Abstract
Biomaterials are essential to medical device production, play a critical role in health care and affect the quality and longevity of human life. The ever-increasing demand for the use of medical devices and implants offering improved function and ease of life makes the development of new biomaterials a challenging task. The design and development of biomaterials are quite a complicated procedure, which involves the manipulation of composition and structure to combine multiple mechanical and biological properties, sometimes even conflicting, in the desired biomaterial, simultaneously. The use of computational techniques can help in the evaluation of designed materials without the need to make any physical object and spend too much money, and more importantly reduce the risks to the development efforts. This chapter overviews the design of orthopaedic biomaterials for total hip and knee replacements, and bone scaffolds. The computational methods involved in their design scenarios are presented, and some efficient tools are introduced to aid in the development of more reliable and optimal biomaterials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The quality of human life can be dramatically improved with the use of biomaterials. Rapidly advancing technologies are allowing new and improved biomaterials to be developed with unprecedented performance behaviour. These materials are being regularly used to make permanent and temporary implants for repairing different parts of the human body, particularly orthopaedic prostheses. The permanently implanted prostheses such as those replacing damaged hip and knee joints are typically made of metals, ceramics and engineering polymers and as the name implies should perform acceptably for a lifetime. The temporarily implanted prostheses such as bone scaffolds are usually based on biodegradable polymers and ceramics that degrade over time. To increase the success of these implants, new biomaterials need to be judiciously designed to rapidly evolve from concept to clinical reality. However, in the design of new biomaterials, costly protocols and long-term experiments are involved (i.e. in vitro and in vivo tests and clinical trials) that must be undertaken before any new product is brought to market (see Fig. 1). This may contain significant commercial risk and uncertainty of outcome for all the efforts made for developing new biomaterials.
To design a new biomaterial, advanced manufacturing techniques [1, 2] and computational approaches [3, 4] should be exploited. The computer modelling of designed materials accelerates novel materials discovery and reduces the risk associated with producing new materials. It does this by providing new opportunities to appreciate how particular structures can be generated in materials, how the structures formed relate to the properties of interest, how the materials respond under real operating conditions and what the design variables of materials are and their optimal values. Also, the consequences of having changes in processing and structure may be assessed and understood, such that the structure–property–performance relationship can be indicated and a basis for specifying the manufacturing approach provided. Meanwhile, the effect of geometry (i.e. shape) variables on the mechanical behaviour of the designed material and the related processing route can be evaluated and it is useful to take into account the concurrent optimization of material and geometry. Further, the computational approaches are capable of estimating the parameters that cannot be readily measured by experiments and of predicting multiple responses, which is the nature of most material design problems, particularly for biomedical applications. Unfortunately, the application of computational methods in the field of biomaterials has been infrequent and fragmented [5], especially when the interactions of biomaterials and the host adjoining tissues are involved. This is probably due to the challenges of establishing suitable computational models that can properly define the complex interactions between biomaterials and living tissues. As a result, research on biomaterial design has mostly been restricted to experimental development, with limited comparisons of several material properties with those of existing materials. Therefore, the generalizing of such trial-and-error approaches on this issue is problematic. However, a more systematic approach is by computational biomaterial design.
2 Rules, Procedures and Methods Used in Computational Design of Biomaterials for Orthopaedic Implants
The investigation of biomaterial functionality is a multifaceted problem and depends on many factors and constraints. Therefore, several design rules should be fulfilled, and some tools should be utilized to develop an efficient biomaterial with enhanced properties. One of the main principles is that the structure and characteristics of a biomaterial should be close to those of the replaced or surrounding tissue to avoid any mismatch that may cause failure of the device either prematurely or in the long term. For example, the stiffness of designed biomaterials connecting bone should be similar to that of the bone in order to avoid adverse load transfer and subsequent bone loss. The other important point is to recommend using an inverse approach to develop the desired product as shown in Fig. 2. The conventional approach to new materials design begins with the synthesis of a new material or with a change in the synthesizing process of an existing material, followed by the characterization and measurement of the properties. This leads to the question, how far are the characteristics obtained from those required for the specific application? This method, therefore, is based on a refined trial-and-error scheme in which new materials are discovered experimentally in laboratories, resulting in spending too much time and expenditure. The inverse approach, however, starts by identifying the required properties for the desired performance. This then becomes the motivation for indicating the synthesizing method required to achieve a suitable microstructure that gives the properties identified. For example, a novel layered material design based on the inverse approach for replacement of damaged cartilage of knee joint [6] can be made of three layers: a layer made of wear-resistant metal alloy such as stainless steel at the articular surface, a layer composed of bioactive ceramic or glass at the bone interface and an intermediate layer made of a mixture of the two materials placed between the two layers (Fig. 3).
The other issue in a biomaterials design scenario is that it sometimes contains multi-scales, such as in tissue engineering for the design of a bone scaffold material. Therefore, the use of computational methods in the field of biomaterials is rather different but similar design process steps are taken. Most of the computational biomaterials design conducted to date has been based on finite element analysis (FEA), which examines new biomaterials by defining calculated properties (as inputs) under different loading conditions.
The following subsections describe the general procedures and techniques used in the computational biomaterials design of hip and knee prostheses because of the numerous and frequent studies conducted and published, and bone scaffolds because of the increasing use in bone surgery.
2.1 Computational Methods for Biomaterial Design in Hip and Knee Replacements
In hip and knee joint implants, the main reason for failure can be aseptic loosening of the prosthetic components, predominantly due to wear, micro-motion or stress shielding effect [7, 8]. However, there may be other causes for the implant to not perform successfully or even fail such as peri-prosthetic fracture, mal-positioning, extensor mechanism failure, infection and pain [9,10,11,12,13]. Usually, a complex mechanical and biological process occurs, causing the failure that ends up needing revision surgery, possibly due to implant design [14], patient condition or surgical factors [15, 16]. In implant design, the material is one of the key elements that can contribute to various failure modes of orthopaedic devices, including adverse body reaction to the particulates released from the implant material, failed bonding due to lack of bioactivity, stress shielding phenomenon as a result of high stiffness (modulus of elasticity) and destructive wear caused by a high coefficient of friction. Therefore, material engineers have focused on the development of novel orthopaedic biomaterials [17,18,19,20] and some with the help of computational design [21,22,23,24].
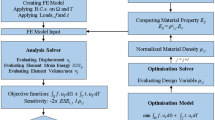
Almost all new orthopaedic biomaterials have been tailored to possess a hierarchical or functionally graded composition and/or structure in order to achieve several properties simultaneously in a single component that are close to those of the natural organ. A number of mathematical methodologies have been adopted to calculate the gradation in properties and effective material properties (mostly Young’s modulus and Poisson’s ratio in biomedical applications) of functionally graded materials (FGMs). This includes exponential functions, power law, sigmoid law, volume fraction and rule of mixture, Hashin–Shtrikman-type bounds and Mori–Tanaka-type models [25]. The exponential functions are usually used for an analytical solution. However, the volume fraction and rule of mixture, which provide a more realistic means of representing the continuous FGM properties, may complicate the analytical solution to FGM problems. Therefore, it is usually used in conjunction with finite element modelling (FEM). For examining new orthopaedic biomaterials in real operating situations (i.e. skeletal systems), three-dimensional (3D) models of the bone segment and implant are obtained by computed tomography (CT) data. These models are then imported to finite element (FE) software, the calculated properties of new biomaterial are assigned, and the loading and boundary conditions are applied. To ensure the function of a new biomaterial, several performance metrics and their ideal values are identified based on the target application. These must be obtained from FEA, although it is rather complicated, particularly when simulating the whole bone‐implant system and when multiple performance metrics should be gained. The performance metrics that represent aseptic loosening are usually defined to estimate wear, implant stability and stress shielding effect (bone loss). For example, the performance metric for the assessment of primary stability of implant component due to lack of osseointegration and bioactivity can be obtained by estimating the micro-motion at the interface of the bone-implant [26,27,28], where the ideal values would be less than 50 μm (bone formation) and the non-ideal values are known to exceed 150 μm (fibrous tissue formation) [29]. The performance metrics can be volumetric/linear wear, maximum stress in contacting surfaces (von Mises and/or contact pressure), wear depth and contact area as measures of wear [30,31,32,33], and von Mises stress distribution in the bone (mean, first quartile and standard deviation of stresses), strain energy density and bone mineral density [26, 34,35,36,37,38] as measures of stress shielding in the hip and knee prostheses. When the early design of new biomaterials proves the proposed concept based on the performance metrics (via comparative analyses with the benchmark materials or if applicable by experiments), optimization of new material design variables such as pore size, amount of porosity, volume fraction of constituents and configuration can be done to achieve the best function. The optimization also can be done by FEA via a parametric study.
The use of FEA to investigate the joint system of a new implant design (i.e. biomaterial and implant geometry) is either based on time-independent analyses or time-dependent/time adaptive modelling techniques. In the latter, a parameter of interest (response) is estimated under an initial condition and then used to adapt the FE model by modification of the implant design (geometry and/or material properties) followed by performing a new analysis and recalculating the parameter of interest again. This is an iterative approach that will end when the solution converges or a predefined time period elapses or the bone-implant system fails. These modelling techniques contain the following simulations [39], which are also conveniently summarized in Fig. 4.
-
Bone remodelling, which is usually used to calculate stress shielding by means of bone mineral density around the femoral component of hip prostheses and tibial and femoral components of knee prostheses [40,41,42,43].
-
Tissue differentiation that is implemented to envisage the differentiation of granulation tissue to fibrous tissue, fibrocartilage or bone based on the micromechanical environment. FEA has been applied to predict tissue differentiation both around implants [44,45,46], and for modelling the fracture healing process [47, 48].
-
Damage accumulation of bone cement, which is conducted according to continuum damage mechanics to estimate fatigue failure of the bone cement [49,50,51].
-
Cement—implant and cement—bone interface de-bonding that is a consecutive detachment process of the cement from the implant based on a static failure criterion initially defined [52, 53].
-
Wear behaviour is mostly based on Archard’s law or a modified version of it. Recently, new wear formulas have been used in computational wear models. In these approaches, wear is related to the contact stress, contact area, wear coefficient and sliding distance. The wear simulation of prosthetic hip and knee joints is aimed at estimating material loss in the softer polyethylene components (i.e. acetabular liner and tibial insert, respectively) and in other types of articular joints such as metal-on-metal joints [30, 54, 55].
2.2 Bone Scaffolds
The technology of bone scaffolds is a rapidly developing area of regenerative medicine. A bone scaffold is a temporary porous structural support used to guide the generation of new bone when filling a defect in the skeletal or bone tissue. This is done by transplanting a bio-factor such as stem cells, genes and proteins, within the porous scaffold, which subsequently should be delivered to regenerate natural tissue. It is expected that the bone scaffold tolerates early loads and provides temporary mechanical stability while it degrades over time, so as the defect can be completely filled with new bone tissue. Also, the porous structure is needed for mass transport (means permeability and diffusion) requirements to nourish cells and to provide channels for cell migration and surface features for cell to be attached [56]. In situations where the size of bone defect is large and/or the bone scaffold alone cannot fully support the early loads, an additional temporary implant in the form of metal plate(s) and/or pin(s), or external fixation is used to stabilize the bone. This is later removed when sufficient strength is obtained in the newly formed bone. The usual procedure for using a porous bone scaffold is as follows [57]:
-
The mesenchymal stem cells and bone cells are usually taken from the bone marrow and the periosteum of the patient or a suitable donor.
-
Since the number of these cells in fresh adult bone marrow is small, culture expansion and cell seeding tasks must be conducted.
-
After the bone scaffold implantation, bone growth takes place in the vicinity of the bony defect filled with the bone scaffold.
-
Then, scaffold degradation occurs by a mechanism related to bulk erosion arising due to a hydrolysis process.
Several factors including porous architecture (i.e. pore size, pore shape, pore interconnectivity and amount of porosity), resorption kinetics, mechanical and flow properties, biocompatibility, hydrophilicity and pre-seeding influence the success of a designed bone scaffold. One of the challenges in bone scaffold design is achieving a balance between the mechanical function and the bio-factor delivery, which varies between a denser or a more porous bone scaffold [58]. This has encouraged material engineers to develop hierarchical porous bone scaffold (functionally graded porous bone scaffold) structures to achieve the required mechanical properties and mass transport characteristics simultaneously [59]. It has been indicated that heterogeneous bone scaffolds with graded elastic modulus and permeability inversely can minimize the fibrous tissue formation when compared to homogeneous bone scaffolds with constant elastic modulus and permeability [60].
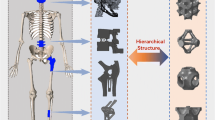
To obtain the hierarchical structure, one can generate libraries of unit cells, as primary components, and assemble them to form the whole desired bone scaffold design as shown in Fig. 5. What is important in the design of bone scaffolds is that the final exterior shape of overall bone scaffold needs to match the complex 3D shape of the bone defect surface, which is achievable by use of medical imaging methods, particularly computed tomography and magnetic resonance imaging (MRI). Structured voxel data sets of patient anatomy, which are based on density distribution, are produced in both CT and MRI. Therefore, the defect shape of interest can be outlined and isolated from these images and a voxel data subset is generated and subsequently used in the design process. The voxel anatomic data can be either converted into solid geometrical models for use in computer-aided design (CAD) [61, 62] or directly used in image-based methods [58, 63, 64]. Next, the internal pore architecture (unit cell) is created and repeated as many times as possible to provide the architecture image. The identified anatomic defect shape is then combined with the internal architecture image through Boolean mathematical techniques, which results in the final bone scaffold design (see Fig. 6). The best functional properties can be obtained by using optimized unit cell architectures that should be as dense as possible for mechanical stability (adequate stiffness or elastic modulus) and as porous as possible for bio-factor delivery. There is consequently conflict in the design requirements because porosity reduces the stiffness. In addition, the porosity used should provide sufficient space for bio-factor seeding and be interconnected for desirable cell nutrition and connective tissue growth. Also, varying the architecture design changes both the elastic modulus and the permeability properties of the bone scaffold. Therefore, bone scaffold design problems involve a number of different design variables and multiple objective functions, thus requiring systematic design tools to be used.
Examples of designed unit cells (CUB NS: regular cubic cells, CUB S: single staggered cubic cells, CUB 2S: double staggered cubic cells, CYL NS: regular cylindrical cells, CYL S: single staggered cylindrical cells and CYL 2S: double staggered cylindrical cells) [65]
To predict the behaviour of implanted bone scaffolds under various environments, computer modelling and mathematical-based approaches can be very helpful [66]. With respect to the literature, two well-differentiated spatio-temporal scales exist for tissue regeneration of bone scaffolds in vivo, which are the tissue (macroscopic) level and the pore scaffold (microscopic) level. Usually, for the former scale, the macroscopic mechanical properties, and diffusive and flow characteristics are obtained using the theory of homogenization [67], whereas for the latter scale, bone regeneration at the surface of bone scaffolds is simulated by the use of a bone growth model based on bone remodelling theory [68, 69], and bone scaffold degradation can be implemented via a model describing the hydrolysis process [70]. What is important is that when new bone is generated in situ onto the bone scaffold surface or once the bone scaffold is degraded, the macroscopic mechanical properties (e.g. stiffness) also vary. Also, bone growth depends on a particular microscopic mechanical stimulus that can be obtained by solving the macroscopic problem. This indicates that a coupling between the two scales should exist. Therefore, multi-scale modelling is required to define these phenomena properly. Traditionally, modelling of tissue regeneration within bone scaffolds has been confined to one of these scales [60, 70,71,72,73]. However, more recent works have applied a micro–macro-mathematical approach that accounts for both scales [74, 75].
There are several tools that can be used in the design process and some of which can be combined with the computational approaches to improve the quality of the output (designed biomaterial).
3 Efficient Tools in the Biomaterial Design Process
Computational modelling and analysis play an essential role in understanding the behaviour of new biomaterials. However, it should be born in mind that computational materials models used for goal-oriented design are highly different from those employed traditionally in materials science research activities, which attempt to realize general phenomena [76]. The models for goal-oriented design are aimed at controlling a material system and its optimization to reach one or more desired outcomes to decide between competitive materials (trade-offs) for a specific product. Furthermore, usually, a new biomaterial tailoring starts with general and abstract concept and ends with concrete and detailed configuration. To accomplish this, one can employ several aiding tools such as Design Failure Modes and Effects Analysis (DFMEA) [77], Quality Function Deployment (QFD) [34, 78], Multi-attribute Decision Making (MADM) [79], Multi-objective Decision Making (MODM) [35, 80] and Design of Experiments (DoE) [81].
The modern material design process usually starts with the identification of design requirements for a specific application, which can be efficiently conducted by DFMEA and QFD. The replacement or modification (tailoring) of a biomaterial might be undertaken if poor performance or failure occurs in the synthesized artificial organ. The failure analysis might indicate that the existing biomaterial could be one of the influential causes. Therefore, it is considered to be a prerequisite to the development of any new material (using the inverse approach). The latter aiding tool (QFD) can help in documenting the voice of the customer (VOC) about the product and translating them into the engineering aspects and technical terms associated with material properties. The customers in the case of biomedical can be patients and complementary team of medical experts and designers. The patients’ expectations may include general needs such as lightness of the implant, non-toxicity and strength which possibly can be complemented by the requirements detected by the medical and design team such as bioactivity and osseointegration, appropriate load transfer and low wear between coupling parts. Then, these functional requirements are translated/related to several engineering and technical requirements, i.e. material properties and/or performance metrics. The former aiding tool (DFMEA) can seek for what goes wrong and how the failure of in-use products relates to the material design aspects. For example, what are the causes behind an implant revision? Are the leading causes related to material characteristics such as implant stiffness, hardness, corrosion or bioactivity? Based on this, a set of engineering requirements also can be identified by considering the product needs in terms of the mechanical, physical, chemical, etc., requirements. Thereafter, the material designers need to tailor these properties in the component(s) of interest. Figure 7 shows an example of DFMEA and QFD for femoral component of knee implants.
In biomedical applications, the monolithic materials usually do not satisfy the desired properties and there is a need for manipulating and orienting the constituent materials (multiple materials and/or porosity) in a hierarchical configuration. This involves a selection process for best constituent materials usually with respect to the material properties identified in DFMEA and QFD as selection criteria, which can be accomplished by MADM. For example, a pure metallic component probably suffers from corrosion and mechanically assisted corrosion as well which leads to adverse local tissue reaction. While it is combined with a non-metallic material like ceramic, the concerns of ion release of metallic component can be alleviated. The metal constituent also can decline the brittleness of bulk ceramic component. However, among the very broad list of available metals and ceramics, the choice of appropriate ones is of importance and deals with many criteria including strength, corrosion resistance, fracture toughness, wear resistance, stiffness, biocompatibility issues and many others. Therefore, multi-attribute decision-making approaches can be very useful tools. Meanwhile, this step requires formulating the properties of new material to generate the conceptual material models which can be evaluated by computational approaches such as FEA. When there are a large number of conceptual material models, MADM can be used again to select the best material considering the outputs of FEA. There are usually some material design variables (e.g. volume fraction of constituents and direction of gradient), and an optimization procedure is needed to set the material design variables at the appropriate levels. In this stage, FEA can be utilized and effectively combined with DoE to run a parametric study in a reduced number of computational experiments. This enables statistical interpretation on main, quadratic and interaction effects of variables and an adequate search of the design space to save time and reduce cost. Finally, MODM can be applied which is necessary for the biomaterials design problem that seeks to satisfy multiple goals concurrently, e.g. adequate stiffness and degradation properties in bone scaffolds. Thus, optimization may offer more than one solution, which can be tested by simulation and comparison with the benchmark biomaterials. The optimization process has been conducted in the literature, but very little has been done using a multi-objective optimization method systematically by applying DoE and the related software packages.
At the end, a selection/ranking process of all alternatives including well-established and optimum computational biomaterials using MADM may require. To ensure the most suitable material, it is essential to provide a material property database with the characteristics of both existing biomaterials and promising ones whose properties are theoretically calculated by computational design. Hence, wide-ranging biomaterials libraries provide a substantial amount of information and enable careful interrogation for a biomaterial with the most preferable properties [82]. Therefore, rather than relying on the intuition and/or experience of biomaterials designers, the most suitable biomaterial can be selected based on rational design criteria. The last stage required is a verifying step which can be done by close corroboration of in vitro and preclinical testing of the devices made of the new biomaterial.
When these tools are used systematically and the above steps are taken in sequence, the design of biomaterial will have “discovery–simulation–optimization–selection–verification” (DSOSV) steps. Following these steps will enable the designers to do the design process within a structured road map. Figure 8 shows these steps schematically, and Fig. 9 presents the biomaterial design of joint replacements along with the aiding tools.
4 Conclusions
Biomaterials design is a multifaceted problem in which several aspects such as the use of inverse material design approach, dependence of required properties to the site of implant in the human body and dealing with macroscopic and microscopic scales should be considered. The biomaterial design process cannot be conducted efficiently if it is left entirely to trial-and-error experimentation. The emphasis was on the role of computational approaches in biomaterials design. Several tools were also introduced to make the biomaterial design process more efficient. The aim of using computational approaches along with these tools is to take into consideration time, cost and quality in the design process. The role of aiding tools is to make the process more logical and intelligible by recognizing the contributions of all inputs, where they fit into the material design process. The motivation for such an approach is to meet a need for biomaterials performance in the context of a given application, where combinations of properties and inherent trade-offs must be simultaneously taken into account.
References
Banerjee S et al (2014) Emerging technologies in arthroplasty: additive manufacturing. J Knee Surg 27(3):185–191
Huang Y et al (2015) Additive manufacturing: current state, future potential, gaps and needs, and recommendations. J Manuf Sci Eng 137(1):014001
Kanouté P et al (2009) Multiscale methods for composites: a review. Arch Comput Methods Eng 16(1):31–75
Boccaccio A et al (2018) A computational approach to the design of scaffolds for bone tissue engineering. Advances in bionanomaterials. Springer, Berlin, pp 111–117
Kohn J (2004) New approaches to biomaterials design. Nat Mater 3(11):745–747
Nygren M et al (2009) Dual-sided joint implant having a wear resistant surface and a bioactive surface. In: International application published under the patent cooperation treaty (PCT), W.I.P. Organization, Editor. Google Patents, US
Bahraminasab M et al (2012) Aseptic loosening of femoral components—a review of current and future trends in materials used. Mater Des 42:459–470
Melvin JS et al (2014) Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty 29(6):1285–1288
Brown NM et al (2015) Extensor mechanism allograft reconstruction for extensor mechanism failure following total knee arthroplasty. J Bone Joint Surg 97(4):279–283
Morrey MC (2015) Revision total knee arthroplasty: management of periprosthetic femur fracture around total knee arthroplasty. Complex primary and revision total knee arthroplasty. Springer, Berlin, pp 129–142
Nishii T et al (2015) Fluctuation of cup orientation during press-fit insertion: a possible cause of malpositioning. J Arthroplasty 30(10):1847–1851
Liddle AD, Rodríguez-Merchán EC (2015) Periprosthetic fractures. Total knee arthroplasty. Springer, Berlin, pp 219–227
Jiranek WA et al (2015) Surgical treatment of prosthetic joint infections of the hip and knee: changing paradigms? J Arthroplasty 30(6):912–918
Bahraminasab M et al (2013) Aseptic loosening of femoral components-materials engineering and design considerations. Mater Des 44:155–163
Katz JN et al (2012) Twelve-year risk of revision after primary total hip replacement in the US Medicare population. J Bone Joint Surg 94(20):1825–1832
Marius N et al (2015) Biomaterials view on the complications associated with hip resurfacing arthroplasty. In: Advanced materials research, vol 1114
Oshkour A et al (2015) Mechanical and physical behaviour of newly developed functionally graded materials and composites of stainless steel 316L with calcium silicate and hydroxyapatite. J Mech Behav Biomed Mater 49:321–331
Doni Z et al (2015) Tribocorrosion behaviour of hot pressed CoCrMo—HAP biocomposites. Tribol Int 91:221–227
Dehaghani MT, Ahmadian M, Beni BH (2015) Fabrication and characterization of porous Co–Cr–Mo/58S bioglass nano-composite by using NH 4 HCO 3 as space-holder. Mater Des 88:406–413
Patel AK, Balani K (2015) Dispersion fraction enhances cellular growth of carbon nanotube and aluminum oxide reinforced ultrahigh molecular weight polyethylene biocomposites. Mat Sci Eng C 46:504–513
Bahraminasab M et al (2013) Material tailoring of the femoral component in a total knee replacement to reduce the problem of aseptic loosening. Mater Des 52:441–451
Enab TA, Bondok NE (2013) Material selection in the design of the tibia tray component of cemented artificial knee using finite element method. Mater Des 44:454–460
Hedia H et al (2014) A new design of cemented stem using functionally graded materials (FGM). Bio-med Mater Eng 24(3):1575–1588
Mehboob H, Chang S-H (2015) Optimal design of a functionally graded biodegradable composite bone plate by using the Taguchi method and finite element analysis. Compos Struct 119:166–173
Gupta A, Talha M (2015) Recent development in modeling and analysis of functionally graded materials and structures. Progr Aerosp Sci 79:1–14
Oshkour A et al (2015) Parametric study of radial functionally graded femoral prostheses with different geometries. Meccanica 1–22
Oshkour AA et al (2013) Finite element analysis on longitudinal and radial functionally graded femoral prosthesis. Int J Numer Methods Biomed Eng 29(12):1412–1427
Oshkour AA et al (2015) Effect of geometrical parameters on the performance of longitudinal functionally graded femoral prostheses. Artif Organs 39(2):156–164
Taylor M, Barrett DS, Deffenbaugh D (2012) Influence of loading and activity on the primary stability of cementless tibial trays. J Orthop Res 30(9):1362–1368
Willing R, Kim IY (2009) Three dimensional shape optimization of total knee replacements for reduced wear. Struct Multi Optim 38(4):405–414
Abdelgaied A et al (2011) Computational wear prediction of artificial knee joints based on a new wear law and formulation. J Biomech 44(6):1108–1116
Mattei L, Di Puccio F, Ciulli E (2013) A comparative study of wear laws for soft-on-hard hip implants using a mathematical wear model. Tribol Int 63:66–77
Netter J et al (2015) Prediction of wear in crosslinked polyethylene unicompartmental knee arthroplasty. Lubricants 3(2):381–393
Jahan A, Bahraminasab M (2015) Multicriteria decision analysis in improving quality of design in femoral component of knee prostheses: influence of interface geometry and material. In: Advances in materials science and engineering
Bahraminasab M et al (2014) Multi-objective design optimization of functionally graded material for the femoral component of a total knee replacement. Mater Des 53:159–173
Bahraminasab M et al (2014) On the influence of shape and material used for the femoral component pegs in knee prostheses for reducing the problem of aseptic loosening. Mater Des 55:416–428
Van Lenthe GH et al (2002) Stemmed femoral knee prostheses: effects of prosthetic design and fixation on bone loss. Acta Orthop 73(6):630–637
Completo A et al (2009) Relationship of design features of stemmed tibial knee prosthesis with stress shielding and end-of-stem pain. Mater Des 30(4):1391–1397
Taylor M, Prendergast PJ (2015) Four decades of finite element analysis of orthopaedic devices: where are we now and what are the opportunities? J Biomech 48(5):767–778
Rezaei F et al (2015) Carbon/PEEK composite materials as an alternative for stainless steel/titanium hip prosthesis: a finite element study. Australas Phys Eng Sci Med 1–12
Gillies RM et al (2007) Adaptive bone remodelling of all polyethylene unicompartmental tibial bearings. ANZ J Surg 77(1–2):69–72
Andersen MR, Petersen MM (2015) Adaptive bone remodeling of the femoral bone after tumor resection arthroplasty with an uncemented proximally hydroxyapatite-coated stem. J Clin Densitometry 19(2):202–207
Pérez M et al (2014) Bone remodeling in the resurfaced femoral head: Effect of cement mantle thickness and interface characteristics. Med Eng Phys 36(2):185–195
Mukherjee K, Gupta S (2015) Bone ingrowth around porous-coated acetabular implant: a three-dimensional finite element study using mechanoregulatory algorithm. Biomech Model Mechanobiol 1–15
Waide V et al (2004) Modelling the fibrous tissue layer in cemented hip replacements: experimental and finite element methods. J Biomech 37(1):13–26
Puthumanapully PK, Browne M (2011) Tissue differentiation around a short stemmed metaphyseal loading implant employing a modified mechanoregulatory algorithm: a finite element study. J Orthop Res 29(5):787–794
Miramini S et al (2015) Computational simulation of the early stage of bone healing under different configurations of locking compression plates. Comput Methods Biomech Biomed Eng 18(8):900–913
Miramini S et al (2015) The relationship between interfragmentary movement and cell differentiation in early fracture healing under locking plate fixation. Australas Phys Eng Sci Med 1–11
Stolk J et al (2002) Finite element and experimental models of cemented hip joint reconstructions can produce similar bone and cement strains in pre-clinical tests. J Biomech 35(4):499–510
Coultrup OJ et al (2010) Computational assessment of the effect of polyethylene wear rate, mantle thickness, and porosity on the mechanical failure of the acetabular cement mantle. J Orthop Res 28(5):565–570
Bouziane M et al (2015) Analysis of the behaviour of cracks emanating from bone inclusion and ordinary cracks in the cement mantle of total hip prosthesis. J Braz Soc Mech Sci Eng 37(1):11–19
Caouette C et al (2015) Influence of the stem fixation scenario on load transfer in a hip resurfacing arthroplasty with a biomimetic stem. J Mech Behav Biomed Mater 45:90–100
Van de Groes S, de Waal-Malefijt M, Verdonschot N (2014) Probability of mechanical loosening of the femoral component in high flexion total knee arthroplasty can be reduced by rather simple surgical techniques. Knee 21(1):209–215
Abdelgaied A et al (2014) The effect of insert conformity and material on total knee replacement wear. Proc Inst Mech Eng Part H J Eng Med 228(1):98–106
Gao L, Dowson D, Hewson RW (2015) Predictive wear modeling of the articulating metal-on-metal hip replacements. J Biomed Mater Res Part B Appl Biomater 105(3):497–506
Bellucci D et al (2011) A new generation of scaffolds for bone tissue engineering. In: Advances in science and technology. Trans Tech Publ
Sanz-Herrera J, García-Aznar J, Doblaré M (2009) On scaffold designing for bone regeneration: a computational multiscale approach. Acta Biomater 5(1):219–229
Hollister SJ et al (2005) Engineering craniofacial scaffolds. Orthod Craniofac Res 8(3):162–173
Hollister SJ (2005) Porous scaffold design for tissue engineering. Nat Mater 4(7):518–524
Kelly DJ, Prendergast PJ (2006) Prediction of the optimal mechanical properties for a scaffold used in osteochondral defect repair. Tissue Eng 12(9):2509–2519
Hutmacher DW, Sittinger M, Risbud MV (2004) Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol 22(7):354–362
Chu T-MG et al (2002) Mechanical and in vivo performance of hydroxyapatite implants with controlled architectures. Biomaterials 23(5):1283–1293
Hollister SJ et al (2000) An image-based approach for designing and manufacturing craniofacial scaffolds. Int J Oral Maxillofac Surg 29(1):67–71
Feinberg SE et al (2001) Image-based biomimetic approach to reconstruction of the temporomandibular joint. Cells Tissues Organs 169(3):309–321
Dallago M et al (2018) Fatigue and biological properties of Ti-6Al-4V ELI cellular structures with variously arranged cubic cells made by selective laser melting. J Mech Behav Biomed Mater 78:381–394
Sengers BG et al (2007) Computational modelling of cell spreading and tissue regeneration in porous scaffolds. Biomaterials 28(10):1926–1940
Sanchez-Palencia E, Zaoui A (1987) Homogenization techniques for composite media. In: Homogenization techniques for composite media
Beaupré G, Orr T, Carter D (1990) An approach for time-dependent bone modeling and remodeling—theoretical development. J Orthop Res 8(5):651–661
Van Lenthe G, De Waal Malefijt M, Huiskes R (1997) Stress shielding after total knee replacement may cause bone resorption in the distal femur. J Bone Joint Surg Br 79(1):117–122
Adachi T et al (2006) Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials 27(21):3964–3972
Sanz-Herrera J, Garcia-Aznar J, Doblare M (2008) A mathematical model for bone tissue regeneration inside a specific type of scaffold. Biomech Model Mechanobiol 7(5):355–366
Hollister SJ, Maddox R, Taboas JM (2002) Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 23(20):4095–4103
Adachi T et al (2001) Trabecular surface remodeling simulation for cancellous bone using microstructural voxel finite element models. J Biomech Eng 123(5):403–409
Sanz-Herrera J, García-Aznar J, Doblaré M (2008) Micro–macro numerical modelling of bone regeneration in tissue engineering. Comput Methods Appl Mech Eng 197(33):3092–3107
Chen Y, Zhou S, Li Q (2011) Microstructure design of biodegradable scaffold and its effect on tissue regeneration. Biomaterials 32(22):5003–5014
Kuehmann C, Olson G (2009) Computational materials design and engineering. Mater Sci Technol 25(4):472–478
Thapa N, Prayson M, Goswami T (2015) Case studies in engineering failure analysis
Santiago A et al (2015) Design of an impulsion prosthetic system for prosthetic foot. In: VI Latin American congress on biomedical engineering CLAIB 2014, Paraná, Argentina 29, 30 and 31 Oct 2014. Springer
Jahan A, Edwards KL, Bahraminasab M (2016) Multi-criteria decision analysis for supporting the selection of engineering materials in product design. Butterworth-Heinemann, Boston
Alaimo G et al (2017) Multi-objective optimization of nitinol stent design. Med Eng Phys 47:13–24
Aherwar A, Singh A, Patnaik A (2016) Study on mechanical and wear characterization of novel Co30Cr4Mo biomedical alloy with added nickel under dry and wet sliding conditions using Taguchi approach. Proc Inst Mech Eng Part L J Mater Des Appl. https://doi.org/10.1177/1464420716638112
Curtarolo S et al (2013) The high-throughput highway to computational materials design. Nat Mater 12(3):191–201
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bahraminasab, M., Edwards, K.L. (2019). Computational Tailoring of Orthopaedic Biomaterials: Design Principles and Aiding Tools. In: Bains, P., Sidhu, S., Bahraminasab, M., Prakash, C. (eds) Biomaterials in Orthopaedics and Bone Regeneration . Materials Horizons: From Nature to Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-13-9977-0_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-9977-0_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9976-3
Online ISBN: 978-981-13-9977-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)