Abstract
TiO2 is known for its photocatalytic activity. It has ability to degrade a number of organic pollutants. In the current study, TiO2 has been prepared in situ on cotton fabric, using its precursor through a modified sol-gel method. The in situ prepared TiO2 on fabric was further given heat treatments via different routes. The crystalline form, surface morphology and Ti content of the samples coated with TiO2 have been evaluated by XRD, FESEM and ICP-MS. The UV absorption behavior of the samples was studied using UV-visible spectroscopy. The photocatalytic degradation of Rhodamine B dye was conducted under UV light, using TiO2 coated cotton fabric prepared via different routes. It has been found that the sample treated with TiO2 via pad-dry-solvothermal route shows the highest rate of decolorization in comparison with samples prepared via pad-dry-cure and pad-dry-hydrothermal route. The mechanism of dye decolorization using TiO2 coated fabric has also been discussed.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Coloration of textile material requires a huge amount of water, in addition to dyes and chemical auxiliaries. About 15% of the total dye used in the coloration of textiles remains in un-reacted form. This eventually goes into wastewater and becomes a cause of high effluent load. This effluent load can be reduced either by removal or by degradation of the un-used dyes from the wastewater [1, 2]. Various chemical, electrochemical and biological processes have been used for the reduction of effluent from the wastewater. However, these processes suffer from low degradation efficiency. Sometimes, these processes require further addition of chemicals and thus become the cause of generation of secondary pollutants.
In recent years, photocatalysis has emerged as a simple, economic and efficient process for destruction of various dyes from the aqueous medium. Photocatalysis is an advanced oxidation process (AOP) by which degradation or even complete mineralization of many complex organic species takes place [3].
Rhodamine B (RhB) dye is one of the organic dyes which are widely used in coloring paper, laser printing and dyeing of textile materials [4]. Rhodamine B belongs to triphenylmethane family and is among the most notorious pollutant dyes used in textile and paper industry. The chemical structure of RhB consists of four N-ethyl groups at either side of the xanthene ring [5,6,7]. The traces of dye can persist for a long period in the aquatic environment due to its resistivity toward chemical and biological attacks. This makes the removal or degradation of this hazardous dye a great challenge [8, 9].
Among the transition metal oxides, TiO2 has been extensively used as photocatalyst due to its strong oxidizing power, non-toxicity, low cost, chemical stability and high photocatalytic activity [10, 11]. It has been utilized in various applications like sterilization, sanitation, air purification and water treatment [12, 13]. TiO2 has also been used by many researchers for the decolorization of Rhodamine B dye solution. But in most of the studies, the powder form of TiO2 is used directly for decolorization of dye wastewater in the presence of UV light. However, repeated use of TiO2 powder and its recovery from the dye solution remains a challenge. Even the centrifugation process does not ensure a complete recovery of TiO2 particles from the aqueous bath [14].
On providing a suitable template like cotton fabric, the deposited photocatalyst on it can be re-used. However, there are problems associated with the deposition of TiO2 particles on cotton fabric. TiO2 in its particle form does not have any affinity with the cotton substrate. Application of binders to hold the TiO2 particles with fabric shields the effectiveness of the photocatalyst. These problems have further been resolved by using low-temperature sol-gel method of preparation of TiO2 from its precursor and its subsequent coating on textile substrate.
The present study demonstrates three different process routes for application of TiO2 on cotton fabric. The role of the process routes (pad-dry-cure, pad-dry-hydrothermal and pad-dry-solvothermal) on the development of TiO2 morphology on fabric and its effect on rate of dye decolorization have also been studied in details.
2 Experimental Section
2.1 Materials
Cotton fabric with ends per inch = 124, picks per inch = 64 and gsm = 150 was supplied by Vardhman fabrics, Budhani. Titanium Tetra Isopropoxide (TTIP) was used as a precursor for synthesis of TiO2 sol. TTIP was purchased from Spectrochem, Mumbai. Analytical grade glacial acetic acid and ethyl alcohol were used in sol-gel preparation. Deionized water was used, whenever required.
2.2 Preparation of TiO2 Sol
Preparation of 1% v/v TTIP sol was done in 50:50 ethanol/DI water solvent system. One mL of pure TTIP was stirred with 10 mL of pure ethanol for 10 min. One mL of glacial acetic acid was stirred with 10 mL of pure ethanol separately for 5 min and then added in the 1st mixture dropwise. 30 mL of pure ethanol was mixed separately with 50 mL DI water and this aqueous ethanol was added dropwise to acidified TTIP/ethanol solution with continuous stirring. Finally, the prepared sol was stirred for another 4 h.
2.3 Application of TiO2 on Cotton Fabric
\(15 \times 15\;{\text{cm}}^{2}\) (approx. 3 g wt) samples of untreated cotton fabric were taken. Each sample was dipped in prepared sol of TTIP, followed by sonication for a period of 10 min. Further, each sonicated sample was padded at a pressure of 2.75 bar and dried at 80 °C. The padding pressure of 2.75 bar ensures a uniform percentage expression of 100% in all the samples. The heat treatment to each fabric sample was subsequently given by curing (sample A), hydrothermal (sample B) or solvothermal method (sample C).
Curing was conducted at 120 °C in hot air for a period of 3 min. Hydrothermal treatment was given by transferring the sample in IR dyeing cylinder containing 100 mL of DI water. It was treated for a period of 3 h at 120 °C. Solvothermal treatment was given by transferring the sample in IR dyeing cylinder containing 100 mL of TiO2 sol. It was also treated for a period of 3 h at 120 °C.
2.4 Characterization
The crystallographic phase of the prepared samples was investigated in a Panalytical X’Pert X-ray diffractometer (XRD) using Cu-K radiation. The surface deposition of TiO2 on cotton fabric was studied using scanning electron microscope (SEM), model ZEISS EVO 50. Energy dispersive X-ray (EDX) analysis was carried out in EDX system Model QuanTax 200 to analyze the elemental composition.
The total amount of Ti in treated samples was determined by high-resolution inductively coupled plasma mass spectrometry (ICP-MS, make: Agilent 7900). A known weight of sample was digested in a microwave-assisted acidic digester at 280 °C for about 30 min. Spectral interferences arising from the plasma gases or the major components of the samples were identified and removed. Eventually, the amount of Titanium present in fabric was determined in terms of microgram of Ti per gram of the fabric (ppm) [15].
ISO 105 C10: 2006—B was adopted to evaluate the performance of the treated samples after standard wash. The treated samples were washed in laundro-meter at 50 °C temperature using 5 gpl of soap. The material to liquor ratio was kept as 50:1 and the washing cycle was completed in 45 min.
UV-visible spectra of samples were recorded on a UV-VIS-NIR Photo-spectrometer, model LAMBDAL6020087 keeping air as a reference.
2.5 Setup Used for Decolorization of Rhodamine B Dye Solution
The photocatalytic activities of untreated and TiO2 treated cotton fabrics were evaluated for decolorization of Rhodamine B dye under UVB light. Figure 1 shows the schematic diagram of setup used for dye decolorization studies. It consists of detachable trays containing UVB tube lights. UVB light of very narrow waveband emission with a peak at 311 nm is supplied with the help of 6 UVB lamps of 9 W each (make—Philips PL-S 9W/01). Dye solution of Rhodamine B with an initial concentration of 20 mg/L was used in the entire study. All the fabric samples were cut in a circular disk shape of diameter 7.5 cm. The individual samples were dipped in the Petri dishes containing 100 mL of prepared dye solution. The vertical distance between the detachable trays containing light source and the dye solutions was kept 45 cm.
3 Results and Discussion
3.1 XRD Analysis
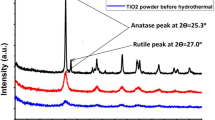
The XRD scans of untreated fabric and fabric impregnated with TiO2 precursor and subsequently prepared via different routes were analyzed. As can be seen from Fig. 2, the untreated fabric has only characteristic peaks of cellulose I at 2θ = 16.5°, 22.5° and 34.6°. Among other samples, samples B and C show a distinct hump at around 25.5°, indicative of anatase form of TiO2. However, sample A in which TiO2 is applied via Pad-dry-cure route, crystalline peak of TiO2 could not be seen. In case of sample A, the curing time given was only 3 min. It is expected that in such a short duration of thermal treatment, transformation of amorphous phase to crystalline phase could not occur in sample A. Therefore, no crystalline phase of TiO2 could be observed in sample A. On the other hand, sufficient time of 3 h and temperature of 120 °C has been able to develop crystalline peak of TiO2 in samples B and C.
3.2 SEM of TiO2 Coated Cotton Samples
The SEM images of TiO2 coated cotton samples are shown in Fig. 3. It can be seen that PDC method results in formation of a layer of TiO2 on fabric surface. In PDH method, the texture of TiO2 turns to be flat-granular, whereas in case of PDS higher concentration of these granules can be seen. Therefore, it can be inferred that the texture of TiO2 deposited on cotton surface can be altered depending upon the method used for application of heat treatment in the samples.
3.3 Measurement of Titania Content in Fabric
The content of Ti in treated cotton fabrics has been measured using ICP-MS. The results are shown in Table 1, which suggests that the add-on level of Ti is similar in case of PDC and PDH and higher with PDS. Moreover, comparing the Ti content of samples after 3 washes with their 1st standard wash samples, it can be observed that wash fastness of samples B and C are better than that of sample A. Prolonged heat treatment given to the samples in hydrothermal or solvothermal process help not only in development of crystalline TiO2 coating, it also ensures the deposition of nano-particulates TiO2 of the size around 100–250 nm on the cotton fabric surface. Therefore, hydrothermal or solvothermal process may be considered to provide a more durable deposition of TiO2 than a pad-dry-cure process.
The Ti content in treated fabrics has also been measured through EDX technique. It can be observed from Fig. 4 that values of Ti content obtained by EDX technique are much higher than the values obtained from ICP-MS method for the same set of samples. This anomaly can be attributed to the surface deposition of TiO2 by these routes. While the ICP method calculates the values on cotton fabric weight, in case of EDX, it is calculated taking into consideration only the surface layer deposition.
3.4 UV Absorption Studies
UV absorption spectra of all the samples were done to understand the absorption behavior of the fabric samples in UV light. The UV-visible absorption spectra of different samples are shown in Fig. 5. The untreated cotton has almost no ability to absorb UV light. On the other hand, the TiO2 treated fabric samples exhibit good absorption of UV light, which confirms that TiO2 is well deposited on samples A, B and C [16]. With respect to the TiO2 coated fabric sample A, sample B and sample C show a comparatively higher absorption of UV light. Higher absorption of UV light facilitates the TiO2 coating to exhibit better photocatalytic activity due to the ease in generation of charge carriers. Therefore, this could be one of the reasons for getting the best results of dye decolorization with sample C.
3.5 Decolorization Study of Rhodamine B Dye Solution
The photo-induced decolorization of dye solution was measured through UV-visible absorption spectroscopy. The characteristic wavelength maxima of Rhodamine B dye solution were observed at 553 nm. Therefore, change in absorbance of the dye solution at 553.6 nm peak was used to calculate the decolorization % of dye solution.
During the photocatalytic degradation of Rhodamine dye solution, the characteristic absorption band of RhB at 553.6 nm decreases. Later, in addition to this a hypsochromic shift of absorption maxima also occurs from 553.6 to 534 nm. This shift in wavelength can be regarded as the formation of many N-de-ethylated intermediates of RhB dye molecule in the process of its photocatalytic degradation [3].
The percentage decolorization of Rhodamine B solution in the presence of cotton fabric as a function of UV light exposure is represented in Fig. 6.
Another experiment was separately performed to study the effect of exposure of UV light directly on Rhodamine B solution of the same concentration in the absence of fabric. However, a direct exposure of light in dye solution in the absence of TiO2 catalyst did not show any change in absorbance peak of dye solution. This confirms that aqueous solutions of RhB dye are stable in UV radiation.
Under UV light, the dye solution shows a significant decrease in the absorbance value in the presence of TiO2 coated cotton fabrics. The absorbance peak of Rhodamine B dye solution at wavelength value of 553.6 nm has been made the basis of calculation of total dye decolorization in the entire study. Figure 7 represents continuous curves between absorbance and wavelength for all the samples. The ultimate decrease in absorbance value of the dye solutions after a complete cycle of exposure of 8 h under UV light has been recorded. Using the calibration curve, it has been found that in comparison with untreated fabric, the TiO2 coated fabric sample prepared via PDC route (sample A) showed a total decolorization of 32.2% after a period of 8 h. Further, in comparison with untreated fabric, samples B and C showed a total decolorization of 76.1 and 88.4%, respectively.
Among the TiO2 coated samples, sample A showed the lowest percentage of overall dye decolorization. As shown in Table 1, the Ti content in samples A and B is comparable to each other. Therefore, Ti content cannot be considered as a cause for this effect. The other reasons like the difference in the XRD pattern and UV absorbance behavior of sample A in comparison with samples B and C can be assigned for the lowest percentage of overall dye decolorization in sample A. As can be seen from the XRD pattern, the coating of TiO2 is amorphous in sample A and hence the pad-dry-cure treatment ends up with a low degree of photocatalytic activity. Also, the UV light absorption of sample A was the lowest among all the TiO2 coated fabrics (Fig. 5).
In contrast, the coating of TiO2 has been stabilized effectively in samples B and C, where sufficient crystallinity in TiO2 coating is developed by prolonged exposure to higher temperature. The other obvious reason for showing the highest decolorization % by sample C may be the highest percentage of titania deposited in sample C.
The overall mechanism involved in photocatalytic degradation of Rhodamine B dye solution can be understood as under-TiO2 nano-particles have a tendency to absorb UV light and generate reactive chemical species. These reactive chemical species take part in mineralization of various organic species. After the exposure of UV light, the electron of the valence band of titanium dioxide present in TiO2 coated fabric gets excited to the conduction band. This leaves behind a positively charged hole (h+) in the valence band. The excited e− reacts with the atmospheric oxygen to convert itself to superoxide radical anion. The positively charged hole can react with water or hydroxyl ions and produces super hydroxyl radical. Super hydroxyl (HO2·) and superoxide O2°(−) radical anions are considered as the reactive species, which oxidize the dye molecule adsorbed on the oxide surface. These generated radicals are the responsible active species for complete mineralization of the hazardous dyes [17,18,19,20].
3.6 Reusability of Samples for Dye Decolorization
The repeated use of the dye decolorization ability of TiO2 coated samples is essential for its practical viability. Therefore, the evaluation of the reusability of the samples was carried out. At the end of 1st decolorization cycle, the fabric samples were washed with deionized water and dried at 50 °C before their use for the next cycle. The results of the three consecutive cycles of dye decolorization with all the fabric samples are shown in Fig. 8. In comparison with the decolorization of 76.1 and 88.4% in 1st cycle, samples B and C showed 71.4 and 83.6% decolorization in the 3rd repeated cycle. However, there was a huge reduction in decolorization ability of sample A from 32.2% in the 1st cycle to 22.5% in the 3rd cycle. This reduction in decolorization ability of sample A may be assigned to the regular loss in titania content on the fabric after each cycle. In contrast, such loss in decolorization efficiency was not shown by samples B and C probably due to the more stable TiO2 coating on these samples.
4 Conclusions
The present study demonstrates coating of TiO2 on cotton fabric using its precursor. This has been done using three different process routes, i.e., pad-dry-cure, pad-dry-hydrothermal and pad-dry-solvothermal. The morphological structures of the coatings confirmed that the process routes play a critical role in deposition behavior of TiO2 on fabric surface. The extent of titania deposition depends upon the process route followed, which in turn determines the extent of dye decolorization. The overall dye decolorization % also depends on the UV light absorption behavior of the prepared samples. The results showed that a uniform coating of TiO2 on cotton fabric prepared by hydrothermal or solvothermal route gives better photocatalytic decolorization of Rhodamine B dye solution.
References
Vinu, R., Akki, S.U., Madras, G.: Investigation of dye functional group on the photocatalytic degradation of dyes by nano-TiO2. J. Hazard. Mater. 176, 765–773 (2010)
Chatterjee, S., Tyagi, A.K., Ayyub, P.: Efficient photocatalytic degradation of rhodamine B dye by aligned arrays of self-assembled hydrogen titanate nanotubes. J. Nanomater. 2014, 7 p (2014)
Aarthi, T., Madras, G.: Photocatalytic degradation of rhodamine dyes with nano-TiO2. Ind. Eng. Chem. Res. 46(1), 7–14 (2007)
Abdel-Messih, M.F., Ahmed, M.A., El-Sayed, A.S.: Photocatalytic decolorization of Rhodamine B dye using novel mesoporous SnO2–TiO2 nano mixed oxides prepared by sol–gel method. J. Photochem. Photobiol. A 260, 1–8 (2013)
Yu, K., Yang, S., He, H., Sun, C., Gu, C., Ju, Y.: Visible light-driven photocatalytic degradation of Rhodamine B over NaBiO3: Pathways and mechanism. J. Phys. Chem. A 113, 10024–10032 (2009)
Ayed, L., Chaieb, K., Cheref, A., Bakhrouf, A.: Biodegradation of triphenylmethane dye Malachite Green by Sphingomonas paucimobilis. World J. Microbiol. Biotechnol. 25(4), 705–711 (2009)
Li, L., Dai, W., Yu, P., Zhao, J., Qu, Y.: Decolorisation of synthetic dyes by crude laccase from Rigidoporus lignosus. J. Chem. Technol. Biotechnol. 84(3), 399–404 (2009)
Mishra, G., Parida, K.M., Singh, S.K.: Solar light driven Rhodamine B degradation over highly active β-SiC–TiO2 nanocomposite. RSC Adv. 4(25), 12918–12928 (2014)
Chen, Y., Li, Y., Zhu, A., Huang, Y., Liu, Z., Yan, K.: Degradation of aqueous Rhodamine B by plasma generated along the water surface and its enhancement using nanocrystalline Fe-, Mn-, and Ce-doped TiO2 films. Environ. Sci. Pollut. Res. 21(16), 9948–9958 (2014)
Di Paola, A., Cufalo, G., Addamo, M., Bellardita, M., Campostrini, R., Ischia, M., Ceccato, R., Palmisano, L.: Photocatalytic activity of nanocrystalline TiO2 (brookite, rutile and brookite-based) powders prepared by thermohydrolysis of TiCl4 in aqueous chloride solutions. Colloids Surf. A 317(1), 366–376 (2008)
Chen, X., Mao, S.S.: Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107(7), 2891–2959 (2007)
Fujishima, A., Rao, T.N., Tryk, D.A.: Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 1(1), 1–21 (2000)
Winkler, J.: Nano‐scaled titanium dioxide–properties and use in coatings with special functionality. In: Macromolecular Symposia, pp. 317–324. Wiley‐VCH, Verlag (2002)
Zhang, Q., Joo, J.B., Lu, Z., Dahl, M., Oliveira, D.Q., Ye, M., Yin, Y.: Self-assembly and photocatalysis of mesoporous TiO2 nanocrystal clusters. Nano Res. 4(1), 103–114 (2011)
Pranaitytė, B., Padarauskas, A., Naujalis, E.: Application of ICP-MS for the determination of trace metals in textiles. Chemija 18, 16–19 (2007)
Koziej, D., Fischer, F., Kranzlin, N., Caseri, W.R., Niederberger, M.: Nonaqueous TiO2 nanoparticle synthesis: a versatile basis for the fabrication of self-supporting, transparent and UV-absorbing composite films. ACS Appl. Mater. Interfaces 1(5), 1097–1104 (2009)
Akpan, U.G., Hameed, B.H.: Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J. Hazard. Mater. 170, 520–529 (2009)
Mahmoodi, N.M., Arami, M., Limaee, N.Y., Tabrizi, N.S.: Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J. Colloid Interface Sci. 295, 159–164 (2006)
Hasnat, M.A., Siddiquey, I.A., Nuruddin, A.: Comparative photocatalytic studies of degradation of a cationic and an anionic dye. Dyes Pigm. 66(3), 185–188 (2005)
Laid, N., Bouanimba, N., Zouaghi, R., Sehili, T.: Comparative study on photocatalytic decolorization of an anionic and a cationic dye using different TiO2 photocatalysts. Desalin. Water Treat. 57(41), 19357–19373 (2016)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Mishra, A., Butola, B.S. (2019). Photocatalytic Decolorization of Rhodamine B Dye Solution Using TiO2 Coated Cotton Fabric. In: Majumdar, A., Gupta, D., Gupta, S. (eds) Functional Textiles and Clothing. Springer, Singapore. https://doi.org/10.1007/978-981-13-7721-1_13
Download citation
DOI: https://doi.org/10.1007/978-981-13-7721-1_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7720-4
Online ISBN: 978-981-13-7721-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)