Abstract
We examined the detailed in situ expression pattern of thymosin beta 4 (Tβ4) in the developing mouse mandibular first molar. Tβ4 mRNA was expressed in the presumptive dental epithelium at embryonic day 10.5 (E10.5) and in the thickened dental epithelium at E12. An in situ signal was observed in the invaginated epithelial bud at E13, in the enamel organ at E14 and E14.5, and in the primary enamel knot (PEK) at E14.5. The signal was localized in the epithelial cells of the outer layer of the enamel organ at E15 and E15.5. No signal was found in the PEK at these stages. Tβ4 mRNA was expressed in the inner enamel epithelium, cervical loop and dental lamina at E16 and E17. The expression of Tβ4 mRNA was observed in the polarized inner epithelial cells at E18, newborn day 1 (N1) and N2. However, the signal intensity decreased markedly at N3. We herein report for the first time that Tβ4 is distinctly expressed in developing tooth germ, and it may also play functional roles in the initiation, growth and differentiation of tooth germ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian tooth development is mediated by the sequential and reciprocal epithelial-mesenchymal interactions of various kinds of molecules like other organs e.g. hair, glands, lung, kidney etc. (Thesleff et al. 1995). Various kinds of signaling molecules have been studied, and found to be related to the tooth morphogenesis (Thesleff and Åberg 1999; Thesleff 2003; Pispa and Thesleff 2003). However, the precise molecular mechanism of tooth germ development is still unclear. Recently, we performed cDNA subtraction between at embryonic day 10.5 (E10.5) and E12 mouse mandibles and thus found several genes to be differentially expressed in the early developmental course of mandible (Yamaza et al. 2001a). Among these genes, we previously reported that mRNAs of set-α and heat shock proteins were expressed in the developing tooth germ by means of an in situ hybridization method, thus suggesting these factors are related to tooth germ morphogenesis (Yamaza et al. 2001b; Wada et al. 2002). Thymosin beta 4 (Tβ4) is another gene that was found to be differentially expressed in the E12 mouse mandible (Yamaza et al. 2001a).
Tβ4 is known to be a 4.9kDa actin sequestering peptide, which is the most abundant β-tthymosin in mammals. It forms a 1:1 complex with ATP-G-actin (monomeric actin) and inhibits the polymerization of actin (Safer et al. 1991). Tβ4 has been reported to play an important role in cell motility due to its participation in the rapid polymerization/depolymerization process of actin (Pantaloni and Carlier 1993; Kang et al. 1999).
Recently, the diverse functional roles of Tβ4 have also been reported to be involved in the process of angiogenesis (Grant et al. 1995; Grant et al. 1999), wound healing (Philp et al. 2004) and tumorigenesis (Wang et al. 2004). The expression of Tβ4 has also been studied in chick embryogenesis, especially regarding the development of the nervous system, cardio vascular system and feather buds (Dathe and Brand-Saberi 2004). The expression of Tβ4 has been detected in the mouse embryonic nervous system and during the differentiation of embryonic cells into cardiac cells, and the developing rat brain, thus indicating that the expression of Tβ4 is involved in the development of the heart and nervous system, (Gómez-Márquez et al. 1993; Gómez-Márquez et al. 1996; Anadón et al. 2001). It may therefore be possibly that Tβ4 is also related to the morphogenesis of the tooth germ. However, the expression of Tβ4 in mammalian tooth development has not yet been reported. A recent study also showed that Tβ4 shared a high degree of sequence homology with Tβ10 (Anadón et al. 2001). Both Tβ4 and Tβ10 showed over a 70% identical homology regarding the amino acid level and nucleotide level of amino acid coding region based on the available data base.
In this study, we performed membrane hybridization of in situ probes to exclude the possibility of a cross reaction, and then investigated the detailed expression pattern of Tβ4 in developing tooth germ using in situ hybridization to disclose any possible functional roles that this gene may play in odontogenesis
Materials and methods
Animal
Three embryos of BALB/c mice at E10.5, 12, 13, 14, 14.5, 15, 15.5, 16, 17 and 18 after gestation, and at newborn day 1 (N1), 2, 3 were used in this study. Adult mice were obtained from Charles River Japan Incorporated (Yokohama, Japan). All experimental procedures were performed according to the Animal Care and Use Review Committee at Kyushu University. Adult female mice (15 weeks) were caged together with male mice. After 3 hours, successful insemination was determined based on the presence of a postcopulatory plug in the vagina. The embryonic day was defined as E0 after such a plug was recognized.
In situ hybridization
Gene specific probes for Tβ4 (405 bases long) and Tβ10 (389 bases long) mRNAs were designed according to the cDNA sequence provided by the GenBank (GenBank accession no.: TB4=X16053 and TB10=NM025284). The sequences of the primer pairs for RNA probes are shown in Table 1. RNA probes for in situ hybridization was made according to our previous studies (Yamaza et al. 2001b; Wada et al. 2002). Briefly, sense and anti-sense RNA probes were generated by in vitro transcription from a linearized plasmid encompassing Tβ4 cDNA, which was digested at the different site. Tβ10 RNA probes were also made in the same way. The specific day old embryos were removed from pregnant female mice of each gestational age under ether anesthesia.
The removed embryos were fixed in 4% paraformaldehyde (PFA) in diethylpyrocarbonate (DEPC) treated phosphate-buffer saline (PBS, pH 7.4) for 12 h. The heads were dissected from each embryo, and then were embedded in OCT compound. Serial frontal cryosections of the heads were cut in 8-μm-thick slices and mounted on silane-coated glass slides. The sections were fixed with 4% PFA in DEPC treated PBS (pH 7.4) for 10 min, treated with 20 μg/ml proteinase K for 2 min at room temperature (RT), and then were immersed in 0.25 %(v/v) acetic anhydride for 10 min at RT to avoid any background signals. Hybridization was carried out overnight in a humidified chamber at 55°C. The hybridization mixture consisted of 50% deionized formamide, 10% dextran sulfate, 1% Denhardt’s solution, 250 μg/ml yeast tRNA, 0.3 mM NaCl, 20 mM Tris-HCl (pH 8.0), 5 mM EDTA, 10 mM NaH2PO4, 1% N−lauroylsarcosine, and 1 ng/μl digoxigenin (DIG)-labeled RNA probe. The sections were then washed twice in 2×standard saline citrate (SSC) containing 50% formamide, for 30 min at 65°C, followed by incubation with 20 μg/ml RNase A for 30 min at 37°C to avoid any nonspecific binding of the probe. They were then washed twice in 2×SSC containing 50% formamide for 20 min at 65°C, followed by 2×SSC for 15 min at 37°C, and 0.1×SSC for 15 min at 37°C. They were then further washed in PBST (PBS+0.1% polyoxyethylene sorbitan monolaurate; Tween 20) for 15 min at RT and treated with 10% normal goat serum for 1 h at RT. The DIG-labeled probes were visualized by alkaline phosphatase-conjugated anti-DIG antibody using 300 μl/section of BM Purple color substrate (Roche Molecular Biochemicals) containing 0.5 mg/ml levamisole (Wako Osaka, Japan).
To confirm the specificity of the in situ probe of Tβ4, membrane hybridization of in situ probes was performed. As shown in Fig. 1a, dose-dependent decrease of binding activity of anti-sense Tβ4 probe to RNAs was demonstrated. The binding activity of anti-sense Tβ4 probe to sense Tβ4 probe was inhibited by adding excessive unlabeled sense or anti-sense probe in the reaction mixture (Fig. 1b). The in situ expression pattern of Tβ4 mRNA at E15.5 was apparently different from that of Tβ10. Tβ10 mRNA was demonstrated in the dental papilla cells as well as in the enamel organ (Fig. 1d). Meanwhile, no detectable in situ signal of Tβ4 was found in this area (Fig. 1c). No in situ signal was found when the tissue sections were pre-treated with excess amount (10 ng/ μl) of either unlabeled homologous or heterologous probe (Fig. 1e and f). No hybridization signals were also detected in the control tissue specimens to which a sense probe was applied at any of the investigated developmental stages (data not shown).
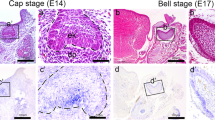
Membrane hybridization of the in situ probes, and the in situ expression of Tβ4 and Tβ10 in the tooth germ at E15.5. a An anti-sense probe for Tβ4 shows dose-dependent hybridization with Tβ4 sense RNA. No hybridization of the labeled Tβ4 antisense probe is observed with Tβ4 antisense or Tβ10 sense/antisense RNAs. b The binding activity of anti-sense Tβ4 probe to sense Tβ4 probe is inhibited by adding excessive unlabeled sense or anti-sense probe in the reaction mixture. c, d Tβ10 mRNA is demonstrated in the dental papilla cells (d) as well as in the enamel organ (arrows). Meanwhile, no detectable in situ signal of Tβ4 is found in the dental papilla cells (c). e, f No in situ signal was found when the tissue sections were pre-treated with excess amount (10 ng/ μl) of either unlabeled homologous (e) or heterologous (f) probe. Scale bars 100 μm
Results
In order to describe the different expression patterns of Tβ4, the developmental stages of tooth germ were classified into the initiation (E10.5), the thickening stage of the dental epithelium (E12), the bud stage (E13-14), the cap stage (E14.5-15.5), the bell stage (E16-18), and the postnatal development stage (N1-3).
Initiation (E10.5)
Expression of Tβ4 mRNA in the E10.5, in which tooth germ had not yet formed in the mandible, was found in both the oral epithelium and mesenchymal cells (Fig. 2a). Although the expression was found through the oral mucosal epithelial layer, an intense in situ signal was found in the limited area of the epithelial layer (Fig. 2a).
In situ expression of Tβ4 in the tooth germ. a Expression of Tβ4 in the E10.5 is observed in the oral epithelial layer. An intense in situ signal was expressed in the oral epithelial layer at the site where the tooth germ would be formed (arrow). b–e Tβ4 is expressed in the tooth germ from E12 to E14.5 (arrows). In situ signal is found in the PEK in the tooth germ at E14.5 (small arrow, PEK). At E15, the tooth germ further developed, and the expression of Tβ4 is observed in the outer layer of the enamel organ and cells in the dental lamina. f, g No Tβ4 expression is observed in the inner epithelial layer of the enamel organ facing the dental mesenchymal tissue and odontogenic epithelial cells in the stellate reticulum at E15 and E15.5. The expression of Tβ4 in the PEK also disappears at these times (small arrow, PEK). Tβ4 is expressed in the outer enamel epithelial cells (arrows, OEE), and in the dental lamina cells. h, i Tβ4 is expressed the enamel epithelial cells at E16 (arrows, IEE). j–m From the day E18 to the day N2, an intense expression of Tβ4 is demonstrated in the polarized ameloblasts, especially the cells facing the enamel matrix formation sites (arrows, IEE). Intense expression is also observed in the cervical loop (small arrows, CL) at N1 and N2. The intensity of the expression in the inner enamel epithelial cells decreases and the expression of Tβ4 is demonstrated in the cervical loop (small arrows, CL) at N3. Scale bars 50 μm (a–j), 100 μm (k–m)
Thickening of dental epithelium (E12)
At E12, small sized tooth bud was formed in the oral epithelial layer. The intense expression of Tβ4 mRNA was seen in the tooth bud. In situ signal was also found in the mucosal epithelial layer, although the signal intensity was weaker than that in the tooth germ (Fig. 2b).
Bud stage (E13-E14)
At E13 and E14, an apparent invagination of the tooth bud into the submucosal mesenchymal tissue was observed, thus forming an early enamel organ. An intense expression was detected in the enamel organ at E13 and E14 (Fig. 2c, 2d). The enamel organ developed up until the early cap stage at E14.5. The expression of Tβ4 mRNA was also demonstrated in the primary enamel knot (PEK), as well as in the odontogenic epithelial cells in the enamel organ (Fig. 2e).
Cap stage (E14.5-E15.5)
At E15, the expression of Tβ4 mRNA was observed in the outer layer of the enamel organ and cells in the dental lamina (Fig. 2f). Meanwhile, the Tβ4 mRNA expression was absent in the inner epithelial layer of the enamel organ facing the dental mesenchymal tissue and odontogenic epithelial cells in the stellate reticulum. The expression of Tβ4 mRNA in the PEK disappeared at E15. Similar expression pattern was also observed at E15.5 (Fig. 2g).
Bell stage (E16-E18)
The enamel organ developed to an apparent bell stage by E16, E17 and E18. Tβ4 mRNA was expressed in the inner enamel epithelial cells (Fig. 2h, 2i, 2j), and in the dental lamina cells (Fig. 2h, 2i). The outer enamel epithelial cells were also weakly and partly positive for Tβ4 mRNA (Fig. 2h, 2i).
Postnatal development (N1-N3)
From day N1 to the day N2, a strong expression of Tβ4 mRNA was demonstrated in the polarized ameloblasts, especially in the cells facing to enamel matrix formation sites. Tβ4 mRNA was localized on the basal side of polarized ameloblasts. An intense expression was also observed in the cervical loop (Fig. 2l, 2m). However, the intensity of the expression in the inner enamel epithelial cells decreased at N3. The expression of Tβ4 mRNA was found in the odontogenic epithelial cells localized at the cervical loop at N3 (Fig. 2m).
In situ expression of Tβ4 mRNA in cranio-facial
Tβ4 was also expressed in the brain (Fig. 3a), nasal epithelium (Fig. 3a), eye (Fig. 3b), salivary duct epithelium (Fig. 3c), hair follicles (Fig. 3d), and lacrimal gland (Fig. 3e). However, the intensity of signals markedly decreased in the brain, eye, and nasal epithelium after birth. An intense expression was preserved in the hair follicles and salivary duct epithelium at N3.
Discussion
In this study, we examined the temporal expression pattern of Tβ4 mRNA in the developing mouse lower first molar. First, we performed membrane hybridization and comfirmed the specificity of in situ probe. In addition, the expression pattern of each Tβ4 and Tβ10 on the tooth germ at E15.5 was different thus indicating the specific hybridization of Tβ4 probe to mRNA. Differential expression of Tβ4 and Tβ10 has been already reported in developing rat cerebellum (Voisin et al. 1995; Anadón et al. 2001). Tβ4 mRNA was found to be expressed in the oral epithelium at a site where the tooth germ is formed at E10.5 because a strong in situ signal was found in the tooth germ on embryonic days E12, E13, E14, and E14.5 days. The in situ expression of Tβ4 subsequently persisted in the enamel organ of the developing tooth germ from E12 to N2. The expression pattern of Tβ4 mRNA in the epithelial tissue of the tooth germ in the early stages of development closely corresponded with the localization of BrdU-positive cells in our previous study (Shigemura et al. 1999; Shigemura et al. 2001). Therefore, the expression of Tβ4 mRNA in the enamel organ seems to be related to cell proliferation in the enamel organ. The expression of Tβ4 mRNA was also demonstrated in the PEK at E14.5. However, the in situ signal of Tβ4 mRNA in the PEK disappeared at E15. We previously examined the precise spatial and tempotal distribution of apoptotic cells in the developing tooth germ and demonstrated that apoptosis occurred in the PEK (Shigemura et al. 1999). A previous report indicated the amount of Tβ4 mRNA in HL60 cells to markedly decrease within 48 hours after induction of apoptosis with araC (Müller et al. 2003). Both our present findings and those of previous studies, thus, suggest the expression of Tβ4 mRNA to be related with the apoptosis in the PEK. PEK is well known to be a signaling center of various kinds of factors that are related to the morphogenesis of tooth germ (Jernvall et al. 1998). Therefore, it seems likely that the expression of Tβ4 mRNA in the PEK plays an important role in the modulation of the signaling pathway in the course of tooth morphogenesis.
The intense expression of the Tβ4 mRNA was also observed in the epithelial elements of the tooth germ from E12 to N2. A previous report demonstrated Tβ4 to actively participate in the hair-promoting process, such as stem cell migration, extracellular matrix degrading enzyme production, and differentiation (Philip et al. 2004). Hair follicle formation is known to start the invagination of epithelial layer into underlying mesenchymal tissue, which is the same as that in the tooth germ. In addition, we found Tβ4 mRNA to be expressed in the hair follicles. Therefore, the expression of Tβ4 mRNA in the epithelial bud of the tooth germ in the very early stages may also be related to the proliferation of odontogenic stem cell.
The gene regulation of Tβ4 has not yet been clarified. Many genes have already been reported to be related to odontogenesis. Most of these genes have been listed in a www database (http://bite-it.helsinki.fi). Among such odontogenesis-related genes, HGF has been shown to be related to the expression of Tβ4. An increase in the Tβ4 expression has been reported to be observed in hepatocyte growth factor (HGF)-treated human umbilical vein endothelial cells (HUVECs) (Oh et al. 2002). HGF is considered to be one of the mediators of epithelial-mesenchymal interactions during early organogenesis (Ohmichi et al. 1998) and to be also involved in the development of the murine teeth (Tabata et al. 1996). In the developing tooth, HGF is expressed in the cells of the dental papillae. Meanwhile, the expression of c-Met, a receptor of HGF, was demonstrated in the cells of the dental epithelium, thus suggesting that the HGF signal released from the dental papilla cells influences the biology of odontogenic epithelial cells (Tabata et al. 1996). Interestingly, the expression pattern of Tβ4 mRNA in our present study at a late stage (E16-N2) of tooth development closely coincided with the expression of c-Met in the previous report. Since HGF up-regulates Tβ4 (Oh et al. 2002), the co-localization of Tβ4 and c-Met in the inner enamel epithelium may represent the up-regulation of Tβ4 by HGF through c-Met. On the other hand, Tβ4 has been reported to mediate load-enhanced synthesis and the activation of matrix metalloproteinases (MMPs) 2 and 9 in the articular cartilage (Blain et al. 2002). MMP-2 was also up-regulated by HGF in HUVECs either directly or indirectly via the induction of Tβ4 (Oh et al. 2002). MMP-2 and -9 play an important role in organogenesis including tooth development by reorganizing the basement membrane and thus helping in the process of the budding of epithelial layer into the underlying mesenchyme (Heikinheimo and Salo 1995; Sahlberg et al. 1999). MMPs including MMP-2 and -9 also take part in the differentiation of ondontogenic cells and they also played a role in the early formation of dentin and enamel (Fanchon et al. 2004). Together with our present study and previous reports, it is possible to hypothesize that the HGF signal induces the expression of Tβ4 through c-Met, and Tβ4 could thereby stimulate the differentiation of ameloblasts and odontoblasts by modulating the expression of MMP-2 and -9.
In this study, we demonstrated the temporal expression pattern of Tβ4 mRNA in the developing tooth in the mouse embryo and neonate, and thus showed the close association of this gene with dentinogenesis. Furthermore, Tβ4 is seemed to be related to the development of various cranio-facial organs. Based on the expression pattern in the developing tooth germ, Tβ4 is considered to be related with cell proliferation in the early stage. Meanwhile, Tβ4 also appears to participate in cell differentiation. However, the essential functional roles of Tβ4 on the development of tooth still remain unknown. Further examinations of the protein level are needed because Tβ4 might be secreted to extracellular space, and thereafter internalized into cells (Bock-Marquette et al. 2004). Thus, Tβ4 possesses diverse functional properties regarding its cellular biology we presently plan to continue yeast two-hybrid experiments to detect any specific proteins that may interact with Tβ4 in the developing tooth germ.
References
Anadón R, Moldes IR, Carpintero P, Evangelatos G, Livianou E, Leondiadis L, Quintela I, Cervino MC, Gómez-Márquez J (2001) Differential expression of thymosins β4 and β10 during rat cerebellum postnatal development. Brain Res 894:255–265
Blain EJ, Mason DJ, Duance VC (2002) The effect of thymosin beta4 on articular cartilage chondrocyte matrix metalloproteinase expression. Biochem Soc Trans 30:879–882
Bock-Marquette L, Saxena A, White MD, Dimalo JM, Srivastava D (2004) Thymosin β4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 432:466–472
Dathe V, Brand-Saberi B (2004) Expression of thymosin beta4 during chick development. Anat Embryol 208:27–32
Fanchon S, Bourd K, Septier D, Everts V, Beertsen W, Menashi S, Goldberg M (2004) Involvement of matrix metalloproteinases in the onset of dentin mineralization. Eur J Oral Sci 112:171–176
Gómez-Márquez J, Pedrares JI, Otero A, Anadón R (1993) Prominent expression of the actin-sequestering peptide Fx gene in the hippocampal region of rat brain. Neurosci Lett 152:41–44
Gómez-Márquez J, del Amo FF, Carpintero P, Anadón R (1996) High levels of mouse thymosin β4 mRNA in differentiating P19 embryonic cells and during development of cardiovascular tissues. Biochim Biophys Acta 1306:187–193
Grant DS, Kinsella JL, Kibbey MC, LaFlamme S, Burbelo PD, Goldstein AL, Kleinman HK (1995) Matrigel induces thymosin beta 4 gene in differentiating endothelial cells. J Cell Sci 108:3685–3694
Grant DS, Rose W, Yaen C, Goldstein A, Martinez J, Kleinman H (1999) Thymosin β4 enhances endothelial cell differentiation and angiogenesis. Angiogenesis 3:125–135
Heikinheimo K, Salo T (1995) Expression of basement membrane type IV collagen and type IV collagenases (MMP-2 and MMP-9) in human fetal teeth. J Dent Res 74:1226–1234
Huff T, Müller CS, Otto AM, Netzker R, Hannappel E (2001) β-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol 33:205–220
Jernvall J, Åberg T, Kettunen P, Keranen S, Thesleff I (1998) The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development 125:161–169
Kang F, Purich DL, Southwick FS (1999) Profilin promotes barbed-end actin filament assembly without lowering the critical concentration. J Biol Chem 274:36963–36972
Mina M, Kollar EJ (1987) The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Boil 32:123–127
Müller CSG, Huff T, Hannappel E (2003) Reduction of thymosin β4 and actin in HL60 cells during apoptosis is preceded by a decrease of their mRNAs. Mol Cell Biochem 250:179–188
Oh IS, So SS, Jahng KY, Kim HG (2002) Hepatocyte growth factor upregulates thymosin β4 in human umbilical vein endothelial cells. Biochem Biophys Res Commun 296:401–405
Ohmichi H, Koshimizu U, Matsumoto K, Nakamura T (1998) Hepatocyte growth factor (HGF) acts as a mesenchyme-derived morphogenic factor during fetal lung development. Development 125:1315–1324
Pantaloni D, Carlier MF (1993) How profiling promotes actin filament assembly in the presence of thymosin β4. Cell 75:1007–1014
Philp D, Goldstein AL, Kleinman HK (2004) Thymosin β4 promotes angiogenesis, wound healing, and hair follicle development. Mech Ageing Dev 125(2):113–115
Philp D, Nguyen M, Scheremeta B, St.-Surin S, Villa AM, Orgel A, Kleinman HK, Elkin M (2004) Thymosin β 4 increases hair growth by activation of hair follicle stem cell. FASEB J 18:385–387
Pispa J, Thesleff I (2003) Mechanism of ectodermal organogenesis. Develop Biol 262:195–205
Safer D, Elzinga M, Nachmias VT (1991) Thymosin beta 4 and Fx, an actin-sequestering peptide, are indistinguishable. J Biol Chem 266:4029–4032
Sahlberg C, Reponen P, Tryggvason K, Thesleff I (1999) Timp-1, −2 and −3 show coexpression with gelatinases A and B during mouse tooth morphogenesis. Eur J Oral Sci 107:121–130
Sanders MC, Goldstein AL, Wang YL (1992) Thymosin beta 4 (Fx peptide) is a potent regulator of actin polymerization in living cells. Proc Natl Acad Sci USA 89:4678–4682
Shigemura N, Kiyoshima T, Kobayashi I, Matsuo K, Yamaza H, Akamine A, Sakai H (1999) The distribution of BrdU- and TUNEL-positive cells during odontogenesis in mouse lower first molars. Histochem J 31:367–377
Shigemura N, Kiyoshima T, Sakai T, Matsuo K, Momoi T, Yamaza H, Kobayashi I, Wada H, Akamine A, Sakai H (2001) Localization of activated caspase-3 positive and apoptotic cells in the developing tooth germ of the mouse lower first molar. Histochem J 33:253–258
Tabata MJ, Kim K, Liu JG, Yamashita K, Matsumura T, Kato J, Iwamoto M, Wakisaka S, Matsumoto K, Nakamura T, Kumegawa M, Kurisu K (1996) Hepatocyte growth factor is involved in the morphogenesis of tooth germ in murine molars. Development 122:1243–1251
Thesleff I (2003) Epithelial-messenchymal signaling regulating tooth morphogenesis. J Cell Sci 116:1647–1648
Thesleff I, Åberg T (1999) Molecular regulation of tooth development. Bone 25:123–125
Thesleff I, Vaahtokari A, Kettunen P, Åberg T (1995). Epithelial-mesenchymal signaling during tooth development. Connect Tissue Res 32:9–19
Voisin PJ, Pardue S, Morrison-Bogorad M (1995) Developmental characterization of thymosin β4 and β10 expression in enriched neuronal culture from rat cerebella. J Neurochem 64:109–120
Wada H, Kobayashi I, Yamaza H, Matsuo K, Kiyoshima T, Akhtar M, Sakai T, Koyano K, Sakai H (2002) Expression of heat shock proteins, Hsc73, Hsj2 and Hsp86 during the morphogenesis of the mouse lower first molar. Histochem J 34:105–109
Wang WS, Chen PM, Hsiao HL, Wang HS, Liang WY, Su Y (2004) Overexpression of the thymosin β-4 gene is associated with increased invasion of SW480 colon carcinoma cells and the distant metastasis of human colorectal carcinoma. Oncogene 23:6666–6671
Yamaza H, Matsuo K, Kiyoshima T, Shigemura N, Kobayashi I, Wada H, Akamine A, Sakai H (2001a) Detection of differentially expressed genes in the early developmental stages of the mouse mandible. Int J Dev Biol 45:675–680
Yamaza H, Matsuo K, Kobayashi I, Wada H, Kiyoshima T, Akhtar M, Ishibashi Y, Sakai T, Akamine A, Sakai H (2001b) Expression of Set-α during morphogenesis of mouse lower first molar. Histochem J 33:437–441
Acknowledgements
This research was supported in part by Grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (number 13470384).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akhter, M., Kobayashi, I., Kiyoshima, T. et al. Possible functional involvement of thymosin beta 4 in developing tooth germ of mouse lower first molar. Histochem Cell Biol 124, 207–213 (2005). https://doi.org/10.1007/s00418-005-0040-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0040-x