, nerve regeneration, and biofilm formation.
10.3 Composite Scaffold
Composite approach of combining natural or synthetic biomaterial (biopolymers and/or bioceramic) is a method to reduce the disadvantage and combine the advantages of each individual biomaterial. Composite materials often show excellent equilibrium in strength and toughness and improved characteristics compared to their individual components [81]. Hence, ceramic/polymer composite scaffolds have emerged. These composite scaffolds enhance the mechanical properties and osteoconductivity. Polymer–bioglass composite shows improvement in cell adhesion and growth of viable cells. Calcium phosphorus composite is more effective in tooth tissue regeneration, and PGLA/TCP scaffold is preferable for dentin–pulp regeneration. Bone reconstruction and regeneration can be done with zirconia hydroxyapatite composite scaffold as it has cellular/tissue compatibility and excellent mechanical properties.
11 Conclusions
The era of biomaterials and biomaterial engineering is focused toward improving the treatment outcomes of the patient. However, immense research is going on to regenerate dental tissues aiming for clinically appropriate structures and function. The application of stem cells and biomaterial sciences for tooth regeneration needs to be understood deeply. Since the current or emerging paradigms in tooth regeneration are showing limited and variable outcome with no true biological tissue formation, the translation of this tooth tissue engineering into clinical practices is still a question.
References
Sharma S, Srivastava D, Grover S, Sharma V (2014) Biomaterials in tooth tissue engineering: a review. J Clin Diagn Res 8(1):309–315
Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC (2002) Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res 81(10):695–700
Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC (2004) Bioengineered teeth from cultured rat tooth bud cells. J Dent Res 83(7):523–528
Honda MJ, Sumita Y, Kagami H, Ueda M (2005) Histological and immune histochemical studies of tissue engineered odontogenesis. Arch Histol Cytolo 68(2):89–101
Duailibi SE, Duailibi MT, Zhang W, Asrican R, Vacanti JP, Yelick PC (2008) Bioengineered dental tissues grown in the rat jaw. J Dent Res 87(8):745–750
Ahsan T (2007) Tissue engineering and regenerative medicine: advancing toward clinical therapies. Trans Approaches Tissue Eng Regener Med 3–16
Kim K, Lee CH, Kim BK, Mao JJ (2010) Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res 89(8):842–847
Yuan Z, Nie H, Wang S, Lee CH, Li A, Fu SY, Zhou H, Chen L, Mao JJ (2011) Biomaterial selection for tooth regeneration. Tissue Eng Part B 17(5):373–388
Kim SG, Zheng Y, Zhou J, Chen M, Embree MC, Song K, Jiang N, Mao JJ (2013) Dentin and dental pulp regeneration by the patient’s endogenous cells. Endod Top 28(1):106–117
Huang GJ, Garcia-Godoy F (2014) Missing concepts in de novo pulp regeneration. J Dent Res 93(8):717–724
Xiao L, Nasu M (2014) From regenerative dentistry to regenerative medicine: progress, challenges, and potential applications of oral stem cells. Stem Cells Cloning Adv Appl 7:89–99
Ohazama A, Modino SAC, Miletich I, Sharpe PT (2004) Stem-cell-based tissue engineering of murine teeth. J Dent Res 83(7):518–522
Arakaki M, Ishikawa M, Nakamura T, Iwamoto T, Yamada A, Fukumoto E, Saito M, Otsu K, Harada H, Yamada Y, Fukumoto S (2012) Role of epithelial-stem cell interactions during dental cell differentiation. J Biol Chem 287(13):10590–10601
Lee JH, Lee DS, Choung HW, Shon WJ, Seo BM, Lee EH, Cho JY, Park JC (2011) Odontogenic differentiation of human dental pulp stem cells induced by preameloblast-derived factors. Biomaterials 32(36):9696–9706
Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T (2007) The development of a bioengineered organ germ method. Nat Methods 4(3):227
Ikeda E, Tsuji T (2008) Growing bioengineered teeth from single cells: potential for dental regenerative medicine. Expert Opin Biol Ther 8(6):735–744
Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, Ogawa M, Mizuno M, Kasugai S, Tsuji T (2009) Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci 106(32):13475–13480
Oshima M, Mizuno M, Imamura A, Ogawa M, Yasukawa M, Yamazaki H, Morita R, Ikeda E, Nakao K, Takano-Yamamoto T, Kasugai S (2011) Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PloS One 6(7):p.e21531
Hartgerink JD, Beniash E, Stupp SI (2001) Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294(5547):1684–1688
Xu R, Zhou Y, Zhang B, Shen J, Gao B, Xu X, Ye L, Zheng L, Zhou X (2015) Enamel regeneration in making a bioengineered tooth. Curr Stem Cell Res Ther 10(5):434–442
Yu JH, Shi JN, Deng ZH, Zhuang H, Nie X, Wang RN, Jin Y (2006) Cell pellets from dental papillae can reexhibit dental morphogenesis and dentinogenesis. Biochem Biophys Res Commun 346(1):116–124
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A (2004) Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein. J Dent Res 83(8):590–595
Fukumoto S, Miner JH, Ida H, Fukumoto E, Yuasa K, Miyazaki H, Hoffman MP, Yamada Y (2006) Laminin α5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J Biol Chem 281(8):5008–5016
Liu L, Liu YF, Zhang J, Duan YZ, Jin Y (2016) Ameloblasts serum free conditioned medium: bone morphogenic protein 4 induced odontogenic differentiation of mouse induced pluripotent stem cells. J Tissue Eng Regener Med 10(6):466–474
Zheng LW, Linthicum L, DenBesten PK, Zhang Y (2013) The similarity between human embryonic stem cell-derived epithelial cells and ameloblast-lineage cells. Int J Oral Sci 5(1):1–6
He P, Zhang Y, Kim SO, Radlanski RJ, Butcher K, Schneider RA, Den-Besten PK (2010) Ameloblast differentiation in the human developing tooth: effects of extracellular matrices. Matrix Biol 29(5):411–419
Kollar EJ, Fisher C (1980) Tooth induction in chick epithelium: expression of quiescent genes for enamel synthesis. Science 207(4434):993–995
Bing Hu, Nadiri Amal, Bopp Sabine Kuchler (2006) Tissue engineering of tooth crown, root, and periodontium. Tissue Eng Part A 12(8):2069–2075
Storrie H, Guler MO, Abu-Amara SN, Volberg T, Rao M, Geiger B, Stupp SI (2007) Supramolecular crafting of cell adhesion. Biomaterials 28(31):4608–4618
Stupp SI, Hartgerink JD, Beniash E, Northwestern University (2009) Self-assembly and mineralization of peptide-amphiphile nanofibers. US Patent 7,491,690
Huang Z, Sargeant TD, Hulvat JF, Mata A, Bringas-Jr P, Koh CY, Stupp SI, Snead ML (2008) Bioactive nanofibers instruct cells to proliferate and differentiate during enamel regeneration. J Bone Miner Res 23(12):1995–2006
Borovjagin AV, Dong J, Passineau MJ, Ren C, Lamani E, Mamaeva OA, Wu H, Keyser E, Murakami M, Chen S, MacDougall M (2011) Adenovirus gene transfer to amelogenesis imperfecta ameloblast-like cells. PloS One 6(10):p.e24281
Sharp T, Wang J, Li X, Cao H, Gao S, Moreno M, Amendt BA (2014) A pituitary homeobox 2 (Pitx2): microRNA-200a-3p: beta-catenin pathway converts mesenchyme cells to amelogenin-expressing dental epithelial cells. J Biol Chem 289(39):27327–27341
Akhter M, Kobayashi I, Kiyoshima T, Matsuo K, Yamaza H, Wada H, Honda JY, Ming X, Sakai H (2005) Possible functional involvement of thymosin beta 4 in developing tooth germ of mouse lower first molar. Histochem Cell Biol 124(3–4):207–213
Shiotsuka M, Wada H, Kiyoshima T, Nagata K, Fujiwara H, Kihara M, Hasegawa K, Someya H, Takahashi I, Sakai H (2014) The expression and function of thymosin beta 10 in tooth germ development. Int J Dev Biol 57(11–12):873–883
Wang B, Li L, Du S, Liu C, Lin X, Chen Y, Zhang Y (2010) Induction of human keratinocytes into enamel-secreting ameloblasts. Dev Biol 344(2):795–799
Inoue T (1986) Induction of chondrogenesis in muscle, skin, bone marrow, and periodontal ligament by demineralaized dentin and bone matrix in vivo and in vitro. J Dent Res 65:15–21
Bessho K, Tanaka N, Matsumoto J, Tagawa T, Murata M (1991) Human dentin-matrix-derived bone morphogenetic protein. J Dent Res 70(3):171–175
Rutherford RB, Wahle J, Tucker M, Rueger D, Charette M (1993) Induction of reparative dentine formation in monkeys by recombinant human osteogenic protein-1. Arch Oral Biol 38(7):571–576
Shinmura Y, Tsuchiya S, Hata KI, Honda MJ (2008) Quiescent epithelial cell rests of Malassez can differentiate into ameloblast like cells. J Cell Physiol 217(3):728–738
Murray PE, Garcia-Godoy F, Hargreaves KM (2007) Regenerative endodontics: a review of current status and a call for action. J Endod 33(4):377–390
Fouad AF (2011) The microbial challenge to pulp regeneration. Adv Dent Res 23(3):285–289
Sun HH, Jin T, Yu Q, Chen FM (2011) Biological approaches toward dental pulp regeneration by tissue engineering. J Tissue Eng Regener Med 5(4):e1–e16
Morse DR, O’Larnic J, Yesilsoy C (1990) Apexification: review of the literature. Quintessence Int 21(7):58–598
Suzuki T, Lee CH, Chen M, Zhao W, Fu SY, Qi JJ, Chotkowski G, Eisig SB, Wong A, Mao JJ (2011) Induced migration of dental pulp stem cells for in vivo pulp regeneration. J Dent Res 90(8):1013–1018
Pan S, Dangaria S, Gopinathan G, Yan X, Lu X, Kolokythas A, Niu Y, Luan X (2013) SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling-potential applications as a homing factor in dental pulp regeneration. Stem Cell Rev Rep 9(5):655–667
Takeuchi N, Hayashi Y, Murakami M, Alvarez FJ, Horibe H, Iohara K, Nakata K, Nakamura H, Nakashima M (2015) Similar in vitro effects and pulp regeneration in ectopic tooth transplantation by basic fibroblast growth factor and granulocyte colony stimulating factor. Oral Dis 21(1):113–122
Yang JW, Zhang YF, Wan CY, Sun ZY, Nie S, Jian SJ, Zhang L, Song GT, Chen Z (2015) Autophagy in SDF-1α-mediated DPSC migration and pulp regeneration. Biomaterials 44:11–23
Zhang LX, Shen LL, Ge SH, Wang LM, Yu XJ, Xu QC, Yang PS, Yang CZ (2015) Systemic BMSC homing in the regeneration of pulp-like tissue and the enhancing effect of stromal cell-derived factor-1 on BMSC homing. Int J Clin Exp Pathol 8(9):10261
Li L, Wang Z (2016) PDGF-BB, NGF and BDNF enhance pulp-like tissue regeneration via cell homing. RSC Adv 6(111):109519–109527
Wang HL, Greenwell H, Fiorellini J, Giannobile W, Offenbacher S, Salkin L, Townsend C, Sheridan P, Genco RJ (2005) Periodontal regeneration. J Periodontol 76(9):1601–1622
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci 97(25):13625–13630
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S (2005) The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res 8(3):191–199
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81(8):531–535
Koyama N, Okubo Y, Nakao K, Bessho K (2009) Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Sur 67(3):501–506
Bressan E, Ferroni L, Gardin C, Pinton P, Stellini E, Botticelli D, Sivolella S, Zavan B (2012) Donor age-related biological properties of human dental pulp stem cells change in nanostructured scaffolds. PLoS One 7(11):49146
d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G (2007) Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 14(6):1162
Yu J, He H, Tang C, Zhang G, Li Y, Wang R, Shi J, Jin Y (2010) Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol 11(1):32
Kaushik SN, Kim B, Walma AMC, Choi SC, Wu H, Mao JJ, Jun HW, Cheon K (2016) Biomimetic microenvironments for regenerative endodontics. Biomater Res 20(1):14
Wongwatanasanti N, Jantarat J, Sritanaudomchai H, Hargreaves KM (2018) Effect of bioceramic materials on proliferation and odontoblast differentiation of human stem cells from the apical papilla. J Endod 44(8):1270–1275
Hong S, Chen W, Jiang B (2018) A comparative evaluation of concentrated growth factor and platelet-rich fibrin on the proliferation, migration, and differentiation of human stem cells of the apical papilla. J Endod 44(6):977–983
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364(9429):149–155
Yoo JH, Lee SM, Bae MK, Lee DJ, Ko CC, Kim YI, Kim HJ (2018) Effect of orthodontic forces on the osteogenic differentiation of human periodontal ligament stem cells. J Oral Sci 17:0310
Wang Y, Zhou Y, Jin L, Pang X, Lu Y, Wang Z, Yu Y, Yu J (2018) Mineral trioxide aggregate enhances the osteogenic capacity of periodontal ligament stem cells via NFκB and MAPK signaling pathways. J Cell Physiol 233(3):2386–2397
Yan XZ, Beucken JJ, Yuan C, Jansen JA, Yang F (2018) Spheroid formation and stemness preservation of human periodontal ligament cells on chitosan films. Oral Dis 24(6):1083–1092
Handa K, Saito M, Yamauchi M, Kiyono T, Sato S, Teranaka T, Narayanan AS (2002) Cementum matrix formation in vivo by cultured dental follicle cells. Bone 31(5):606–611
Guo L, Li J, Qiao X, Yu M, Tang W, Wang H, Guo W, Tian W (2013) Comparison of odontogenic differentiation of human dental follicle cells and human dental papilla cells. PLoS One 8(4):62332
Batouli S, Miura M, Brahim J (2003) Comparison of stem-cell- mediated osteogenesis and dentinogenesis. J Dent Res 82:976–981
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nor JE (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34(8):962–969
Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M (2009) Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod 35(11):1536–1542
Albrektsson T, Johansson C (2001) Osteoinduction, osteoconduction and osseointegration. Eur Spine J 10(2):S96–S101
Bhui AS, Singh G, Sidhu SS, Bains PS (2018) Experimental investigation of optimal ED machining parameters for Ti-6Al-4V biomaterial. FU Ser Mech Eng 16(3):337–345
Granito RN, Custodio MR, Renno ACM (2017) Natural marine sponges for bone tissue engineering: the state of art and future perspectives. J Biomed Mater Res Part B Appl Biomater 105(6):1717–1727
Ganesh N, Hanna C, Nair SV, Nair LS (2013) Enzymatically cross-linked alginic-hyaluronic acid composite hydrogels as cell delivery vehicles. Int J Biol Macromol 55:289–294
Husain S, Al-Samadani KH, Najeeb S, Zafar MS, Khurshid Z, Zohaib S, Qasim SB (2017) Chitosan biomaterials for current and potential dental applications. Materials 10(6):602
Varoni EM, Vijayakumar S, Canciani E, Cochis A, De-Nardo L, Lodi G, Rimondini L, Cerruti M (2018) Chitosan-based trilayer scaffold for multitissue periodontal regeneration. J Dent Res 97(3):303–311
Srinivasan S, Jayasree R, Chennazhi KP, Nair SV, Jayakumar R (2012) Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydr Polym 87(1):274–283
Uhrich KE, Rutgers State University of New Jersey (2010) Polyanhydride linkers for production of drug polymers and drug polymer compositions produced thereby. US Patent 7,666,398
Conte R, Di-Salle A, Riccitiello F, Petillo O, Peluso G, Calarco A (2018) Biodegradable polymers in dental tissue engineering and regeneration. Mater Sci 5(6):1073–1101
Hasturk H, Kantarci A, Ghattas M, Dangaria SJ, Abdallah R, Morgan EF, Diekwisch TG, Ashman A, Van-Dyke T (2014) The use of light/chemically hardened polymethylmethacrylate, polyhydroxylethylmethacrylate, and calcium hydroxide graft material in combination with polyanhydride around implants and extraction sockets in minipigs: part II: histologic and micro CT evaluations. J Periodontol 85(9):1230–1239
Bains PS, Sidhu SS, Payal HS (2016) Fabrication and machining of metal matrix composites: a review. Mater Manuf Process 31(5):553–573
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pushpalatha, C., Nagaraja, S., Sowmya, S.V., Kamala, C. (2019). Biomaterials in Tooth Tissue Engineering. In: Bains, P., Sidhu, S., Bahraminasab, M., Prakash, C. (eds) Biomaterials in Orthopaedics and Bone Regeneration . Materials Horizons: From Nature to Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-13-9977-0_7
Download citation
DOI: https://doi.org/10.1007/978-981-13-9977-0_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9976-3
Online ISBN: 978-981-13-9977-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)