Abstract
Positron emission tomography/computed tomography (PET/CT) has become an important method for the diagnosis/identification, staging, follow-up monitoring, and efficacy and prognosis determination of malignant tumors. The glucose analog 18F-deoxyglucose (FDG) is the most mature and widely used imaging agent for PET. Since most malignant cells show a high glucose metabolism, they can accumulate more 18F-FDG than do normal tissues and organs, which can be detected by PET/CT and distinguished from normal tissues. 18F-FDG-PET/CT imaging has good sensitivity and accuracy for the diagnosis and evaluation of most malignant tumors. However, 18F-FDG-PET/CT has limited application in primary urinary tumors (prostate cancer, renal clear-cell carcinoma, and bladder cancer [BC]). Initial studies evaluating the role of 18F-FDG-PET in kidney and bladder malignancies showed disappointingly low sensitivity. The main reason is that FDG is mainly excreted through the urinary system, thereby masking the FDG uptake of primary renal and BCs. Many researchers are trying to improve the sensitivity and specificity of PET/CT in urological malignancies through a variety of techniques, including bladder irrigation and delayed imaging. Recent studies using PET/CT instead of PET and some improved protocol techniques have shown better sensitivity and specificity, and PET/CT now appears to be more useful in the staging and follow-up of urinary malignancies than originally reported.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
5.1 Introduction
Positron emission tomography/computed tomography (PET/CT) has become an important method for the diagnosis/identification, staging, follow-up monitoring, and efficacy and prognosis determination of malignant tumors. The glucose analog 18F-deoxyglucose (FDG) is the most mature and widely used imaging agent for PET. Since most malignant cells show a high glucose metabolism, they can accumulate more 18F-FDG than do normal tissues and organs, which can be detected by PET/CT and distinguished from normal tissues. 18F-FDG-PET/CT imaging has good sensitivity and accuracy for the diagnosis and evaluation of most malignant tumors. However, 18F-FDG-PET/CT has limited application in primary urinary tumors (prostate cancer, renal clear-cell carcinoma, and bladder cancer [BC]). Initial studies evaluating the role of 18F-FDG-PET in kidney and bladder malignancies showed disappointingly low sensitivity. The main reason is that FDG is mainly excreted through the urinary system, thereby masking the FDG uptake of primary renal and BCs. Many researchers are trying to improve the sensitivity and specificity of PET/CT in urological malignancies through a variety of techniques, including bladder irrigation and delayed imaging. Recent studies using PET/CT instead of PET and some improved protocol techniques have shown better sensitivity and specificity, and PET/CT now appears to be more useful in the staging and follow-up of urinary malignancies than originally reported.
5.2 Bladder Cancer
5.2.1 Overview of Bladder Cancer
BC is the ninth most common cancer in the world and the most common type of cancer of the urinary system. Painless hematuria is the most common symptom of BC. More than 90% of BC cases are of urinary tract epithelial (transitional cell) cancer, 5% of squamous cell carcinoma, and less than 2% of adenocarcinoma [1]. The main method for the diagnosis of BC is cystoscopy and biopsy under cystoscopy for pathological analysis. Approximately 30% of patients show muscle invasion. Prolonged metastases of BC are common, and the most important metastatic sites include the liver, lung, bone, and adrenal gland. In general, BC with muscle invasion is treated with cystectomy and pelvic lymphadenectomy (PLND), while metastatic disease is treated with cisplatin-based combination chemotherapy.
5.2.2 Glucose Metabolism in Bladder Cancer
The metabolism of malignant tumor cells mainly comprises anaerobic glycolysis of glucose; these cells also show decreased TCA activity. Cancer cells in the urinary tract rely on a special transformation of aerobic glycolysis-dependent metabolism (Warburg effect) to provide a rapid supply of ATP, thereby maintaining the primary source of energy for uncontrolled proliferation. Therefore, high glycolysis flux is dependent on glycolysis-related genes (glucose transporter type 1 [GLUT1], lactate dehydrogenase A [LDHA], hexokinase 1 [HK1], pyruvate kinase type M [PKM2], hypoxia-inducible factor 1-α [HIF-1α]), etc., resulting in excessive production of pyruvic acid and lactic acid. In addition, activation of the PTEN/PI3K/AKT/mTOR signaling pathway in BC also activates tumor glucose metabolism and suppresses oxidative phosphorylation by promoting anaerobic glycolysis.
5.2.3 Clinical Utility of 18F-FDG-PET/CT Scanning in BC
5.2.3.1 18F-FDG-PET/CT for Diagnosis of Primary BC
In the past, the use of FDG-PET/CT for the diagnosis of primary BC was limited because it was difficult to distinguish the radiotracer activity from the urine from the tumor activity in the bladder. There are several ways to overcome radioactive interference in the urine, such as delayed imaging after urination, diuretic interventional imaging, and bladder irrigation after catheterization. These methods significantly reduce the physiological uptake of FDG in the bladder and improve the detection rate of the primary BC (Figs. 5.1 and 5.2). A recent meta-analysis evaluated the accuracy of FDG PET/CT in the diagnosis of bladder lesions. Six studies met the inclusion criteria. The sensitivity and specificity of BC for PET or PET/CT detection are 80.0% and 84.0%, respectively [2]. Compared to magnetic resonance imaging (MRI) and CT, FDG-PET/CT showed no advantage in detecting local bladder lesions.
5.2.3.2 18F-FDG-PET/CT for Staging of BCs
CT and MRI can help determine whether lymph node (LN) metastasis is mainly based on the size and shape of the LNs. However, the rate of misdiagnosis of LNs with normal-sized metastasis is very high. Therefore, both the imaging methods have very low sensitivity for the detection of LN metastases. The specificity of the two methods for the detection of LN metastases is also low. Moreover, the rate of misdiagnosis is higher, because lymphadenopathy may be caused by benign diseases, such as inflammatory hyperplasia of the LNs. Metastases of BC, such as LN metastasis, also have metabolic properties similar to those of the primary tumor; therefore, LNs or distant metastatic lesions with high metabolic activity outside the bladder are detected by PET (Fig. 5.3). A recent meta-analysis of FDG-PET/CT for BC staging and re-segmentation found that the sensitivity, specificity, and accuracy of PET/CT for BC staging were 82%, 89%, and 92%, respectively [3]. In 20–40% of patients, FDG-PET/CT detected more malignant diseases than conventional CT/MRI, and FDG-PET/CT may change the clinical treatment plan in 68% of patients [4]. Some authors believe that FDG-PET/CT diagnosis of LN metastasis is not significantly better than CT. Swinnen et al. reported that the accuracy, sensitivity, and specificity of FDG-PET/CT for diagnosing LN metastasis of BC were 84%, 46%, and 97%, respectively [5]. The corresponding values when CT results were analyzed separately were 80, 46, and 92%. The study considered that compared to CT alone, the combination of FDG-PET/CT afforded no advantage in the staging of invasive BC and LN metastases.
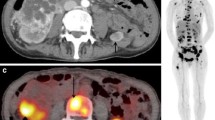
Images of a patient with diagnosed muscle-invasive bladder cancer. (a) The MIP of FDG-PET; (b) CT of FDG-PET/CT and (c) PET/CT showed high FDG uptake of primary tumor in the bladder wall after diuresis. A lymph node metastasis is seen in the right side of the pelvis on CT of FDG-PET/CT (d) and PET/CT (e). Bone metastasis in pubic bone can be seen on CT of FDG-PET/CT (f) and PET/CT (g)
5.2.3.3 18F-FDG-PET/CT for Restaging of BCs
The prognosis of recurrent BC is very poor. Early detection and active treatment remain very important for prolonging the survival time of patients and improving their quality of life. Considering that recurrent BC patients may require expensive targeted therapies and immunotherapy, precise re-segmentation is particularly important. FDG-PET/CT can be used for whole-body imaging, and in most instances of recurrence, BC shows higher FDG uptake; thus, FDG-PET/CT can be used for an accurate diagnosis of BC (Fig. 5.4). Jadvar et al. retrospectively evaluated the ability of FDG-PET or PET/CT to diagnose recurrent and metastatic BC. In their study, all 35 patients had previously been treated for the primary disease. The sites of metastasis detected in the study included the mediastinum, lungs, and bone. FDG-PET/CT had an impact on the clinical treatment of 17% of patients by facilitating the proposal of additional treatment or waiting for an observational strategy [6]. FDG-PET can also be used to detect recurrent tumors in the pelvis and distinguish between local recurrence and post- or postradiation fibrosis/necrosis and distant metastasis.
5.2.3.4 18F-FDG-PET/CT for Determining the Response to Therapy
In bladder muscle-invasive BC, neoadjuvant chemotherapy is the standard treatment, which can improve the overall survival rate of patients with BC. Patients with significantly reduced LNs after neoadjuvant chemotherapy can further choose surgery and reduce the recurrence rate. However, we should also recognize that some BCs are not responsive to neoadjuvant chemotherapy. It is often difficult and inaccurate to use conventional imaging modalities such as CT to assess the responsiveness of LN to neoadjuvant chemotherapy. This is mainly due to the difficulty in identified surviving tumors in residual (necrotic) masses and small-tumor deposits in normal-sized LNs. FDG-PET/CT is currently used to monitor the response of pelvic LN metastasis to BC neoadjuvant chemotherapy. According to the European Cancer Research and Treatment Organization (EORTC) recommendations, metabolic responses are assessed based on changes in FDG uptake. Reactivity was assessed on CT according to Reaction Assessment Criteria in Solid Tumors (RECIST). All patients underwent a histopathological examination for PLND. PET/CT and CT correctly distinguished between nonresponders (79%) and complete responders (68%) among disabled patients (63%). At present, it is commonly believed that PET/CT is suitable for evaluating the response of LN to neoadjuvant chemotherapy. However, this belief needs to be confirmed in large clinical trials [7] (Fig. 5.5).
A 66-year-old male patient with bladder cancer (cT3N1) developed lymph node metastasis and bone metastasis after neoadjuvant chemotherapy and radical cystectomy. (a) The maximum intensity projection (MIP) of FDG-PET shows two regions of abnormal FDG uptake in the pelvis. (b) CT of FDG-PET/CT and (c) PET/CT showed moderate FDG uptake (SUVmax, 7.2) corresponding to a 1.5-cm enlarged para-aortic lymph node (arrow), suggesting lymph node recurrence. The little FDG uptake on the left side of the lymph node recurrence lesion is a physiological excretion in the left ureter. CT of FDG-PET/CT and (e) PET/CT showed strong FDG uptake (SUVmax, 13.0) corresponding to a mild sclerosis of the left pubic symphysis (arrow), suggesting bone metastasis. It is difficult to detect this bone metastasis only by (d) CT
5.3 Renal Cell Carcinoma
5.3.1 Overview of Renal Cell Carcinoma
Renal cell carcinoma (RCC) accounts for about 3% of human cancers, most of which are well differentiated and cured by surgical resection. However, poorly differentiated kidney cancer is highly invasive and remains the main cause of cancer-related death. Kidney cancer is divided into clear-cell carcinomas (60–80%), papillary carcinomas (10%), chromophobe cell carcinoma (5%), and other tumors such as sarcomatoid carcinomas, squamous cell carcinomas, and leiomyosarcomas [8]. Because some RCCs are highly fatal, it is important to identify, recognize, and grade them and then to monitor them during treatment. Ten years ago, PET had little to do with RCC. In recent years, a more comprehensive understanding of the clinical application of FDG-PET in RCC has been gained.
5.3.2 Glucose Metabolism of Renal Cell Carcinoma
RCC glucose metabolism is active at the cellular level and exhibits the classic Warburg effect. Increased levels of GLUT 1 in clear-cell renal cell carcinoma (ccRCC) tumors compared to those in normal control tissues indicate increased glucose uptake. In addition, metabolomics, proteomics, and transcriptomics studies have shown increased levels of glycolytic metabolites and enzymes, such as hexokinase, pyruvate kinase 2, and lactate dehydrogenase A (LDHA) in ccRCC cells and tissues, indicating increased glucose utilization in renal cancer; the lactic acid product of anaerobic glycolysis increases accordingly. On the other hand, the kidney is an important organ of gluconeogenesis, and fructose 1,6-bisphosphatase 1 (FBP1) is highly expressed in the kidney and can antagonize glycolysis. However, the expression of FBP1 that antagonizes glycolysis in renal cancer is reduced or even disappears in the tumor. Studies have shown that overexpression of FBP1 in tumors can inhibit ccRCC tumor growth, indicating glycolysis and the closely related gluconeogenesis play an important role in the occurrence and development of kidney cancer. In addition, many studies have confirmed that the levels of pyruvate carboxylase and pyruvate dehydrogenase (i.e., enzymes that catabolize pyruvate to facilitate the TCA cycle) also significantly reduce in ccRCC, further indicating that these tumors are dependent on glycolysis (Table 5.1) [9].
5.3.3 Clinical Utility of 18F-FDG-PET/CT Scanning in RCC
5.3.3.1 18F-FDG-PET/CT for Diagnosis of Primary RCC
It is generally believed that the value of FDG-PET in the diagnosis of primary RCC is little. The accuracy of CT-based diagnosis of renal cancer can reach 95%. CT scans of RCC are characterized by a soft tissue mass in the renal parenchyma, showing a uniform or uneven density, a slightly higher density, or a slightly lower density lesion, which may have cystic changes and calcification. FDG-PET/CT sensitivity is only about 60% in RCC, although its specificity for malignancy is 90% [4]. In clinical practice, RCC has relatively low FDG uptake, especially in well-differentiated ccRCC. The low sensitivity of 18F-FDG-PET/CT for renal cancer diagnosis may be related to the following factors: RCC is mostly clear-cell carcinoma, especially of grades I–II; low expression of GLUT-1 in tumor tissue, which reduces the glucose uptake ability of the tumor; gluconeogenesis in some well-differentiated renal cancers, and high glucose-6-phosphatase activity in the tumor, so that the phosphorylated FDG regenerates free FDG and discharges the tumor cells; and the effect of intrarenal urine radioactivity on tumor detection. These factors make it difficult to distinguish between FDC uptake in RCC tumor tissue and physiological FDG uptake in renal parenchyma. In addition, the uptake of FDG in renal tumors also depends on tumor size (Fig. 5.6). FDG is sensitive and specific for poorly differentiated clear-cell carcinomas, such as those of grades III and IV, which may be related to decreased gluconeogenesis and enhanced glycolysis in poorly differentiated renal cancer. Ferda et al. found that patients with the highest FDG intake had a tumor grade of IV and SUVmax was more than 10 in these patients [10].
5.3.3.2 18F-FDG-PET/CT for the Staging of RCC
FDG-PET plays a minor role in the diagnosis of primary renal cancer. Enhanced CT and/or MRI usually detects the primary lesion well, and if identification is difficult, it can be further punctured to pinpoint the pathology. FDG plays an important role in the diagnosis of specific kidney cancer subtypes such as type II papillary and sarcomatoid cancers, and FDG-PET plays a relatively minor role in the initial staging of RCC. Currently, there is a higher risk, and larger tumors are more studied in FDG-PET. For local staging, FDG-PET has been found to be able to determine whether the thrombus in the renal vein and inferior vena cava is malignant or benign. When isolated lesions are detected, FDG-PET can diagnose metastatic disease based on the metabolic properties of the lesion (Figs. 5.7 and 5.8). However, FDG PET is not widely used in RCC staging.
Clear-cell renal cancer recurrence after operation. (a) The MIP of FDG-PET shows regions of abnormal FDG uptake on whole body. Lymph node metastasis is seen in the side of abdominal aorta on (b) CT of FDG-PET/CT and (c) PET/CT. Liver metastasis is seen in the right lobe of liver on (d) CT of FDG-PET/CT and (e) PET/CT. Bone metastasis is seen in sacrum on (f) CT of FDG-PET/CT and (g) PET/CT
5.3.3.3 18F-FDG-PET/CT for Response to Therapy
FDG-PET is also commonly used to monitor and evaluate RCC sensitivity to novel targeted therapies. Kidney cancer does not respond to conventional chemotherapy, so this treatment approach is rarely used. A variety of targeted therapies, including tyrosine kinase inhibitors such as sorafenib and sunitinib (with anti-angiogenic mechanisms) and mTOR inhibitors (such as everolimus), have shown good curative effects. Although measurement of the tumor size with the RECIST criteria has been attempted to monitor chemotherapy effects, tumor size typically does not change much in targeted therapies. Evaluation of FDG-PET metabolic activity can allow better monitoring of the effectiveness of targeted therapy with tyrosine kinase inhibitors. Ferda et al. demonstrated that FGF uptake was associated with the prognosis in pre-treatment tumors, and patients with a renal tumor SUVmax >10 had a significantly lower survival rate than those with an SUVmax <10. A reduction in FDG uptake is also closely related to the effect of targeted therapy, regardless of the location of the lesion (e.g., bone, lung, LNs, etc.). The extent of the SUVmax decline was associated with progression-free survival and overall survival (OS). The advantage of FDG-PET over conventional methods is most pronounced in bone and musculoskeletal metastases, which are difficult to assess on CT and MRI because bone damage persists even if targeted therapy was effective for the lesion. Some investigators used the total metabolic mass and total disease glycolysis (TLG) as an overall measure of disease burden rather than a separate SUVmax score. This is calculated by multiplying by SUVmax and the number of lesions. Therefore, FDG-PET is being increasingly used to monitor the therapeutic effects in patients with metastatic disease receiving tyrosine kinase inhibitors and other targeted therapies.
5.4 Prostate Cancer
5.4.1 Overview of Prostate Cancer
Prostate cancer is the second most common malignant tumor in men and the fifth leading cause of cancer-related death in men worldwide. At present, a prostate biopsy is still the only way to provide a definitive diagnosis of prostate cancer. Other diagnostic methods for prostate cancer include measurement of serum prostate-specific antigen (PSA) levels, ultrasound (US) and MRI examinations, and PET. The current clinical challenge in the management of this disease is improving the accuracy of biopsy for diagnosis and identification of aggressive prostate cancer to avoid overdiagnosis and overtreatment. Accurate staging is another major challenge in the diagnosis of prostate cancer. Accurate staging of prostate cancer on the basis of imaging data can avoid unnecessary LN dissection and reduce the related complications.
5.4.2 Glucose Metabolism in Prostate Cancer
Prostate tissue cells show lower levels of anaerobic glycolysis. Early prostate cancer cells rely more on lipids and other energy molecules to produce energy than they do on aerobic respiration. Therefore, the Warburg effect is not consistent in the pathogenesis of prostate cancer, and most prostate tumor cells do not show a high glycolytic ability. Clinically, many prostate tumor lesions show negative findings on FDG-PET scans. Only some poorly differentiated, advanced prostate cancers will begin to exhibit the Warburg effect and show high glucose uptake. Late prostate cancer lesions show increased lactate concentrations, expressing monocarboxylate transporters (MCTs) to transport intracellular lactate. Studies have shown that the phenotype of MCT is associated with the aggressiveness of the cancer and the prognosis. By blocking MCT activity, tumor cells can accumulate toxic metabolites at a faster rate. Thus, expression of the lactate shuttle in prostate cancer represents a potential therapeutic target as well as a potential diagnostic and prognostic indicator.
5.4.3 Clinical Utility of 18F-FDG-PET/CT Scanning in Prostate Cancer
5.4.3.1 18F-FDG-PET/CT for Diagnosis of Primary Prostate Cancer
In 2008, Jadvar et al. [11] studied the glucose metabolism of the normal prostate in 145 normal male patients who underwent FDG-PET/CT imaging. They found a mean SUV of 1.3 for the prostate and an average SUVmax of 1.6. In 2010, Minamimoto et al. [12] performed FDG-PET examinations before prostate biopsy in 50 patients with elevated PSA levels. The patients’ median age was 68 years and their median PSA level was 15.9 ng/mL. The investigators reported that FDG-PET assessments diagnosed prostate cancer with a sensitivity of 51.9% and a specificity of 75.7%. Shiiba et al. [13] reported a prospective study comparing 20 male patients with elevated PSA levels who were assessed for prostate cancer. These patients underwent 11 C-methionine and FDG imaging. Their median age was 72.4 years, and the median PSA level was 181.3 ng/mL. In their study, the sensitivity and specificity of FDG-PET/CT for the diagnosis of prostate cancer were 35.8% and 92.3%, respectively. The researchers concluded that the two tracers performed the same in patients with a high Gleason score, although FDG-PET/CT findings were poor when the Gleason score was lower than 8. In 2012, Minamimoto and colleagues [14] reported the findings for a total of 155,456 investigations, and FDG-PET/CT showed positive findings in 16,955. Of these, 1912 were diagnosed with cancer, and 165 of them had prostate cancer. The overall sensitivity of FDG-PET/CT for detecting cancer was 78% (1491/1912), but the detection rate for prostate cancer was only 37%. Therefore, FDG-PET is not suitable as the main diagnostic method for prostate cancer (Fig. 5.9).
5.4.3.2 18F-FDG-PET/CT for the Staging of Prostate Cancers
FDG-PET shows some value in the staging of prostate cancer (Fig. 5.10). In 2010, Tiwari et al. [15] examined 16 cases involving FDG-PET/CT and bone imaging and found 197 bone lesions by FDG-PET/CT imaging, of which 97 (49%) were also detected using conventional bone imaging. Most of the lesions (i.e., 95%) shown by FDG-PET/CT showed matched lesions in bone imaging. Yu et al. [16] compared the findings of FDG and 11C-acetate PET/CT imaging in eight patients. In their study, 11C-acetate PET/CT showed more metastases than FDG-PET/CT in most patients. In 2013, Damle et al. [17] compared FDG-PET/CT, 18F-sodium fluoride PET/CT, and bone imaging for their effectiveness in detecting bone metastasis of prostate cancer, lung cancer, and breast cancer. In assessments for 49 patients with prostate cancer, they found that the sensitivity, specificity, and accuracy of FDG-PET/CT were 71.9%, 100%, and 81.6%, respectively.
Patients diagnosed with prostate cancer. (a) The MIP of FDG-PET. Primary tumors in the prostate can be visualized on CT (b) and FDG PET/CT (c). Lymph nodes on the metastatic side of the iliac arteries can be seen on CT (d) and FDG PET/CT (e). Pubic bone metastases can be seen on CT (f) and FDG PET/CT (g)
5.4.3.3 18F-FDG-PET/CT for Restaging of Prostate Cancers
Many clinical studies have assessed the use of FDG-PET/CT for prostate cancer re-segmentation. Most of these studies compared FDG to another PET tracer. Garcia et al. [18] studied 38 patients and found that FDG-PET/CT diagnosed recurrent lesions with a sensitivity of 34% and 11C-choline PET/CT showed a sensitivity of 68%. Therefore, the researchers concluded that FDG-PET/CT has limited value for prostate cancer re-segmentation. However, a study by the National Cancer PET Registry is worth mentioning in this context. This study used scans for pathologically confirmed cancer patients to determine the stage or suspected recurrence (Fig. 5.11). A total of 40,863 FDG-PET and FDG-PET/CT scans were included, of which 5309 were performed in men with prostate cancer (i.e., 2042 for initial staging, 1477 for re-segmentation, and 1790 for suspected recurrence) [19]. These scans resulted in an expected cancer treatment change in 35.1% of cases: 25.3% from untreated to treatment and 9.7% from treatment to untreated. The rates of staging, reclassification, or detection of subgroup changes in treatment plans due to suspected recurrence were 32.0%, 34.0%, and 39.4%, respectively.
5.4.3.4 18F-FDG-PET/CT to Determine the Prognosis of Prostate Cancers
One study explored the value of FDG-PET/CT in determining the prognosis of prostate cancer. The study was published in 2013 by Jadvar et al. and included 87 patients with metastatic prostate cancer after castration and antiandrogen therapy [20]. The median follow-up period was 22.2 months, and 61 patients died. The authors tested the SUVmax values by univariate and multivariate Cox regression analyses of continuous PET parameters adjusted for the standard clinical parameters (age, serum PSA levels, alkaline phosphatase levels, analgesics, previous chemotherapy, and Gleason score at initial diagnosis). In their study, the sum of the SUVmax and average SUVmax influenced the OS, and the sum of the SUVmax values of all metabolically active lesions (after deducting patient-specific background—the average SUV of the liver) provided independent prognostic information for the OS of metastatic prostate cancer in castrated men. This information may be useful in assessing various traditions and the effectiveness of emerging treatment strategies.
5.5 Summary
At present, FDG-PET/CT is not the main imaging method for diagnosing urinary system tumors, mainly because of its low sensitivity, which may be caused by disturbances in physiological excretion and the low level of tumor glucose metabolism in these tumor cells. In kidney cancer, FDG-PET/CT may play a new role in the selection of surgery and targeted therapy during the initiation and follow-up periods in TKI treatment. In BC, FDG-PET/CT can be used for staging and re-segmentation, and its clinical value in prostate cancer remains doubtful. With regard to the three main types of cancers of the urinary system, there is a need to study the potential of FDG or other tracers in PET/CT more systematically.
References
Apolo AB, Riches J, Schoder H, Akin O, Trout A, Milowsky MI, Bajorin DF (2010) Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol 28(25):3973–3978
Avery R, Kuo PH (2013) 18F sodium fluoride PET/CT detects osseous metastases from breast cancer missed on FDG PET/CT with marrow rebound. Clin Nucl Med 38(9):746–748
Bouchelouche K (2012) PET/CT and MRI in bladder cancer. J Cancer Sci Ther s14(1):7692–7709
Caldarella C, Muoio B, Isgro MA, Porfiri E, Treglia G, Giovanella L (2014) The role of fluorine-18-fluorodeoxyglucose positron emission tomography in evaluating the response to tyrosine-kinase inhibitors in patients with metastatic primary renal cell carcinoma. Radiol Oncol 48(3):219–227
Ferda J, Ferdova E, Hora M, Hes O, Finek J, Topolcan O, Kreuzberg B (2013) 18F-FDG-PET/CT in potentially advanced renal cell carcinoma: a role in treatment decisions and prognosis estimation. Anticancer Res 33(6):2665–2672
Garcia JR, Soler M, Blanch MA, Ramirez I, Riera E, Lozano P, Perez X, Delgado E, Carrio I, Lomena F (2009) [PET/CT with (11)C-choline and (18)F-FDG in patients with elevated PSA after radical treatment of a prostate cancer]. Rev Esp Med Nucl 28(3):95–100
Hillner BE, Siegel BA, Shields AF, Liu D, Gareen IF, Hunt E, Coleman RE (2008) Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the national oncologic PET registry. J Nucl Med 49(12):1928–1935
Sahni VA, Silverman SG (2014) Imaging management of incidentally detected small renal masses. Semin Intervent Radiol 31(1):9–19
Jadvar H, Quan V, Henderson RW, Conti PS (2008) [F-18]-Fluorodeoxyglucose PET and PET-CT in diagnostic imaging evaluation of locally recurrent and metastatic bladder transitional cell carcinoma. Int J Clin Oncol 13(1):42–47
Lu YY, Chen JH, Liang JA, Wang HY, Lin CC, Lin WY, Kao CH (2012) Clinical value of FDG PET or PET/CT in urinary bladder cancer: a systemic review and meta-analysis. Eur J Radiol 81(9):2411–2416
Jadvar H, Ye W, Groshen S, Cibtut PS (2008) [F-18]-fluorodeoxyglucose PET-CT of the normal prostate gland. Ann Nucl Med 22:787–793
Minamimoto R, Uemura H, Sano F, Terao H, Nagashima Y, Yamanaka S, Shizukuishi K, Tateishi U, Kubota Y, Inoue T (2011) The potential of FDG-PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med 25(1):21–27
Minamimoto R, Uemura H, Sano F, Terao H, Nagashima Y, Yamanaka S, Shizukuishi K, Tateishi U, Kubota Y, Inoue T (2011) The potential of FDG-PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med 25(1):21–27
Minamimoto R, Senda M, Jinnouchi S, Terauchi T, Yoshida T, Murano T, Fukuda H, Iinuma T, Uno K, Nishizawa S, Tsukamoto E, Iwata H, Inoue T, Oguchi K, Nakashima R, Inoue T (2013) The current status of an FDG-PET cancer screening program in Japan, based on a 4-year (2006-2009) nationwide survey. Ann Nucl Med 27(1):46–57
Tiwari BP, Jangra S, Nair N, Tongaonkar HB, Basu S (2010) Complimentary role of FDG-PET imaging and skeletal scintigraphy in the evaluation of patients of prostate carcinoma. Indian J Cancer 47(4):385–390
Yu EY, Muzi M, Hackenbracht JA, Rezvani BB, Link JM, Montgomery RB, Higano CS, Eary JF, Mankoff DA (2011) C11-acetate and F-18 FDG PET for men with prostate cancer bone metastases: relative findings and response to therapy. Clin Nucl Med 36(3):192–198
Damle NA, Bal C, Bandopadhyaya GP, Kumar L, Kumar P, Malhotra A, Lata S (2013) The role of 18F-fluoride PET-CT in the detection of bone metastases in patients with breast, lung and prostate carcinoma: a comparison with FDG PET/CT and 99mTc-MDP bone scan. Jpn J Radiol 31(4):262–269
Wang N, Jiang P, Lu Y (2014) Is fluorine-18 fluorodeoxyglucose positron emission tomography useful for detecting bladder lesions? A meta-analysis of the literature. Urol Int 92(2):143–149
Wettersten HI, Aboud OA, Lara PJ, Weiss RH (2017) Metabolic reprogramming in clear cell renal cell carcinoma. Nat Rev Nephrol 13(7):410–419
Jadvar H, Desai B, Ji L, Conti PS, Dorff TB, Groshen SG, Pinski JK, Quinn DI (2013) Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med 54(8):1195–1201
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd. and Shanghai Jiao Tong University Press
About this chapter
Cite this chapter
Zhou, X., Huang, G. (2019). Clinical Utility of 18F-FDG PET/CT Scanning in Urological Cancers Management. In: Huang, G. (eds) Nuclear Medicine in Oncology. Springer, Singapore. https://doi.org/10.1007/978-981-13-7458-6_5
Download citation
DOI: https://doi.org/10.1007/978-981-13-7458-6_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7457-9
Online ISBN: 978-981-13-7458-6
eBook Packages: MedicineMedicine (R0)