Abstract
There is no question that new antibiotics are sorely needed as bacteria become more and more resistant. However, another important cause of antibiotic failure is the tolerance bacteria exhibit to treatment when they are present within a biofilm. In contrast to the heritable changes bacteria undergo when acquiring new resistance determinants, tolerance is a reversible phenotype that is dependent upon being in a biofilm. Therefore, approaches that can successfully disrupt biofilms are likely to potentiate the efficacy of antibiotics. Biofilm removal by mechanical means is the conventional and most straightforward approach and includes desloughing and debridement. However, newer experimental approaches that target integral components of the biofilm itself are now showing promise.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In their simplest definition, biofilms are communities of microorganisms, adhered to a surface or to each other, and surrounded by an extracellular matrix (ECM). This ECM can be comprised of materials originating from the microbes themselves, or from the host or the environment, and serves as an excellent defensive strategy that enables microorganisms to survive in harsh environments. While ECM components vary widely depending on the biofilm environment and the microorganisms present, they are typically very hydrated and composed of polysaccharides, proteins, and extracellular DNA and RNA (Flemming et al. 2000; Dötsch et al. 2012).
Microorganisms make up less than 10% of the dry mass of most biofilms, with the ECM representing the remainder (Flemming and Wingender 2010). Living within the confines of the ECM not only protects the resident microbes from the environment, but also from the host immune system and any antimicrobials that have been administered. In fact, it has been shown that biofilm-dwelling bacteria can be up to 1000 times more tolerant to antibiotic agents than free-living planktonic cells (Mah and O’Toole 2001). Thus, as an alternative approach to traditional therapies, which directly target the causative pathogens, many researchers have turned their attention towards degrading the protective ECM, giving traditional antimicrobials and host defenses a fighting chance against the liberated, more-susceptible pathogens.
The central dogma concerning biofilm development begins with the attachment of a single planktonic cell to a surface, which is usually exposed to an aqueous medium. After attachment, microcolony formation is characterized by the growth of non-motile colonies that begin producing an ECM. Through quorum sensing pathways, other planktonic cells can be attracted to a microcolony in the subpopulation stage. This quickly gives rise to the macrocolony stage with the development of large towers or mushroom-shaped colonies. A macrocolony can become activated by the introduction of stressful environmental factors or even signal molecules produced by other cells that will lead to the final stage of biofilm development known as dispersion (Yang et al. 2011; Yang et al. 2012). However, it should be noted that most of what we know concerning biofilm development is derived from in vitro studies conducted with a few select microorganisms, and it is clearly very different from what occurs in vivo for many different types of infections. For example, to our knowledge, there have been no observations of the characteristic macrocolony shapes (e.g., towers and mushroom-shaped structures) in clinical biofilm samples. And while some biofilms found in the body are surface-associated and quite large (e.g., dental plaque or implant-associated biofilms), most biofilms seen in samples taken from tissue-associated infections, such as lung, wound, and middle ear, tend to be small aggregates that are surrounded by both bacterial and host-derived ECM (DeLeon et al. 2014; Watters et al. 2014; Sonderholm et al. 2017). Despite their size and structural differences, these biofilms are still exceptionally tolerant to antimicrobials and contribute significantly to a chronically infected state.
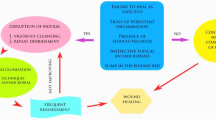
Biofilm-associated infections are so difficult to treat that once a biofilm forms in vivo, the only real treatment option is to remove it. However, removal of biofilms from an infection site is always problematic. If the biofilm is located on a foreign body, for example, removal could be as simple as taking out a catheter, or as difficult as replacing an artificial joint. While this can involve the expense and risk of a revision surgery, it is still more straightforward than the removal of a tissue-associated biofilm. In these infections, biofilms can be located deep within the tissue, muscle, or bone and often amputation is the only option. In this chapter, we will discuss the current standards of care and experimental strategies for the removal of biofilm from tissue-associated infections. In most cases, biofilm removal or disruption is not in itself antimicrobial, but is simply a way to reduce bacterial load and/or weaken biofilms so that conventional antimicrobials are more effective.
2 Mechanical Techniques to Remove Biofilms
Despite the enormous effort and resources devoted to combatting biofilms, in some cases, the simplest of means are still the most effective. For example, some studies show that mechanical treatment is the most commonly used and/or effective method of biofilm removal (Sladek et al. 2007; Barbara and Cogan 2015). Mechanical removal of biofilms is traditionally very effective while also being relatively simple, and includes any method that removes biofilm by physical force. This includes everything from surgical debridement of tissue to ablation via non-thermal plasma jet.
While tissue-associated biofilm infections encompass a broad spectrum of infections (Lebeaux et al. 2013), mechanical removal of biofilm is most often associated with wounds. Therefore, most of the focus of this section will be in regard to wound infections, for which the most commonly used methods for removal of biofilm-infected tissue fall under two blanket terms, desloughing and debridement.
2.1 Desloughing
The “slough” present in wounds is composed of devitalized tissue, white blood cells, fibrin, and debris and can present in several forms (Young 2014). It is normally yellow or yellow/brown and can be superficial and wet, thick and slimy, or become dry and encrusted. Removal of slough is an important step for minimizing infection risk because it acts as a safe haven for bacterial colonization and subsequent biofilm formation (Milne 2015, Percival and Suleman 2015). If the slough is hydrated, it can be mechanically scraped, washed, or sucked off a wound. If slough presents itself as a dry crust, then the first step is to hydrate it to allow autolysis to naturally occur. To hydrate the wound, a moist environment can be created by using either an occlusive dressing to trap moisture in the wound, or by applying a hydrating topical treatment, such as, hydrogel, alginate, or hydrofoam (Percival and Suleman 2015). Additionally, hydrating dressings infused with antimicrobials can be used to prevent bacterial colonization of slough.
2.1.1 Wet-to-Dry Gauze
If an individual’s wound is not capable of autolytic desloughing, then a series of mechanical desloughing methods can be employed (Young 2014; Milne 2015; Percival and Suleman 2015). A method known as wet-to-dry gauze slough removal has been popular among healthcare providers in the past and, depending on the wound, can also act as a debridement method. Wet-to-dry gauze debridement is implemented through the addition of a saline-soaked gauze bandage to a wound, where it is left to dry. While the bandage dries, it incorporates with outer layers of necrotic tissue, exudate, and wound scab. After the gauze is given ample time to dry, a healthcare provider will remove the bandage, thereby debriding the wound (Cowan and Stechmiller 2009). While often effective, this method is time-consuming, painful for the patient, and can take some time to show improvement (Milne 2015). New methods, such as monofilament debridement pads, negative pressure wound therapy (NPWT), or slough-trapping wound dressings have gained prevalence in recent years as the beneficial effects of clean, efficient desloughing are being realized (Percival and Suleman 2015).
2.1.2 Monofilament Debridement Pads
Monofilament debridement pads (MDP) are composed of polyester fibers that bind to loose material on the wound surface. They are used to clean wounds in a similar manner to a sponge when cleaning dishes. Laboratory and clinical studies using a commercially available MDP, called Debrisoft®, have shown that it is able to effectively remove wound slough and decrease the bacterial load of both planktonic and biofilm-associated Pseudomonas aeruginosa (Wilkinson et al. 2016; Schultz et al. 2018). Additionally, clinical studies have revealed that patient pain is significantly lower when using Debrisoft® in comparison to the wet-to-dry gauze method (Schultz et al. 2018).
2.1.3 Slough-Trapping Dressings
Slough is rich in inflammatory material and can act as a safe-haven for infectious bacteria (Percival and Suleman 2015), so the expedient removal of slough can enhance tissue regranulation and decrease risk of infection (Milne 2015; Percival and Suleman 2015; Young et al. 2016). In addition, molecular methods for detecting bacteria in patient wounds suggests that anaerobic bacteria may play a larger role in chronic and necrotizing soft tissue infections (NSTI) than previously thought (Dowd et al. 2008; Zhao-Fleming et al. 2017). From these findings, we can reasonably surmise that by exposing the underlying tissue to air through slough removal, healthcare providers may be inadvertently impairing infectious anaerobic bacteria. To minimize these risks, healthcare providers must regularly deslough high slough-producing chronic wounds, and to help automate this process, researchers have developed slough absorbent wound dressings. A 2017 study on KytoCel®, a commercially available slough absorbent dressing, showed that 19 of 27 high slough-producing patients exhibited enhanced healing when treated with the chitosan-composed dressing (Stephen-Haynes et al. 2018). A similar dressing composed of chitosan was imbued with silver nanoparticles to give the slough absorbent dressing antimicrobial properties (Liang et al. 2016). As shown in their study, chitosan alone had no antimicrobial properties, but the inclusion of silver nanoparticles provided significant antibacterial activity against common pathogens, such as Staphylococcus aureus and P. aeruginosa (Liang et al. 2016).
2.1.4 Negative Pressure Wound Therapy (NWPT)
NPWT is an emerging technique used in the treatment of acute and chronic wounds. Since the early 2000s, NPWT has gained increasing popularity for the treatment of chronic and burn wounds due to a number of published studies describing increased healing rates and decreases in biofilm formation within the wound (Langer et al. 2015; Young et al. 2016). NPWT works by maintaining constant low vacuum pressure on the wound. This vacuum pressure removes wound slough and bacteria within the slough or on the surface of the wound, and also aids in increasing blood flow to the wound bed. For this reason, NPWT is especially effective in treating wounds, such as diabetic foot ulcers, where peripheral blood flow is low and the risk of chronic infection is high. Alone, NPWT does not usually exhibit a significant reduction in bacterial load within infected wounds (Yang et al. 2017). However, when used in conjunction with topical antimicrobial treatments, NPWT has been shown to aid in a significant reduction of bacterial load (Yang et al. 2017). This is likely due to NPWT’s ability to mechanically disrupt biofilm and remove obstacles such as wound slough from the wound bed.
2.2 Surgical Debridement
Wound debridement is the surgical removal of necrotic, infected, or otherwise compromised tissue (Milne 2015), which can be toxic to the surrounding tissue, cause slower wound healing rates, and provide accessible nutrients for bacterial colonization. Surgical removal of compromised tissues is considered invasive when compared to alternative methods of debridement or desloughing, and can also be very painful. For these reasons, surgical debridement is typically only performed on patients with a high risk of sepsis by well-trained personnel. The tools used in surgical debridement have not advanced significantly in recent years, and most healthcare providers still use standard surgical scissors, scalpels, and forceps (Percival and Suleman 2015). Plasma scalpels have been available since the mid-1970s, and while they do offer the benefit of immediate cauterization of the wound and maintenance of sterility, they are cost prohibitive (Guild et al. 2017). Regardless of methodology, early intervention of surgical debridement can prevent significant future morbidity and/or mortality for chronically infected patients (Hsu et al. 2015).
2.2.1 Maggot Wound Therapy (MWT)
As archaic as it sounds, using maggot debridement therapy (MDT) to treat chronic wounds has proven to be an effective method for both desloughing and debridement, resulting in quicker healing rates among a variety of wound types (Sun et al. 2014). Maggots used in MDT are sterile and derived from the Phaenicia (Lucilia) sericata fly (Sun et al. 2014). They clean infected wounds by consuming necrotic tissue with a high degree of fidelity and have recently been found to secrete antimicrobials effective at killing a variety of pathogens, most notably methicillin-resistant Staphylococcus aureus (MRSA) (Bowling et al. 2007). With this newly realized potential of MDT, researchers have begun experimenting with maggots genetically engineered to secrete new varieties of antimicrobials or wound-healing factors (Linger et al. 2016). Utilizing maggots as both a wound cleaner and producer of healing factors can bring treatment options to the masses that are normally not affordable, as long-term antimicrobial or growth factor regimens are extremely expensive.
2.2.2 Sound Energy
Sonication is the application of sound energy to an environment. In respect to biofilm disruption, varying intensities of ultrasonication have been used to disrupt a biofilm, or even target and kill the bacteria within a biofilm. Ultrasonication during wound treatment uses frequencies above 20 kHz. Low-intensity sonication is generally nonspecific because it employs non-focused pulses of ultrasound over a broad area of tissue. Results show that low-intensity ultrasound pulses have the ability to disrupt a variety of bacterial biofilms in vitro (Crone et al. 2015). For example, high ultrasound frequency of 580 kHz is optimal for the disintegration of bacterial aggregates without causing cell death, while using lower frequencies of 20 and 40 kHz resulted in colony forming unit (CFU) reduction of Escherichia coli and Klebsiella pneumonia (Ermolaeva et al. 2011). Alternatively, low-intensity ultrasound can be used to optimize antibiotic treatment of P. aeruginosa biofilm infections, resulting in increased CFU reduction over antibiotic or ultrasound treatments alone (Kopel et al. 2011).
High-intensity ultrasound does not actually use higher frequencies of ultrasound than low-intensity ultrasound in most cases, as the name suggests. Instead, high-intensity ultrasound differs from low-intensity in that it is usually composed of multiple ultrasound sources that converge on a single point. This technique allows healthcare providers to focus treatment within a 3D space for the destruction of necrotic tissue by creating a heating effect at the convergence point. High-intensity ultrasound has also been successfully used to destroy E. coli biofilms, and the bacteria within them (Bigelow et al. 2009). Treatment using this method raises the effectiveness of ultrasound therapy against biofilm, and even makes targeting infections below the surface of healthy tissue possible. Unfortunately, collateral tissue damage via the heating effect created through converging ultrasound frequencies may limit the number of cases for which this therapy can be considered a viable option.
2.2.3 Cold Plasma
Non-thermal plasma (NTP), or cold plasma, is being investigated as a new method for aiding in the sterilization and debridement of wound infections. While there are many plasma types, NTP denotes plasma operating near normal atmospheric pressure and ambient temperature. The majority of previous investigations into using NTP to fight biofilm contamination has centered on treating delicate surfaces, including heat-sensitive plastics and food items prone to contamination, such as lettuce. A recent study reported that a quick 15-second treatment of Salmonella biofilms on food contact surfaces resulted in over a 2-log reduction in CFU (Niemira et al. 2014). Promising results such as these have led to increased interest in applying NTP technology to dentistry and medicine.
2.2.3.1 NTP Treatment of Dental Biofilms
In regard to removing the biofilm (plaque) from teeth, traditional methods include tooth brushing and antimicrobial mouth rinses, such as chlorhexidine (CHX). However, NTP therapy has been adapted for disinfecting dental biofilms, and shows great promise in clinically relevant studies against biofilms grown from the mouth microbiota of healthy volunteers through saliva collection. Researchers often choose to study biofilms grown by isolates collected from saliva because the oral microbiota is very complex and is known to contain a wide variety of pathogenic microorganisms that contribute to severe mouth infection, such as Pseudomonas, Staphylococci, Enterococci, Candida, and some species of anaerobic bacteria (Leonhardt et al. 2003; Botero et al. 2005). Proof of concept studies have shown that treating S. mutans and mixed-population saliva-derived biofilms grown on Ti disks with NTP resulted in a CFU decrease of over 5 logs for both biofilm types, while CHX decreased S. mutans and saliva-derived biofilms by only 3.36 and 1.5 logs, respectively (Koban et al. 2011). Increasingly clinically relevant studies have been performed on surfaces that mimic tooth enamel, and have shown a 96% reduction in S. mutans biofilm when a multimodal approach was taken that paired CHX with NTP (Hong et al. 2016).
2.2.3.2 NTP Treatment of Wound Biofilms
With the increasing prevalence of multi-drug-resistant pathogens, the search for new ways to combat biofilm-associated wound infections is becoming a popular endeavor. Naturally, many researchers have begun characterizing NTP efficacy against the most common infection-associated pathogens. Short NTP treatments significantly reduce the bacterial load of all ESKAPE pathogens (Flynn et al. 2015). However, treatment effect is dependent on species and strain (Ermolaeva et al. 2011; Flynn et al. 2015). Moreover, Gram-positive bacteria are more susceptible to NTP than Gram-negative bacteria, but lack of efficacy can be remedied using longer treatment times or higher intensity treatments (Ermolaeva et al. 2011). The consensus is that NTP antimicrobial attributes primarily stem from the production of reactive oxygen species (ROS), which disrupt bacterial membranes (Ermolaeva et al. 2011; Flynn et al. 2015; Xu et al. 2015). In addition to the production of ROS, researchers have noticed that NTP exhibits ablative properties against the outermost surface of the biofilm (Xu et al. 2015; Xu et al. 2017). NTP also effects more than just the bacterial cells themselves, as evidence suggests that NTP may be able to target and significantly disrupt specific quorum sensing-controlled factors such as pyocyanin and elastase production (Ziuzina et al. 2015).
Concerns of biocompatibility issues between NTP and host tissue have also been noted. Reductions in cell epithelial viability have been seen in several cell and NTP types (Haertel et al. 2012, Wende et al. 2014). However, short treatments of approximately 10 seconds resulted in no distinguishable decreases in survival (Wende et al. 2014). Correlating with cell viability, data show that treatment times greater than 10 seconds can result in a significant decrease in DNA synthesis through the disruption of ssDNA (Wende et al. 2014). These results should provide a cautionary tale to researchers investigating NTP for medical applications, and further investigation into the effects of NTP on host cells is of great importance so that we can carefully optimize therapeutic treatments with the patient’s safety in mind.
3 Degrading and Disrupting the ECM
One of the major challenges that researchers are faced with when developing ECM-specific therapeutics is that the composition of the matrix is highly variable, depending on the microorganisms present, the age and maturity of the biofilm, nutrient and substrate availability, environmental forces, and a plethora of other applicable conditions of the host environment (Koo et al. 2017a, b). Considering the variable makeup of the ECM, any realistic approaches to effectively disrupting it should be multifaceted. The most practical and effective strategies, especially for inherently complex, highly polymicrobial biofilms such as those present in chronic wound infections (Wolcott et al. 2015), are those that will target highly conserved ECM components that are produced by a broad spectrum of pathogens, while at the same time avoiding collateral damage to the host. Clinically, such a therapy could be implemented, regardless of the specific microbial makeup of each individual infection and be expected to augment traditional antimicrobial agents.
Targeting specific ECM components as a means of therapeutic intervention is not a novel concept. In 2002, Whitchurch et al. were the first to show that exogenously added DNase I could inhibit the formation of, and degrade established P. aeruginosa biofilms in vitro, leading to dispersal (Whitchurch et al. 2002). Similarly, in 2003, Kaplan et al. found that dispersin B, a glycoside hydrolase produced by A. actinomycetemcomitans, could degrade the polysaccharide, poly(1,6)-N-acetyl-D-glucosamine (PNAG), by hydrolyzing β(1,6) glycosidic linkages, leading to structural collapse of the biofilm in vitro (Kaplan et al. 2003). However, despite increasing research efforts on medical biofilm dispersal (Fleming and Rumbaugh 2017), clinical application is virtually non-existent, with the exception of Dornase alpha (Pulmozyme®), an FDA-approved therapy for the treatment of lung biofilms presenting in cystic fibrosis patients (Wagener and Kupfer 2012a, b). Thus, despite the promising future of ECM targeting for medical biofilm eradication, there is still quite a bit of work to be done, especially in handling the diverse compositional makeup of biofilms from species to species, and case to case. In this section, we provide an overview of the current state of ECM-targeting strategies, and discuss the logistics of future clinical application.
Active degradation of mature biofilm ECM can be accomplished by various means, and in this section, we discuss three particularly promising approaches: the utilization of ECM-targeted enzymes, antibiofilm peptides, and dispersal molecules. In the interest of broad-spectrum applicability, only the strategies which can be practically effective against the ECM of a range of microbial species will be considered.
3.1 ECM-Targeted Enzymes
Some of the most extensive work in targeting structural ECM components has been the exogenous addition of enzymes that have one or more ECM-integral targets (Fleming and Rumbaugh 2017). Three of the most ubiquitous targets of interest have been proteins, polysaccharides, and extracellular DNA (eDNA).
3.1.1 Exoprotein-Targeting
For many pathogens, proteins constitute a significant percentage of total ECM mass (Lasa and Penades 2006; Jiao et al. 2010; Muthukrishnan et al. 2011; Speziale et al. 2014). Exoproteins are important not only for the role they play in maintaining and modifying the matrix (Zhang and Bishop 2003; Kaplan 2010), but also for surface and ECM scaffolding adhesion and the overall structural stability of the biofilm (Lasa and Penades 2006; Hobley et al. 2015). Therefore, proteases are a potential tool of interest for the deconstruction of the ECM matrix. For example, proteinase K is a serine protease that cleaves peptide bonds adjacent to the carboxylic group of aliphatic and aromatic amino acids, and has been experimentally effective against the biofilms of multiple Gram-positive and Gram-negative bacterial species (Chaignon et al. 2007; Patterson et al. 2007; Fredheim et al. 2009; Izano et al. 2009; Medina and Kadouri 2009; Kumar Shukla and Rao 2013; Nguyen and Burrows 2014; Cui et al. 2016). Another protease with proven broad-spectrum antibiofilm efficacy is trypsin, which targets peptides at the carboxyl side of the positively charged amino acids, lysine, and arginine (Chaignon et al. 2007; Patterson et al. 2007; Niazi et al. 2014; Banar et al. 2016). It should be noted that, while both proteinase K and trypsin are well-documented antibiofilm agents, potential host toxicity due to the presence of target peptides within eukaryotic tissue must be considered, as is the case with many other proteases.
3.1.2 eDNA-Targeting
Another vital ECM component for the adhesion and stability of biofilms is eDNA, which can play an important role in the initial stages of biofilm development, as well as in protecting mature biofilms from physical stress and antibiotics as both an internal scaffolding and a protective cap (Whitchurch et al. 2002; Das et al. 2013; Jakubovics et al. 2013; Alhede et al. 2014; Okshevsky and Meyer 2015; Sena-Velez et al. 2016). Ribonucleases that can break down eDNA represent an extensively studied and clinically applicable class of antibiofilm agent. In fact, Dornase Alpha, marketed as Pulmozyme®, represents the only widely used ribonuclease currently approved by the FDA (Wagener and Kupfer 2012a, b). Although Dornase alpha is used medically to break up DNA-rich mucus in the lungs of cystic fibrosis patients, it has the potential to treat a wide range of biofilm infections and has been shown effective against multiple pathogens (Hall-Stoodley et al. 2008; Kaplan et al. 2012; Claudius et al. 2015). Similarly, DNAse 1, first shown to break down established biofilms in 2002, has also shown promise as a prospective broad-spectrum antibiofilm agent (Whitchurch et al. 2002; Inoue et al. 2003; Nemoto et al. 2003; Rice et al. 2007; Thomas et al. 2008; Fredheim et al. 2009; Izano et al. 2009; Medina and Kadouri 2009; Svensson et al. 2009; Tetz et al. 2009; Harmsen et al. 2010; Martins et al. 2010; Tetz and Tetz 2010; Conover et al. 2011; Godeke et al. 2011; Seper et al. 2011; Sahu et al. 2012; Rajendran et al. 2013; Waryah et al. 2016).
3.1.3 Exopolysaccharide-Targeting
The major ECM component for a wide range of biofilm-dwelling microbes are exopolysaccharides (Flemming and Wingender 2010). Their ubiquity is such that “Exopolysaccharide” and “Extracellular Polymeric Substance” are often times used as interchangeable, if not erroneous, descriptors for the “ECM” abbreviation. In addition to being a major biofilm structural component that is vital to overall mechanical stability, polysaccharides play a host of other roles for biofilm initiation and persistence, including but not limited to surface and scaffolding adhesion, microbial aggregation, desiccation tolerance, nutrient sorption and storage, enzyme binding, and physical protection against antimicrobials and host defenses (Wingender et al. 2001; Flemming and Wingender 2010; Bales et al. 2013; Limoli et al. 2015; Watters et al. 2016). Considering their pervasiveness and utility, polysaccharides are a common target for anti-biofilm researchers, and glycoside hydrolases are at the forefront of prospective biofilm dispersal strategies. For example, Dispersin B, a glycoside hydrolase specific for the polysaccharide, PNAG, has been shown to degrade the ECM of both Gram-positive and Gram-negative pathogens that contain the polysaccharide (Kaplan et al. 2004; Itoh et al. 2005; Izano et al. 2007a, b; Yakandawala et al. 2011; Fazekas et al. 2012; Gawande et al. 2014; Waryah et al. 2016) and is currently being pursued by Kane Biotech Inc. as an antibiofilm agent for use in wound, oral, and lung infections in combination with DNAse 1 (Biotech 2017).
Even with highly conserved targets, however, a single glycoside hydrolase may not be universally efficacious, especially when considering the exceedingly complex, polymicrobial infections that can contain dozens of different microbial species coexisting within one biofilm, as is often the case in chronic wound infections (Wolcott et al. 2016). Thus, combinatorial therapies that contain enzymes with differing targets are of interest. For example, in 2017, researchers showed that α-amylase and cellulase, each targeting a separate, conserved, glycosidic linkage, were able to degrade polymicrobial biofilms and increase antibiotic effectiveness (Fleming et al. 2017). Such multi-glycoside hydrolase combinations, especially when coupled with additional classes of enzymes that target other conserved structural components, such as the previously mentioned exoproteins and eDNA, have the potential to greatly bolster broad-spectrum applicability and clinical practicality. However, it must be considered that the more multifaceted the therapy, the more cautious we must be to avoid collateral damage to the host.
3.1.4 Antibiofilm Peptides
In response to the rapid rise of antibiotic resistance, antimicrobial peptides (AMPs) have received a lot of attention as alternative microbicidal agents, and to date, more than 2600 AMPs have been discovered (Wang et al. 2016). Most of these peptides, however, have only been tested against planktonic cells, with their efficacy against biofilm-dwelling microbes still understudied. Additionally, due in large part to the fact that they are microbicidal, there is the possibility of resistance arising against AMPs in a similar fashion to antibiotics. Antibiofilm peptides (ABPs), however, possess distinct structure–activity relationships when compared to AMPs and are defined as AMPs that exhibit antibiofilm activity below their minimal inhibitory concentration (MIC) (Pletzer et al. 2016; Pletzer and Hancock 2016). The ability of ABPs to degrade biofilms at sub-microbicidal levels make them promising adjunctive agents to existing antibiotic therapies. An example of an ABP that has shown considerable promise is the human cathelicidin, LL-37, which has demonstrated antibiofilm activity far below MIC. Similarly, innate defense regulator peptide 1018 (IDR-1018), a 12-amino acid synthetic peptide that triggers the degradation of the (p)ppGpp bacterial stringent response signal, is another ABP that has exhibited broad-range efficacy against both Gram-positive and Gram-negative organisms, dispersing the bacteria and augmenting antibiotic efficacy against them (de la Fuente-Nunez et al.2014; Reffuveille et al. 2014; Wang et al. 2015).
3.1.5 Dispersal Molecules
Other molecules that are detrimental to the ECM matrix, but do not exhibit enzymatic activity, and are not derivatives of AMPs, can be classified as dispersal molecules. These are molecules that can directly act on ECM constituents by multiple means, including binding to and destabilizing ECM scaffolding, and acting as a detergent that can disrupt the adhesion of biofilm cells and matrix components. For example, rhamnolipids are a type of biosurfactant that are produced by a number of bacterial species, including P. aeruginosa (Boles et al. 2005), that exhibit bimodal activity in biofilms. At lower concentrations, rhamnolipids promote the biofilm mode of life by driving fluid channel maintenance and allowing for robust ECM structure, with rhamnolipid-negative biofilms appearing flat and formless (Davey et al. 2003; Pamp and Tolker-Nielsen 2007; Glick et al. 2010). At higher concentrations, however, rhamnolipids trigger biofilm dispersal for a range of bacterial species by increasing cellular motility, thus inhibiting adherence to the substratum (Boles et al. 2005; Quinn et al. 2013; Bhattacharjee et al. 2016; De Rienzo and Martin 2016). Another example of a potential biofilm dispersal molecule is urea which has been shown effective against the biofilms of both Gram-positive and Gram-negative bacteria, possibly by disrupting structurally important ECM hydrogen bonds (Chen and Stewart 2000, Brindle et al. 2011).
In summary, specifically targeting extracellular ECM components represents a promising approach to degrading biofilms. Additionally, attacking highly conserved biofilm targets with a multifaceted, non-microbicidal therapy may be the most practical way to combat complex, polymicrobial infections with an eclectic, chimeric mix of matrix constituents, while at the same time carrying the decreased risk of the pathogens developing additional resistances. In this way, traditional antimicrobial therapies and host defenses can be augmented, and infections can be more readily cleared.
3.1.6 Dispersal Signal Manipulation
Another avenue of research for the eradication of pathogenic biofilm infections is the manipulation of dispersal signals. Dispersal signals are mediated by certain molecules whose decreased or increased presence triggers active ECM degradation by biofilm microbes. By harnessing our knowledge of active biofilm dispersal mechanisms, we can theoretically design clinical interventions involving the addition or sequestration of key molecules involved in biofilm dispersal pathways (Fleming and Rumbaugh 2017).
One method of biofilm dispersal signal manipulation is the sequestration or degradation of molecules whose increased concentration is associated with biofilm formation and maintenance. Cyclic di-GMP (c-di-GMP) is a nearly ubiquitous intracellular secondary messenger molecule with broad-spectrum involvement in the biofilm life cycle of both Gram-positive and Gram-negative organisms (Jenal et al. 2017). Universally, increased c-di-GMP levels are almost always associated with the biofilm mode of life, and a plethora of studies over the last decade have focused on the modulation of c-di-GMP as a means of biofilm inhibition and dispersal (Jakobsen et al. 2017). Many of these focus on small-molecule inhibition of diguanylate cyclases, which synthesize c-di-GMP from two molecules of GTP, or by direct degradation by certain phosphodiesterases that deconstruct c-di-GMP into the linear dinucleotide, pGpG (Jakobsen et al. 2017).
An essential nutrient required for the formation and persistence of biofilms in a range of pathogens is iron (Banin et al. 2005; Lin et al. 2012; Oglesby-Sherrouse et al. 2014). Thus, iron chelators and competitive inhibitors (such as gallium) serve as potential agents that can trigger biofilm dispersal (Koo et al. 2017a, b). For example, lactoferrin, a globular, iron-binding glycoprotein vital to the innate immune system, so named because of its high abundance in milk, can sequester iron away from the biofilm microbes and has been shown effective against a variety of pathogens (Ammons and Copie 2013; Reffuveille et al. 2014; Fleming and Rumbaugh 2017).
For other molecules, an increase in concentration can trigger inhibitory changes in ECM-production pathways (i.e., c-di-GMP) or induce the production of matrix degradation enzymes. One example is nitric oxide, which has been shown to regulate the levels of c-di-GMP in a conserved manner across microbial species (Barraud et al. 2009; Barraud et al. 2015; Howlin et al. 2017). While biofilm pathogens produce nitric oxide endogenously in order to trigger dispersal, exogenously added nitric oxide has shown promise as a potential biofilm therapeutic agent, which largely acts by stimulating phosphodiesterase activity (Barraud et al. 2015).
Another molecule whose decreased concentration is associated with dispersal in multiple species is Cis-2-decenoic acid (CDA) (Davies and Marques 2009; Rahmani-Badi et al. 2014, 2015; Sepehr et al. 2014). CDA is a fatty acid cross-kingdom signaling molecule, or diffusible signal factor. As an extracellular signaling molecule recognized by both bacteria and fungi, CDA has the potential to trigger the dispersal of complex, polymicrobial biofilms, by inducing the expression of matrix-degrading enzymes by both prokaryotes and eukaryotes (Davies and Marques 2009). It should be noted that, just as with enzymatic therapy, the most applicable therapies are those that would induce multiple dispersal signals, thereby reducing the effect of redundant signaling pathways.
4 Conclusions
The tendency of pathogens to live within the protection of a biofilm affords them greatly increased tolerance to antimicrobials, the host immune system, and environmental stressors, and is a compounding factor in the alarming rise of antibiotic resistance. Therefore, as an alternative to the traditional approach of directly targeting the infecting microbes, many researchers have turned their attention toward anti-biofilm-specific strategies. In this chapter, we highlighted many of the mechanical and chemical approaches to the removal of tissue-associated biofilms, from simple, age-old techniques such as surgical debridement and maggot wound therapy, to cutting-edge technologies like non-thermal plasma, monofilament debridement pads, and small-molecule dispersal signal manipulation. As we better understand the enormous impact that biofilm-associated infections have on medicine, such strategies offer a way forward in their effective management.
References
Alhede, M., Bjarnsholt, T., Givskov, M., & Alhede, M. (2014). Pseudomonas aeruginosa biofilms: Mechanisms of immune evasion. Advances in Applied Microbiology, 86, 1–40.
Ammons, M. C., & Copie, V. (2013). Mini-review: Lactoferrin: A bioinspired, anti-biofilm therapeutic. Biofouling, 29(4), 443–455.
Bales, P. M., Renke, E. M., May, S. L., Shen, Y., & Nelson, D. C. (2013). Purification and characterization of biofilm-associated EPS exopolysaccharides from ESKAPE organisms and other pathogens. PLoS One, 8(6), e67950.
Banar, M., Emaneini, M., Satarzadeh, M., Abdellahi, N., Beigverdi, R., Leeuwen, W. B., & Jabalameli, F. (2016). Evaluation of mannosidase and trypsin enzymes effects on biofilm production of Pseudomonas aeruginosa isolated from burn wound infections. PLoS One, 11(10), e0164622.
Banin, E., Vasil, M. L., & Greenberg, E. P. (2005). Iron and Pseudomonas aeruginosa biofilm formation. Proceedings of the National Academy of Sciences of the United States of America, 102(31), 11076–11081.
Barbara, S., & Cogan, N. G. (2015). Modelling mechanical and chemical treatment of biofilms with two phenotypic resistance mechanisms. Environmental Microbiology, 17(6), 1870–1883.
Barraud, N., Schleheck, D., Klebensberger, J., Webb, J. S., Hassett, D. J., Rice, S. A., & Kjelleberg, S. (2009). Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic Di-GMP levels, and enhanced dispersal. Journal of Bacteriology, 191(23), 7333–7342.
Barraud, N., Kelso, M. J., Rice, S. A., & Kjelleberg, S. (2015). Nitric oxide: A key mediator of biofilm dispersal with applications in infectious diseases. Current Pharmaceutical Design, 21(1), 31–42.
Bhattacharjee, A., Nusca, T. D., & Hochbaum, A. I. (2016). Rhamnolipids mediate an interspecies biofilm dispersal signaling pathway. ACS Chemical Biology, 11, 3068–3076.
Bigelow, T. A., Northagen, T., Hill, T. M., & Sailer, F. C. (2009). The destruction of Escherichia coli biofilms using high-intensity focused ultrasound. Ultrasound in Medicine & Biology, 35(6), 1026–1031.
Biotech, K. (2017). “Kane Biotech – Business and Intellectual Property Update.” Retrieved March 30, 2018, from http://kanebiotech.com/kane-biotech-business-and-intellectual-property-update/
Boles, B. R., Thoendel, M., & Singh, P. K. (2005). Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Molecular Microbiology, 57(5), 1210–1223.
Botero, J. E., Gonzalez, A. M., Mercado, R. A., Olave, G., & Contreras, A. (2005). Subgingival microbiota in peri-implant mucosa lesions and adjacent teeth in partially edentulous patients. Journal of Periodontology, 76(9), 1490–1495.
Bowling, F. L., Salgami, E. V., & Boulton, A. J. (2007). Larval therapy: A novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers. Diabetes Care, 30(2), 370–371.
Brindle, E. R., Miller, D. A., & Stewart, P. S. (2011). Hydrodynamic deformation and removal of Staphylococcus epidermidis biofilms treated with urea, chlorhexidine, iron chloride, or DispersinB. Biotechnology and Bioengineering, 108(12), 2968–2977.
Chaignon, P., Sadovskaya, I., Ragunah, C., Ramasubbu, N., Kaplan, J. B., & Jabbouri, S. (2007). Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Applied Microbiology and Biotechnology, 75(1), 125–132.
Chen, X., & Stewart, P. S. (2000). Biofilm removal caused by chemical treatments. Water Research, 34(17), 4229–4233.
Claudius, C., Perner, A., & Møller, M. H. (2015). Nebulised dornase alfa versus placebo or hypertonic saline in adult critically ill patients: A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Systematic Reviews, 4, 153.
Conover, M. S., Mishra, M., & Deora, R. (2011). Extracellular DNA is essential for maintaining Bordetella biofilm integrity on abiotic surfaces and in the upper respiratory tract of mice. PLoS One, 6(2), e16861.
Cowan, L. J., & Stechmiller, J. (2009). Prevalence of wet-to-dry dressings in wound care. Advances in Skin & Wound Care, 22(12), 567–573.
Crone, S., Garde, C., Bjarnsholt, T., & Alhede, M. (2015). A novel in vitro wound biofilm model used to evaluate low-frequency ultrasonic-assisted wound debridement. Journal of Wound Care, 24(2)., 64, 66–69, 72.
Cui, H., Ma, C., & Lin, L. (2016). Co-loaded proteinase K/thyme oil liposomes for inactivation of Escherichia coli O157:H7 biofilms on cucumber. Food & Function, 7(9), 4030–4040.
Das, T., Sehar, S., & Manefield, M. (2013). The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environmental Microbiology Reports, 5(6), 778–786.
Davey, M. E., Caiazza, N. C., & O’Toole, G. A. (2003). Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. Journal of Bacteriology, 185(3), 1027–1036.
Davies, D. G., & Marques, C. N. (2009). A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. Journal of Bacteriology, 191(5), 1393–1403.
de la Fuente-Nunez, C., Reffuveille, F., Haney, E. F., Straus, S. K., & Hancock, R. E. (2014). Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathogens, 10(5), e1004152.
De Rienzo, M. A., & Martin, P. J. (2016). Effect of mono and Di-rhamnolipids on biofilms pre-formed by Bacillus subtilis BBK006. Current Microbiology, 73(2), 183–189.
DeLeon, S., Clinton, A., Fowler, H., Everett, J., Horswill, A. R., & Rumbaugh, K. P. (2014). Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infection and Immunity, 82(11), 4718–4728.
Dötsch, A., Eckweiler, D., Schniederjans, M., Zimmermann, A., Jensen, V., Scharfe, M., Geffers, R., & Häussler, S. (2012). The Pseudomonas aeruginosa transcriptome in planktonic cultures and static biofilms using RNA sequencing. PLoS One, 7(2), e31092.
Dowd, S. E., Sun, Y., Secor, P. R., Rhoads, D. D., Wolcott, B. M., James, G. A., & Wolcott, R. D. (2008). Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiology, 8, 43.
Ermolaeva, S. A., Varfolomeev, A. F., Chernukha, M. Y., Yurov, D. S., Vasiliev, M. M., Kaminskaya, A. A., Moisenovich, M. M., Romanova, J. M., Murashev, A. N., Selezneva, I. I., Shimizu, T., Sysolyatina, E. V., Shaginyan, I. A., Petrov, O. F., Mayevsky, E. I., Fortov, V. E., Morfill, G. E., Naroditsky, B. S., & Gintsburg, A. L. (2011). Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. Journal of Medical Microbiology, 60(Pt 1), 75–83.
Fazekas, E., Kandra, L., & Gyemant, G. (2012). Model for beta-1,6-N-acetylglucosamine oligomer hydrolysis catalysed by DispersinB, a biofilm degrading enzyme. Carbohydrate Research, 363, 7–13.
Fleming, D., & Rumbaugh, K. P. (2017). Approaches to dispersing medical biofilms. Microorganisms, 5(2), E15.
Fleming, D., Chahin, L., & Rumbaugh, K. (2017). Glycoside hydrolases degrade Polymicrobial bacterial biofilms in wounds. Antimicrobial Agents and Chemotherapy, 61(2), e01998–e01916.
Flemming, H. C., & Wingender, J. (2010). The biofilm matrix. Nature Reviews. Microbiology, 8(9), 623–633.
Flemming, H.-C., Wingender, J., & Griegbe, M. C. (2000). Physico-chemical properties of biofilms. Macmillan Publishers Limited, 8, 19–34.
Flynn, P. B., Higginbotham, S., Alshraiedeh, N. H., Gorman, S. P., Graham, W. G., & Gilmore, B. F. (2015). Bactericidal efficacy of atmospheric pressure non-thermal plasma (APNTP) against the ESKAPE pathogens. International Journal of Antimicrobial Agents, 46(1), 101–107.
Fredheim, E. G., Klingenberg, C., Rohde, H., Frankenberger, S., Gaustad, P., Flaegstad, T., & Sollid, J. E. (2009). Biofilm formation by Staphylococcus haemolyticus. Journal of Clinical Microbiology, 47(4), 1172–1180.
Gawande, P. V., Leung, K. P., & Madhyastha, S. (2014). Antibiofilm and antimicrobial efficacy of DispersinB(R)-KSL-W peptide-based wound gel against chronic wound infection associated bacteria. Current Microbiology, 68(5), 635–641.
Glick, R., Gilmour, C., Tremblay, J., Satanower, S., Avidan, O., Deziel, E., Greenberg, E. P., Poole, K., & Banin, E. (2010). Increase in rhamnolipid synthesis under iron-limiting conditions influences surface motility and biofilm formation in Pseudomonas aeruginosa. Journal of Bacteriology, 192(12), 2973–2980.
Godeke, J., Paul, K., Lassak, J., & Thormann, K. M. (2011). Phage-induced lysis enhances biofilm formation in Shewanella oneidensis MR-1. The ISME Journal, 5(4), 613–626.
Guild, G. N., 3rd, Runner, R. P., Castilleja, G. M., Smith, M. J., & Vu, C. L. (2017). Efficacy of hybrid plasma scalpel in reducing blood loss and transfusions in direct anterior Total hip arthroplasty. The Journal of Arthroplasty, 32(2), 458–462.
Haertel, B., Volkmann, F., von Woedtke, T., & Lindequist, U. (2012). Differential sensitivity of lymphocyte subpopulations to non-thermal atmospheric-pressure plasma. Immunobiology, 217(6), 628–633.
Hall-Stoodley, L., Nistico, L., Sambanthamoorthy, K., Dice, B., Nguyen, D., Mershon, W. J., Johnson, C., Hu, F. Z., Stoodley, P., Ehrlich, G. D., & Post, J. C. (2008). Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiology, 8, 173.
Harmsen, M., Lappann, M., Knochel, S., & Molin, S. (2010). Role of extracellular DNA during biofilm formation by Listeria monocytogenes. Applied and Environmental Microbiology, 76(7), 2271–2279.
Hobley, L., Harkins, C., MacPhee, C. E., & Stanley-Wall, N. R. (2015). Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiology Reviews, 39(5), 649–669.
Hong, Q., Dong, X., Chen, M., Xu, Y., Sun, H., Hong, L., Wang, Y., & Yu, Q. (2016). Disinfection of Streptococcus mutans biofilm by a non-thermal atmospheric plasma brush. Japanese Journal of Applied Physics, 55(7S2):07LG02.
Howlin, R. P., Cathie, K., Hall-Stoodley, L., Cornelius, V., Duignan, C., Allan, R. N., Fernandez, B. O., Barraud, N., Bruce, K. D., Jefferies, J., Kelso, M., Kjelleberg, S., Rice, S. A., Rogers, G. B., Pink, S., Smith, C., Sukhtankar, P. S., Salib, R., Legg, J., Carroll, M., Daniels, T., Feelisch, M., Stoodley, P., Clarke, S. C., Connett, G., Faust, S. N., & Webb, J. S. (2017). Low-dose nitric oxide as targeted anti-biofilm adjunctive therapy to treat chronic Pseudomonas aeruginosa infection in cystic fibrosis. Molecular Therapy, 25(9), 2104–2116.
Hsu, C. R., Chang, C. C., Chen, Y. T., Lin, W. N., & Chen, M. Y. (2015). Organization of wound healing services: The impact on lowering the diabetes foot amputation rate in a ten-year review and the importance of early debridement. Diabetes Research and Clinical Practice, 109(1), 77–84.
Inoue, T., Shingaki, R., Sogawa, N., Sogawa, C. A., Asaumi, J., Kokeguchi, S., & Fukui, K. (2003). Biofilm formation by a fimbriae-deficient mutant of Actinobacillus actinomycetemcomitans. Microbiology and Immunology, 47(11), 877–881.
Itoh, Y., Wang, X., Hinnebusch, B. J., Preston, J. F., 3rd, & Romeo, T. (2005). Depolymerization of beta-1,6-N-acetyl-D-glucosamine disrupts the integrity of diverse bacterial biofilms. Journal of Bacteriology, 187(1), 382–387.
Izano, E. A., Sadovskaya, I., Vinogradov, E., Mulks, M. H., Velliyagounder, K., Ragunath, C., Kher, W. B., Ramasubbu, N., Jabbouri, S., Perry, M. B., & Kaplan, J. B. (2007a). Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microbial Pathogenesis, 43(1), 1–9.
Izano, E. A., Wang, H., Ragunath, C., Ramasubbu, N., & Kaplan, J. B. (2007b). Detachment and killing of Aggregatibacter actinomycetemcomitans biofilms by dispersin B and SDS. Journal of Dental Research, 86(7), 618–622.
Izano, E. A., Shah, S. M., & Kaplan, J. B. (2009). Intercellular adhesion and biocide resistance in nontypeable Haemophilus influenzae biofilms. Microbial Pathogenesis, 46(4), 207–213.
Jakobsen, T. H., Tolker-Nielsen, T., Givskov, M. (2017). Bacterial biofilm control by perturbation of bacterial signaling processes. International Journal of Molecular Sciences, 18(9):1970. Published 2017 Sep 13.https://doi.org/10.3390/ijms18091970
Jakubovics, N. S., Shields, R. C., Rajarajan, N., & Burgess, J. G. (2013). Life after death: The critical role of extracellular DNA in microbial biofilms. Letters in Applied Microbiology, 57(6), 467–475.
Jenal, U., Reinders, A., & Lori, C. (2017). Cyclic di-GMP: Second messenger extraordinaire. Nature Reviews. Microbiology, 15(5), 271–284.
Jiao, Y., Cody, G. D., Harding, A. K., Wilmes, P., Schrenk, M., Wheeler, K. E., Banfield, J. F., & Thelen, M. P. (2010). Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Applied and Environmental Microbiology, 76(9), 2916–2922.
Kaplan, J. B. (2010). Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. Journal of Dental Research, 89(3), 205–218.
Kaplan, J. B., Ragunath, C., Ramasubbu, N., & Fine, D. H. (2003). Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous β-Hexosaminidase activity. Journal of Bacteriology, 185(16), 4693–4698.
Kaplan, J. B., Ragunath, C., Velliyagounder, K., Fine, D. H., & Ramasubbu, N. (2004). Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrobial Agents and Chemotherapy, 48(7), 2633–2636.
Kaplan, J. B., LoVetri, K., Cardona, S. T., Madhyastha, S., Sadovskaya, I., Jabbouri, S., & Izano, E. A. (2012). Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. Journal of Antibiotics (Tokyo), 65(2), 73–77.
Koban, I., Holtfreter, B., Hubner, N. O., Matthes, R., Sietmann, R., Kindel, E., Weltmann, K. D., Welk, A., Kramer, A., & Kocher, T. (2011). Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro – Proof of principle experiment. Journal of Clinical Periodontology, 38(10), 956–965.
Koo, H., Allan, R. N., Howlin, R. P., Stoodley, P., & Hall-Stoodley, L. (2017a). Targeting microbial biofilms: Current and prospective therapeutic strategies. Nature Reviews Microbiology, 15(12), 740–755.
Koo, H., Allan, R. N., Howlin, R. P., Stoodley, P., & Hall-Stoodley, L. (2017b). Targeting microbial biofilms: Current and prospective therapeutic strategies. Nature Reviews. Microbiology, 15(12), 740–755.
Kopel, M., Degtyar, E., & Banin, E. (2011). Surface acoustic waves increase the susceptibility of Pseudomonas aeruginosa biofilms to antibiotic treatment. Biofouling, 27(7), 701–710.
Kumar Shukla, S., & Rao, T. S. (2013). Dispersal of Bap-mediated Staphylococcus aureus biofilm by proteinase K. Journal of Antibiotics (Tokyo), 66(2), 55–60.
Langer, V., Bhandari, P. S., Rajagopalan, S., & Mukherjee, M. K. (2015). Negative pressure wound therapy as an adjunct in healing of chronic wounds. International Wound Journal, 12(4), 436–442.
Lasa, I., & Penades, J. R. (2006). Bap: A family of surface proteins involved in biofilm formation. Research in Microbiology, 157(2), 99–107.
Lebeaux, D., Chauhan, A., Rendueles, O., & Beloin, C. (2013). From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens, 2(2), 288–356.
Leonhardt, A., Dahlen, G., & Renvert, S. (2003). Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. Journal of Periodontology, 74(10), 1415–1422.
Liang, D., Lu, Z., Yang, H., Gao, J., & Chen, R. (2016). Novel asymmetric Wettable AgNPs/chitosan wound dressing: In vitro and in vivo evaluation. ACS Applied Materials & Interfaces, 8(6), 3958–3968.
Limoli, D. H., Jones, C. J., & Wozniak, D. J. (2015). Bacterial extracellular polysaccharides in biofilm formation and function. Microbiology Spectrum, 3(3). https://doi.org/10.1128/microbiolspec.MB-0011-2014.
Lin, M. H., Shu, J. C., Huang, H. Y., & Cheng, Y. C. (2012). Involvement of iron in biofilm formation by Staphylococcus aureus. PLoS One, 7(3), e34388.
Linger, R. J., Belikoff, E. J., Yan, Y., Li, F., Wantuch, H. A., Fitzsimons, H. L., & Scott, M. J. (2016). Towards next generation maggot debridement therapy: Transgenic Lucilia sericata larvae that produce and secrete a human growth factor. BMC Biotechnology, 16(1), 30.
Mah, T. F., & O’Toole, G. A. (2001). Mechanisms of biofilm resistance to antimicrobial agents. Trends in Microbiology, 9(1), 34–39.
Martins, M., Uppuluri, P., Thomas, D. P., Cleary, I. A., Henriques, M., Lopez-Ribot, J. L., & Oliveira, R. (2010). Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia, 169(5), 323–331.
Medina, A. A., & Kadouri, D. E. (2009). Biofilm formation of Bdellovibrio bacteriovorus host-independent derivatives. Research in Microbiology, 160(3), 224–231.
Milne, J. (2015). Wound-bed preparation: The importance of rapid and effective desloughing to promote healing. The British Journal of Nursing, 24(Suppl 20), S52–S58.
Muthukrishnan, G., Quinn, G. A., Lamers, R. P., Diaz, C., Cole, A. L., Chen, S., & Cole, A. M. (2011). Exoproteome of Staphylococcus aureus reveals putative determinants of nasal carriage. Journal of Proteome Research, 10(4), 2064–2078.
Nemoto, K., Hirota, K., Murakami, K., Taniguti, K., Murata, H., Viducic, D., & Miyake, Y. (2003). Effect of Varidase (streptodornase) on biofilm formed by Pseudomonas aeruginosa. Chemotherapy, 49(3), 121–125.
Nguyen, U. T., & Burrows, L. L. (2014). DNase I and proteinase K impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilms. International Journal of Food Microbiology, 187, 26–32.
Niazi, S. A., Clark, D., Do, T., Gilbert, S. C., Foschi, F., Mannocci, F., & Beighton, D. (2014). The effectiveness of enzymic irrigation in removing a nutrient-stressed endodontic multispecies biofilm. International Endodontic Journal, 47(8), 756–768.
Niemira, B. A., Boyd, G., & Sites, J. (2014). Cold plasma rapid decontamination of food contact surfaces contaminated with Salmonella biofilms. Journal of Food Science, 79(5), M917–M922.
Oglesby-Sherrouse, A. G., Djapgne, L., Nguyen, A. T., Vasil, A. I., & Vasil, M. L. (2014). The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa. Pathogens and Disease, 70(3), 307–320.
Okshevsky, M., & Meyer, R. L. (2015). The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Critical Reviews in Microbiology, 41(3), 341–352.
Pamp, S. J., & Tolker-Nielsen, T. (2007). Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. Journal of Bacteriology, 189(6), 2531–2539.
Patterson, J. L., Girerd, P. H., Karjane, N. W., & Jefferson, K. K. (2007). Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. American Journal of Obstetrics and Gynecology, 197(2), 170 e171–170 e177.
Percival, S. L., & Suleman, L. (2015). Slough and biofilm: removal of barriers to wound healing by desloughing. Journal of Wound Care, 24(11), 498, 500–493–498, 506–410.
Pletzer, D., & Hancock, R. E. W. (2016). Antibiofilm peptides: Potential as broad-Spectrum agents. Journal of Bacteriology, 198(19), 2572–2578.
Pletzer, D., Coleman, S. R., & Hancock, R. E. W. (2016). Anti-biofilm peptides as a new weapon in antimicrobial warfare. Current Opinion in Microbiology, 33, 35–40.
Quinn, G. A., Maloy, A. P., Banat, M. M., & Banat, I. M. (2013). A comparison of effects of broad-spectrum antibiotics and biosurfactants on established bacterial biofilms. Current Microbiology, 67(5), 614–623.
Rahmani-Badi, A., Sepehr, S., Mohammadi, P., Soudi, M. R., Babaie-Naiej, H., & Fallahi, H. (2014). A combination of cis-2-decenoic acid and antibiotics eradicates pre-established catheter-associated biofilms. Journal of Medical Microbiology, 63(Pt 11), 1509–1516.
Rahmani-Badi, A., Sepehr, S., & Babaie-Naiej, H. (2015). A combination of cis-2-decenoic acid and chlorhexidine removes dental plaque. Archives of Oral Biology, 60(11), 1655–1661.
Rajendran, R., Williams, C., Lappin, D. F., Millington, O., Martins, M., & Ramage, G. (2013). Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryotic Cell, 12(3), 420–429.
Reffuveille, F., de la Fuente-Nunez, C., Mansour, S., & Hancock, R. E. (2014). A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrobial Agents and Chemotherapy, 58(9), 5363–5371.
Rice, K. C., Mann, E. E., Endres, J. L., Weiss, E. C., Cassat, J. E., Smeltzer, M. S., & Bayles, K. W. (2007). The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proceedings of the National Academy of Sciences of the United States of America, 104(19), 8113–8118.
Sahu, P. K., Iyer, P. S., Oak, A. M., Pardesi, K. R., & Chopade, B. A. (2012). Characterization of eDNA from the clinical strain Acinetobacter baumannii AIIMS 7 and its role in biofilm formation. ScientificWorldJournal, 2012, 973436.
Schultz, G. S., Woo, K., Weir, D., & Yang, Q. (2018). Effectiveness of a monofilament wound debridement pad at removing biofilm and slough: Ex vivo and clinical performance. Journal of Wound Care, 27(2), 80–90.
Sena-Velez, M., Redondo, C., Graham, J. H., & Cubero, J. (2016). Presence of extracellular DNA during biofilm formation by Xanthomonas citri subsp. citri strains with different host range. PLoS One, 11(6), e0156695.
Sepehr, S., Rahmani-Badi, A., Babaie-Naiej, H., & Soudi, M. R. (2014). Unsaturated fatty acid, cis-2-decenoic acid, in combination with disinfectants or antibiotics removes pre-established biofilms formed by food-related bacteria. PLoS One, 9(7), e101677.
Seper, A., Fengler, V. H., Roier, S., Wolinski, H., Kohlwein, S. D., Bishop, A. L., Camilli, A., Reidl, J., & Schild, S. (2011). Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Molecular Microbiology, 82(4), 1015–1037.
Sladek, R. E., Filoche, S. K., Sissons, C. H., & Stoffels, E. (2007). Treatment of Streptococcus mutans biofilms with a nonthermal atmospheric plasma. Letters in Applied Microbiology, 45(3), 318–323.
Sonderholm, M., Bjarnsholt, T., Alhede, M., Kolpen, M., Jensen, P. O., Kuhl, M., & Kragh, K. N. (2017). The consequences of being in an infectious biofilm: Microenvironmental conditions governing antibiotic tolerance. International Journal of Molecular Sciences, 18(12), 2688.
Speziale, P., Pietrocola, G., Foster, T. J., & Geoghegan, J. A. (2014). Protein-based biofilm matrices in staphylococci. Frontiers in Cellular and Infection Microbiology, 4, 171.
Stephen-Haynes, J., Toner, L., & Jeffrey, S. (2018). Product evaluation of an absorbent, antimicrobial, haemostatic dressing. The British Journal of Nursing, 27(6), S24–s30.
Sun, X., Jiang, K., Chen, J., Wu, L., Lu, H., Wang, A., & Wang, J. (2014). A systematic review of maggot debridement therapy for chronically infected wounds and ulcers. International Journal of Infectious Diseases, 25, 32–37.
Svensson, S. L., Davis, L. M., MacKichan, J. K., Allan, B. J., Pajaniappan, M., Thompson, S. A., & Gaynor, E. C. (2009). The CprS sensor kinase of the zoonotic pathogen Campylobacter jejuni influences biofilm formation and is required for optimal chick colonization. Molecular Microbiology, 71(1), 253–272.
Tetz, V. V., & Tetz, G. V. (2010). Effect of extracellular DNA destruction by DNase I on characteristics of forming biofilms. DNA and Cell Biology, 29(8), 399–405.
Tetz, G. V., Artemenko, N. K., & Tetz, V. V. (2009). Effect of DNase and antibiotics on biofilm characteristics. Antimicrobial Agents and Chemotherapy, 53(3), 1204–1209.
Thomas, V. C., Thurlow, L. R., Boyle, D., & Hancock, L. E. (2008). Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. Journal of Bacteriology, 190(16), 5690–5698.
Wagener, J., & Kupfer, O. (2012a). Dornase alfa (Pulmozyme). Current Opinion in Pulmonary Medicine, 18(6), 609–614.
Wagener, J. S., & Kupfer, O. (2012b). Dornase alfa (Pulmozyme). Current Opinion in Pulmonary Medicine, 18(6), 609–614.
Wang, Z., de la Fuente-Nunez, C., Shen, Y., Haapasalo, M., & Hancock, R. E. (2015). Treatment of Oral multispecies biofilms by an anti-biofilm peptide. PLoS One, 10(7), e0132512.
Wang, G., Li, X., & Wang, Z. (2016). APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Research, 44(D1), D1087–D1093.
Waryah, C. B., Wells, K., Ulluwishewa, D., Chen-Tan, N., Gogoi-Tiwari, J., Ravensdale, J., Costantino, P., Gokcen, A., Vilcinskas, A., Wiesner, J., & Mukkur, T. (2016). In vitro antimicrobial efficacy of tobramycin against Staphylococcus aureus biofilms in combination with or without DNase I and/or Dispersin B: A preliminary investigation. New York: Microbial Drug Resistance.
Watters, C., Everett, J. A., Haley, C., Clinton, A., & Rumbaugh, K. P. (2014). Insulin treatment modulates the host immune system to enhance Pseudomonas aeruginosa wound biofilms. Infection and Immunity, 82(1), 92–100.
Watters, C., Fleming, D., Bishop, D., & Rumbaugh, K. P. (2016). Host responses to biofilm. Progress in Molecular Biology and Translational Science, 142, 193–239.
Wende, K., Strassenburg, S., Haertel, B., Harms, M., Holtz, S., Barton, A., Masur, K., von Woedtke, T., & Lindequist, U. (2014). Atmospheric pressure plasma jet treatment evokes transient oxidative stress in HaCaT keratinocytes and influences cell physiology. Cell Biology International, 38(4), 412–425.
Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C., & Mattick, J. S. (2002). Extracellular DNA required for bacterial biofilm formation. Science, 295(5559), 1487.
Wilkinson, H. N., McBain, A. J., Stephenson, C., & Hardman, M. J. (2016). Comparing the effectiveness of polymer debriding devices using a porcine wound biofilm model. Advances in Wound Care, 5(11), 475–485.
Wingender, J., Strathmann, M., Rode, A., Leis, A., & Flemming, H. C. (2001). Isolation and biochemical characterization of extracellular polymeric substances from Pseudomonas aeruginosa. Methods in Enzymology, 336, 302–314.
Wolcott, R. D., Hanson, J. D., Rees, E. J., Koenig, L. D., Phillips, C. D., Wolcott, R. A., Cox, S. B., & White, J. S. (2015). Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair and Regeneration, 24(1), 163–174.
Wolcott, R. D., Hanson, J. D., Rees, E. J., Koenig, L. D., Phillips, C. D., Wolcott, R. A., Cox, S. B., & White, J. S. (2016). Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair and Regeneration, 24(1), 163–174.
Xu, Z., Shen, J., Zhang, Z., Ma, J., Ma, R., Zhao, Y., Sun, Q., Qian, S., Zhang, H., Ding, L., Cheng, C., Chu Paul, K., & Xia, W. (2015). Inactivation effects of non-thermal atmospheric-pressure helium plasma jet on Staphylococcus aureus biofilms. Plasma Processes and Polymers, 12(8), 827–835.
Xu, Z., Shen, J., Cheng, C., Hu, S., Lan, Y., & Chu, P. (2017). In vitro antimicrobial effects and mechanism of atmospheric-pressure He/O2 plasma jet on Staphylococcus aureus biofilm. Journal of Physics D: Applied Physics, 50, 10.
Yakandawala, N., Gawande, P. V., LoVetri, K., Cardona, S. T., Romeo, T., Nitz, M., & Madhyastha, S. (2011). Characterization of the poly-beta-1,6-N-acetylglucosamine polysaccharide component of Burkholderia biofilms. Applied and Environmental Microbiology, 77(23), 8303–8309.
Yang, L., Hu, Y., Liu, Y., Zhang, J., Ulstrup, J., & Molin, S. (2011). Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environmental Microbiology, 13(7), 1705–1717.
Yang, L., Liu, Y., Wu, H., Song, Z., Hoiby, N., Molin, S., & Givskov, M. (2012). Combating biofilms. FEMS Immunology and Medical Microbiology, 65(2), 146–157.
Yang, C., Goss, S. G., Alcantara, S., Schultz, G., & Lantis Ii, J. C. (2017). Effect of negative pressure wound therapy with instillation on bioburden in chronically infected wounds. Wounds, 29(8), 240–246.
Young, S. (2014). Management of slough in diabetic foot wounds. Diabetic Foot Journal, 17(1), 29–33.
Young, C. N. J., Ng, K. Y. B., Webb, V., Vidow, S., Parasuraman, R., & Umranikar, S. (2016). Negative pressure wound therapy aids recovery following surgical debridement due to severe bacterial cellulitis with abdominal abscess post-cesarean: A case report (CARE-compliant). Medicine, 95(50), e5397.
Zhang, X., & Bishop, P. L. (2003). Biodegradability of biofilm extracellular polymeric substances. Chemosphere, 50(1), 63–69.
Zhao-Fleming, H., Dissanaike, S., & Rumbaugh, K. (2017). Are anaerobes a major, underappreciated cause of necrotizing infections? Anaerobe, 45, 65–70.
Ziuzina, D., Boehm, D., Patil, S., Cullen, P. J., & Bourke, P. (2015). Cold plasma inactivation of bacterial biofilms and reduction of quorum sensing regulated virulence factors. PLoS One, 10(9), e0138209.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Fell, C., Fleming, D., Rumbaugh, K.P. (2019). Approaches for Disrupting Tissue-Associated Biofilms. In: Ahmad, I., Ahmad, S., Rumbaugh, K. (eds) Antibacterial Drug Discovery to Combat MDR. Springer, Singapore. https://doi.org/10.1007/978-981-13-9871-1_23
Download citation
DOI: https://doi.org/10.1007/978-981-13-9871-1_23
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9870-4
Online ISBN: 978-981-13-9871-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)