Abstract
Curcumin (CUR) is the bio-active agent found in turmeric and has been used for centuries as a flavouring and colouring agent in the Southeast Asian cuisine. It possesses a wide range of biological activities, such as anti-inflammatory, antioxidant, antimicrobial, antirheumatic and anticancer characteristics. CUR is a desirable anticancer agent as it selectively induces apoptosis in cancer cells without affecting healthy cells. It has been shown to directly interfere with numerous signalling molecules involved in the growth, promotion and angiogenesis of cancer cells. Furthermore, the consumption of large amounts of CUR of up to 12 g per day has demonstrated acceptable tolerance and safety for human consumption. However, the in vivo application of CUR is limited by its low water solubility, stability and bioavailability. The encapsulation of CUR in nano-formulations has been shown to improve the efficacy of CUR as an anticancer agent by increasing its solubility in aqueous media, prolonging in vivo circulation time and decreasing the rate of degradation. Nano-formulations open a door for developing CUR as an anticancer drug with minimal side effects. In the present chapter, anticancer efficacy of various CUR nano-formulations, such as polymeric nanoparticles, polymeric micelles, liposomes, dendrimers, solid lipid nanoparticles, protein-based nanoparticles, mesoporous silica nanoparticles, inorganic nanoparticles and magnetic nanoparticles has been discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Curcumin (CUR) is a natural polyphenolic compound derived from Curcuma longa Linn., rhizomes found in the tropical regions of Asia. As a powder, it is referred to as turmeric and has been most commonly used for imparting colour and flavour in Southeast Asian cuisine (Debnath et al. 2013). Turmeric has also been used as folk medicine in both traditional Indian and Chinese medicinal therapies for over 2000 years for various diseases due to its extensive range of pharmacological activities such as anti-inflammatory, antioxidant, antimicrobial, antirheumatic and anticancer effects (Verderio et al. 2013; Yoon et al. 2015; Zeighamian et al. 2016). Turmeric powder is made of approximately 2–6% (w/w) of curcuminoids, which consists mostly of CUR, followed by demethoxy CUR(<20%) and bis-demethoxy CUR (2%). Miłobȩdzka et al. (1910) first determined the chemical structure of CUR as diferuloylmethane or 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) (Fig. 7.1).
Cancer cells arise from genetic and epigenetic mutations as well as disruptions in cell cycle resulting in the dysregulation of multiple cellular pathways involved in the regulation of cell proliferation (Stefanska et al. 2012). Experimental studies have shown that CUR has the ability to modulate various signal transduction pathways and molecular targets that have been implicated in cancer development (López-Lázaro 2008). Various studies have reported that CUR both alone and in combination with other drugs can play a role in preventing and treating various forms of cancer such as breast (Liu et al. 2013; Farhangi et al. 2015), prostate (Ide et al. 2010; Kakarala et al. 2010), pancreatic (Durgaprasad et al. 2005; Kanai 2014), colon (Carroll et al. 2011; He et al. 2011) and lungs cancers (Gupta et al. 2013). Preclinical studies in animal models of carcinogenesis have shown that CUR has the potential to inhibit tumour formation (Gou et al. 2011; Dhule et al. 2012; Alizadeh et al. 2015; Bisht et al. 2016; Zaman et al. 2016). CUR is also a desirable anticancer agent in comparison to other conventional anticancer agents due to its ability to induce apoptosis in cancer cells without affecting healthy cells (Verderio et al. 2013). Furthermore, clinical studies by Lao and colleagues reported that the consumption of large amounts of CUR of up to 12 g per day was well tolerated and considered safe with no adverse effects (Lao et al. 2006), therefore indicating that CUR is safe and tolerable for human consumption with low intrinsic toxicity at high doses. In 2012, it was estimated that approximately 1.67 million new cases of breast cancer were diagnosed worldwide, claiming over 500,000 deaths and making it the main cause of cancer-related deaths in women (Smith et al. 2016). Various in vitro and in vivo studies have demonstrated CUR’s ability to interfere with breast carcinogenesis in three stages: tumour growth, promotion and angiogenesis. The biological activities of CUR on signalling pathways in breast cancer cells have been summarized in Fig. 7.2. To date, there is only one published Phase I clinical trial by Bayet-Robert et al. (2010) who explored the combinational use of docetaxel (DTX) in combination with CUR in patients diagnosed with advanced metastatic breast cancer. DTX is a microtubule-targeting agent that arrests cells at their G2/M transition, disrupting cell division resulting in cellular toxicity. Patients were administered with a 1 h intravenous infusion of DTX (100 mg/m2) every 3 weeks for six cycles. CUR was orally administered starting from a dose of 500 mg/day for 7 consecutive days by cycle with a dose escalation until a point that dose-limiting toxicity occurred. The study found that the recommended dose of 6000 mg/day of CUR for 7 consecutive days every 3 weeks in combination with the standard DTX dose of 100 mg/m2 is required for anticancer activity. Both DTX and CUR were reported to inhibit angiogenesis in vitro and in vivo via suppression of VEGF levels. This clinical trial showed that this combination of anti-cancer therapy has the potential to significantly reduce VEGF levels resulting in the possible reduction of tumour size and metastasis in breast cancer patients. Phase II randomized clinical trials on this combination in advanced and metastatic breast cancer patients are currently being carried out to confirm the efficacy of this combination. Despite showing promising characteristics as an anticancer agent in breast cancer, a major problem with CUR is its poor solubility in an aqueous environment and instability in an alkaline environment which restricts its clinical efficacy (Yoon et al. 2015; Khosropanah et al. 2016). In vivo studies have previously reported that the compound exhibits poor absorption and undergoes rapid metabolism and elimination resulting in poor bioavailability (Suwannateep et al. 2011). Yang et al. (2007) reported that administration of 10 mg/kg of CUR intravenously in rats achieved the maximum plasma concentration (0.36 μg/ml), due to its poor solubility.
To overcome these limitations, scientists have adopted various approaches and strategies to enhance the bioavailability of CUR, including the fabrication of nanoparticles (NP). Nanotechnology is a rapidly developing field especially in drug delivery as it overcomes problems associated with the delivery of hydrophobic drugs. In recent years, numerous nano-formulations of CUR have been prepared in the form of, but not limited to, polymeric NP, liposomes, dendrimers and solid lipid NP (SLN) in an attempt to improve the efficacy of CUR as an anticancer agent (Fig. 7.3). These nano-formulations enhance CUR’s anticancer properties by improving its solubility in an aqueous media, prolonging in vivo circulation time and decreasing the rate of degradation. A comparative study of nanoCUR (theracurmin) and CUR powder by Sasaki and colleagues in humans found that nanoCUR brought about a 27-fold higher blood level of CUR in comparison to native CUR powder, indicating that the nano-formulation significantly enhanced CUR solubility (Sasaki et al. 2011). Besides enhancing the solubility of poorly soluble drugs, NP are also beneficial in drug delivery by directing anticancer agents towards the tumour through passive targeting NP that range around ~200 nm in size have been shown to be subjected to the enhanced permeation and retention (EPR) effect when administered intravenously into the bloodstream (Bertrand et al. 2014; Nikpoor et al. 2015). This phenomenon occurs as when a solid tumour reaches a certain size, the delivery of oxygen and nutrients by simple diffusion becomes insufficient for tumour cell proliferation, and the tumour becomes diffusion limited. To overcome this, the tumour undergoes the process of angiogenesis, the formation of new blood vessels which occurs under a strict control of molecular factors. However, in tumour cells, this process is abnormal due to an imbalance of molecular factors influencing the process of angiogenesis (Bertrand et al. 2014). As a result, the basal membrane of the formed blood vessels is either discontinuous or absent, thus resulting in irregular capillaries with large fenestrations ranging from 200 to 2000 nm in size. Therefore, administration of NP which are smaller than the fenestration and larger than the tight endothelial junctions of normal capillaries may result in the extravasation of the NP through the fenestration and accumulation within the tumour interstitium. This phenomenon denotes the enhanced permeation portion of the EPR effect. At the same time, tumours also lack well-defined lymphatic drainage as a consequence of the rapidly proliferating tumour cells (Baeza et al. 2016). Therefore, NP that extravasate out into the tumour interstitium tend to be retained, representing the enhanced retention portion of the EPR effect. The combined effect of leaky vasculature and poor drainage comprises the EPR effect, which leads to preferential accumulation of NP at the tumour site over time. Apart from passive targeting through the EPR effect, active targeting has been widely studied as certain receptors have been found to be up-regulated on the surface of tumour cells. During angiogenesis, various receptors have been reported to be up-regulated on the surface of tumour tissues in comparison to normal healthy tissues to accommodate for the enhanced rate of cell proliferation. The concept of active targeting involves conjugation of cell-specific or tissue-specific ligands to the surface of the NP. In turn, this allows for the preferential accumulation of NP into the desired target site. In the present chapter, anticancer efficacy of various CUR nano-formulations such as polymeric NP, polymeric micelles, liposomes, dendrimers, solid lipid NP, protein-based NP, mesoporous silica NP, inorganic NP and magnetic NP has been discussed.
7.2 CUR Nano-formulations
These are of the following types.
7.2.1 Polymeric Nanoparticles
For over 40 years, polymeric NP have been the interest of many research groups due to its favourable properties such as biodegradability, biocompatibility, non-toxicity, prolonged circulation and wide payload spectrum of therapeutic agents (Duan et al. 2012; Yoon et al. 2015). Polymeric NP are solid colloidal particles with sizes ranging from 1 to 1000 nm and are desired carriers in the field of anti-cancer therapy due to their ability to control the release of drug molecules at selective target sites. Polymeric NP can be easily produced through various techniques such as solvent evaporation, salting out, dialysis, supercritical fluid technology, microemulsion, mini-emulsion, surfactant-free emulsion and interfacial polymerization (Jawahar and Meyyanathan 2012).
7.2.1.1 Curcumin-Loaded Polymeric Nanoparticles
One of the most researched and commonly utilized polymeric nano-formulation is poly(lactic-co-glycolide) (PLGA) NP due to their biocompatibility, biodegradability, in vivo stability as well as particle diameter which gives them the ability to accumulate within the tumour environment via the EPR mechanism (Verderio et al. 2013; Yoon et al. 2015). Yallapu et al. (2010) fabricated CUR-PLGA-NP to improve the therapeutic effects of CUR. Studies performed in MDA-MB-231 cells displayed enhanced accumulation in cancer cells with dose-dependent cytotoxic effects through apoptosis in comparison to free CUR (in DMSO). Similarly, Verderio et al. (2013) synthesized CUR-loaded PLGA-NP (CUR-PLGA-NP) through a single-emulsion process. The CUR-PLGA-NP were found to release CUR following Fickian-law diffusion and suppressed the proliferation of cells in a time- and dose-dependent manner through specific G2/M phase block (Verderio et al. 2013). Yoon et al. (2015) also formulated PLGA-NP for the intravenous delivery of CUR. In vitro anticancer efficacy of the CUR-PLGA-NP in MDA-MB-231 cells was found to be comparable to that of free CUR. The authors postulated that the similarity was attributed to the presence of different cellular uptake mechanisms for CUR-PLGA-NP and free CUR; CUR-PLGA-NP are expected to enter cells via endocytosis, whereas free CUR enters cells via simple passive diffusion. As CUR is gradually released from the PLGA-NP, the anticipated higher cytotoxicity in comparison to CUR solution was not seen. In vivo studies in rats showed that CUR-PLGA-NP had prolonged circulation in the bloodstream in comparison to CUR in solution. Thus, they deduced that CUR-PLGA-NP would be more cytotoxic towards cancer cells due to the prolonged systemic exposure and pH-dependent release capability (Yoon et al. 2015). However, further in vivo studies on the suppression of tumour size are required to confirm their hypothesis.
A major biological obstacle in the delivery of NP is the recognition of hydrophobic particles by the reticuloendothelial system (RES), which effectively removes NP from the bloodstream to be taken up by the liver and spleen (Tabatabaei Mirakabad et al. 2016). To overcome this limitation, NP are commonly modified by coating the surface of the particles with hydrophilic molecules to render the particles invisible to the RES. The most common hydrophilic molecule used for surface modification is non-ionic polymer, polyethylene glycol (PEG). In in vitro studies, PEG surface-modified PLGA-NP encapsulating CUR (CUR-PLGA-PEG-NP) exhibited a greater cytotoxic effect in MCF-7 cells in comparison to free CUR (in methanol) (Tabatabaei Mirakabad et al. 2016).
Metastasis involves the migration of cancer cells through the lymphatic system or vascular compartment from the primary malignant site to a distant organ followed by the generation of a secondary tumour (Palange et al. 2014). These circulating tumour cells are postulated to overexpress certain receptor molecules that are easily recognized by counter-molecules found on endothelial cells in distant organs, thereby allowing them to be captured from the bloodstream through the stable formation of cellular molecular bonds (Palange et al. 2014). The vascular docking of circulating tumour cells has also been associated with certain adhesion molecules associated with vascular inflammation such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion protein 1 (VCAM-1) (Kawai et al. 2008; Liang and Dong 2008). CUR-loaded NP comprising of PLGA and mixture of lipids (1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phospho ethanolamine-N-[succinyl(PEG)-2000 (DSPE-PEG)) were synthesized by Palange et al. (2014). Confocal microscopy analysis of MDA-MB-231 cells exposed to CUR-loaded NP had shown CUR to be distributed uniformly within the cytosol. Upon internalization of CUR-loaded NP into the cells, CUR is released through the gradual degradation of the PLGA matrix in the lysosomal environment. In comparison to free CUR, CUR-NP were found to be more cytotoxic to MDA-MB-231 cells. The effect of CUR on the vascular adhesion of circulating tumour cells was also investigated. MDA-MB-231 cells treated with 10 μM of CUR-loaded NP and 10 μM of free CUR exhibited a reduction in cancer cell vascular adhesion propensity by 70 and 50%, respectively. Immunohistochemistry analysis of the cancer cells displayed the presence of Mucin1 molecule, which has been reported to be involved in the adhesion process of tumour cells to vascular walls. Overall, the prepared CUR-loaded NP showed good potential to interfere with the cell adhesion process which plays a critical role in the metastatic cascade.
Raja et al. (2016) fabricated CUR-loaded oleate alginate ester (OAE) NP through a simple sonication method to investigate the cell uptake and cytotoxicity of the NP. In vitro studies in MCF-7 cells found that cell uptake of CUR from CUR-OAE-NP was both time- and concentration-dependent, and the NP displayed a sustained cytotoxic effect on the cells. Similarly, several other studies that have displayed enhanced suppression of cancer cell proliferation by CUR-loaded polymeric NP which include CUR-loaded PEG-poly(lactic acid) (PLA) nanospheres (Liang et al. 2017) in MDA-MB-231 and dipolymeric ethylcellulose and methylcellulose nanospheres (Suwannateep et al. 2011) and poly(N-isopropylacrylamide-co-methacrylic acid) (PNIPAAm-MAA) NP (Zeighamian et al. 2016) in MCF-7 cells.
Chun et al. (2012) formulated CUR polymer NP using N-isopropyl acrylamide, vinylpyrrolidone and acrylic acid through a free-radical reaction. The aim of the study was to determine if CUR administered intraductally would have the ability to reduce the incidence of mammary tumour following chemical carcinogenesis by M-methyl-N-nitrosourea (MNU). Treatment of rats post-MNU exposure with CUR-NP and free CUR (in corn oil) revealed that the CUR-NP at a dose of 2 mg CUR significantly reduced the incidence of mammary tumours in comparison to free CUR, with protection effects comparable to that of rats administered with 30–40 mg of CUR orally. An alternative type of polymeric NP involves the use of polyelectrolytes which contain ionizable groups in its polymer structure, enabling them to either partially or completely dissociate in aqueous solution. In comparison to neutral polymers, which typically have random coil formations, polyelectrolytes are more stretched out due to the presence of charges which cause repulsion of the chains (Sarika and James 2016). A polyelectrolyte complex carrier was formulated from cationically modified gelatin (CG) and sodium alginate (Alg) for the delivery of CUR (Sarika and James 2016). They performed a comparative study of the anticancer activity of free CUR (in DMSO) and CUR-CG/Alg-NP in MCF-7 cells and reported that at a CUR dose of 50 μg/ml, CUR-CG/Alg-NP showed comparable toxicity to free CUR. At lower doses of 12.5μg/ml, free CUR was shown to have greater cytotoxicity than CUR-CG/Alg-NP due to the high solubility of CUR in DMSO, allowing it to be fully available to the cells to cause severe cytotoxicity. Another well-known polymer that has been widely used in the field of biomedical sciences is chitosan (CS), a naturally occurring polymer derived from the shells of crustaceans. An advantage of using CS in the formulation of polymeric NP is its ability to undergo phase transition in response to external stimuli such as temperature. Rejinold et al. (2011) formulated a thermo-responsive CS-g-poly(N-isopropylacrylamide) NP (TRCS-NP) for the delivery of CUR to cancer cells. Cytotoxicity studies in MCF-7 cells showed that CUR-TRCS-NP exhibited specific dose-dependent toxicity to cancer cells through apoptotic cell death, while empty TRCS-NP showed no significant cytotoxicity.
7.2.1.2 Synergistic Curcumin Polymeric Nanoparticle Combinations
Synergistic relationships of CUR with well-established anticancer agents have been shown to exhibit enhanced efficacy in the treatment of breast cancer. An example of an established anticancer agent used for the treatment of breast cancer is doxorubicin (DOX). The therapeutic efficacy of DOX in certain breast cancer subtypes is limited due to the active efflux of drug molecules by transporters found on cancer cells, leading to multi-drug resistance (MDR) (Duan et al. 2012). On the other hand, CUR has been reported to cause the downregulation of intracellular-level efflux transporters such as P-gp, MDR-associated protein 1 and mitoxantrone resistance protein (ABCG2). Therefore, to overcome this phenomenon, Duan et al. (2012) have formulated poly(butyl cyanoacrylate) (PBCA) NP co-encapsulating CUR and DOX (CUR+DOX-PBCA-NP). In vitro studies showed that co-encapsulation of DOX and CUR achieved the highest cytotoxicity with downregulation of P-gp in MCF-7 cells resistant to Adriamycin (MCF-7/ADR) in comparison to either free drug combination or one free drug/another agent-loaded combination (Duan et al. 2012). Similarly, Guo et al. (2014) synthesized NP consisting of a CUR-loaded poly(L-lactide) (PLLA) core and heparin shell adsorbed with DOX. Cell studies on 4 T1 cells demonstrated enhanced cellular uptake and cytotoxicity in comparison to the combination of drugs in solution or drug alone. Furthermore, the co-delivery of CUR and DOX in NP also inhibited the growth of tumour and prolonged the survival of mice inoculated with 4 T1 cells. Another potent anticancer agent, docetaxel (DTX), is similar to DOX, whereby its clinical success is also limited by MDR. Co-encapsulation of DTX with CUR has been shown to be able to overcome this problem through the downregulation of P-gp and MDR protein expression (Pawar et al. 2016). They fabricated a DTX+CUR-PLGA-NP via a modified emulsion solvent evaporation technique. DTX+CUR-PLGA-NP showed enhanced cellular uptake in MCF-7 cells with an initial burst release of DTX and CUR followed by a sustained release for 5 days. DTX+CUR-PLGA-NP also showed a reduction in haemolytic toxicity in comparison with commercial DTX intravenous injection, Taxotere®. When administered in rats, DTX+CUR-PLGA-NP showed prolonged residence time in the systemic blood circulation, therefore allowing for a longer duration of action and reduction in dose and frequency of administration. The co-encapsulation of DTX with CUR may also aid in overcoming MDR associated with DTX through the reduction of P-gp protein expression, allowing for a safe plasma concentration of DTX to be achieved.
7.2.2 Polymeric Micelles
Polymeric micelles consist of a lipid-soluble hydrophobic core and an external water-soluble hydrophilic surface. During the formulation process, amphiphilic block copolymers spontaneously form a self-assembled micellar structure in an aqueous environment at critical micellar concentration (CMC) (Guzzarlamudi et al. 2016). The macromolecules in contact with both the inner hydrophobic region and outer hydrophilic regions are made up of distinct block domains. Upon exposure to an aqueous environment, the block copolymers aggregate to form an entropically favoured supramolecular assembly (Biswas et al. 2016). Polymeric micelles are advantageous because their hydrophobic core allows for the solubilization of hydrophobic drug molecule, while their hydrophilic segments allow for the compatibility with an aqueous environment (Guzzarlamudi et al. 2016). Furthermore, size manipulation of size of polymeric micelles allows for the EPR mechanism, thereby resulting in enhanced accumulation within tumour sites. Some common methods used for the preparation of polymeric micelles include oil/water emulsion, water/oil/water emulsion, dialysis, co-solvent evaporation and lyophilization (Miller et al. 2013).
7.2.2.1 Curcumin-Loaded Polymeric Micelles
Liu et al. (2013) performed a comparative study to evaluate the anticancer activity of free CUR and CUR-loaded methoxy PEG-poly(ɜ-caprolactone) (mPEG-PCL) copolymer micelles (Liu et al. 2013). The formulated CUR-mPEG-PCL micelles were found to inhibit the growth of 4 T1 cells more significantly in comparison to free CUR in vitro. The authors also evaluated the inhibition of tumour growth and spontaneous pulmonary metastasis of subcutaneous 4 T1 cells in vivo. The mice were administered CUR-mPEG-PCL micelles and free CUR, each at 30 mg/kg body weight, and 100 μL blank mPEG-PCL micelles for a span of 10 days. While blank mPEG-PCL micelles showed no signs of tumour growth inhibition, CUR-mPEG-PCL micelles were found to inhibit tumour growth more efficiently, resulting in significantly lower tumour weight (0.97 ± 0.29 g) in comparison to the group treated with free CUR (2.15 ± 0.60 g). To determine the pro-apoptotic potential of CUR-mPEG-PCL micelles, immunofluorescent TUNEL staining assay was carried out, and a higher number of apoptotic cells in tumour tissues were observed in the CUR-mPEG-PCL micelles treated group. Following an immunofluorescence CD31 staining assay to analyse 4 T1 tumour angiogenesis, CUR-mPEG-PCL micelles treated cells displayed the least immunoreactive microvessels in tumour tissues among all formulations. Immunohistochemical staining of tumours also showed weak Ki-67 immunoreactivity in CUR-mPEG-PCL micelles treated group, indicating a reduction in tumour cell proliferation. The results of the study indicate that CUR-mPEG-PCL micelles have the potential to induce apoptosis, inhibit tumour angiogenesis and suppress tumour cell proliferation, which are all essential components of the metastatic cascade. Yu et al. (2014) constructed a multistage drug delivery system for CUR by synthesizing a series of amphiphilic and pH-sensitive mPEG-PLA-PAE copolymers. The CUR-mPEG-PLA-PAE micelles were found to be cytotoxic towards MCF-7 cells in vitro. Interestingly, when exposed to weakly acidic pH of the tumour microenvironment, the drop in pH caused protonation of PAE, resulting in an increase in the surface charge as well as shrinking of the micelles size, allowing for enhanced cellular uptake and prolonged circulation time and accumulation at tumours sites (Yu et al. 2014). The results of the in vivo studies correlated with that of the in vitro studies, with greater accumulation and uptake of CUR-mPEG-PLA-PAE micelles by the tumour cells and enhanced tumour growth suppression.
Alizadeh et al. (2015) synthesized a diblock copolymer micelles encapsulating CUR from the esterification of oleoyl chloride and mPEG 2000 for the delivery of CUR in vitro and in vivo. The CUR-loaded micelles suppressed the cell proliferation of 4 T1 cells in vitro and significantly suppressed the growth of tumour in vivo. Immunohistochemistry studies further revealed that breast tumours of mice treated with CUR-loaded micelles had an increase in Bax (pro-apoptotic) protein expression in comparison with the control group treated with normal saline. A reduction in Bcl-2 (anti-apoptotic) protein expression and activity was also observed in the CUR-loaded micelle-treated group.
In the pursuit of fabricating an alternative treatment from the existing breast cancer treatment, Guzzarlamudi et al. (2016) synthesized CUR-loaded mPEG and linoleic acid conjugated (Cla) polymeric micelles via dialysis method. Cla, most commonly found in dairy products, are a family of stereo and positional isomers of linoleic acid that have been shown to affect the growth of mammary tumours in mice (Visonneau et al. 1997; Wong et al. 1997). They also inhibit the proliferation of oestrogen receptor (ER)-positive MCF-7 cells by interfering with the hormone-regulated mitogenic pathway (Durgam and Fernandes 1997). This formulation not only overcomes the solubility issue associated with the delivery of CUR but also presents a synergistic effect with Cla against MCF-7 cells. Cell viability studies found that CUR-mPEG-Cla micelles were 5.83- and 1.34-fold more cytotoxic than free CUR (in PBS) and blank PM, respectively. This enhanced cytotoxicity was attributed to the synergistic effect of CUR with Cla. Cell cycle arrest studies found that the highest percentage of cells treated with CUR-mPEG-Cla micelles was in the G1 phase. Flow cytometry carried out 36 h postexposure to blank PM, free CUR and CUR-mPEG-Cla micelles revealed 2.54, 7.46 and 11.74% of cells in early apoptosis phase, respectively, and 0.46, 0.30 and 9.22% in late state apoptosis phase, respectively. This implies that CUR-mPEG-Cla micelles significantly improved CUR-dependent apoptosis in MCF-7 cells. Pharmacokinetics studies in rats showed enhanced biodistribution and half-life of CUR-mPEG-Cla micelles in vivo in comparison to free CUR. The improved retention time of the CUR-mPEG-Cla micelles as a result of micellar structure also allowed controlled release of CUR from the shell. Furthermore, the presence of PEG may have also aided in the avoidance of the RES, thereby improving the circulation time. Cai et al. (2016) synthesized pluronic P123-poly(β-amino ester) (P123-PAE) micelles encapsulating CUR and found that the CUR-P123-PAE micelles exhibited similar anticancer effects against MCF-7 cells compared to CUR in solution as a result of the sustained release of CUR from the NP. The P123-PAE micelles had significantly prolonged the retention time of CUR in vivo. However, further studies are warranted to determine if the P123-PAE micelles have superior anticancer activity in comparison to CUR solution in vivo.
Alternatively, Kumar et al. (2014) formulated a polymer-/lipid-based NP consisting of polyhydroxyethylmethacrylate (PHEMA)/stearic acid (SA) via the emulsification-solvent evaporation method. SA is a non-toxic, biocompatible fatty acid. The interaction of SA with hydrophilic polymer, PHEMA, during the assembly of NP has been shown to exhibit good physical and chemical stability, providing protection of the encapsulated drug against degradation. The authors constructed PHEMA-SA-NP encapsulating CUR for the evaluation of efficacy in MCF-7 cell in vitro. CUR-PHEMA-SA micelles and CUR alone (in PBS) had IC50 values of 7 μg/mL and 10.72 μg/mL, respectively, indicating a better cellular uptake and enhanced efficacy of the CUR-PHEMA-SA micelles in MCF-7 cells. Cell cycle analysis reported a higher percentage of cell in the G1 phase when treated with CUR-PHEMA-SA micelles. The results were in agreement with an earlier study carried out by Srivastava et al. (2007). Apoptosis analysis of the cells was also carried out using flow cytometry; the cells treated with CUR-PHEMA-SA micelles had a higher number of cells in the necrotic and late apoptotic stages than CUR-treated and untreated cells. Collectively, the studies show that CUR-PHEMA-SA micelles had higher localization into cells with greater apoptotic activity in comparison to CUR alone in the MCF-7 cell line. Alternatively, Lee et al. (2015) engineered monodispersed CUR micellar NP coated with polyvinyl alcohol (PVA) of varying sizes for the qualitative and quantitative studies on cellular uptake to determine the effect of size on potency of the NP as an anticancer agent. While the study showed that the CUR-NP was not as potent against MCF-7 cells in comparison to melanoma cancer MM96L and bone osteosarcoma MG-63 cells, the CUR micelles still had a 2.53-fold times lower IC50 with higher cellular uptake than CUR alone (in DMSO). The study also found the cellular uptake to be size dependent with smaller CUR micelles demonstrating greater internalization through endocytosis in the following order: 205, 106 and 28 nm.
7.2.2.2 Synergistic Curcumin Polymeric Micelles Combinations
As previously mentioned, P-gp-mediated drug efflux poses as an obstacle in anticancer therapy of certain cancer types. To improve the anticancer efficacy of DOX, Wang et al. (2015) fabricated D-α-tocopherol polyethylene glycol succinate and (TPGS) 2000 and PEG 2000-DSPE polymeric micelles for the co-delivery of DOX and CUR. While both DOX+CUR micelles and DOX micelles alone showed significant cytotoxicity in vitro, DOX+CUR micelles showed the highest cell growth inhibition in MCF-7 cells. Cellular uptake studies carried out in MCF-7/ADR cells overexpressing P-gp showed that DOX+CUR micelles significantly improved cellular internalization of DOX in comparison to DOX micelles and DOX micelles administered with CUR in solution. The authors concluded that DOX+CUR micelles reversed MDR through two pathways. Firstly, upon endocytosis of the micelles, P-gp efflux pump is inhibited by TPGS 2000, thereby improving DOX uptake. This is followed by the simultaneous inhibition of P-gp by CUR released from DOX+CUR micelles, which further reduces the efflux of DOX. DOX+CUR micelles were also shown to have greater tumour growth inhibitory effects in 4 T1 tumours in vivo in comparison to DOX and CUR in solution or DOX micelles, therefore indicating that the co-administration of DOX+CUR micelles can achieve a synergistic therapeutic effect in anticancer therapy.
7.2.3 Dendrimers
Analogous to polymers, dendrimers are repeating branched polymeric molecules which first emerged in 1978 (Dykes 2001). It was only in the early 1990s that dendrimers gained popularity, and their potential as a drug delivery system began to be the focus of many researchers. Dendrimers consist of three distinct parts: initiator core, branches and terminal functional groups attached to the outermost branching unit (Mollazade et al. 2013; Kesharwani et al. 2015a). Dendrimers are usually globular in shape and have sizes within the range of 1–100 nm (Kesharwani et al. 2015b). Poly(amidoamine) (PAMAM) dendrimer is the most frequently used and most studied dendrimer due to its widespread application in drug and gene delivery (Kesharwani et al. 2015b). PAMAM possesses highly functionalized terminal surface polyvalently displaying amine groups and has an exceptional degree of molecular uniformity in an aqueous environment (Debnath et al. 2013). Other than PAMAM’s good water solubility, which can enhance the solubility of poor water-soluble drugs, it also exploits the EPR effect due its larger size and the presence of leaky vasculatures in tumours.
7.2.3.1 Curcumin-Loaded Dendrimers
In the pursuit to enhance CUR delivery to breast cancer cells, Mollazade et al. (2013) fabricated CUR-loaded PAMAM dendrimers and carried out a comparative study with free CUR. Cytotoxicity studies revealed that CUR-loaded PAMAM had greater inhibition of T47D cell growth in comparison to free CUR (in DMSO) with IC50 values of 10.5 and 22.5 μM, respectively, at 24 h. It was hypothesized that CUR interferes with cancer cells via the suppression of telomerase activity, thereby suppressing cancer cell proliferation. The authors investigated the telomerase activity in the treated T47D cells and reported that both free CUR and CUR-loaded PAMAM exhibited concentration-dependent inhibition of telomerase activity. At same CUR concentrations, CUR-loaded PAMAM displayed greater inhibition implying that the PAMAM dendrimer has a profound effect on enhancing CUR’s potential in inhibiting telomerase activity. Similarly, Debnath et al. (2013) synthesized a PAMAM dendrimer-CUR conjugate to increase the water solubility and cytotoxic effects of CUR against breast cancer cell lines. Cell studies revealed a reduction of IC50 values by 3.77-fold and 10.8-fold in SKBr3 and BT549 cells through apoptosis in comparison to free CUR (in DMSO) as measured by caspase-3 activation, indicating enhanced cytotoxicity by the PAMAM dendrimer-CUR conjugate.
7.2.4 Lipid Vesicles
Lipid vesicles, best known as liposomes were discovered over 50 years ago and are made up of amphiphilic phospholipids molecules consisting of a hydrophilic head and a hydrophobic tail (Hasan et al. 2014). In an aqueous environment, the phospholipids self-associate to form an enclosed lipid bilayer membrane with an aqueous compartment (Nguyen et al. 2016). Based on their size and lamellarity, liposomes exist as multilamellar vesicles (500–5000 nm), large unilamellar vesicles (200–800 nm) and small unilamellar vesicles (~100 nm) (Fig. 7.4) (Maherani et al. 2011). Liposomes have been widely researched due to their biocompatibility, long circulating half-lives, ability to entrap both hydrophobic and hydrophilic drugs and ease of modification of their physical properties (size, zeta potential) through addition of new ingredients or method of preparation (Torchilin 2005). Common methods of preparing liposomes include vortexing or handshaking method, extrusion method, homogenization, ultrasonication, thin-film hydration method, injection method, reverse evaporation, gel exclusion chromatography and dialysis (Maherani et al. 2011).
7.2.4.1 Curcumin-Loaded Liposomes
Dhule et al. (2012) formulated nano-liposomes and CUR-2-hydroxypropyl-γ-cyclodextrin (HPγCD) nano-liposomes using phospholipids (1,2-dimyristoyl-sn-glycero-3-(phospho-rac-(1-glycerol)) (DMPG) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC)) via thin-film hydration method. The study reported that both nano-liposomes suppressed the cell viability of MCF-7 cells more significantly compared to non-liposomal formulations. It was reported that MCF-7 cells were most susceptible to the anticancer effects of CUR at a concentration range of 4–28 μg/ml. In another study, Hasan et al. (2014) formulated various nano-liposomes using lecithin from various sources via high-pressure homogenization. The CUR-loaded nano-liposomes formulated with lecithin showed dose-dependent cytotoxicity on MCF-7 cells at 12 and 20 mM. However, at 5 mM, no significant difference in cell viability was observed. Alternatively, Kangarlou et al. (2017) formulated CUR-loaded nano-liposomes from oleyl-peptide and lecithin via thin-film hydration method and conjugated the nano-liposomes with integrin-homing peptide and neuropilin-1 for targeted delivery and receptor-mediated internalization, respectively. In vitro cytotoxicity tests of CUR-loaded nano-liposomes on MCF-7 and MDA-MB-468 cells showed a significant reduction in cell viabilities in both cell lines with IC50 values of 3.8 μM and 5.4 μM, respectively. The higher IC50 values seen in MDA-MB-468 cells is due to its more aggressive nature with greater metastatic potential.
7.2.4.2 Curcumin-Loaded Dendrosomes
Analogous to liposomes, dendrosomes are an upcoming nano-formulation characterized by its spherical, amphipathic and biodegradable nature. Dendrosomes are liposomal systems containing an entrapped dendrimer-DNA complex for the enhancement of gene/DNA delivery (Babaei et al. 2012). Briefly, dendrimers interact with nucleic acids through electrostatic interactions forming dendriplexes, which are smaller in size than liposomes. Dendrimers condense nucleic acids, thereby enhancing DNA expression while also acting as an endosomal pH buffer (Movassaghian et al. 2011). However, Farhangi et al. (2015) formulated dendrosomal CUR (DNC) and reported significant time- and dose-dependent suppression of 4 T1 cells with IC50 values of 32.5, 25.0 and 17.5 μM after 24, 48 and 72 h, respectively. In contrast, the viability of the cells were reported to only be affected by free CUR (in acetone) at 72 h signifying enhanced cellular uptake of the DNC by the cancer cells. Scratch assay and adhesion assay studies also found DNC to cause inhibition of migration and adhesion of 4 T1 cells in vitro. Meanwhile, in vivo analysis in BALB/c mice dosed with 40 and 80 mg/kg of DNC for 35 days showed a significant reduction in size and weights of tumours as well as a reduction in metastasis incidences. Polymerase chain reaction (PCR) analysis revealed a reduction in NF-κβ p105 in 4 T1 breast tumours as well as a reduction in metastases in the lungs, liver, brain and spleen tissue, therefore indicating the potential of DNC as an anti-metastatic agent. The same group of authors continued their study on DNC by investigating the M1/M2 macrophage balance in the tumour microenvironment of metastatic 4 T1 cells (Shiri et al. 2015). M1 macrophages have a high capacity for antigen presentation and secretion of IL-12 (antitumour cytokine) that activates signal transducer and activator of transcription 4 (STAT4), a transcription factor involved in anticancer immune responses. In contrast, M2 macrophages are better known as tumour promoters as they do not present antigens or elicit immune responses. M2 macrophages secrete high levels of IL-10, resulting in the activation of STAT3, which are found to be up-regulated in a variety of tumours including breast cancer. To determine the role of these macrophages in the anticancer effects of CUR, the researchers administered 40 to 80 mg/kg of DNC into mice for 35 days, 3 days after the tumour injection, and euthanized the mice 42 days post cell injection. PCR analysis of the tumour microenvironment demonstrated that mice treated with DNC displayed an increase in mRNA expression of IL-12 and STAT4 with a more pronounced effect in mice dosed with 80 mg/kg of CUR. The dose-dependent increase in gene expressions indicated high levels of M1 macrophages in the tumour and spleen tissues. Furthermore, there was also a reduction in tumour-promoting M2 macrophages in the tumour and spleen as indicated by the suppression of STAT3, IL-10 and arginase 1 (M2 macrophage marker) gene expression. Overall, the study demonstrated that DNC has good potential as an anti-metastatic agent, but further immunohistochemistry and immunofluorescence studies are required in order to determine the characteristics, differentiation and quantity of M1 and M2 subpopulations.
7.2.5 Solid Lipid Nanoparticles
Introduced in 1991, solid lipid NP (SLN) have since gained its fame as a drug delivery system and as an alternative to the conventional colloidal carriers such as vesicular systems, polymeric NP and polymeric micelles. SLN not only maintains the advantages associated with the traditional drug delivery systems but also overcomes some of their disadvantages such as physical instability associated with drug loading, cytotoxicity associated with degradation of certain polymers and lack of a method for large-scale productions (Müller et al. 2000). There has since been a shift of research efforts from various areas of drug formulation research to SLN due to their unique size-dependent properties, efficient release profiles and good physical stability (Mukherjee et al. 2009; Naseri et al. 2015). SLN are spherical in shape and typically range between 40 and 1000 nm. SLN are mainly composed of a solid lipid phase with surfactants which act as emulsifiers. SLN are commonly produced by high-pressure homogenization under hot or cold temperatures, high-shear homogenization, breaking of oil/water microemulsion, double emulsion, solvent emulsification-evaporation, solvent injections or spray drying (Naseri et al. 2015).
7.2.5.1 Curcumin-Loaded Solid Lipid Nanoparticles
Sun et al. (2013) aimed to enhance the chemical stability and dispersibility of CUR through encapsulation within SLN prepared from triglycerides Dynasan 114® and Sefsol-218® via high-pressure homogenization. The authors compared proliferation inhibition efficacy of CUR-encapsulated SLN (CUR-SLN) against free CUR (in DMSO) in MCF-7 cells. Concentration- and time-dependent inhibitory effects were observed in both CUR-SLN and free CUR at 24, 48 and 72 h. They found that free CUR exhibited greater anticancer activity in comparison to CUR-SLN at 24 and 48 h. However, beyond 72 h, the free CUR-treated cells exhibited significant recovery, whereas CUR-SLN-treated cells maintained the same level of inhibition as that observed at 48 h, indicating that CUR-SLN exhibited prolonged inhibitory activity in cancer cells.
Transferrin (Tf) is an iron-binding protein found on the surface of glycoproteins necessary for cell proliferation. Due to the increased rate of proliferation in cancer cells and the need for a higher iron demand, these receptors are commonly up-regulated in tumour cells. Therefore, Mulik et al. (2010) fabricated a Tf-targeted CUR-loaded SLN (Tf-CUR-SLN) to enhance the cellular uptake and cytotoxicity in breast cancer cells. The study found that at a dose of 9 μM, the MCF-7 cell viability was 14.5 ± 0.7%, 41.3 ± 1.3% and 64.2 ± 1.2% for Tf-CUR-SLN, CUR-SLN and free CUR (solubilized in 0.1% (w/v) poloxamer 188), respectively, indicating that Tf-CUR-SLN were the most cytotoxic, among all. However, at a dose of 27 μM, there was no significant difference between cell viabilities of those treated with CUR in solution and CUR-SLN with cell viabilities of 28.91 ± 1.21% and 22.45 ± 1.44%, respectively, while the cell viability of Tf-CUR-SLN was significantly reduced to 4.01%. The authors conjectured that while CUR-SLN and CUR in solution entered the cells similarly via non-specific pathway, Tf-CUR-SLN entered the cells via Tf receptor-mediated endocytosis, thereby increasing their therapeutic potency in cancer cells.
7.2.5.2 Synergistic Curcumin Solid Lipid Nanoparticle Combinations
Pawar et al. (2016) synthesized a folic acid (FA)-targeted SLN encapsulating DTX and CUR (FA-DTX+CUR+SLN) to improve the pharmacokinetic efficacy of DTX therapy. FA, also known as vitamin B9 is essential in nucleic acid metabolism, amino acid production as well as prevention of DNA changes. Rapidly growing cancer cells express high surface levels of FA receptors to obtain enough FA for their DNA replication during cell division and cell proliferation (Pawar et al. 2016). By measuring the fluorescence intensity, the authors discovered that the FA-DTX+CUR-SLN had significantly greater cell uptake in comparison to nontargeted DTX+CUR-SLN in MCF-7 cells. They also investigated the in vitro anticancer effects of the targeted and nontargeted SLN in MCF-7 cells and found that FA-DTX+CUR-SLN decreased cell viability to a maximum extent followed by nontargeted DTX+CUR-SLN and DTX-SLN with the order of cytotoxicity of FA-DTX+CUR-SLN > DTX+CUR-SLN > DTX-SLN. The authors concluded that the presence of FA significantly enhanced the cellular uptake of the SLN resulting in greater reduction in cell viability compared to the nontargeted SLN.
7.2.6 Protein-Based Nanoparticles
Proteins are a class of natural macromolecules that are essential in order for cells and organisms to function properly (Zaman et al. 2014). Protein-based NP are favourable in the field of nanomaterials due to their biodegradability, non-toxicity, nonantigenicity and metabolizable nature in vivo into naturally occurring components (Elzoghby et al. 2012; Jahanshahi and Babaei 2008). The unique primary structure of protein molecules also offers the possibility of surface modification and covalent attachments allowing for drug loading and functionalization with targeting ligands (Zaman et al. 2014). Proteins are also less likely to undergo opsonization by the RES due to the presence of an aqueous steric barrier on their surface (Elzoghby et al. 2012). Common proteins used in the formulation of protein-based NP include albumin, gelatin and soy.
7.2.6.1 Curcumin-Loaded Albumin Nanoparticles
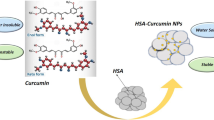
In the early 2000s, Abraxis BioScience developed a novel patented albumin-bound NP known as Abraxane®, approved by the Food and Drug Administration (FDA) for the treatment of breast cancer (Gong et al. 2015; Thadakapally et al. 2016). It was a revolutionary finding as this technology offered a solvent-free, nonantigenic, safe and efficient delivery of drug through the exploitation of the natural properties of albumin (Thadakapally et al. 2016). Albumin can be easily obtained from various sources such as egg white (ovalbumin), bovine serum albumin (BSA) and human serum albumin (HSA). It is a major soluble plasma protein found in the circulation system (Jahanshahi and Babaei 2008). The presence of reactive groups (thiol, amino and carboxylic groups) on the surface of albumin makes it an attractive NP as high drug loading can be achieved in the particle matrix (Jahanshahi and Babaei 2008). CUR has been reported to have a high loading efficiency in albumin NP with minimal toxic effects due to the endogenous nature of albumin which metabolizes in vivo to produce innocuous degradation products (Zaman et al. 2014). In addition to its enhanced endocytic uptake of drugs, albumin NP can also exploit the EPR mechanism and retain itself within the tumour microenvironment, a characteristic attributed to its large size of about ~200 nm (Jithan et al. 2011). Jithan et al. (2011) prepared CUR-albumin NP by desolvation and showed that CUR-albumin NP inhibited MDA-MB-231 cell proliferation more significantly than free CUR (dissolved in ethanol and PEG). Rats administered with 10 mg of CUR-albumin NP and free CUR were found to have a maximum serum concentration of 425 and 276 ng/ml, respectively. Moreover, CUR-albumin NP were detected in the rat body for a longer period of about 25 days, in comparison to free CUR which was only detectable for 24 h. The increased bioavailability of CUR observed was a result of sustained release of CUR from the NP. Furthermore, the CUR-albumin NP also demonstrated better tissue-targeting ability with higher CUR concentration found in the brain and lungs which are common sites of breast cancer metastases. On the other hand, Gong et al. (2015) engineered human serum albumin (HSA) CUR-NP (HSA-Cur-NP) by β-mercaptoethanol (β-ME) denaturation. The authors investigated the tumour-targeting capabilities of HSA-Cur-NP in vitro and discovered its accumulation within the cytoplasm of MCF-7 cells. The HSA-Cur-NP were also labelled with NIR-797 isothiocyanate for in vivo imaging. It was also observed that after 10 and 16 days, accumulation of NIR-797-HSA-Cur-NP was detected at the tumour sites, while free NIR-797 could not be detected indicating improved tumour-targeting capability by HSA-Cur-NP. More recently, Thadakapally et al. (2016) prepared a CUR-loaded PEG-albumin NP (CUR-PEG-albumin-NP) to increase the solubility, permeability and tumour accumulation of CUR in breast cancer cells. In vitro studies in BT549 and MDA-MB-231 cells showed that CUR-PEG-albumin-NP had enhanced cell cytotoxicity in comparison to free CUR (dissolved in ethanol and PEG).
7.2.6.2 Curcumin-Loaded Silk-Fibroin Nanoparticles
Another protein-based NP that has been garnering attention is silk-fibroin (SF)-derived NP due to its biocompatible and biodegradable properties. Fibroin proteins are naturally occurring copolymers, consisting of repetitive hydrophobic and hydrophilic peptide sequences. Hydrophobic peptides such as alanine, glycine and tyrosine can interact with hydrophobic drugs such as CUR through hydrophobic interactions. On the other hand, hydrophilic peptides give the NP water solubility and the ability to form NP in aqueous solutions (Kasoju and Bora 2012; Li et al. 2016). Gupta et al. (2009) engineered CUR-loaded SF and CS NP (CUR-SF-CS-NP) and CUR-SF-NP. Cell uptake studies on MCF-7 and MDA-MB-231 cells showed that CUR-SF-NP had a higher CUR intracellular uptake and cell cytotoxicity in comparison to CUR-SF-CS-NP. It was postulated that the presence of CS had increased the hydrophilic character of the NP, resulting in decreased entrapment efficiency of hydrophobic CUR, thereby resulting in lower cell cytotoxicity. More recently, Li et al. (2016) prepared 5-fluorouracil-(5-FU) and CUR-loaded SF NP (5-FU+CUR-SF-NP) for the treatment of breast cancer. Being a highly potent anticancer agent, 5-FU interrupts DNA replication via suppression of the methylation reaction of deoxyuridylic acid to thymidylic acid. Cellular morphological analysis revealed that 4 T1 cells treated with 5-FU+CUR-SF-NP underwent changes in cell shape from spindle form to spherical form, indicating cell apoptosis. There was also a significant increase in reactive oxygen species (ROS) levels in the 5-FU+CUR-SF-NP treated group, indicating that apoptosis of cells may have occurred as a result of ROS generations within the cells. The efficacy of the 5-FU+CUR-SF-NP was also investigated in vivo, with results showing that it was effective in reducing tumour size more significantly than free CUR (in ethanol) and with close to no changes in tumour size for the control group.
7.2.7 Inorganic Nanoparticles
Inorganic NP have been widely utilized in the field of diagnostics and as potential therapeutic agents in vitro and in vivo. Inorganic NP have gained significant attention due to their size-dependent physicochemical properties, biocompatibility, inertness, stability and ease of functionalization for targeted delivery (Kim and Hyeon 2014). In comparison to their organic and polymeric counterparts, inorganic NP also possess several unique material-dependent characteristics (Huang et al. 2011). For example, certain inorganic NP have the ability to respond to specific external stimuli such as near-infrared light (NIR) or magnetic fields to facilitate the delivery of drugs to desired regions (Anselmo and Mitragotri 2015).
7.2.7.1 Curcumin-Loaded Mesoporous Silica Nanoparticles
In recent years, mesoporous silica (MS) NP have gained a great deal of attraction as a nanoparticulate drug delivery system in the field of biomedical applications. MS-NP have desirable properties as a drug carrier, such as well-defined pores with narrow diameter distribution and high pore volume, facile surface multi-functionalization and more importantly chemical inertness, biocompatibility and biodegradability (Ma’mani et al. 2014; Kotcherlakota et al. 2016; Taebnia et al. 2016). The surface of the MS-NP is hydrophilic in nature, and simple chemical modification of the surfaces with suitable functional groups can provide binding sites for hydrophobic drugs to be loaded into the particle matrix (Taebnia et al. 2016).
Ma’mani et al. (2014) integrated novel guanidine functionalized PEGylated Ia3d mesoporous silica NP KIT-6 (Gu@PEGylated KIT-6) for the delivery of CUR to breast cancer cells. The three-dimensional cubic Ia3d mesoporous silica NP was functionalized with PEG due to its ability to increase circulation half-life through the avoidance of RES, enhance cellular uptake as well as ability to impart colloidal stability to molecules in aqueous dispersions (Ma’mani et al. 2014). CUR was loaded onto the MS-NP pores through electrostatic interactions between the carbonyl functional groups on CUR and the guanidine functional groups and PEG groups on the MS. The cytotoxicity of CUR-loaded Gu@PEGylated KIT-6 evaluated in MCF-7 and 4 T1 cells displayed significant inhibition of cell proliferation in a dose- and time-dependent manner in comparison to CUR alone. Flow cytometry analysis performed on MCF-7 cells at 12, 24 and 48 h posttreatment showed that the percentage of viable cells were 89.0, 73.2 and 7.6% proving the potent cytotoxic effects of the CUR-loaded Gu@PEGylated in vitro. However, Kotcherlakota et al. (2016) developed KIT-6, MSU-2 and MCM-41 MS-NP that have been functionalized with amine groups through 3-aminopropyltriethoxysilane (APTES) followed by the conjugation of MS-NP to CUR. Studies in MCF-7 cells showed that only CUR-loaded MSU-2 MS-NP caused significant suppression of cell viability in comparison to CUR-loaded KIT-6 and MCM-41 MS-NP and free CUR as a result of enhanced cellular uptake in cancer cells. In contrast, KIT-6 and MCM-41 MS-NP only exhibited slight suppressions which may be a result of the functionalization effect. The study also reported that the CUR uptake caused apoptosis of cancer cells as a result of generation of intracellular ROS and downregulation of poly ADP-ribose polymerase (PARP) enzymes levels. Alternatively, Zhang et al. (2015) engineered a novel NIR-responsive DNA-hybrid-gated NP based on MS-coated Cu1.8S NP (Cu1.8S@MS-NP). In the recent years, aptamers have emerged as a novel class of molecule rivaling antibodies for active targeting. AS1411 is a 26-base DNA oligonucleotide that possesses high selectivity and affinity for protein nucleolin allowing for cell membrane penetration through receptor-mediated endocytosis. The Cu1.8S@MS-NP were conjugated with aptamer (AS1411)-modified GC-rich DNA-helix to allow for the loading of DOX and targeted delivery. Under a 980 nm laser, the AS1411-Cu1.8S NP possesses high photothermal conversion efficiency, allowing for the denaturation of DNA double strands and triggered release of DNA-helix-loaded DOX and MS-loaded CUR. Cellular uptake studies in MCF-7 cells demonstrated that DOX+CUR-Cu1.8S@MS-NP had a greater affinity for MCF-7 cells in comparison to nontargeted DOX+CUR-Cu1.8S@MS-NP due to the overexpression of nucleolin on the surface of MCF-7 cells. Cell toxicity studies showed that upon exposure to λ = 980, the AS1411-DOX+CUR-Cu1.8S@MS-NP had a sixfold decrease in IC50 value (based on DOX concentration) compared to the combination of free CUR and DOX (in DMSO) in MCF-7 cells. However, negligible cell deaths of MCF-7 cells were observed in the absence of NIR irradiation. The authors inferred that NIR light was required to uncap the mesopores via denaturation of double-strand DNA and trigger the release of drugs from the MS-NP. Flow cytometry analysis revealed that the cytotoxic effect of the aptamer-conjugated AS1411-DOX+CUR-Cu1.8S@MS-NP was elicited through mitochondria-mediated apoptosis as indicated by the levels of pro-caspase 3 expression and enhanced ratio of Bax/Bcl-2.
7.2.7.2 Curcumin-Loaded Magnetic Nanoparticles
Iron oxide NP (Fe2O3 NP) also known as magnetic NP (MNP) have been utilized for a variety of biomedical applications such as magnetic resonance imaging (MRI) (Briley-Saebo et al. 2004), magnetic drug delivery (Alexiou et al. 2002; Ito et al. 2004; Mahmoudi et al. 2010), magnetic hyperthermia treatment (MHT) (Hergt et al. 2004; Fortin et al. 2007), biosensors (Safarik and Safaríková 1997) and magnetofection. MNP are typically ‘superparamagnetic’, where they possess magnetism in the presence of an external magnetic field but lose their magnetism when the field is removed. MNP have the benefit that they are ultrasmall in size (<20 nm), and under the presence of an external magnetic field, MNP can be drawn to a specific site allowing for the preferential accumulation within a tumour site (Balasubramanian et al. 2014; Saikia et al. 2016).
A study by Yallapu et al. (2012) prepared CUR-loaded MNP (CUR-MNP) by the chemical precipitation method. In vitro studies in MDA-MB-231 cells exhibited internalization by endocytosis of CUR-MNP after 6 h. CUR-MNP were shown to have enhanced anticancer properties with amplified loss of mitochondrial membrane potential integrity and generation of ROS in comparison to free CUR (in DMSO). CUR-MNP had enhanced cellular uptake of CUR in the presence of magnetic field, indicating that MNP increases the targeting capability of CUR. Saikia et al. (2016) fabricated CUR-loaded aminated starch coated MNP via co-precipitation method, while, in vitro studies found the CUR-MNP to be compatible with human lymphocyte cells, it was found to significantly inhibit the growth of MCF-7 cells. The same authors then continued their study of MNP by synthesizing a FA-targeted aminated starch and zinc oxide (ZnO)-coated MNP for the delivery of CUR. The FA-targeted CUR-MNP showed no toxicity in human lymphocytes but was found to suppress the cell viability of MCF-7 cells more significantly than free CUR with cell viabilities of 58 and 80%, respectively. While the MNP increased the internalization of CUR, the presence of FA on the surface further enhanced the cell internalization of CUR, resulting in enhanced cytotoxicity. Alternatively, Balasubramanian et al. (2014) synthesized a FA- and Tf-targeted MNP-encapsulated PLGA-NP for the co-delivery of CUR and 5-FU [(CUR+5-FU)-PLGA-MNP]. The dual-targeted CUR+5-FU-PLGA-MNP were found to have enhanced accumulation in MCF-7 cells in comparison to single-targeted CUR+5-FU-PLGA-MNP with enhanced dose-dependent and pH-responsive cytotoxic activity.
7.2.7.3 Curcumin-Loaded Gold Nanoparticles
Gold NP (AuNP) have earned the status as a nanoparticulate drug delivery system due to their good biocompatibility and stability in vitro and in vivo, facile synthetic methods and ease of surface functionalization for the attachment of ligands for active targeting (Dey et al. 2016; Haume et al. 2016). An advantage of AuNP is their ability to achieve much smaller particle size diameters which is not only advantageous for passive targeting via EPR but also for the avoidance of clearance by the RES. The synthesis of AuNP typically involves two distinct steps: firstly, the reduction of Au3+ to Au0 in the presence of a reducing agent and, secondly, stabilization of the AuNP to direct the shape of the formed AuNP. This is to avoid agglomeration as AuNP have been reported to be highly unstable with the tendency to form aggregates upon slight pH and electrolyte concentration changes (Muddineti et al. 2016). Common reducing agents used for the formation of AuNP include sodium borohydrate and trisodium citrate, whereas common stabilizing agents used are a variety of natural gums, surfactants, polymers and carbohydrates. Dey et al. (2016) synthesized methotrexate (MTX)- and CUR-loaded AuNP stabilized by biopolymer, alginate (Alg). MTX has a structure analogous to FA and is a potent anticancer agent as it interrupts the synthesis of DNA and RNA in cancer cells resulting in cellular apoptosis. The study found that MTX+CUR-Alg-AuNP were more cytotoxic against MCF-7 cells in comparison to individual drugs alone. The authors concluded that the enhanced cytotoxicity of MTX+CUR-Alg-AuNP was attributed to the presence of MTX, the ‘anti-folate’ drug, which facilitated active targeting of the AuNP in MCF-7 cells which overexpress FA receptors.
7.2.7.4 Curcumin-Loaded Zirconium Nanoparticles
Another promising inorganic nanoparticulate drug carrier is zirconium phosphate (ZrP) due to its high temperature stability, biocompatibility and chemical and biological inertness (Kalita et al. 2016). While only limited reports on ZrP-NP are currently available, common methods of preparation include solvothermal (Feng et al. 2014), chemical precipitation (Hajipour and Karimi 2014) and water-in-oil-microemulsion (Bellezza et al. 2006). A study by Kalita et al. (2016) prepared a pH-sensitive ZrP-NP for the delivery of CUR through a simple sonication method. The IC50 values of CUR-loaded ZrP-NP and free CUR in MDA-MB-231 cells were found to be 13.24 and 16.41 μg/ml, respectively. This indicated higher suppression efficiency of the CUR-loaded ZrP-NP as a result of the triggered CUR release from the pH-sensitive ZrP-NP in the acidic intracellular environment of the cancer cells.
7.2.7.5 Curcumin-Loaded Copper Nanoparticles
The most common method for the preparation of metal NP includes chemical reduction, photochemical reduction, electrochemical techniques and physical vapour condensation. It has been previously reported that CUR-copper (Co) complexes exhibit enhanced antioxidant and superoxide scavenging activities in comparison to CUR alone (Barik et al. 2005). Kamble et al. (2016) formulated a CUR-capped Co-NP (CUR-Co-NP) via the Creighton method using sodium borohydride (NaBH4) as the reducing agent for the possible suppression of breast cancer cells and angiogenesis. Interestingly, it was found that the MDA-MB-231 cells treated with CUR-Cu-NP had an increase in cell proliferation, whereas cells treated with free CUR alone (in ethanol) resulted in the suppression of cell proliferation. Furthermore, while free CUR alone was found to possess anti-angiogenic potential, CUR-Cu-NP did not demonstrate such activity in a significant manner. Similarly, free CUR alone was found to suppress the migration of MDA-MB-231 cells in a concentration-dependent manner, whereas CUR-Cu-NP did not arrest cell migration despite increases in concentration. The results of the study clearly demonstrate that native CUR had more anticancer activity than CUR-Cu-NP. The authors postulated that the carbonyl group (C=O) is the key functional group in the biological activity of CUR, and the reduction of the C=O group by NaBH4 during the synthesis may be responsible for the lack of biological activity of CUR-Cu-NP. Hence Co-NP may be of limited benefit for the delivery of CUR in this sense.
7.2.7.6 Curcumin-Loaded Titanium Oxide Nanoparticles
Titanium oxide (TiO2) NP has also attracted a lot of attention in the field of cancer drug delivery due to its ability to generate highly reactive ROS under ultraviolet (UV) light radiation causing cancer cell death (Ding et al. 2016). TiO2 NP are ultrasmall in size with large surface areas, which increase their chemical reactivity and cell penetration. Ding et al. (2016) engineered FA-targeted PEG-modified TiO2 NP co-encapsulating salvianolic acid B and CUR (FA-SalB+CUR-PEG-TiO2) NP. SalB, a polyphenolic acid, has been shown to not only have antioxidant, anti-inflammatory and anti-coagulant effects but has also been shown to have potent anticancer effects against a variety cancer types. In addition to the synergistic anticancer activity with CUR, SalB also protects against myocardial damage caused by the TiO2 NP. The FA-SalB+CUR-PEG-TiO2 NP were found to be more cytotoxic with enhanced cellular uptake in MCF-7 and MDA-MB-231 cells in comparison to the nontargeted group and free CUR (in dichloromethane) and SalB (in ultrapure water). In vivo studies in mice bearing MDA-MB-231 tumours also found FA-SalB+CUR-PEG-TiO2 NP (5 mg/kg CUR, 1.35 mg/kg Sal B, and 1 mg/kg cisplatin) to have significantly greater tumour suppressive effects in comparison to the anticancer drug cisplatin, nontargeted TiO2 NP and free CUR and SalB in solution due to the FA receptor-mediated targeted function. Furthermore, histopathological studies on the heart tissue of the targeted and nontargeted TiO2 NP showed normal heart morphological structures in comparison to that with the cisplatin group which had signs of local inflammatory cell infiltration. Therefore, co-encapsulation of SalB can be a promising delivery approach in the delivery of CUR-loaded TiO2 NP for the treatment of breast cancer due to the dual mechanism of SalB, providing cardioprotection from the TiO2 NP.
7.2.8 Other Nano-Formulations
7.2.8.1 Curcumin-Loaded Nano-droplets
Ultrasound-responsive nano-droplet is a new emerging class of smart drug delivery systems. The concept of ultrasound-mediated anticancer modality is based on the systemic administration of a phase-shift drug-loaded nano-droplets (Baghbani et al. 2017). Under the presence of ultrasound, the nano-droplets vaporize and convert into microbubbles via acoustic droplet vaporization resulting in the release of encapsulated drugs. Upon acoustic droplet vaporization, the nano-droplets undergo expansion which also leads to mechanical tissue erosion and consequently cell damage, thereby promoting vascular permeability and ablation of the tumour tissue. Baghbani et al. (2017) set out to formulate novel ultrasound-responsive chitosan/perfluorohexane (PFH) nano-droplets to enhance the bioavailability and anticancer efficiency of CUR through an image-guided ultrasound-mediated triggered drug release. From the experiments, no CUR was released from the nano-droplets over a period of 10 min in the absence of ultrasound exposure, demonstrating good drug retention of the nano-droplets shell. In contrast, ultrasound exposure resulted in the triggered release of 63.5% of CUR within 4 mins, indicating that the nano-droplets had undergone acoustic droplet vaporization. The cytotoxicity of the CUR-loaded nano-droplets in the presence and absence of ultrasound and free CUR (in DMSO) was also examined in 4 T1 cells. All formulations showed dose-dependent cytotoxicity with CUR-loaded nano-droplets in the presence of 1 MHz ultrasound having the highest cytotoxicity (92.1% at 0.4 μg/ml), followed by CUR-loaded nano-droplets in the absence of ultrasound (55.5% at 0.4 μg/ml). The cytotoxicity of free CUR was significantly lower than the CUR-loaded nano-droplets as a result of poor stability in its free form and lipophilic nature resulting in lower cellular uptake.
7.2.8.2 Curcumin-Loaded Nanocrystal Suspension
Another method used to increase the solubility of poorly soluble drugs is the formulation of nanocrystal suspension (nano-suspension) formed from pure drug crystals in the presence of a surfactant or polymer for stabilization. Gao et al. (2011) formulated a CUR nano-suspension using soya lecithin and sodium deoxycholate (SDS) by high-pressure homogenization for the intravenous delivery of CUR. In vitro cytotoxicity studies in MCF-7 cells demonstrated that CUR-nano-suspension had superior anticancer activity to free CUR (in DMSO) with IC50 values of 26.45 and 36.64 μg/ml, respectively (Gao et al. 2011).
7.2.8.3 Curcumin-Loaded Nano-emulsion
Steuber et al. (2016) developed a CUR-loaded tocotrienol nano-emulsion to improve the solubility and enhance the delivery and anticancer activity of CUR. The authors reported that the CUR-loaded nano-emulsion demonstrated pronounced anticancer activity against MCF-7 cells with maximum cell apoptosis (>90%) when the concentration of CUR was 30 μM. The IC50 of CUR nano-emulsion was also found to be 3 and 49 times more potent than free CUR (in hydroalcoholic solution) and empty tocotrienol nano-emulsion, respectively.
7.2.8.4 Curcumin-Loaded Nano-fibres
Magnesium oxide (MgO) NP has been reported to possess antibacterial, antioxidant and anticancer activities against a variety of cell lines (Heydary et al. 2015; Mahmoud et al. 2016; Sudakaran et al. 2017). Sudakaran et al. (2017) formulated MgO NP in poly (L-lactic acid-co-ε-caprolactone) (PLACL) nano-fibres for the co-delivery of CUR and β-cyclodextrin for anticancer therapy. The CUR-loaded nano-fibres and CUR and β-cyclodextrin-loaded nano-fibres were shown to be cytotoxic to MCF-7 cells as the cells displayed a shrunken shape indicating apoptosis. In contrast, the cells of the empty nano-fibres exhibited a distorted polygonal shape, therefore indicating little to no apoptotic effect in comparison to the drug-loaded nano-fibres. The study established that interaction of CUR with MgO resulted in higher suppression of MCF-7 cells in comparison to all other nano-fibrous scaffolds that were fabricated, thereby indicating that CUR-loaded MgO nano-fibres may be a promising biocomposite material system for breast cancer treatment.
7.2.8.5 Curcumin-Loaded Nanoparticles with Surfactants
Dev et al. (2012) prepared CUR-NP with didodecyl-dimethylammonium bromide (DDAB) and pluronic F127 through a continuous flow microfluidic rotating tube processor (RTP). The anticancer activity of the CUR-NP and free CUR (in DMSO) was evaluated in MCA-MB-468 and MCF-7 cells. At CUR concentrations of 25 and 50 μg/ml, the anticancer effects of CUR-NP were found to be significantly higher than free CUR indicating that the nano-formulation more effectively delivered CUR to cells. CUR-NP were prepared by the desolvation method and surface modified with Tf and gelatin (GT) through adsorption to enhance their targeting ability and stability (Choi 2016). In comparison to CUR-NP, Tf-CUR-NP and GT-CUR-NP showed significantly higher cellular uptake in MCF-7 cells. The cytotoxicity studies were consistent with the findings of the cellular uptake studies which showed that at a CUR concentration of 20 μg/ml, GT-CUR-NP, Tf-CUR-NP, CUR-NP and free CUR (pure) had cytotoxicities of 40.8, 30.1, 13.9 and 9.5% in MCF-7 cells, respectively.
7.2.8.6 Curcumin-Loaded β-Cyclodextrin Inclusion Complex
Kazemi-Lomedasht et al. (2013) engineered a β-cyclodextrin-CUR (CD-CUR) inclusion complex and evaluated its effect on human telomerase reverse transcriptase (hTERT) gene expression in T47D cell line in comparison to free CUR. The IC50 values of CD-CUR and free CUR (in DMSO) after 24 h of treatment was 18 and 22 μM, respectively, signifying enhanced cellular uptake of CD-CUR with respect to free CUR. As telomerase activity has been closely linked to most cancers, targeting of telomerase could be a promising strategy in anticancer treatment. The authors reported that CD-CUR suppressed the proliferation of T47D cells and decreased the level of hTERT mRNA expression more significantly than free CUR proving that CD-CUR was more cytotoxic than free CUR in the breast cancer cell line. Zhang et al. (2011) aimed to enhance the solubility of CUR by formulating CUR-NP with rubusoside (RUB), a steviol glycoside that possesses solubilizing properties. The anticancer activity of formulated CUR-RUB-NP and free CUR (in DMSO) was evaluated in MDA-MB-231 cells and was found to have no significant differences in IC50 values. The authors interpreted this as CUR-RUB-NP being as equally as bioavailable and efficacious as CUR solubilized in DMSO indicating that the bioactivity of CUR can be maintained completely when formulated as water-soluble RUB-NP.
7.3 Conclusions and Future Prospects
CUR holds great potential as an anticancer agent for the treatment of breast cancer that affects women in both developed and developing countries. CUR modulates various molecular pathways involved in the growth, promotion and metastatic pathways of breast cancer. However, CUR’s potential in in vivo application is limited by its poor solubility in an aqueous environment, rapid degradation and elimination resulting in poor bioavailability. Nanotechnology provides a pathway to overcome this limitation by improving CUR solubility, efficacy in vitro and in vivo and pharmacokinetic stability in vivo. During the formulation process, special considerations should be taken into the structure of CUR and the mechanism of formulation of the nano-formulation as to prevent loss of CUR activity in the final product. While a few papers reported comparable results to that of free CUR and CUR nano-formulation, a majority of studies conducted in cell lines and preclinical animal models have proven the cytotoxic efficacy of CUR nano-formulation. All animal studies also reported CUR nano-formulations to have an enhanced biodistribution with a prolonged half-life in vivo in comparison to that of free CUR. The synergistic effect of CUR when administrated with other more potent anticancer drugs has also shown to be highly beneficial in not only enhancing the efficacy of anticancer drugs by overcoming MDR but also allowing safe and effective plasma concentrations to be achieved with minimal systemic toxicity.
While hundreds of papers have demonstrated the potential CUR nano-formulations as an anticancer agent in vitro and in vivo, there are currently only a total of four clinical trials in the field of CUR as a treatment for breast cancer, none of which are delivered in the form of a CUR nano-formulation. A published Phase I clinical trial study by Bayet-Robert et al. (2010) on the dose escalation of DTX plus CUR taken orally in patients with advanced and metastatic breast cancer was previously discussed. Phase II clinical trial is currently ongoing at the Centre Jean Perrin in Clermont-Ferrand, France, to determine the response rate of breast cancer recurrence in metastatic breast cancer patients treated with DTX and CUR in comparison to DTX alone. There is also an ongoing Phase I trial at the National Center of Oncology in Yerevan, Armenia, to determine the response rate of CUR administered intravenously with paclitaxel in patients with advanced breast cancer. Another Phase II clinical trial is currently being carried out to investigate if CUR administered orally can reduce NF-κβ DNA binding in peripheral blood mononuclear cells of chemotherapy-treated breast cancer patients undergoing radiotherapy at Emory University in Atlanta, United States.
Moving forward from a laboratory setting, future perspectives should include further preclinical and clinical studies focusing on the targeted delivery of CUR nano-formulations. Future researchers in this field should also carry out more studies investigating the chemical stability of CUR nano-formulations with a specific focus on the kinetics and degradation of CUR in vivo. There is currently also a lack of data of the safety and toxicity profiles of CUR nano-formulations, limiting its justification to move forward with human clinical trials. Lastly, more consideration should also be given into the feasibility in terms of large-scale industrial production for the commercial drug product manufacture of CUR nano-formulations.
References
Alexiou C, Schmidt A, Klein R, Bergemann C, Arnold W (2002) Magnetic drug targeting: biodistribution and dependency on magnetic field strength. J Magn Magn Mater 252:363–366
Alizadeh AM, Sadeghizadeh M, Najafi F, Ardestani SK, Erfani-Moghadam V, Khaniki M, Rezaei A, Zamani M, Khodayari S, Khodayari H, Mohagheghi MA (2015) Encapsulation of curcumin in diblock copolymer micelles for cancer therapy. Biomed Res Int 2015:824746. https://doi.org/10.1155/2015/824746
Anselmo AC, Mitragotri S (2015) A review of clinical translation of inorganic nanoparticles. AAPS J 17:1041–1054
Babaei E, Sadeghizadeh M, Hassan ZM, Feizi MAH, Najafi F, Hashemi M (2012) Dendrosomal curcumin significantly suppresses cancer cell proliferation in vitro and in vivo. Int Immunopharmacol 12:226–234
Baeza A, Manzano M, Collila M, Vallet-Regi M (2016) Recent advances in mesoporous silica nanoparticles for antitumor therapy: our contribution. Biomater Sci 4:803–813
Baghbani F, Chegeni M, Moztarzadeh F, Hadian-Ghazvini S, Raz M (2017) Novel ultrasound-responsive chitosan/perfluorohexane nanodroplets for image-guided smart delivery of an anticancer agent: curcumin. Mater Sci Eng C 74:186–193
Balasubramanian S, Girija AR, Nagaoka Y, Iwai S, Suzuki M, Kizhikkilot V, Yoshida Y, Maekawa T, Nair SD (2014) Curcumin and 5-fluorouracil-loaded, folate- and transferrin-decorated polymeric magnetic nanoformulation: a synergistic cancer therapeutic approach, accelerated by magnetic hyperthermia. Int J Nanomedicine 9:437–459
Barik A, Mishra B, Shen L, Mohan H, Kadam RM, Dutta S, Zhang HY, Priyadarsini KI (2005) Evaluation of a new copper(II)-curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radic Biol Med 39:811–822
Bayet-Robert M, Kwiatowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, Mouret-Reynier MA, Durando X, Barthomeuf C, Chollet P (2010) Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther 9:8–14
Bellezza F, Cipiciani A, Costantino U, Marmottini F, Quotadamo MA (2006) Zirconium phosphate nanoparticles from water-in-oil microemulsions. Colloid Polym Sci 285:19–25
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC (2014) Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 66:2–25
Bisht S, Schlesinger M, Rupp A, Schubert R, Nolting J, Wenzel J, Holdenrieder S, Brossart P, Bendas G, Feldmann G (2016) A liposomal formulation of the synthetic curcumin analog EF24 (Lipo-EF24) inhibits pancreatic cancer progression: towards future combination therapies. J Nanobiotechnol 14:57. https://doi.org/10.1186/s12951-016-0209-6
Biswas S, Kumari P, Lakhani PM, Ghosh B (2016) Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci 83:184–202
Briley-Saebo K, Bjørnerud A, Grant D, Ahlstrom H, Berg T, Kindberg GM (2004) Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: implications for magnetic resonance imaging. Cell Tissue Res 316:315–323
Cai X, Liu M, Zhang C, Sun D, Zhai G (2016) pH-responsive copolymers based on pluronic P123-poly(beta-amino ester): synthesis, characterization and application of copolymer micelles. Colloids Surf B Biointerfaces 142:114–122
Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C, Meyskens FL, Brenner DE (2011) Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res 4:354–364
Choi JS (2016) Development of surface curcumin nanoparticles modified with biological macromolecules for anti-tumor effects. Int J Biol Macromol 92:850–859
Chun YS, Bisht S, Chenna V, Pramanik D, Yoshida T, Hong SM, de Wilde RF, Zhang Z, Huso DL, Zhao M, Rudek MA (2012) Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis 33:2242–2249
Debnath S, Saloum D, Dolai S, Sun C, Averick S, Raja K, Fata JE (2013) Dendrimer-curcumin conjugate: a water soluble and effective cytotoxic agent against breast cancer cell lines. Anti Cancer Agents Med Chem 13:1531–1539
Dev S, Prabhakaran P, Filgueira L, Iyer KS, Raston CL (2012) Microfluidic fabrication of cationic curcumin nanoparticles as an anti-cancer agent. Nanoscale 4:2575–2579
Dey S, Sherly MC, Rekha MR, Sreenivasan K (2016) Alginate stabilized gold nanoparticle as multidrug carrier: evaluation of cellular interactions and hemolytic potential. Carbohydr Polym 136:71–80
Dhule SS, Penfornis P, Frazier T, Walker R, Feldman J, Tan G, He J, Alb A, John V, Pochampally R (2012) Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine 8:440–451
Ding L, Li J, Huang R, Liu Z, Li C, Yao S, Pi J (2016) Salvianolic acid B protects against myocardial damage caused by nanocarrier TiO2; and synergistic anti-breast carcinoma effect with curcumin via codelivery system of folic acid-targeted and polyethylene glycol-modified TiO2 nanoparticles. Int J Nanomedicine 11:5709–5727
Duan J, Mansour HM, Zhang Y, Deng X, Chen Y, Wang J, Pan Y, Zhao J (2012) Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm 426:193–201
Durgam VR, Fernandes G (1997) The growth inhibitory effect of conjugated linoleic acid on MCF-7 cells is related to estrogen response system. Cancer Lett 116:121–130
Durgaprasad S, Pai CG, Kumar V, Alvres JF, Namitha S (2005) A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res 122:315–318
Dykes GM (2001) Review dendrimers: a review of their appeal and applications. J Chem Technol Biotechnol 76:903–918
Elzoghby AO, Samy WM, Elgindy NA (2012) Protein-based nanocarriers as promising drug and gene delivery systems. J Control Release 161:38–49
Farhangi B, Alizadeh AM, Khodayari H, Khodayari S, Dehghan MJ, Khori V, Heidarzadeh A, Khaniki M, Sadeghiezadeh M, Najafi F (2015) Protective effects of dendrosomal curcumin on an animal metastatic breast tumor. Eur J Pharmacol 758:188–196
Feng YJ, He W, Zhang XD, Jia XT, Zhao HS (2014) The preparation of nanoparticle zirconium phosphate. Mater Lett 61:3258–3261
Fortin JP, Wilhelm C, Servais J, Ménager C, Bacri JC, Gazeau F (2007) Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J Am Chem Soc 129:2628–2635
Gao Y, Li Z, Sun M, Guo C, Yu A, Xi Y, Cui J, Lou H, Zhai G (2011) Preparation and characterization of intravenously injectable curcumin nanosuspension. Drug Deliv 18:131–142
Gong G, Pan Q, Wang K, Wu R, Sun Y, Lu Y (2015) Curcumin-incorporated albumin nanoparticles and its tumor image. Nanotechnology 26:045603. https://doi.org/10.1088/0957-4484/26/4/045603
Gou M, Men K, Shi H, Xiang M, Zhang J, Song J, Long J, Wan Y, Luo F, Zhao X, Qian Z (2011) Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale 3:1558–1567
Guo O, Li X, Yang Y, Wei J, Zhao Q, Luo F, Qian Z (2014) Enhanced 4T1 breast carcinoma anticancer activity by co-delivery of doxorubicin and curcumin with core-shell drug-carrier based on heparin modified poly(L-lactide) grafted polyethylenimine cationic nanoparticles. J Biomed Nanotechnol 10:227–237
Gupta SC, Kismali G, Aggarwal BB (2013) Curcumin, a component of turmeric: from farm to pharmacy. Biofactors 39:2–13
Gupta V, Aseh A, Ríos CN, Aggarwal BB, Mathur AB (2009) Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int J Nanomed 4:115–122
Guzzarlamudi S, Singh PK, Pawar VK, Singh Y, Sharma K, Paliwal SK, Chourasia MK, Ramana MV, Chaurasia M (2016) Synergistic chemotherapeutic activity of curcumin bearing methoxypolyethylene glycol-G-linoleic acid based micelles on breast cancer cells. J Nanosci Nanotechnol 16:4180–4190
Hajipour AR, Karimi H (2014) Synthesis and characterization of hexagonal zirconium phosphate nanoparticles. Mater Lett 116:356–358
Hasan M, Belhaj N, Benachour H, Barberi-Heyob M, Kahn CJ, Jabbari E, Linder M, Arab-Tehrany E (2014) Liposome encapsulation of curcumin: physico-chemical characterizations and effects on MCF7 cancer cell proliferation. Int J Pharm 461:519–528
Haume K, Rosa S, Grellet S, Śmiałek MA, Butterworth KT, Solov’yov AV, Prise KM, Golding J, Mason NJ (2016) Gold nanoparticles for cancer radiotherapy: a review. Cancer Nanotechnol 7:8
He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J (2011) Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Investig 29:208–213
Hergt R, Hiergeist R, Hilger I, Kaiser WA, Lapatnikov Y, Margel S, Richter U (2004) Maghemite nanoparticles with very high AC-losses for application in RF-magnetic hyperthermia. J Magn Magn Mater 270:345–347
Heydary V, Navaei-Nigjeh M, Rahimifard M, Mohammadirad A, Baeeri M, Abdollah M (2015) Biochemical and molecular evidences on the protection by magnesium oxide nanoparticles of chlorpyrifos-induced apoptosis in human lymphocytes. J Res Med Sci 20:1021–1031
Huang HC, Barua S, Sharma G, Dey SK, Rege K (2011) Inorganic nanoparticles for cancer imaging and therapy. J Control Release 155:344–357
Ide H, Tokiwa S, Sakamaki K, Nishio K, Isotani S, Muto S, Hama T, Masuda H, Horie S (2010) Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 70:1127–1133
Ito A, Takizawa Y, Honda H, Hata K, Kagami H, Ueda M, Kobayashi T (2004) Tissue engineering using magnetite nanoparticles and magnetic force: heterotypic layers of cocultured hepa- tocytes and endothelial cells. Tissue Eng 10:833–840
Jahanshahi M, Babaei Z (2008) Protein nanoparticle: A unique system as drug delivery vehicles. Afr J Biotechnol 7:4926–4934
Jawahar N, Meyyanathan SN (2012) Polymeric nanoparticles for drug delivery and targeting: a comprehensive review. Int J Health Allied Sci 1:217
Jithan A, Madhavi K, Madhavi M, Prabhakar K (2011) Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int J Pharm Investig 1:119–125
Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS (2010) Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat 122:777–785
Kalita H, Prashanth Kumar BN, Konar S, Tantubay S, Mahto MKR, Mandal M, Pathak A (2016) Sonochemically synthesized biocompatible zirconium phosphate nanoparticles for pH sensitive drug delivery application. Mater Sci Eng C 60:84–91
Kamble S, Utage B, Mogle P, Kamble R, Hese S, Dawane B, Gacche R (2016) Evaluation of curcumin capped copper nanoparticles as possible inhibitors of human breast cancer cells and angiogenesis: a comparative study with native curcumin. AAPS Pharm Sci Tech 17:1030–1041
Kanai M (2014) Therapeutic applications of curcumin for patients with pancreatic cancer. World J Gastroenterol 20:9384–9391
Kangarlou S, Ramezanpour S, Balalaie S, Roudbar Mohammadi S, Haririan I (2017) Curcumin-loaded nanoliposomes linked to homing peptides for integrin targeting and neuropilin-1-mediated internalization. Pharm Biol 55:277–285
Kasoju N, Bora U (2012) Fabrication and characterization of curcumin-releasing silk fibroin scaffold. J Biomed Mater Res B 100:1854–1866
Kawai Y, Kaidoh M, Ohhashi T (2008) MDA-MB-231 produces ATP-mediated ICAM-1-dependent facilitation of the attachment of carcinoma cells to human lymphatic endothelial cells. Am J Physiol Cell Physiol 295:C1123–C1132
Kazemi-Lomedasht F, Rami A, Zarghami N (2013) Comparison of inhibitory effect of curcumin nanoparticles and free curcumin in human telomerase reverse transcriptase gene expression in breast cancer. Adv Pharm Bull 3(1):127–30
Kesharwani P, Banerjee S, Padhye S, Sarkar FH, Iyer AK (2015a) Parenterally administrable nano-micelles of 3,4-difluorobenzylidene curcumin for treating pancreatic cancer. Colloids Surf B Biointerfaces 132:138–145
Kesharwani P, Xie L, Banerjee S, Mao G, Padhye S, Sarkar FH, Iyer AK (2015b) Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloid Surf B Biointerfaces 1:413–423
Khosropanah M, Dinarvand A, Nezhadhosseini A, Haghighi A, Hashemi S, Nirouzad F, Khatamsaz S, Entezari M, Hashemi M, Dehghani H (2016) Analysis of the antiproliferative effects of curcumin and nanocurcumin in MDA-MB231 as a breast cancer cell line. Iran J Pharm Res 15:231–239
Kim T, Hyeon T (2014) Applications of inorganic nanoparticles as therapeutic agents. Nanotechnology 25:012001
Kotcherlakota R, Barui AK, Prashar S, Fajardo M, Briones D, Rodríguez-Diéguez A, Patra CR, Gómez-Ruiz S (2016) Curcumin loaded mesoporous silica: an effective drug delivery system for cancer treatment. Biomater Sci 4:448–459
Kumar SSD, Mahesh A, Mahadevan S, Mandal AB (2014) Synthesis and characterization of curcumin loaded polymer/lipid based nanoparticles and evaluation of their antitumor effects on MCF-7 cells. Biochim Biophys Acta 1840:1913–1922
Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE (2006) Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 17:10
Lee WH, Bebawy M, Loo CY, Luk F, Mason RS, Rohanizadeh R (2015) Fabrication of curcumin micellar nanoparticles with enhanced anticancer activity. J Biomed Nanotechnol 11:1093–1105
Li H, Tian J, Wu A, Wang J, Ge C, Sun Z (2016) Self-assembled silk fibroin nanoparticles loaded with binary drugs in the treatment of breast carcinoma. Int J Nanomedicine 11:4373–4380
Liang S, Dong C (2008) Integrin VLA-4 enhances sialyl-Lewisx/a-negative melanoma adhesion to and extravasation through the endothelium under low flow conditions. Am J Physiol Cell Physiol 295:C701–C707
Liang H, Friedman JM, Nacharaju P (2017) Fabrication of biodegradable PEG-PLA nanospheres for solubility, stabilization, and delivery of curcumin. Artif Cells Nanomed Biotechnol 45:297–304
Liu L, Sun L, Wu Q, Guo W, Li L, Chen Y, Li Y, Gong C, Qian Z, Wei Y (2013) Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int J Pharm 443:175–182
López-Lázaro M (2008) Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res 52:S103–S127
Ma’mani L, Nikzad S, Kheiri-Manjili H, al-Musawi S, Saeedi M, Askarlou S, Foroumadi A, Shafiee A (2014) Curcumin-loaded guanidine functionalized PEGylated I3ad mesoporous silica nanoparticles KIT-6: practical strategy for the breast cancer therapy. Eur J Med Chem 83:646–654
Maherani B, Arab-Tehrany E, Mozafari MR, Gaiani C, Linder M (2011) Liposomes: a review of manufacturing techniques and targeting strategies. Curr Nanosci 7:436–452
Mahmoud A, Ezqi O, Merve A, Ozhan G (2016) In vitro toxicological assessment of magnesium oxide nanoparticle exposure in several mammalian cell types. Int J Toxicol 35:429–437
Mahmoudi M, Simchi A, Imani M, Shokgozar MA, Milani AS, Häfeli UO, Stroeve P (2010) A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf B Biointerfaces 75:300–309
Miller T, van Colen G, Sander B, Golas MM, Uezquen S, Weigandt M, Goepferich A (2013) Drug loading of polymeric micelles. Pharm Res 30:584–595
Miłobȩdzka J, Kostanecki SV, Lampe V (1910) Zur kenntnis des curcumins. Ber Deut Chem Ges 43:2163–2170
Mollazade M, Nejati-Koshki K, Akbarzadeh A, Zarghami N, Nasiri M, Jahanban-Esfahlan R, Alibakhshi A (2013) PAMAM dendrimers augment inhibitory effects of curcumin on cancer cell proliferation: possible inhibition of telomerase. Asian Pac J Cancer Prev 14:6925–6928
Movassaghian S, Moghimi HR, Shirazi FH, Torchilin VP (2011) Dendrosome-dendriplex inside liposomes: as a gene delivery system. J Drug Target 19:925–932
Muddineti OS, Kumari P, Ajjarapu S, Lakhani PM, Bahl R, Ghosh B, Biswas S (2016) Xanthan gum stabilized PEGylated gold nanoparticles for improved delivery of curcumin in cancer. Nanotechnology 27:325101
Mukherjee S, Ray S, Thakur RS (2009) Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci 71:349–358
Mulik RS, Monkkonen J, Juvonen RO, Mahadik KR, Paradkar AR (2010) Transferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosis. Int J Pharm 398:190–203
Müller RH, Mäder K, Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm 50:161–177
Naseri N, Valizadeh H, Zakeri-Milani P (2015) Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull 5:305–313
Nguyen TA, Tang QC, Doan DCT, Dang MC (2016) Micro and nano liposome vesicles containing curcumin for a drug delivery system. Adv Nat Sci Nanosci Nanotechnol 7:035003
Nikpoor AR, Tavakkol-Afshari J, Gholizadeh Z, Sadri K, Babaei MH, Chamani J, Badiee A, Jalali SA, Jaafari MR (2015) Nanoliposome-mediated targeting of antibodies to tumors: IVIG antibodies as a model. Int J Pharm 495:162–170
Palange AL, Di Mascolo D, Carallo C, Gnasso A, Decuzzi P (2014) Lipid-polymer nanoparticles encapsulating curcumin for modulating the vascular deposition of breast cancer cells. Nanomedicine 10:991–1002
Pawar H, Wankhade SR, Yadav DK, Suresh S (2016) Development and evaluation of co-formulated docetaxel and curcumin biodegradable nanoparticles for parenteral administration. Pharm Dev Technol 21:725–736
Raja MA, Zeenat S, Arif M, Liu C (2016) Self-assembled nanoparticles based on amphiphilic chitosan derivative and arginine for oral curcumin delivery. Int J Nanomedicine 11:4397–4412
Rejinold NS, Sreerekha PR, Chennazhi KP, Nair SV, Jayakumar R (2011) Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarrier for curcumin drug delivery. Int J Biol Macromol 49:161–172
Safarik I, Safaríková M (1997) A high-sensitivity micromachined biosensor. Proc IEEE Inst Electr Electron Eng 85:672–680
Saikia C, Das MK, Ramteke A, Maji TK (2016) Effect of crosslinker on drug delivery properties of curcumin loaded starch coated iron oxide nanoparticles. Int J Biol Macromol 93:1121–1132
Sarika PR, James NR (2016) Polyelectrolyte complex nanoparticles from cationised gelatin and sodium alginate for curcumin delivery. Carbohydr Polym 148:354–361
Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K (2011) Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull 34:660–665
Shiri S, Alizadeh AM, Baradaran B, Farhanghi B, Shanehbandi D, Khodayari S, Khodayari H, Tavassoli A (2015) Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing M1/M2 macrophage balance in the tumor microenvironment. Asian Pac J Cancer Prev 16:3917–3922
Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, Horne R, Wardle J, Cuzick J (2016) Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol 27:575–590
Srivastava RK, Chen Q, Siddique I, Sarva K, Shankar S (2007) Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21 (WAF1/CIP1). Cell Cycle 6:2953–2961
Stefanska B, Karlic H, Varga F, Fabianowska-Majewska K, Haslberger AG (2012) Epigenetic mechanisms in anti-cancer actions of bioactive food components–the implications in cancer prevention. Br J Pharmacol 167:279–297
Steuber N, Vo K, Wadhwa R, Birch J, Iacoban P, Chavez P, Elbayoumi TA (2016) Tocotrienol nanoemulsion platform of curcumin elicit elevated apoptosis and augmentation of anticancer efficacy against breast and ovarian carcinomas. Int J Mol Sci 17:1792
Sudakaran SV, Venugopal JR, Vijayakumar GP, Abisegapriyan S, Grace AN, Ramakrishna S (2017) Sequel of MgO nanoparticles in PLACL nanofibers for anti-cancer therapy in synergy with curcumin/beta-cyclodextrin. Mater Sci Eng C 71:620–628
Sun J, Bi C, Chan HM, Sun S, Zhang Q, Zheng Y (2013) Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf B Biointerfaces 111:367–375
Suwannateep N, Banlunara W, Wanichwecharungruang SP, Chiablaem K, Lirdprapamongkol K, Svasti J (2011) Mucoadhesive curcumin nanospheres: biological activity, adhesion to stomach mucosa and release of curcumin into the circulation. J Control Release 151:176–182
Tabatabaei Mirakabad FS, Akbarzadeh A, Milani M, Zarghami N, Taheri-Anganeh M, Zeighamian V, Badrzadeh F, Rahmati-Yamchi M (2016) A comparison between the cytotoxic effects of pure curcumin and curcumin-loaded PLGA-PEG nanoparticles on the MCF-7 human breast cancer cell line. Artif Cells Nanomed Biotechnol 44:423–430
Taebnia N, Morshedi D, Yaghmaei S, Aliakbari F, Rahimi F, Arpanaei A (2016) Curcumin-loaded amine-functionalized mesoporous silica nanoparticles inhibit α-synuclein fibrillation and reduce its cytotoxicity-associated effects. Langmuir 32:13394–13402
Thadakapally R, Aafreen A, Aukunuru J, Habibuddin M, Jogala S (2016) Preparation and characterization of peg-albumin-curcumin nanoparticles intended to treat breast cancer. Indian J Pharm Sci 78:65–72
Torchilin VP (2005) Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–160
Verderio P, Bonetti P, Colombo M, Pandolfi L, Prosperi D (2013) Intracellular drug release from curcumin-loaded PLGA nanoparticles induces G2/M block in breast cancer cells. Biomacromolecules 14:672–682
Visonneau S, Cesano A, Tepper SA, Scimeca JA, Santoli DK (1997) Conjugated linoleic acid suppresses the growth of human breast adenocarcinoma cells in SCID mice. Anticancer Res 17:969–973
Wang J, Ma W, Tu P (2015) Synergistically improved anti-tumor efficacy by co-delivery doxorubicin and curcumin polymeric micelles. Macromol Biosci 15:1252–1261
Wong MW, Chew BP, Wong TS, Hosick HL, Boylston TD, Shultz TD (1997) Effects of dietary conjugated linoleic acid on lymphocyte function and growth of mammary tumors in mice. Anticancer Res 17:987–993
Yallapu MM, Gupta BK, Jaggi M, Chauhan SC (2010) Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci 351:19–29
Yallapu MM, Othman SF, Curtis ET, Bauer NA, Chauhan N, Kumar D, Jaggi M, Chauhan SC (2012) Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomedicine 7:1761–1779
Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH (2007) Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B 853:183–189
Yoon IS, Park JH, Kang HJ, Choe JH, Goh MS, Kim DD, Cho HJ (2015) Poly(D,L-lactic acid)-glycerol-based nanoparticles for curcumin delivery. Int J Pharm 488:70–77
Yu Y, Zhang X, Qiu L (2014) The anti-tumor efficacy of curcumin when delivered by size/charge-changing multistage polymeric micelles based on amphiphilic poly(β-amino ester) derivates. Biomaterials 35:3467–3479
Zaman M, Ahmad E, Qadeer A, Rabbani G, Khan RH (2014) Nanoparticles in relation to peptide and protein aggregation. Int J Nanomedicine 9:899–912
Zaman MS, Chauhan N, Yallapu MM, Gara RK, Maher DM, Kumari S, Sikander M, Khan S, Zafar N, Jaggi M, Chauhan SC (2016) Curcumin nanoformulation for cervical cancer treatment. Sci Rep 6:20051. https://doi.org/10.1038/srep20051
Zeighamian V, Darabi M, Akbarzadeh A, Rahmati-Yamchi M, Zarghami N, Badrzadeh F, Salehi R, Tabatabaei Mirakabad FS, Taheri-Anganeh M (2016) PNIPAAm-MAA nanoparticles as delivery vehicles for curcumin against MCF-7 breast cancer cells. Artif Cells Nanomed Biotechnol 44:735–742
Zhang F, Koh GY, Jeansonne DP, Hollingsworth J, Russo PS, Vicente G et al (2011) A novel solubility-enhanced curcumin formulation showing stability and maintenance of anticancer activity. J Pharm Sci 100:2778–2789
Zhang Y, Hou Z, Ge Y, Deng K, Liu B, Li X, Li Q, Cheng Z, Ma PA, Li C, Lin J (2015) DNA-hybrid-gated photothermal mesoporous silica nanoparticles for NIR-responsive and aptamer-targeted drug delivery. ACS Appl Mater Interfaces 7:20696–20706
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
De Silva, L., Goh, BH., Lee, LH., Chuah, LH. (2019). Curcumin-Loaded Nanoparticles and Their Potential as Anticancer Agents in Breast Cancer. In: Swamy, M., Akhtar, M. (eds) Natural Bio-active Compounds. Springer, Singapore. https://doi.org/10.1007/978-981-13-7205-6_7
Download citation
DOI: https://doi.org/10.1007/978-981-13-7205-6_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7204-9
Online ISBN: 978-981-13-7205-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)