Abstract
Free enzymes do not possess properties of recovery and reusability, and also they are not stable at wide pH and temperature range. Therefore, new ways which can enhance enzyme stability and reusability should be developed, and hence, the immobilization technique is one such approach. These immobilization techniques offer such materials which have the ability to be active in the much wide range of pH and temperature, and also they are more stable than the free enzymes. Immobilization is carried out on the nanosized material either by adsorption, covalent coupling, entrapment, encapsulation or cross-linking. These nanomaterial-immobilized enzymes show several advances over the free enzymes because of large surface area-to-volume ratio, lower mass transfer resistance and high mobility. Several nanomaterials are used for immobilizing the enzymes; however, their recovery from the reaction mixture is very poor. Therefore, the magnetic nanomaterials are more attractively used in immobilization because the enzyme immobilized through magnetic nanomaterial has the tendency to be easily separated out from the reaction mixture. These nanomaterial-immobilized enzymes show wide range of applications in biotechnology, bioanalysis, biomedicine, pathology and biosensors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Enzymes are protein molecules that enhance the rate of biochemical reactions, but they are not used up during the reactions. Enzymes are biological catalyst and show a lot of dominance over any chemical catalyst. Besides having high catalytic efficiency, enzymes are also chemoselective, regioselective and stereoselective, and hence, they are widely used in the industries (Gupta et al. 2011). Enzyme can catalyse several chemical and biochemical reactions. Mankind has used enzyme in the processing of food from several millennia. Various raw materials such as oils, fats, carbohydrates, proteins, vitamins, lignin and amino acids are used for the production of several biorenewables. These compounds are also important in our daily life in the form of wood, paper, starch, rubber, fabrics and some other biorenewable materials. However, the enzymatic actions on these raw materials are needed for their transformation into biorenewables under some mild and sustainable conditions (Franssen et al. 2013).
The present-day development and success widely depend on the synthesis of chemicals and their production at industry level in the presence of enzymes. This success is due to the increased number of enzymes and their applications in a wide range of processes (Hanefeld et al. 2013). Enzymes show diverse applications in dairy products: in the preparation of wine, beer, fruit and vegetable juices and in the preparation of paper and pulp. Enzymatic pathways are cost-effective, sustainable and environment friendly. However, the free enzymes have very poor stability towards high temperature and pH, and hence, there are only restricted possibilities of their recovery as well as reusability. In order to remove these limitations and to turn the enzyme activity into industrial applications, numbers of approaches have been tried, and immobilization technique is one such tool for this purpose. These increase the stability of enzyme, its selectivity and also its reusability with the emerging tools. A number of immobilization techniques are available that can utilize newly developed materials that offer high biocatalytic activity and favourable environments that are necessary for the action of immobilized enzymes. In the presence of these favourable environments, activities and stability of enzymes are improved for any applications in comparison to free enzymes because of much wider range of pH and temperature available (Mateo et al. 2007; Goldberg and Kolibas 1990; Iyer and Ananthanarayan 2008; Brady and Jordaan 2009; Stepankova et al. 2013; Chibata 1978). A powerful approach for immobilization of enzymes is by the use of nanomaterials (Franssen et al. 2013). Materials that range from 1 to 100 nm in particle size and show different characteristics from their bulk precursors are called nanomaterials. According to ISO (2015), nanomaterials are defined as materials having external dimension in nanoscale, or materials having internal structure or their surface structure in the nanoscale. Nanomaterials have extremely small dimensions, and hence, they allow us to take advantage of unique physical, chemical, optical, electronic and mechanical properties that are present at nanoscale. For the immobilization of enzymes, nanomaterials are used because they can make up a novel and attractive matrices. Enzymes immobilized on these matrices are highly impactful in the today’s research due to the high surface-to-volume ratio of nanomaterials and their Brownian motion. Carbon nanotubes, superparamagnetic nanoparticles and some mesoporous materials are used as some important category of matrices.
There are number of reports of micro- and nanomaterials that have various applications in biotechnology and biomedicine due to their incredible potential in delivery systems and targeting, and it also shows advantages in biosubstance binding (Inès and Dhouha 2015; Kuthati and Kankala 2015; Wang et al. 2015; Löhr et al. 1998). There are some examples of different processes occurring on industrial scale that are using immobilized enzymes including high-fructose corn syrup production (Gupta et al. 2011). A number of substrates such as fatty acids, carbohydrates, proteins, rubber and their building blocks are utilized in the creation of biorenewables in the presence of any immobilized enzymes. The renewable produced in this way shows a wide range of applications, that is, they are acting as building blocks for several industries such as in dairy, textile industries, pharmaceutical and polymer industry and the preparation of additives for food industries. They are also showing several bioanalytical and biomedical applications because they are utilizing immobilized antibodies or antigens. They may make use of some immobilized receptors or ligands and also a variety of immobilized cells. Immobilized antigens or antibodies are used in affinity chromatography, and immobilized receptors or ligands are used in biosensors. In the last few decades, biocatalysis has emerged as an important tool in order to meet the demand for green as well as sustainable synthesis of chemicals that mainly involves the production of pharmaceuticals, vitamins, flavours, fragrances and some other specific chemicals. Additionally, the system of the immobilized enzyme is useful either in aqueous or media with low water content in the field of biocatalysis or in resolution of racemates (Wang 2006; Chiang and Sung 2006; Mikhaylova et al. 2004; Kim et al. 2003; Gao et al. 2003; Dyal et al. 2003; Gardimalla et al. 2005; Zhang et al. 2008; Wang et al. 2009; Lee et al. 2008; Netto et al. 2009).
8.2 Synthesis of Nanomaterials
Nowadays, researchers and scientists are working together to develop some new materials having some superior properties, more functionality and lower cost than the on-hand materials. Several physical, chemical and physiological methods have been tried to boost up the performance and behaviour of nanomaterials. Several approaches have been tried for synthesizing nanomaterials in order to get control over distribution of nanoparticles and their particle size (Shibata et al. 1998).
8.2.1 Different Approaches for the Synthesis of Nanomaterials
There are two general approaches that are widely involved in the nanomaterial synthesis and the manufacture of different nanostructures. These two main approaches are represented in Fig. 8.1.
8.2.1.1 Top-Down Approach
This route generally entails breaking of larger particles into smaller ones, and this can be achieved by using several forces like crushing, milling or grinding. These approaches use larger or macroscopic structure as the initial material that is controlled during the processing of nanostructures from the outside. Although this approach is used in the synthesis of nanomaterials, this route is generally not appropriate for the preparation of materials having uniform shape. However, the major problem with this approach is the limitation in the surface configuration. Such limitations or imperfection would have a considerable impact on physical properties and surface chemistry of nanostructures and nanomaterials. These approaches are also slow and hence are not suitable for the production at large scale.
8.2.1.2 Bottom-Up Approach
Bottom-up approach involves the synthesis of material from the bottom: atom-by-atom, molecule-by-molecule or cluster-by-cluster. In these approaches, material components are firstly miniaturized up to their atomic level which later on construct the nanostructures by self-assembling. During the self-assembly process, numerous substantial forces are operating at nanoscale that is used for uniting the fundamental units into the larger established structure. This route is mostly used for the preparation of the nanoscale materials, and it shows the ability for the generation of a uniform size, shape and distribution of the nanomaterials. It has the ability to control the reaction to inhibit the growth of particles further. However, the bottom-up approach is not showing any novelty, but the fabrication and processing of nanostructures and nanomaterials can be easily achieved by these approaches. Researchers always feel challenging to control the particles size distribution, purity, morphology, quality and quantity to a better extent during the synthesis of nanomaterials through environment friendly and economical processes (Cao 2006).
8.2.2 Methods Involved in Nanomaterial Synthesis
The top-down and the bottom-up approaches that are used in the synthesis of nanomaterials offer several diversities in the synthesis methods which result in the diversities in the product. There are several methods, namely, physical, chemical, biological or hybrid, that are widely used in the synthesis of nanomaterials such as nanoparticles, nanotubes, colloids, thin films, quantum dots or nanorods. These different methods are shown in Fig. 8.2.
8.2.2.1 Physical Methods
Nanomaterials can be synthesized via physical methods through evaporation and condensation or by applying mechanical pressure and high-energy radiation or by using thermal or electrical energy for the abrasion of the material. These methods are generally based on the top-down approach. Physical methods that are generally used for the synthesis of the nanomaterials are laser pyrolysis, spray pyrolysis, high-energy ball milling, physical vapour deposition, melt mixing, laser ablation, sputter deposition, electric arc deposition, ion implantation, etc. These methods show several advantages as they can generate uniform monodisperse nanoparticles, and also they are free from the contamination through solvent. However, during the synthesis processes, profuse amount of waste is produced; hence, the physical methods may be treated as uneconomical (Dhand et al. 2015).
8.2.2.1.1 High-Energy Ball Milling (HEBM)
HEBM was first time used by John Benjamin in 1970 for the synthesis of alloys strengthened with the oxide dispersion. This method is capable for synthesizing nanoparticles having a lot of variation in their shape and sizes (Dhand et al. 2015; Xing et al. 2013). The HEBM route involves the transfer of kinetic energy from the moving balls to the milled material. Due to this transfer of kinetic energy, bond breaking will take place, and hence, the new surface will be generated by the breakdown of milled materials into the smaller particles (Fig. 8.3a). HEBM process is also considered a mechanochemical process of synthesis because this method is based on the conditions of temperature and pressure. Nowadays, nanomaterial synthesis using HEBM method is carried out in either the presence or absence of surfactant (Fig. 8.3b) (Dhand et al. 2015; de Carvalho et al. 2013). However, the synthesis of nanoparticles through HEBM method in the presence of surfactant is widely used because in the presence of surfactant, we can get precise particle size and their morphology (Dhand et al. 2015; de Carvalho et al. 2013) (Fig. 8.3).
(a) It represents the high-energy ball milling scheme of nanomaterial synthesis and (b) representation of nanoparticle synthesis through HEBM system in the presence and absence of surfactant. (Dhand et al. 2015)
8.2.2.1.2 Melt Mixing
In this method, nanomaterial synthesis can be carried out by mixing the modified form of nanofillers with the polymer mechanically (Karak 2009). Melt mixing method is widely used because this method is highly compatible for different industrial processes and is also environment friendly (Lin et al. 2006). Melt mixing technique was used by Zuhal et al. for the synthesis of polypyrrole with polypropylene nanocomposite nanoparticles (Sevil and Zuhal 2010). Weiss et al. synthesized hybrid nanoparticles by mixing hydroxybenzoate (HBA) and hydroxynaphthanoate with the zinc salts of sulfonated ionomers of polystyrene. This involves the chemical bond formation between the polyester and the residual zinc acetate as it is carried out at high temperature (Lee et al. 2005).
8.2.2.1.3 Laser Ablation
In laser ablation method, vaporization of material is carried out by using laser light (Chen and Yeh 2002). After vaporizing the material, nanomaterial can be obtained in the colloidal solution form. It is a very easy and fast method for synthesis of the nanomaterials. It is also environment friendly and hence considered a green method. In this method, we are also able to introduce some organic moieties on the surface of the nanoparticles (Dhand et al. 2015; Singh and Gopal 2007).
8.2.2.1.4 Physical Vapour Deposition
Physical vapour deposition consists of group of processes that are used for the production of nanoparticles and for the deposition of thin layers of materials. These thin layers generally lie in the nanometre or micrometre range. In PVD technique, mainly three steps are involved: (1) the first step involves vaporization of the materials that are mainly from solid source; (2) in the second step, vaporized material is transported; and (3) the third step is the nucleation and growth for the generation of thin films and nanoparticles (Okuyama and Lenggoro 2003). Some of the frequently used PVD methods for synthesizing the nanomaterials (Hatakeyama et al. 2011; Veith et al. 2007; Bouchat et al. 2013; Asanithi et al. 2012; Ichida et al. 2014; Ghosh et al. 2007; Veith et al. 2005; Ramalingam et al. 2013; Zhang et al. 2004; Takahashi et al. 2004; Dmitrieva et al. 2006; Stellacci et al. 2002; Hsieh et al. 2010; Naessens et al. 2001; Ong et al. 2008; Andrea et al. 2009; Jing et al. 2014) are (i) sputtering, (ii) vacuum arc, (iii) pulsed laser deposition and (iv) electron beam evaporation.
8.2.2.2 Chemical Method
For the synthesis of nanomaterials, several chemical methods are also used such as hydrothermal synthesis, chemical vapour synthesis, sol-gel method, chemical vapour deposition, microemulsion technique and polyol synthesis.
8.2.2.2.1 Sol-Gel Method
The term sol gel consists of two components: ‘sol’ and ‘gel’. The sol is a colloidal suspension in which the dispersed phases are solid particles while the dispersion medium is liquid. However, the gel is a polymer containing liquid. Hence, in this process, ‘sols’ are formed in the liquid phase. During the first step of the sol-gel process, hydrolysis takes place wherein the presence of water bonds of the precursor molecules is disintegrated (Sol-gel science: the physics and chemistry of sol-gel processing 1990). After hydrolysis, condensation occurs allowing nanomaterials to form. Finally, water is removed for the generation of the final structure of the material (Behnajady et al. 2011). Synthesis of nanoparticles by the sol-gel method is widely dependent on different experimental conditions; therefore, the reflux temperature, reflux time, solvent percentage or calcination temperature should be optimized.
8.2.2.2.2 Microemulsion Method
Microemulsions are homogenous, and transparent dispersion consists of mainly three components: polar phase, nonpolar phase and surfactant. Polar phase generally includes water, and the hydrocarbon liquid or oil is used as nonpolar phase. Surfactant molecules are used so that (i) separating layer may be created between the polar and the nonpolar phase and (ii) interfacial tension may be reduced between the microemulsion and the excess phase. This technique is mainly used for the preparation of inorganic nanomaterials that may be Au, Pt and Pd nanoparticles (metal nanoparticles); BaCO3, SrCO3 and CaCO3 nanoparticles (metal salt nanoparticles); CdS, PbS, CuS, CdSe and Cu2S nanoparticles (semiconducting metal sulphite nanoparticles); and ZrO2, GeO2, TiO2, Fe2O3 and SiO2 nanoparticles (metal oxide nanoparticles) (Solanki and Murthy 2011; Zhang et al. 2006). Au nanoparticles were synthesized from Au(III) ions by its reduction in the presence of alkaline solution of 2,7-dihydroxynaphthalene (DNP).
8.2.2.2.3 Hydrothermal Method
This method generally involves the fabrication of nanoparticles of some metal oxide, lithium iron phosphate and iron oxide. In this method, we have control over the different properties of the particles by varying the conditions of pressure and temperature (Hayashi and Hakuta 2010). This method is carried out in vessel called autoclave that is operated under high pressure, and the reaction is carried out in the aqueous solution. In hydrothermal method, by taking care of the reaction temperature, pressure, solution composition, solvent properties, etc., we have better control over the particle size as well as morphology (Abedini et al. 2013). Hydrothermal synthesis is generally carried out in two types of systems that are continuous hydrothermal and the batch hydrothermal processes. Du et al. synthesized Pt nanoparticles by hydrothermal method through one-pot synthesis (Du et al. 2014). Hydrothermal method is also widely used for the synthesis of iron oxide (Fe3O4, Fe2O3), copper oxide, silver oxide, zinc oxide and nickel oxide nanoparticles (Liu et al. 2015; Tadic et al. 2014; Sue et al. 2011).
8.2.2.3 Biological Method
The biological method or biosynthesis creates low toxicity and is cost-effective and also environment friendly. Hence, it is considered a green synthesis method. In these methods, several bio-organisms such as bacteria, viruses, fungi, yeast, plant extracts, etc. are involved in the production of metal and metal oxide nanoparticles. This biosynthesis method can be categorized into three major parts.
8.2.2.3.1 Biosynthesis Using Microorganisms
In this method, several microorganisms are used in the synthesis of nanoparticles. Target ions from their environment are captured by the microorganisms, and then, the metal ions are converted into their elemental form through several enzymes produced through cellular activities. Depending on the location of nanoparticle synthesis, it is further classified as extracellular and intracellular. In the intracellular method, metal ions are transported into the inside of the microbial cell for the formation of nanoparticles, whereas in extracellular synthesis method, metal ions are trapped on the surface of the cell followed by reduction in the presence of enzymes (Zhang et al. 2011). Bacteria can reduce the metal ions by utilizing several functional groups, proteins, enzymes and also some reducing sugars (Nanda and Saravanan 2009). Biosynthesis by utilizing fungi has several advantages over other microorganisms: (i) it can show high bioaccumulation, (ii) it is economically viable, and (iii) it also shows easy handling of biomass (Mukherjee et al. 2001).
8.2.2.3.2 Nanomaterial Synthesis Using Biomolecules as Templates
Several biomolecules such as nucleic acid, viruses, membranes, etc. are used as templates in the synthesis of nanomaterials. DNA is considered one of the most useful templates in the synthesis process as it shows very strong affinity with the transition metal ions. Au nanoparticles are formed by incorporating gold, Au (III) metal ions to DNA. In this process, reduction of Au (III) occurs, and hence, the Au atoms and metal cluster developed which will result in the formation of nanoparticles on the chain of the DNA (Zinchenko et al. 2014). Similarly, Ag nanoparticles were synthesized by Kundu et al. by using DNA as a template. These Ag nanoparticle clusters that have developed on the surface of the DNA also show good catalytic activities in the reduction of some aromatic nitro compounds (Kundu 2013; Kundu et al. 2008; Kundu and Nithiyanantham 2014).
Biological membranes are also used as templates in the synthesis of nanoparticles because they are having ultrafine pores in their structure. In the synthesis of Au nanoparticles, rubber membrane prepared from the Hevea brasiliensis trees is used as template where reduction of Au (III) takes place at 80 °C (Cabrera et al. 2013).
8.2.2.3.3 Nanomaterial Synthesis Using Plant Extracts
The generation of nanomaterials from the plant extracts is eco-friendly and a rapid process. Hence, this method is widely used for the synthesis of nanoparticles of noble metals, metal oxides and some bimetallic alloys (Iravani 2011). Gold nanotriangles were synthesized by using lemongrass leaf extract. For this preparation, Shankar et al. treated the lemongrass leaf extract with the aqueous solution of the AuCl ions (Shankar et al. 2014). In a similar way, the leaf extract of Aloe vera, Tamarindus indica and Emblica officinalis and leaf extract of some other plants are also used for the synthesis of the Au nanoparticles (Chandran et al. 2006; Ankamwar et al. 2005a; b). Some other nanoparticles such as Pd nanoparticles and Pt nanoparticles were also synthesized by using the extract taken out from different parts of diverse species of plants (Coccia et al. 2012; Sathishkumar et al. 2009). Shankar et al. also synthesized Ag nanoparticles from the leaf extract of Azadirachta indica and also from the fruit extract of Emblica officinalis (Ankamwar et al. 2005b; Shankar et al. 2004).
8.2.2.4 Hybrid Method
In this category of nanomaterial synthesis method, physical, chemical and biological are all involved. Hence, it is considered a hybrid method for the synthesis of the nanomaterial. It involves several methods such as chemical vapour deposition, chemical vapour synthesis or arresting of the particles in the glass or zeolites or polymers.
8.2.2.4.1 Chemical Vapour Deposition and Chemical Vapour Synthesis
In this method, solid films are deposited from the vapour phase through chemical reactions taking place at high temperature. This method was earlier used for the production of thin films. However, nanoparticles can also be produced by this method if the following conditions are optimized:
-
(i)
In the hot wall reactors, the temperature should be kept high.
-
(ii)
The partial pressure of monomers should be kept high so that high supersaturation can be achieved.
-
(iii)
Residence time should be kept slow.
This modified process is then called chemical vapour synthesis (CVS) or chemical vapour reaction (CVR) or chemical vapour precipitation (CVP) or chemical vapour condensation (CVC). During the synthesis process, either solid, liquid or gaseous precursors are converted to their vapour form (Swihart 2003). Chemical vapour synthesis method is generally utilized for the generation of nanoparticles by using several material such as ZnO, iron oxide, silicon oxide and copper oxides, Cr-doped zinc oxide, Al-doped zinc oxide, etc. (Hartner et al. 2009; Jin et al. 2007; Suffner et al. 2010; Lahde et al. 2011; Lee et al. 2009). One disadvantage of this method is that high temperature is required for this method, and hence, there are difficulties in providing such high temperature.
8.2.3 Synthesis of Nanoparticles
Nanoparticles show wide range of applications as they are used in number of optical, electronic, and magnetic devices and also they are used as catalysts, adsorbents, sensors and ferrofluids. Most of these applications of nanoparticles depend on their size as well as their shape, and hence, the synthesis of nanoparticles should be carried out in such a way so that well-defined shape and size of the nanoparticles may be obtained. One of the very common techniques used is the formation of metallic colloid dispersions by the reduction of metal complexes. Sizes of nanoparticles depend on the type of reducing agent used during its synthesis. If the reducing agent is strong, then the rate of reaction will be fast. Hence, the nanoparticles formed are of smaller size, but the nanoparticles formed will be of larger sized when we use a weak reducing agent because it induces slower rate of reaction.
Aggregation of nanoparticles can be prevented by the use of some polymeric stabilizers. These polymeric stabilizers form a single layer on the nanoparticle surface, and in this way aggregation can be prevented. These polymeric stabilizers are also called as capping material. But when we use polymeric stabilizers, the growth of nanoparticles gets inhibited because the monolayer of polymer has the tendency to significantly affect the process of growth by blocking the growth sites. Polymeric stabilizers also have the tendency to affect the shape of nanoparticles when they are used in different amounts.
8.2.4 Synthesis of Nanowires, Nanorods and Nanotubes
Several monodimensional nanomaterials like nanowires, nanorods and nanotubes can be synthesized by different techniques:
-
(a)
Spontaneous growth – It involves various methods such as evaporation-condensation, vapour-liquid-solid growth and stress-induced recrystallization
-
(b)
Template-based synthesis – The methods used here are electroplating, electrophoretic deposition, colloid dispersion, melt or solution filling and chemical reaction.
-
(c)
Electrospinning
-
(d)
Lithography
In spontaneous growth due to the preferential direction for crystal growth, there is formation of nanowires and nanorods in their single-crystal form.
Long ribbon-like nanostructures were synthesized by Pan et al. (2001) by evaporating the metal oxide powders at high temperatures, and for this they used mainly zinc, tin, indium, cadmium and gallium in their oxide form having semiconducting behavior (Pan et al. 2001).
One method for nanowire synthesis involves filling a template having cylindrical holes of size ranging in nanoscale. It is one of the most straightforward and common methods. It involves synthesis of nanowires into the cylindrical pores present at thin membrane by reducing the metal salt. This method has one advantage of producing nanowires and nanorods of controlled size when there is availability of template with homogeneous pores, and for this requirement, there are a number of permeable templates available that can accomplish such necessities (Martin 1994; Thurn-Albrecht et al. 2000).
By using various techniques, several nanochannel silicon membranes are prepared having pores in perfectly structured form, and such membranes of silicon are widely used in the synthesis of modulated-diameter gold nanorods. By using electrodeposition techniques, this method has been adapted for the creation of nanorods having different metal segments. They are widely used for tagging molecules in analytical chemistry and in the field of biology (Ozin and Arsenault 2006).
Some nanorods have functional molecules introduced to its some selective locations, and these functional molecules which anchored nanorods have wide applications in the today’s research. One of the applications of this method involves functionalization of gold nanorod end with thiolated DNA. The complimentary DNA is Rhodamine labelled and is coupled to the nanorods through its end. By using a similar approach, gold nanorods side are also functionalized with DNA, i.e. gold nanorods with DNA side functionalized are also created, and these are directed for self-assembling on complimentary DNA-functionalized gold surface sites with soft lithographic pattern.
8.3 Enzyme Immobilization
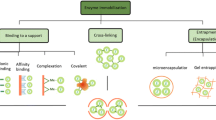
Our main aim for immobilization of enzyme is to improve the stability of enzyme and its handling as well as storage properties. Enzymes are utilized for their several applications where indigenous form of them is not possible. Biosensors based on enzyme reactions are used for their certain therapies, but the enzymes used for biotransformation in industries are based on the use of some stable biocatalysis. These necessities can be fulfilled by immobilizing the enzymes (Sheldon 2007; Tran and Balkus 2011; Garcia-Galan et al. 2011; Hanefeld et al. 2009). There are certain techniques available for effective immobilization of enzymes, and these can be achieved by (i) binding the enzyme molecules with solid carrier (support), (ii) embedding the enzyme in an open matrix (encapsulation) or (iii) cross-linking through physical or chemical interactions (Singh and Gopal 2007). These different techniques are represented in Fig. 8.4.
-
(a)
Binding to a support (carrier) involves several interactions that may be physical (e.g. hydrophobic and van der Waals interactions), ionic or covalent (Sheldon and van Pelt 2013; Cao 2005). However, among these several interactions, physical binding has no sufficient strength to hold the enzyme with the carrier during the rigorous industrial environment such as high concentration of reactant and product and high ionic strength. Ionic interaction is somewhat stronger; however, the covalent bond between the enzyme and the carrier usually does not allow the leaching of enzyme through its surface. However, the covalent bonding between the enzyme and the support has one limitation, that is the enzymes become irreversibly deactivated making the enzymes and the support useless. Some examples of support in immobilizing the enzymes are synthetic resins, biopolymers such as polysaccharides or inorganic solids such as mesoporous silicas or zeolites.
-
(b)
Encapsulation of enzyme can be performed in matrix of organic polymer such as polyacrylamide or in matrix of inorganic polymer such as silica sol gel or in a membrane device such as hollow fibre or a microcapsule. Entrapment can be done after synthesizing the polymer matrix when enzymes are being there. One example that shows this behaviour is that when immobilization of enzymes is performed in mesoporous silica which is already fabricated, the enzyme would be located inside the mesopores but this cannot be supposed as encapsulation; however, when enzyme is being there during the process of synthesis of a silica sol gel, the enzyme would be encapsulated.
-
(c)
The carrierless macroparticles can be prepared by cross-linking the enzyme aggregates or crystals having the bifunctional reagent. Cross-linked enzyme crystals (CLECs) (Roy and Abraham 2004) and cross-linked enzyme aggregates (CLEAs) (Sheldon 2011) are some carrier-free immobilized enzymes. These approaches provide number of advantages such as high stability of enzyme, high enzyme activity or low costs of production, and hence it has the potential to replace other expensive carrier.
8.3.1 Active Nanomaterials in Enzyme Immobilization
The major advantage of nanomaterials in the immobilization of enzyme is that surface area-to-volume ratio is high that increases the effectiveness of the enzyme. Large numbers of nanoparticles are already being employed in immobilizing the enzymes that leads to the construction of some nanostructured systems such as nanofibres, nanotubes, nanomaterials, nano hollow fibre materials and some nanoporous material (Misson et al. 2014).
-
(a)
Sol-gel networks
Enzymes can be immobilized by embedding them through this sol-gel process. During the process of enzyme immobilization, enzyme is added first to the sol materials and due to this structure of enzyme would be frozen. This enzyme becomes entrapped into the sol-gel network and is called xerogels that are fine powdered substance and stable from the mechanical point of view (Avnir et al. 2006).
-
(b)
Protein matrices
During the creation of protein-based matrices, enzymes are connected by covalent binding, and there is a generation of cross-linking between the enzymes by some bifunctional agents. These materials actually do not have any solid carrier, but they are called nanostructured immobilized enzyme. This method shows a lot of advantages as it is simple and cost-effective and can be applicable for a number of enzymes. The major benefit of this method is that immobilized enzyme can be created without any intermediate materials and also without the need for cleaning. However, this method shows some disadvantages also, that is, the enzymes are located on the outer surface, and hence, it may come in contact to the substrate without any problem, but the transport of material by the enzymes located inside the matrix is difficult. Another problem is that binding of cross-linked agents shows some negative effect on the structure of enzyme by disturbing the activity of enzyme, making it inactive.
-
(c)
Nanofibres
Both inside and outside of the nanofibres created by electrostatic fibre production are used to immobilize the enzymes. After creating the nanofibres, the enzyme solution is allowed to mix with the polymer solution when the immobilization is carried out inside the nanofibres. The enzyme immobilization can show stability, security and some better quality for the enzyme functionality when immobilization is carried out inside the nanofibres in comparison to the immobilization of enzyme on the outer side of the nanofibres.
-
(d)
Nanotubes
Nanotubes are used in electronic, optic fibres and in different sensors due to their mechanical, thermal and structural properties. These are among the largely used enzyme carriers that are especially used in the development of biofuel cells. Both the single-walled and multi-walled carbon nanotubes are broadly used for immobilizing the enzymes. Single-walled nanotubes are used because they show high surface area and hence act as successful carrier material for the enzymes, while the multi-walled nanotubes are used because of their ease in dispersion property. The interactions or the type of bonding involved in immobilization are covalent or secondary bonding. Adsorption occurs through H-bonding or hydrophobic or π-π interactions, and the advantage of adsorption is that original behaviour of both the enzymes and the support is conserved. But it may dissolve easily during the applications due to which catalyst may become free; however, the covalent bonding gives more robust and durable association between the enzyme and nanotube (Feng and Ji 2011).
-
(e)
Magnetic nanoparticles
Successful immobilization of enzymes on magnetic nanoparticles is widely utilized for diagnostic purposes such as in measurement of blood sugar, biological transformations with stereoselectivity, etc. Nanosized magnetic particles show number of beneficial behaviour. Therefore, the cross-linked iron oxide particles, monocrystalline iron oxide nanoparticles and ultrasmall superparamagnetic iron oxide are used as an imaging agent in magnetic resonance imaging. Nowadays, magnetic nanoparticles are used for immobilizing the enzymes because they have the tendency to be easily separated out from the reaction mixture, they have the tendency to enhance the stability of bioelement and they can also increase the stability of enzymes. These magnetic nanoparticle-immobilized enzymes illustrate several applications in biotechnology or in analytical devices such as biosensors or in nanomedicine where nanoparticles are used for ease in diagnosis and disease treatments (Govan and Gunko 2014). Due to the advantage of magnetic nanoparticles in the ease of their separation, Liang et al. tried to immobilize the Candida rugosa lipase (CRL) enzyme by using magnetic iron oxide nanoparticles modified with citric acid (CA-Fe3O4 NPs) in the presence of nucleotide-hybrid metal coordination polymers (NMCPs). Hence, the activity as well as stability of the CA-Fe3O4@Zn/AMP nanofibres-immobilized CRL enzyme gets increased (Li et al. 2017). Their separation behaviour is shown in Fig. 8.5.
The behaviour of magnetic nanoparticle-immobilized CRL enzyme, i.e. CA-Fe3O4@Zn/AMP CPs. (a) Before separation and (b) after separation. (Li et al. 2017)
8.3.2 Immobilized Enzymes in Biotechnology
Enzymes show one of the attracting roles, that is, they act as natural biocatalysts due to the ability of enzyme to hasten the rate of all biological reactions, but they themselves are not consumed during the reactions. They also do not affect the equilibrium between the reactants and the products. However, the immobilized enzymes show a number of advantages such as they show ability of easy reusability and lower degradation in comparison to free enzymes available in solution. In addition to these advantages, the rate of reaction can be controlled and also prevent contamination of the substrate with enzyme or protein. Stability of enzymes can also be improved by expanding their half-life through immobilizing the enzymes. Immobilized enzymes can work in a broader range of environments that have the ability to increase the stability of enzymes against temperature, pH, contaminants and impurities. Enzymes after their immobilization show improved biocatalytic activity and efficiency that make them highly attractive and therefore show high applicability in numerous varieties of growing biotechnologies (Gerday et al. 2000; Bradley and Wang 2015; Kell et al. 2015; Joshi and Satyanarayana 2015; Tavano 2013; Hyeon et al. 2013; Yu et al. 2015; Wendisch 2015).
8.3.3 Immobilized Enzymes in Biomedicine
Immobilized enzymes or proteins are widely used for the detection and treatment of several diseases, and these show their applications in the field of medicine. Several immobilized antibodies, receptors and enzymes are used in the form of biosensors for analysing different biologically active compounds in diagnosis. Other use of immobilized enzymes in the field of biomedicine includes synthetic cells and the manufacture of some systems that are used in drug delivery for dosing of proteins or enzymes and are well controlled. The development of some enzyme-based electrodes is another important application of immobilized enzymes in the field of biomedicine. Those enzymes that are highly specific and reactive towards its substrate are widely used in biosensor. Hence, the biosensor developed has high reliability, sensitivity and accuracy, showing ease in their handling, and is of low cost in comparison to the usual analytical methods.
Some important applications of bioreactors that are broadly used in the field of medicine for human are:
-
(a)
In the poisoning of organophosphate, degradation of organophosphate can be done by the use phosphotriesterases (Chatterjee et al. 2014; Petrikovics et al. 1999).
-
(b)
Alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase are used for the conversion of alcohol to its acetate form in the alcohol poisoning (Pei et al. 1995; Magnani et al. 1993).
-
(c)
Repairing of damaged DNA in aging of skin and cancer by the use of DNA repair enzyme (liposome) (Yarosh et al. 1999; Yarosh et al. 1996).
-
(d)
In hypercholesterolemia, hydrolysis of phospholipids can be done by the use of phospholipase A2 (liposome) (Jørgensen et al. 1999).
8.4 Applications of Nanomaterials in Enzyme Immobilization
Nowadays, researchers are using two types of carriers (i.e. microcarriers and nanocarriers) for immobilizing the enzymes so that the immobilized enzymes can be utilized for several biotechnological, biochemical, bioanalytical or biomedical applications. These micro- and nanocarriers are magnetic nanoparticles and cross-linked enzyme aggregates (CLEAs), respectively. However, among these two carriers, the magnetic nanoparticles show a number of applications in biomedical, bioanalytical, biophysical and biomedical field. Magnetic nanoparticles are widely used in these fields because the nanosized structures have smaller size, excellent magnetic properties and large surface area-to-volume ratio (hence, they have higher surface energy). Therefore, they are used as carriers to which different active substances can easily bind. Several enzyme immobilization methods such as adsorption, chelating or metal binding, affinity binding or covalent binding help in the production of some efficient and stable magnetic nanoparticles bounded with enzyme. The development and synthesis of such materials have immense contribution in the present-day research in the form of imaging agents, sensors, drug delivery targets/vehicles and diagnostic tools. The application of nanomaterial in this field has enhanced the scope of studies.
8.4.1 Gold Nanoparticles as Enzyme Immobilization Templates
Gold nanoparticles are widely used in enzyme immobilization. When the aqueous gold nanoparticles are assembled on the surface of polyurethane (PU) spheres, it leads to the formation of [gold nanoparticle shell]-[polyurethane core] structure. The conjugation of gold nanoparticles occurs easily on the microspheres of polymer because nitrogen in the polymer interacts with the nanoparticles. One essential step in the core-shell structure protocols based on the polymer requires performing some additional modification on the surface of the polymer microspheres. Finally, the nanogold-PU material is allowed to make conjugation with enzyme such as pepsin which leads to the creation of a novel category of biocatalyst. In this way, the enzyme-conjugated material shows some enhanced biocatalytic activity and considerably improved stability to withstand broader pH and temperature range in comparison to the free enzyme in solution. One another advantage of this pepsin bioconjugated gold nanoparticle-labelled polyurethane microsphere is that it can be separated out from the reaction medium without any difficulty and it shows higher reusability of the bioconjugate up to six reaction cycles. In DNA immobilization and detection methodologies, the use of such gold nanoparticle shell-PU core structures is currently being envisaged (Phadtare et al. 2003).
8.5 Conclusion
Enzymes show environmental friendliness, high selectivity, high specificity, low cost and mild reaction conditions, and hence, they have the potential to replace several chemical catalysts. Enzymes are used as important biocatalysts, and hence they show wide applications such as in biosensors, pharmaceuticals, chemicals and foods. Due to low operational stability and short lifetime, enzymes have only limited applications. However, these limitations can be overcome by immobilizing the enzymes. Several materials have been tried for immobilizing the enzymes such as carboxyl-functionalized grapheme oxide, polyurethane foam support, SBA-15 and chitosan. However, the nanostructured carriers are attracting much attention of the researchers and are considered as ideal material for immobilizing the enzymes due to their specific surface area, high dispersibility and lower mass transfer resistance. Many nanomaterials have been used for immobilizing the enzymes so that improvement should be achieved in the enzyme activity and stability. However, the recovery of nanomaterials is very difficult, and hence, they cannot be recycled. Therefore, the magnetic nanosupport would be the most excellent alternative so that recovery of nanostructured immobilized enzymes may become easy by using magnet as the separation medium. Therefore , the magnetic nanoparticles such as iron oxide as well as ferric oxide have been tried successfully for immobilizing the enzymes. In this way, the immobilization support has the tendency to increase the enzyme stability, activity, specificity, lifetime, productivity and structural rigidity.
References
Abedini A, Daud AR, Hamid MAA, Othman NK, Saion E (2013) Nanoscale Res Lett 8:1–10
Andrea CD, Neri F, Ossi PM, Santo N, Trusso S (2009) Nanotechnology 20:245606
Ankamwar B, Chaudhary M, Sastry M (2005a) Synth React Inorg Met-Org Nano-Met Chem 35:19–26
Ankamwar B, Damle C, Ahmad A, Sastry M (2005b) J Nanosci Nanotechnol 5:1665–1671
Asanithi P, Chaiyakun S, Limsuwan P (2012) J Nanomater 2012:963609
Avnir D, Coradin T, Lev O, Livage J (2006) J Mater Chem 16:1013–1030
Behnajady MA, Eskandarloo H, Modirshahla N, Shokri M (2011) Desalination 278:10–17
Bouchat V, Moreau N, Colomer JF, Lucas S (2013) J Surf Eng Mater Adv Technol 3:184–189
Bradley RW, Wang B (2015) New Biotechnol 32:635–643
Brady D, Jordaan J (2009) Biotechnol Lett 31:1639–1650
F. C. Cabrera, H. Mohan, R. J. D. Santos, D. L. S. Agostini, R. F. Aroca, M. A. Rodr’ıguez- P’erez and A. E. Job, J Nanomater, 2013, 2013, 1–10
Cao L (2005) Carrier-bound immobilized enzymes, principles, applications and design. Wiley-VCH, Weinheim
Cao G (2006) Nanostructures and nanomaterials. Imperial College Press, London. Chapters 1–6
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Biotechnol Prog 22:577–583
Chatterjee K, Sarkar S, Jagajjanani Rao K, Paria S (2014) Adv Colloid Interf Sci 209:8–39
Chen Y, Yeh C (2002) Colloids Surf A Physicochem Eng Asp 197:133–139
Chiang CL, Sung CS (2006) J Magn Magn Mater 302:7–13
Chibata I (1978) Pure Appl Chem 50:667–675
Coccia F, Tonucci L, Bosco D, Bressand M, Alessandro N (2012) Green Chem 14:1073–1078
de Carvalho JF, de Medeiros SN, Morales MA, Dantas AL, Carrico AS (2013) Appl Surf Sci 275:84–87
Dhand C, Dwivedi N, Loh XJ, Ying ANJ, Verma NK, Beuerman RW, Lakshminarayanan R, Ramakrishna S (2015) RSC Adv 5:105003–105037
Dmitrieva O, Acet M, Dumpich G, Kastner J, Antoniak C, Farle M, Fauth K (2006) J Phys D Appl Phys 39:4741–4745
Du JJ, Chen C, Gan YL, Zhang RH, Yang CY, Zhou XW (2014) Int J Hydrog Energy 39:17634–17637
Dyal A, Loos K, Noto M, Chang SW, Spagnoli C, Shafi KVPM, Ulman A, Cowman M, Gross RA (2003) J Am Chem Soc 125:1684–1685
Feng W, Ji P (2011) Biotechnol Adv 29:889–895
Franssen MCR, Steunenberg P, Scott EL, Zuihofac H, Sanders JPM (2013) Chem Soc Rev 42:6491
Gao X, Yu KMK, Tam KY, Tsang SC (2003) Chem Commun:2998–2999
Garcia-Galan C, Berenguer-Murcia A, Fernandez-Lafuente R, Rodrigues RC (2011) Adv Synth Catal 353:2885–2904
Gardimalla HMR, Mandal D, Stevens PD, Yenb M, Gao Y (2005) Chem Commun 35:4432–4434
Gerday C, Aittaleb M, Bentahir M, Chessa JP, Claverie P, Collins T et al (2000) Trends Biotechnol 18:103–107
Ghosh PK, Ahmed SF, Jana S, Chattopadhyay KK (2007) Opt Mater 29:1584–1590
Goldberg RL, Kolibas LM (1990) Connect Tissue Res 24:265–275
Govan J, Gunko YK (2014) Nano 4:222–241
Gupta MN, Kaloti M, Kapoor M, Solanki K (2011) Artif Cells Blood Substit Biotechnol 39:98–109
Hanefeld U, Gardossi L, Magner E (2009) Chem Soc Rev 38:453–468
Hanefeld U, Cao L, Magner E (2013) Chem Soc Rev 42:6211
Hartner S, Ali M, Schulz C, Winterer M, Wiggers H (2009) Nanotechnology 20:445701
Hatakeyama Y, Onishi K, Nishikawa K (2011) RSC Adv 1:1815–1821
Hayashi H, Hakuta Y (2010) Materials 3:3794–3817
Hsieh T, Chuang C, Chou Y, Shu C (2010) Mater Des:31
Hyeon JE, Jeon SD, Han SO (2013) Biotechnol Adv 31:936–944
Ichida D, Uchida G, Seo H, Kamataki K, Itagaki N, Koga K, Shiratani M (2014) J Phys Conf Ser 518:012002
Inès M, Dhouha G (2015) Carbohydr Res 416:59–69
Iravani S (2011) Green Chem 13:2638–2650
Iyer PV, Ananthanarayan L (2008) Process Biochem 43:1019–1032
Jin W, Lee I, Kompch A, Dorfler U, Winterer M (2007) J Eur Ceram Soc 27:4333–4337
Jing Y, Wang H, Chen X, Wang X, Wei H, Guo Z (2014) Appl Surf Sci 316:66–71
Jørgensen K, Kiebler T, Hylander I, Vermehren C (1999) Int J Pharm 183:21–24
Joshi S, Satyanarayana T (2015) Bioresour Technol 176:273–283
Karak N (2009) Fundamentals of polymers: raw materials to finish products, 1st edn. Prentice-Hall Of India Pvt Ltd, New Delhi
Kell DB, Swainston N, Pir P, Oliver SG (2015) Trends Biotechnol 33:237–246
Kim DK, Mikhaylova M, Zhang Y, Muhammed M (2003) Chem Mater 15:1617–1627
Kundu S (2013) Phys Chem Chem Phys 15:14107–14119
Kundu S, Nithiyanantham U (2014) Ind Eng Chem Res 53:13667–13679
Kundu S, Maheshwari V, Saraf RF (2008) Langmuir 24:551–555
Kuthati Y, Kankala RK (2015) Appl Clay Sci 112–113:100–116
Lahde A, Kokkonen N, Karttunen AJ, Jaaskelainen S, Tapper U, Pakkanen TA, Jokiniemi J (2011) J Nanopart Res 13:3591–3598
Lee HS, Zhu L, Weiss RA (2005) Polymer 46:10841–10853, 1684–1687
Lee J, Lee Y, Youn JK, Na HB, Yu T, Kim H, Lee SM, Koo YM, Kwak JH, Park HG, Chang HN, Hwang M, Park JG, Kim J, Hyeon T (2008) Small 4:143–152
Lee D, Tolochko OV, Turaev FR, Kim D, Kim B (2009) J Nanosci Nanotechnol 9:1–6
Li C, Jiang S, Zhao X, Liang H (2017) Molecules 22:179
Lin B, Sundararaj U, Potschke P (2006) Macromol Mater Eng 291:227–238
Liu XD, Chen H, Liu SS, Ye LQ, Li YP (2015) Mater Res Bull 62:217–221
Löhr M, Müller P, Karle P, Stange J, Mitzner S, Jesnowski R et al (1998) Gene Ther 5:1070–1078
Magnani M, Fazi A, Mangani F, Rossi L, Mancini U (1993) Biotechnol Appl Biochem 18:217–226
Martin CR (1994) Science 266:1961
Mateo C, Palomo JM, Lorente GF, Guisan JM, Lafuente RF (2007) Enzym Microb Technol 40:1451–1463
Mikhaylova M, Kim DK, Berry CC, Zagorodni A, Toprak M, Curtis ASG, Muhammed M (2004) Chem Mater 16:2344–2354
Misson M, Zhang H, Jin B (2014) Interface 12:1–20
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parishcha R, Ajaykumar PV, Alam M, Kumar R, Sastry M (2001) Nano Lett 1:515–519
Naessens K, van Daele P, Baets R (2001) SPIE-Int Soc Opt Eng Proc 4426:124–127
Nanda A, Saravanan M (2009) Nanomedicine 5:452–456
Netto CGCM, Andrade LH, Toma HE (2009) Tetrahedron Asymmetry 20:2299–2304
Okuyama K, Lenggoro IW (2003) Chem Eng Sci 58:537–547
Ong PL, Mahmood S, Zhang T, Lin JJ, Ramanujan RV, Lee P, Rawat RS (2008) Appl Surf Sci 254:1909–1914
Ozin GA, Arsenault AC (2006) Nanochemistry. RSC Publishing, Cambridge. Chapters 1–6
Pan ZW, Dai ZR, Wang ZL (2001) Science 291:1947
Pei L, Petrikovics I, Way JL (1995) Fundam Appl Toxicol 28:209–214
Petrikovics I, Hong K, Omburo G, Hu QZ, Pei L, McGuinn WD et al (1999) Toxicol Appl Pharmacol 156:56–63
Phadtare S, Kumar A, Vinod VP, Dash C, Palaskar DV, Rao M, Shukla PG, Sivaram S, Sastry M (2003) Chem Mater 15:1944–1949
Ramalingam B, Mukherjee S, Mathai CJ, Gangopadhyay K, Gangopadhyay S (2013) Nanotechnology 24:205602
Roy JJ, Abraham TE (2004) Chem Rev 104:3705–3721
Sathishkumar M, Sneha K, Kwak IS, Mao J, Tripathy SJ, Yun YS (2009) J Hazard Mater 171:400–404
Sevil B, Zuhal K (2010) Macromol Symp 295:59–64
Shankar SS, Rai A, Ahmad A, Sastry M (2004) J Colloid Interface Sci 275:496–502
Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M (2014) Synth React Inorg Met-Org Nano-Met Chem 3:482–488
Sheldon RA (2007) Adv Synth Catal 349:1289–1307
Sheldon RA (2011) Appl Microbiol Biotechnol 92:467–477; Sheldon RA (2011) Org Process Res Dev 15:213–223
Sheldon RA, van Pelt S (2013) Chem Soc Rev 42:6223
Shibata S, Aoki K, Yano T, Yamane M (1998) J Sol-Gel Sci Technol 11:279
Singh SC, Gopal R (2007) Bull Mater Sci 30:291–293
Solanki JN, Murthy ZVP (2011) Ind Eng Chem Res 50:12311–12323
Brinker CJ, Scherer GW (eds) (1990) Sol-gel science: the physics and chemistry of sol-gel processing. Academic, New York. e-book, http://depts.washington.edu/solgel/documents/class_docs/MSE502/SolGel_Science_The physics_and_chemistry_of_sol- gel_processing_-_Brinker_1990.pdf
Stellacci F, Bauer CA, Meyer-Friedrichsen T, Wenseleers W, Alain V, Kuebler SM, Pond SJK, Zhang Y, Marder SR, Perry JW (2002) Adv Mater 14:175–198
Stepankova V, Bidmanova S, Koudelakova T, Prokop Z, Chaloupkova R, Damborsky J (2013) ACS Catal 3:2823–2836
Sue K, Kawasaki S, Suzuki M, Hakuta Y, Hayashi H, Arai K, Takebayashi Y, Yoda S, Furuya T (2011) Chem Eng J 166:947–953
Suffner J, Agoston P, Kling J, Hahn H (2010) J Nanopart Res 12:2579–2588
Swihart MT (2003) Curr Opin Colloid Interface Sci 8:127–133
Tadic M, Panjan M, Damnjanovic V, Milosevic I (2014) Appl Surf Sci 320:183–187
Takahashi YK, Koyama T, Ohnuma M, Ohkubo T, Hono K (2004) J Appl Phys 95:2690–2696
Tavano OL (2013) J Mol Catal B Enzym 90:1–11
Thurn-Albrecht T, Schotter J, Kästle GA, Emley N, Shibauchi T, Krusin- Elbaum L, Guarini K, Black CT, Tuominen MT, Russell TP (2000) Science 290:2126
Tran DN, Balkus KJ Jr (2011) ACS Catal 1:956–968
Veith GM, Lupini AR, Pennycook SJ, Ownby GW, Dudney NJ (2005) J Catal 231:151–158
Veith GM, Lupini AR, Pennycook SJ, Villa A, Prati L, Dudney NJ (2007) Catal Today 122:248–253
Wang P (2006) Curr Opin Biotechnol 17:574–579
Wang W, Xu Y, Wang DIC, Li Z (2009) J Am Chem Soc 139:12892–12893
Wang C, Huang L, Song S, Saif B, Zhou Y, Dong C et al (2015) Appl Surf Sci 357,. Part B:2077–2086
Wendisch VF (2015) J Biotechnol 201:1
Xing T, Sunarso J, Yang W, Yin Y, Glushenkov AM, Li LH, Howlett PC, Chen Y (2013) Nanoscale 5:7970–7976
Yarosh D, Klein J, Kibitel J, Alas L, O'Connor A, Cummings B et al (1996) Photodermatol Photoimmunol Photomed 12:122–130
Yarosh DB, O'Connor A, Alas L, Potten C, Wolf P (1999) Photochem Photobiol 69:136–140
Yu G, Rosenberg JN, Betenbaugh MJ, Oyler GA (2015) Biotechnol 36:199–204
Zhang Y, Wan J, Skumryev V, Stoyanov S, Huang Y, Hadjipanayis GC, Weller D (2004) Appl Phys Lett 85:5343–5345
Zhang W, Qia X, Chen J, Wang H (2006) J Colloid Interface Sci 302:370–373
Zhang Y, Li J, Han D, Zhang H, Liu P, Li C (2008) Biochem Biophys Res Commun 365:609–613
Zhang X, Yan S, Tyagi RD, Surampalli RY (2011) Chemosphere 82:489–494
Zinchenko A, Miwa Y, Lopatina LI, Sergeyev VG, Murata S (2014) ACS Appl Mater Interfaces 6:3226–3232
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, A.K., Tiwari, I. (2020). Nanomaterial Synthesis and Mechanism for Enzyme Immobilization: Part II. In: Srivastava, M., Srivastava, N., Mishra, P., Gupta, V. (eds) Nanomaterials in Biofuels Research. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-13-9333-4_8
Download citation
DOI: https://doi.org/10.1007/978-981-13-9333-4_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9332-7
Online ISBN: 978-981-13-9333-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)