Abstract
With the development of scientific exploration in deep space, human activities will become more frequent, and activity time will be longer in the deep space. The environment alteration may cause severe bone changes of human in deep space. The changes of bone mass caused by spatial microgravity are related to the decrease of osteoblast formation and development in bone tissue, and the decrease of osteoblast formation is related to the down-regulation of differentiation of human bone marrow mesenchymal stem cells (hMSCs). Therefore, the study for the biological effects of microgravity on bone cell formation and the relative molecular mechanisms at stem cell level is one of the important subjects to explore the effects of spatial microgravity on bone changes. These studies may provide a scientific basis for the development and the related technologies of target drugs research. Based on exploring the flight conditions on the ground and simulating flight experiments with the automated space experimental device, we utilized a real microgravity environment in the SJ-10 recoverable microgravity experimental satellite (SJ-10 satellite) to examine the effects of space microgravity on transcriptome expression and differentiation potentials of hMSCs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Summarization
1.1 Introduction for Mesenchymal Stem Cells
Stem cells are a population of undifferentiated cells with the potentials of self-renewal and multidirectional differentiation (Cedar 2006). Stem cells can produce two identical stem cells by symmetry splitting or differentiate into terminal cells by asymmetric splitting. In addition, the committed differentiation is regulated by the inherent mechanisms and the microenvironments.
According to their source and development potentials, stem cells can be classified into embryonic stem cells and adult stem cells. Embryonic stem cells derived from animal embryonic cell clusters or primordial reproductive crests can differentiate into all the cell types needed for fetal development. Adult stem cells can produce various types but not all the cell types required for fetal development. Adult stem cells play an important role in repair and regeneration of tissues and organs in adult animals, such as epidermal and hematopoietic systems. Under some conditions, adult stem cells can be proliferated into new stem cells, or form functional cells in accordance with a certain differentiation process to maintain the balance of growth and decay of tissues and organs.
Mesenchymal stem cells (MSCs), one of the pluripotent stem cells, originate from the mesoderm and ectoderm in the early stages of development and are initially found in the bone marrow and subsequently found in other tissues of human body. MSCs can differentiate into hematopoietic cells as well as osteoblasts, chondrocytes, adipose tissue cells, cardiomyocytes, nerve cells, hepatocytes, and other types of cells in vitro or in vivo (Pereira et al. 1995; Tuan et al. 2003; Wang 2008; Pereira et al. 1995; Wang et al. 2007; Fu et al. 2006). For their source, MSCs can be obtained from the donor under certain conditions without involving in ethical issues, which is superior to other stem cells. The superior characteristics of MSCs can be reduced as: (1) MSCs can be easily extracted from cord blood, peripheral blood, adipose tissue, placenta, muscle especially myocardium, and other tissues and organs (Lee et al. 2004; O’Donoghue et al. 2003; Chen et al. 2004; In’t Anker et al. 2004; Warejcka et al. 1996; Nasef et al. 2008). (2) MSCs are easy to be cultured and passaged several times in vitro, and their genetic characteristics is still stable after several passages. Therefore, a large number of MSCs can be obtained in a short time. All the above characteristics meet the needs for tissue engineering and treatment of disease based on stem cells. (3) MSCs have a high efficiency of gene transfection in vitro, and the foreign genes have the capacity of stable and efficient expression in MSCs. (4) MSCs have low immunogenicity and portability (Nasef et al. 2008; Sotiropoulou and Papamichail 2007) and can be delivered to various tissues or organs in the body in a variety of ways and repair the damaged tissues or organs without immune rejection. Because MSCs have the above-mentioned advantages, they have been widely used in experimental and clinical studies in recent years. Bone marrow is an important source of MSCs, MSCs from bone marrow are easier to be isolated, cultured and purified than MSCs from other sources, and this advantage brings a wider prospect of research and treatment of the bone marrow-derived MSCs. In this project, human bone marrow mesenchymal stem cells (hMSCs) were used as cell material to study the potentials of committed differentiation of hMSCs, the signal pathways and the key signal molecules specific for this committed differentiation under space microgravity.
1.2 Microgravity and Bone Loss
1.2.1 Effects of Microgravity on Bone Loss
Due to the disappearance of gravity force, a variety of tissues and organs in the body may produce a series of adaptive changes, which leads to space medical problems and thereby becomes an important obstacle to human long-term space exploration.
As an important inherit sensor organ of gravity, bone is one of organs affected by microgravity. Bone loss caused by long-term space flight mainly performs the reduced bone mineral salt content and sedimentation efficiency as well as the declined bone substance density and biomechanics function, thus gives rise to the symptoms of osteoporosis. Bone loss caused by microgravity exists continuously in the whole space flight progress. Astronauts lose their bone densities at the rate of 1–2% average each month (Tilton et al. 1980) and need a longer time for recovery after they return to earth (Lang et al. 2006) found that the amount of bone cells in astronauts decreased about 12% after space flight from four to six months and was unable to restore completely one year after they returned to earth. Another report also showed that the bone mass of astronauts was still less than preflight even five years later after astronauts had a long-term space flight and returned to earth (Schneider et al. 1995). Many symptoms accompanied with bone loss, such as the increased urine calcium excretion, the elevated blood calcium concentration and the increased risk of kidney stones or vascular sclerosis. In a word, bone loss induced by microgravity environment damages seriously the physical health and the abilities of task execution and re-flight of astronauts.
Although astronauts can adopt some effective measures, such as physical exercise, nutritional supplements and drug prevention, to against the damages caused by microgravity during space flight, bone loss cannot not be prevented completely. Therefore, the study of molecular mechanism of bone loss under microgravity environment and the design of effective countermeasures have the great significance for the health of astronauts in long-term space residence.
1.2.2 Mechanisms of Bone Loss Caused by Microgravity
Bone tissue consists mainly of cells and bone matrix, and the cells in bone include bone mesenchymal cells, bone progenitor cells, osteoblasts, bone cells, and osteoclasts. The bone has been maintained in a dynamic balance between bone resorption and bone formation to accommodate the mechanical environment. On the one hand, osteoclasts migrate to the old bone surface, and then secrete acidic substances to dissolve minerals and secrete protease to digest bone matrix, finally result in the formation of bone resorption lacunae. On the other hand, bone mesenchymal stem cells can differentiate into bone progenitor cells and osteoblasts in turn, and the bone progenitor cells and osteoblasts migrate to bone resorption site and secrete bone matrix, and further differentiate into the mature bone cells to repair the bone tissue. These bone cells can mineralize and finally form new bones (Hill 1998). Combination of bone resorption and bone formation maintains a dynamic balance to keep a normal bone mass under normal circumstances. However, this balance of bone reconstruction may be broken due to the change of mechanical environment, and the destroyed balance may result in microgravity-relative bone loss.
1.2.2.1 Effects of Microgravity on Mesenchymal Stem Cells
Osteoblasts are derived from bone marrow stem cells. Bone formation includes two progresses: (1) Mesenchymal stem cells differentiate into osteoblasts, and (2) osteoblasts differentiate into to the mature bone cells. In vitro, bone mesenchymal stem cells (BMSCs) can be firstly induced to the directional differentiation of bone progenitors and osteoblasts, and finally induced toward the differentiation of bone cells. Differentiation of BMSCs into osteoblasts is a very complex process and regulated by many factors such as cytokines, hormones and mechanical force stimulation. The changes of mechanical force stimulation caused by microgravity may directly affect the function of BMSCs and thereby affect the bone formation.
Previous studies showed that microgravity affects the proliferation ability of BMSCs. BMSCs are harvested from the femur subculture of rats experimented by a seven-day tail hanging and cultured for passage. The results showed that the proliferation of BMSCs under simulated microgravity is slower than that under normal gravity (Pan et al. 2008). Experiments in Cell Rotary have also proved the inhibited effects of simulated microgravity on the proliferation of mesenchymal stem cells (Huang et al. 2009). Furthermore, the mitotic cycle of mesenchymal stem cells was blockaded in G0/G1 stage by microgravity (Dai et al. 2007), which indicates that the proliferation ability of BMSCs was restrained under microgravity environment.
Under specific induction conditions, BMSCs can be induced to differentiate into many types of cells, including osteoblasts, chondrocytes, fat cells, myocardial cells and other kinds of cells, but the differentiation behaviors are changed significantly under microgravity. BMSCs stimulated by microgravity were passaged under normal gravity, and then induced to differentiate into osteoblasts and adipocytes in the conditioned medium. Results showed that the differentiation ability of bone mesenchymal stem cells into adipocytes gradually decreases with the time of microgravity removal, while the differentiation ability into osteoblasts does not increase and remains in a low potential of osteogenesis compared with the control under normal gravity (Pan et al. 2008). Microgravity inhibits the osteogenic differentiation of BMSCs and reduces the expression of RUNX2, COL1A1, ALP and other osteoblast marker genes. At the same time, the expression PPARG2 (peroxisome proliferator activated receptor gamma), a key adipogenic transcription factor, is increased significantly (Huang et al. 2009; Marie and Kaabeche 2006; Zayzafoon et al. 2004) found that PPARG2 can positively regulate the differentiation of BMSCs into adipocytes and negatively regulate the differentiation of BMSCs into osteoblasts. The balance of differentiation into adipocytes and osteoblasts can be restored through foreign importing TGF-β2 to regulate the PPARG2 expression (Lazarenko et al. 2006). RUNX2, a bone specific transcription factor, can activate the expression of osteopontin and osteocalcin, and promote miner deposition. In addition, PPARG2 can inhibit the expression of RUNX2 gene or reduce the concentration of activated RUNX2 by combining with RUNX2 (Platt and El-Sohemy 2009). Therefore, microgravity not only directly weakened the osteogenic ability of BMSCs, but also inhibits the osteogenic differentiation of BMSCs through enhancing their adipogenic differentiation.
1.2.2.2 Effects of Microgravity on Osteoblasts
The data obtained from some space flight experiments illuminated that microgravity inhibits osteoblast differentiation. Previous study showed that microgravity environment not only obviously suppresses the osteoblast differentiation of MG63 cells cultured on “Photon-10” for a nine-day flight experiment but also decreases their responses to 1, 25(OH)2VD3 and TGF-β (Carmeliet et al. 1997). In addition to the decreased number and declined function of osteoblasts, microgravity also changes the morphology of osteoblasts. After space flight, the microtubules and actin filaments of MC3T3 cells are depolymerized, and the middle fiber shows an abnormal morphology. More detection found that the order of cytoskeleton declines, microtubules become shorter, and the reticular structure appears around the nucleus. All results proved that microgravity destroys the morphological characteristics of osteoblasts (Hughes-Fulford et al. 1998)
Gravity plays a key regulatory role in the osteogenic differentiation through changing chemical signals in cells. Dai et al. (2013) found that microfilament cytoskeleton is involved in regulating the response of osteoblasts to BMP2, and microgravity inhibits the response of RUNX2 to BMP2 by promoting depolymerization of microfilaments in osteoblasts, while the promoting effect of BMP2 on osteoblast differentiation can be restored partly after adding the skeleton stabilizer. Another study showed that the mechanical stimulation and cytokines can allegedly regulate the osteoblast differentiation. Membrane surface protein integrin αVβ3 in osteoblasts, one of important molecules in response to gravity, can regulate the osteogenic differentiation through PI3K signaling pathway with cooperating with IGF-1 (Dai et al. 2014). miRNAs also play an important role in regulating the bone loss caused by microgravity. MIR-103 has been proved as a miRNA associated with bone loss caused by microgravity environment. Zuo et al. (2015) found that the expression of MIR-103 significantly increases in mouse during the tail hanging experiment and the expression of RUNX2 significantly decreases. Further study showed that MIR-103 directly targets RUNX2 and inhibits its protein expression, thus exhibits regulatory function on the bone loss caused by microgravity. Injection of MIR-103 inhibitor can reduce the bone loss caused by tail hanging experiment. In addition, Sun et al. (2015a, b) simulated the microgravity effects using a cell rotary system and found that the expression of MIR-103 significantly increases in MC-3T3 cells under cell rotary system. It suggests that microgravity may suppress the expression of Cav1.2, one calcium ion channel, and then inhibit the proliferation of MC-3T3 cells.
MIR-214 is also related with the bone loss caused by microgravity. Wang et al. (2013) found that the expression of OCN and ALP decreases and the bone formation index such as bone volume (BV), bone mineral density (BMD) declines in tail hanging mice models, while the expression of MIR-214 increases. MIR-214 can suppress the bone formation through inhibiting the expression of ATF4. The delivery of MIR-214 antagonists to osteoblasts using osteoblast specific delivery system can significantly inhibit the bone loss caused by tail hanging.
1.2.2.3 Effects of Microgravity on Osteoclasts
Osteoclasts are derived from mononuclear cells, and RANKL secreted by osteoblasts can induce mononuclear cells to differentiate into osteoclasts. In FOTON-M3 space mission, Tamma et al. (2009) inoculated osteoclasts and osteoclast precursors on cow bone chips to examine the differentiation of osteoclasts. They found that the expression of genes specific for osteoclasts are significantly increased, and bone resorption lacunaes are also increased after space flight. These findings suggest that microgravity enhances the osteoclasts activity. Ground experiments showed that simulated microgravity can activate signaling molecules specific for osteoclastic differentiation, such as ERK, P38, PLC2 and NFATc1, and the activated molecules make osteoclast precursors more sensitive to the RANKL-mediated osteoclastic differentiation. After the osteoclasts are cultured in a rotary system for 24 h and treated with RANKL for 3–4 days, the formation of TRAP-positive multinucleated osteoclasts can be enhanced, and the osteoclast marker genes TRAP and cathepsin K raise significantly (Saxena et al. 2011). In addition, the expression of RANKL/OPG significantly increases in osteoblasts under simulated microgravity, and the medium supernatant from this simulated microgravity experiment can promote the differentiation of osteoclasts (Rucci et al. 2007).
1.3 Methods and Techniques of Cell Research in Space
At present, microgravity biology mainly includes two aspects: space flight experiment and ground simulation experiment. Space flight experiment is the most obvious way to obtain a low gravity by space vehicles, such as space shuttle and return satellite. However, flight experiments are expensive and have limited opportunities (Krikorian et al. 1992; Mesland et al. 1996; Katembe et al. 1998a, b). Therefore, the following methods can also be used to reduce or offset 1-g gravity (Claassen et al. 1994; Mesland et al. 1996), drop towers, parabolic flights (tens of microseconds), and sounding rockets (microgravity for a few minutes). However, the duration of microgravity experiment provided by these methods is too short for biological experiments. The device used widely to simulate “weightlessness” in ground is called as the clinostat, which is based primarily on the time required for gravity stimulation by plants (Claassen and Spooner 1994; Hoson et al. 1997).
1.3.1 Parabolic Flights
Various types of aircraft, ranging from small propeller-powered aerobatics to large KC-135 turbojet airliners, have been used for parabolic flight to create a gravity-reducing environment. A small propeller can produce microgravity for 5–8 s. A small non-military helicopter such as a learner jet, the Nagoya Mu-300, can produce 2 × 10−2 × g microgravity for more than 20 s. Parabolic aircraft modified by the European Space Agency (ESA), the Canadian Space Agency and the United States Aeronautics and Space Administration (NASA) are capable to produce 1-3 × 10−2 × g microgravity for 20–30 s. Boeing KC-135 and supersonic F-104 helicopters are used for parabolic flight aircraft in United States. The F-104 parabolic flight, which flight path is from 25,000 to 65,000 feet, produces 3 × 10−2 × g for 30 s or less than 1 × 10−1 × g for 60 s. The parabolic trajectory of the KC-350 (NASA-930) flight is similar to F-104 parabolic flight and the path was from 26,000 to 34,000 feet. Before the 90s, NASA’s parabolic flight was designed to train astronauts and pilot space flight hardware, and it could be used to carry out scientific experiments for 5–6 times one year.
1.3.2 Sounding Rocket Flights
Sounding rockets are suborbital vehicles which normally lands on the area about 100 miles far from the launch site and provides a microgravity of 1 × 10−5 × g for 7–14 min (Gurkin 1992). The cellular biology study by using sounding rocket is similar to those by using parabolic flight. However, the sounding rockets can supply a longer time and a higher level of microgravity, and thereby study extensively the cytology.
1.3.3 Orbital Spaceflights
Orbital aircrafts such as bio-satellites, space crafts and space shuttles, can provide several days to tens of days, as well as higher and more stable level of microgravity.
1.3.4 Space Stations
More than 10 space stations have been established since 1971, such as “Salute” series and Mir Space Stations established by the former Soviet Union, and Sky Laboratory set up by Unite States. In recent years, International Space Station, the biggest space station, which was jointly established by Unite States, ESA, Russia, Canada, has brought a series of opportunities for the development of space life science. Temple Space Station, prepared by China, has completed partial assembly.
1.3.5 Ground Simulators
Gyrator is a device that rotates a biological sample around an axis (usually a horizontal axis), where the organism is still in the gravitational field and is subjected to a constant gravitational vector. However, the direction of organism and gravity is continuously changing in a gyrator. Whenever the relative direction of gravity changes, the biological effects have not yet time to show before the next direction has changed. Since the direction of gravity vector cannot be kept constant for this minimum time, the effect of gravity is always too late to behave as if there is no gravitational force, and this phenomenon is similar to microgravity. Thus, the biological effect under microgravity condition is demonstrated (Wang et al. 2015). The gravity vector is of constant size and continuous change of directions from a reference coordinate system fixed on a biological sample. However, the object is always in a constant gravity role in a rotor. Strictly, gyrator only simulates the effect of microgravity, but not simulates microgravity. The performance of organisms on the gyroscope is just qualitatively similar to the samples under the space microgravity conditions, but not in quantitative level.
1.4 Scientific Significance of This Space Experiment
After performing a variety of experiments under simulated microgravity and developing multiple comparisons with experiments under normal gravity, we have determined the biological effects, molecular mechanisms and key cell signaling molecules specific for these effects during the osteogenic differentiation. Else, we performed the experiments of regulation with small molecule drugs in view of the key signaling molecules, thus identified the feasibility of reversing the effects of microgravity on the osteogenic differentiation. However, any simulated experiment has its limitation and controversy. The effective solution for this problem is to perform scientific experiments in the real space microgravity, so as to truly reflect the biology effects and molecule mechanisms of microgravity on osteogenic differentiation.
The Recoverable Science Satellite SJ-10 of China provided a true microgravity and pressure sealing environment for us to develop biological experiments in space. Two-week flight cycle is just suitable for our experimental period in space, and the timely returned space cell samples can provide a guarantee for analysis on the effects of microgravity. The experiment for committed differentiation of hMSCs was performed in satellite recoverable capsule. Therefore, it is necessary to develop the automatic space experiment equipment that can carry out the stable and meticulous cell culture operation. This space equipment should meet the requirements of space experiment process involving culture, differentiation and fixing of cells and samples preservation at low temperature.
The objective of this space experiment is to study the effects of microgravity on the osteogenic differentiation potentials and molecule mechanisms as well as the key cell signaling molecules specific for osteogenic differentiation. These studies may provide scientific basis for overcoming the bone loss during space exploration and developing the targeted drugs. This project is the first research on the molecule mechanisms specific for osteogenic differentiation of mesenchymal stem cells under real microgravity.
2 Space Culture Device
2.1 Research Basis for Space Culture Device
Due to the disappear of convection, sedimentation and hydrostatic pressure caused by microgravity in the space environment, the limitation of space resources (such as volume, weight, power consumption etc.) and the maneuverability of cells culture in space, the cultivation vessels and devices used on the ground cannot be used in the space experiments. It is necessary to develop a cell bioreactor that is specifically adapted for operation in the space environment. The space cell bioreactor should meet the requirements of loading bearing, temperature controlling, culture fluid supplication, gas exchanging and pH buffering functions as the cell culture device on the ground, and the realization of various functions must be taken account of the mechanical characteristics in the space microgravity environment, especially the change in the fluid mechanics behavior. Else, the bioreactor should substantially depend on the automatic operation.
The space experiment for the committed differentiation of hMSCs is designed as two programs (experiment I and experiment II) according to the arrangement of scientific mission for SJ-10 satellite. The culture proposal included: (1) MSCs in experiment I were induced for two days with osteogenic induction medium (Levis-15 medium supplement with 10% of FBS, 50 μg/mL L-ascorbic acid, 10 mM β-sodium glycerol-phosphate, 10−7 M dexamethasone, 100 U/mL penicillin, and 100 μg/mL streptomycin), then the induction medium was replaced with washing buffer-PBS, finally RNAlater was added after wiping off PBS. Then the cell samples were stored for ten days under ~6 °C until the satellite returned to the ground. After satellite returns to the ground, space samples were harvested, RNA and proteins in samples were extracted, and the effects of microgravity on the expression of genes and the activity of proteins specific for osteogenic or adipogenic differentiation were analyzed. (2) hMSCs in experiment II were induced for seven days with osteogenic induction medium, refreshing induction medium every two days. Then the induced program was terminated, cells were fixed with 4% formaldehyde after being washed with PBS for one time. The fixed sample was kept in ~9 °C until satellite returned to the ground. Samples in experiment II was harvested and detected by histochemistry and immunohistochemistry to analyze the osteogenic differentiation potentials of hMSCs.
According to the object of this space experiment, the automatic device controlled by software must satisfy the following requirement. Firstly, this device should contain the cell culture unit and the liquid storage unit. Moreover, the device can provide airtight environment and suitable temperature for cell culture as well as the lower temperature for storage of cell samples and liquids. Furthermore, the device can timely replace the liquid in cell culture unit, including the induction medium, washing buffer and reagents. The device can also adjust and control the volume and rate of liquid replacement. Finally, the device is able to automatically monitor the humidity, pressure and other conditions inside the device.
2.2 Principle of Space Culture Device
On the basis of the above design requirements, the National Space Science Center of Chinese Scientific Academy designed and prepared an automatic device for culture and induction of hMSCs. In this device, the cultivate unit consists of two cultivation vessels respectively for experiment I (two days) and experiment II (seven days), and three PLGA three-dimensional scaffolds with hMSCs are cultured in each cultivation vessel. The cultivate unit can provide 37 ± 1 °C temperature environment controlled by semiconductor thermostat. hMSCs are cultured in the normal culture medium (α-MEM culture medium supplemented with 10% of FBS, 100 U/mL penicillin, 100 μg/mL streptomycin and 5 ng/mL BFGF) before the orbital implantation, and the normal culture medium is taken out after the orbital implantation, and then the induction medium is injected into cultivation vessels to start the induction program. At the end of induction, the induction medium is aspirated into the washing buffer. Finally, RNAlater (for 2 days) or 4% formaldehyde (for 7 days) is injected into the cultivation vessel to treat cells. Then, the thermostat is switched to cryogenic storage state until the satellite returns to the ground.
The liquids required for space experiment are stored in their respective containers. Induction medium, RNAlater and cell fixing solution are stored at low temperature controlled by a semiconductor thermostat for seven days, and the temperature of washing buffer and waste liquid is not controlled overall the space experiment process. The fluid circuit system containing of the syringe pump and the fluid dispenser implements the liquid withdrawal and injection. In order to prevent the liquid to reflux, three electromagnetic valves are connected with the fixing solution, RNAlater and waste liquid task separately and then connected with the corresponding piping of the fluid distributor, and the liquids are distributed into the cultivation vessel inlets and outlets. Solenoid valve opens only when necessary to prevent fixing solution or waste liquid mixing to the cultivation vessel when the channel switch opens during medium replacing, which can avoid any impact on the normal cell culture. Load system principle is shown as Fig. 1 in chapter “Study on Bone Marrow Box, Radiation Gene Box and Integrated Electrical Control Boxes”.
The measurement and control circuit with regarding a FPGA logic controller as the core completes the process of operation, data detection and storage in the space experiment. Data transformation and command reception are run through a 422 serial port and an integrated electronic control box. Integrated electronic control box controls the experimental process and provides power required by the load according to the present or injection instructions. Load electric control schematic diagram is shown in Fig. 1.
2.3 The Structure and Performance Index of Space Culture Device
2.3.1 Whole and Internal Structure of Space Culture Device
As shown in Fig. 2, the space culture device includes five parts: an external three-stage airtight chassis, 2 cultivation vessels, 6 liquid storage units, 1 automatic liquid change system, and 1 electronic control system. The fluid system tubing is connected by silicone rubber.
2.3.2 The Main Indicators of Space Culture Device
The internal structure designing of space culture device is not only necessary to meet the requirement of scientific experiment and adapt to the characteristic of microgravity environment, but also limited to the satellite space, energy, air pressure and thermal control and other resource. The main performance indicators are showed as Tables 1 and 2.
3 Ground Preparation and Scientific Matching Experiments
Due to the limitation of space, environment, loading capacity and flight program of the satellite, the space experiments are different from the ground experiments. Therefore, many experimental conditions should be improved and optimized on the ground to meet the requirement of the space experiments. A multi-stage and multi-round matching experiment should be performed on the ground to ensure that the cells are kept in a relative good condition of growth and differentiation in the space culture device. The matching experiment is divided into four stages: the matching experiment phase for the component testing of device, the matching experiment phase for preliminary device, the matching experiment phase for the official device, and the matching experiment phase for launching preparation. Each stage had its definite experimental target, process and time node. A systemic matching experiment of osteogenic differentiation is formed through the connection of different processes involving induction of differentiation, washing, fixation and lysis of induced cells in the space culture device.
3.1 Preparation of hMSCs
hMSCs were extracted from healthy donors sponsored by the First Affiliated Hospital of Zhejiang University. The purification of hMSCs is referred to the previous literature (Shi et al. 2010). Briefly, 5 mL human bone marrow was added to the DMEM medium containing heparin to prevent blood coagulation, and then transported to the laboratory at low temperature. Mononuclear leucocytes were isolated using lymphocyte isolation liquid Fillco by centrifuging at 1800 rpm for 10 min. Cells were seeded in the culture dish with a density of 106 cells/mL. hMSCs could be purified through the adherent culture, and the cells at passage 3 or 4 were used for the matching experiment. The properties of hMSCs were determined by detecting the cell surface markers using flow cytometry. hMSCs showed the following phenotype: CD34−, CD14−, CD19−, CD45−, HLA-DR−, CD73+, CD105+, CD90+, which make sure that these cells are MSCs (Fig. 3).
3.2 Preparation of PLGA Scaffold
In order to simulate the three-dimensional environment in vivo, PLGA three-dimensional scaffold was used for cell culture. The preparation of porous poly-lactic-co-glycolic acid (PLGA) scaffold was described previously (Zong et al. 2010). Briefly, the gelatin particles were bound together to form a three-dimensional assembly through a water vapor treatment before the polymer solution was cast. The PLGA scaffolds were prepared as cylinders (ϕ 25 × 1 mm) and had porosity of 85%. The pore diameter was 280–450 μm.
3.3 Culture and Osteogenic Induction of hMSCs in the Condition Without Carbon Dioxide
Gas involving O2 and CO2 is one of the necessary conditions for survival of mammalian cells cultured. O2 is mainly involved in tricarboxylic acid cycle, resulting in production of the energy required for growth and proliferation of cells and synthesis of various components required for cell growth. CO2 is not only one of cell metabolites, but also one of components required for cell growth and reproduction, and its major role in cell culture is to maintain the pH value of medium. Culture medium pH is generally kept between 7.0 and 7.4. Most of culture mediums are based on a balanced salt solution (BSS), which acts as a buffer solution and can greatly stabilize the pH when a small amount of acid, base or water is added. The pH buffer system plays an important role in maintaining the normal pH value of the organism and the normal physiological environment. Most of cells can only be operated in a very narrow pH range, and need a buffer system to counteract the changes of pH that may occur during their metabolic processes. Since the carbonate pH buffering system is a physiological pH buffering system, which is the most important pH buffer system in human blood, this system is used in most of culture mediums to maintain a stable pH value. A given amount of sodium bicarbonate is often added in the preparation of powder culture medium. For carbonate regarding as the basis pH buffering system in most of cell cultures, the concentration of CO2 in the incubator needs to be kept between 2 and 10% to maintain a stable pH value, and the CO2 concentration at 5% in the open culture can maintain the concentration of dissolved carbon dioxide in the medium. In addition, a certain degree of ventilation in the cultivation vessel is needed to facilitate the exchange of gas. It has been found that HCO3-depletion in the culture medium is accelerated if the cells are not incubated in a carbon dioxide incubator due to the low concentration of carbon dioxide in the air and thereby may reduce the proliferation of cells. Therefore, most of animal cells need to be cultured in the carbon dioxide incubator. In the space science experiment, however, CO2 storage tanks could not be loaded due to the constraints for satellite space, payload, device design and safety in SL-10 satellite. Therefore, how hMSCs can normally grow and differentiate in the condition without carbon dioxide is the primary problem we should resolve.
In the previous experiment, we explored whether hMSCs could be induced toward to osteogenic differentiation in the condition without carbon dioxide. Experimental protocol was as following: hMSCs were cultured in the induction medium of osteogenic differentiation for 2 days and 7 days in a cell incubator without carbon dioxide, and the induction medium was changed every 2 days. We collected the cell culture supernatant and measured the pH valve of the supernatant, and found that the pH valve of the cell culture supernatant decreased very rapidly, and this decrease in pH valve became more rapid as the induction time increased (Fig. 4a). The morphological changes were not observed on day 2. However, the cells became rounded on day 4 and cells death occurred on day 5 (Fig. 4b). It is concluded that hMSCs cannot be cultured for normal growth more than 7 days in the condition without carbon dioxide. Therefore, this experimental conditions need to be optimized to meet the requirement of this space experiment.
The influence of Levis’-15 and C-DMEM induction media on the osteogenic differentiation of hMSCs. a The PH valve in the culture of hMSCs with the traditional induction medium for 7 days in the condition without carbon oxygen. b The morphology of hMSCs cultured with the traditional induction medium in the condition without carbon oxygen for 3, 4 and 5 days. c The PH valve in the culture of hMSCs in the traditional induction condition (traditional induction media with 5% carbon oxygen) for 7 days and the PH valve in the culture of hMSCs with Levis’-15 and C-DMEM induction media in the condition without carbon oxygen. d The expression of ALP and RUNX2 mRNA in hMSCs cultured in the traditional induction condition and in Levis’-15 and C-DMEM induction media in the condition without carbon oxygen for 2 days; e ALP staining of hMSCs cultured in the traditional induction condition and in Levis’-15 and C-DMEM induction media in the condition without carbon oxygen for 7 days. Scale: 100 μm. CON: hMSCs cultured in the traditional induction condition; C-DMEM: hMSCs cultured with C-DMEM induction medium; L-15: hMSCs cultured with Levis’-15 induction medium
When the permeable cultivation vessel is taken out of the CO2 incubator, the carbonate buffer system in the culture medium is lost its buffer effects due to the low concentration of carbon dioxide in the air. Therefore, additional pH buffering systems, such as HEPES buffer systems, are often added to the culture medium to increase pH buffering capacity. HEPES has a strong buffering capacity between pH 7.2 and 7.4. We increased the concentration of HEPES buffer system components in the traditional induction medium (L-DMEM medium supplement with 10% of FBS, 50 μg/mL L-ascorbic acid, 10 mM β-sodium glycerol-phosphate, 10−7 M dexamethasone, 100 U/mL penicillin, and 100 μg/mL streptomycin) to study whether the PH valve of induction medium can be maintained in the physiology range for 7 days in the condition without carbon dioxide. However, we found that the HEPES buffer system could stabilize the pH valve of induction medium for a short term but not for a long-term in the condition without carbon dioxide. Therefore, we used a phosphate buffer system to replace the carbonate buffer system in the traditional induction medium. Sodium bicarbonate was added to L-DMEM culture medium without NaHCO3. Sodium dodecahydrate, potassium dihydrogen phosphate, and sodium chloride were used to adjust the osmotic pressure, and then the pH valve of medium was adjusted to 7.2. This medium was designed as Table 3 and named as C-DMEM medium.
In addition, Levis’-15 (hereinafter referred to as L-15 medium) is a commercial medium suitable for cell growth in the condition without carbon dioxide. We performed the experiments of osteogenic differentiation in Levis’-15 and C-DMEM induction media (Levis’-15 or C-DMEM medium supplement with 10% of FBS, 50 μg/mL L-ascorbic acid, 10 mM β-sodium glycerol-phosphate, 10−7 M dexamethasone, 100 U/mL penicillin, and 100 μg/mL streptomycin) in the condition without carbon oxide. As shown in Fig. 4c, no significant difference of cell morphology was detected between cells cultured in these carbon dioxide-free induction media (Levis’-15 and C-DMEM induction media) and the traditional induction medium. During the induction, both carbon dioxide-free induction media maintained the normal physiological PH valve.
In order to investigate the effects of these carbon dioxide-free induction media on the osteogenic differentiation of hMSCs, we examined the genes expression of RUNX2 and ALP specific for osteogenesis after induction for 2 days (Fig. 4d) and the activity of ALP after induction for 7ays (Fig. 4e). The results showed that these carbon dioxide-free induction media can significantly promote the osteogenic differentiation of hMSCs, and there is no difference of differentiation potentials between cells cultured in the carbon dioxide-free induction media and the traditional induction medium.
The above results indicated that both C-DMEM and L-15 induction media are able to maintain the normal PH value for 7 days, and does not change the properties of the cells. These features can meet the requirement for osteogenic induction of hMSCs in the condition without carbon dioxide.
3.4 Storage of Induction Medium in Low Temperature
L-Glutamine is important in cell culture. L-glutamine can be used as the energy source of cells, and participates in protein synthesis and nucleic acid metabolism. However, glutamine is an amino acid particularly easy to be deteriorated. Previous studies have shown that the half-life of glutamine is about 2 weeks at 4 °C, and the stability of glutamine is related with the storage temperature and the PH value. The degradation of glutamine will increase as the increase of temperature.
Cell contamination, usually including bacterial and fungal contamination, is a common problem in cell culture, especially in space experiments of cells. The complexity of operation for cell culture in space is far greater than that on the ground, which may increase the probability of contamination. Therefore, the addition of antibiotics can effectively prevent the contamination of bacterial and fungal in cell culture. Conventional antibiotics, penicillin and streptomycin, are two broad-spectrum bacterial antibiotics used commonly, and amphotericin B is another antifungal antibiotics. But these antibiotics have lower stability. The antibiotics diluted in the culture medium can be usually stored at 4 °C for more than 2 weeks. Subsequently, the antibiotic effect of antibiotics will decrease obviously as the time and temperature increase.
Generally, the optimal storage conditions for cell culture media supplemented with glutamine and antibiotics are 2–8 °C on the ground. In the SJ-10 satellite, however, the lowest temperature in the liquid storage tank of space culture device can be only kept 10 ± 1 °C due to the limitation of energy consumption in the satellite. In order to verify whether the induction medium and other solutions stored at this temperature is still effective for induction of osteogenic differentiation, the prepared L-15 induction medium was placed in 4 °C low-temperature refrigerator and 12 °C incubator respectively. The osteogenic differentiation of hMSCs was induced for 7 days with the stored L-15 induction medium, and the induced cells were subjected to ALP staining. As shown in Fig. 5a, the induction medium stored at 11 °C could induce the osteogenic differentiation of hMSCs, and the expression of ALP was positive. There was no difference of osteogenic potentials between cells induced with the induction medium stored at 11 °C and the induction medium stored at 4 °C. Results showed that 10 ± 1 °C of liquid storage provided by space culture device can meet the scientific requirement for this space experiment.
ALP staining of hMSCs. a ALP staining of hMSCs cultured for 7 days with Levis’-15 induction medium stored at 4 °C or 11 °C; CON: Levis’-15 induction medium stored at 4 °C; EXP: Levis’-15 induction media stored at 11 °C. b ALP staining of hMSCs cultured for 7 days with Levis’-15 induction medium after the mechanical simulation experiments. Scale: 100 μm. CON: control; MF: the mechanical simulation experiment
3.5 Simulation Experiment of Mechanic Environments
During launching and flying, the satellite must be subjected to the dynamic loads such as vibration, shock and noise generated during rocket launching, satellite-rocket separating and orbiting. The animal cells are sensitive to mechanic force. In order to ensure the smoothness of the space experiment, therefore, it is necessary to simulate the mechanic environment caused by dynamic loads to examine the effects of mechanical environment during the satellite launching and flying on the adhesion, growth and differentiation of hMSCs.
We performed the experiments to study the effects of shock, oscillation, and acceleration on cells cultured in the space culture device. The experimental parameters are shown in Table 4. We treated the three-dimensional scaffold surface with gelatin and matrix glue to increase the adhesion of cells. The cells were seeded into the scaffolds, and then the scaffolds were placed in the cultivation vessels. The cultivation vessels were fixed in a culture apparatus before mechanical simulation experiments. After the mechanical simulation experiment, cells in the cultivation vessels were examined. We did not find any floating cells, which showed the good adherence of cells in scaffolds. In addition, the mechanical simulation experiment indicated that the mechanical simulation has no effect on the osteogenic potentials of hMSCs (Fig. 5b). Therefore, our results suggested that hMSCs could withstand the loading of satellite during launching and flight.
4 Space Flight Experiments
4.1 Space Flight Experiments Programs
The above-mentioned matching experiment on the ground solved several major problems that may be encountered in the space experiment, which ensured the smooth running of this space experiment. On this basis, we developed the process for space experiment. The specific process is shown as Fig. 6. The space experiment consisted of two phases: the ground preparation phase pre-launching and the flight phase post-launching. The process for ground preparation was run by manual operation of the inspection device on the ground, and the process for space experiment in flight stage was run by the integrated electronic control program set prior to the operation of the flight sequence. If the process of space experiment program is abnormal, the procedure of operation will be changed by sending the immediate command from the ground.
To better simulate the in vivo environment of hMSC growth and differentiation and reflect the real microgravity environment, a three-dimensional scaffold was used to culture hMSCs instead of regular cell culture. The core diameter and porosity were shown in Sect. 3.2 and hMSCs seeding density was 4 × 105/scaffold.
The space experiment started at 2 am April 6, 2016, and stopped at 2 pm April 18, 2016. The space experiment was lasted for 12 days and 12 h. At 18 min after injection, the induction procedure of osteogenic differentiation was started by the integrative electric control program. The culture medium was extracted from the cultivation vessel, and then the induction medium was injected into the cultivation vessel. After 2 days of space osteogenic induction, cells induced in one of cultivation vessels were lysed, and then the temperature was switched to 6 °C until satellite returned to the ground. After 7 days of space osteogenic induction, cells induced in another cultivation vessel were fixed, and then the temperature was switched to 6 °C until satellite returned to the ground. The temperature in the liquid storage unit was set as 8 ± 2 °C during ground preparation and maintained for 7 days after launching, and then the control of temperature was stopped. The process of space experiment consisted of 13 procedures and the temperature-controlling were shown as Table 5 and Fig. 7.
Temperature curve in space culture device. The temperature in cultivation vessel 1 was maintained at ~37 °C for the first 2 days and then remained at ~6 °C for another 10 days. The temperature in cultivation vessel 2 was maintained at ~37 °C for the first 7 days and then remained at ~9 °C for another 5 days. The temperature in storage tanks was maintained at ~10 °C for the first 8 days and then uncontrolled for the remaining time
4.2 Overviews of Returned Space Culture Device
After the satellite returned to the ground, the external appearance of space culture device dismantled from satellite was intact by visual viewing. Two cultivation vessels were filled with RNAlater and fixative liquids respectively, and the amount of liquids in other tanks was also normal. Two cultivation vessels were confirmed to be free of contamination. The results showed that the space experiment programs were completed satisfactorily and the required cell samples in the space experiment were obtained from the space culture device. Disassembly of space culture device is showed as Fig. 8.
The space experiment samples were brought back to the laboratory at low temperature. At the same time of space experiment, we performed the ground-based control experiments. The cells, reagents, culture device and experimental programs used in the ground-based control experiments were same as the space experiments. Afterwards, the samples from space experiments and ground-based control experiments were analyzed as followings.
4.3 Materials and Methods for Analysis of Induced Cells
4.3.1 RNA Isolation
After RNA samples lysed with RNALater were collected, RNALater was removed by centrifugation at 1000 g for 3 min, and the pellet was then washed three times with PBS. RNA extraction was performed using TRIzol (Sigma). Following a phenol/chloroform extraction and isopropanol precipitation, RNA samples were treated with DNase I using the DNA-free TM kit (Ambion). The prepared RNA samples were subjected to deep sequencing and real-time (reverse transcriptase) polymerase chain reaction (PCR) assays.
4.3.2 Real-Time Polymerase Chain Reaction (PCR) Assays
The PCR assay was performed to determine the expression of genes specific for osteogenesis or adipogenesis in cells under normal gravity or space microgravity. After a lysate from each cell culture compartment was collected, RNALater was removed by centrifugation at 1000 g for 3 min, and then the pellet was washed with PBS three times. RNA extraction was performed using the TRIzol reagent. Reverse-transcription was conducted using 1 μg of total RNA with the RevertAid First Strand cDNA Synthesis kit. Primers were designed using the Primer Premier 6.0 Demo and Oligo 7.36 Demo Software as shown in Table 6. Quantitative real-time PCR reactions were run in triplicate on a Roche 480 II Real-Time PCR Detection System using the 480 SYBR Green qPCR Supermix. The cycling conditions of the real-time PCR were as follows: 40 cycles at 95 °C for 15 s and 60 °C for 1 min. GAPDH served as the reference gene of classical real-time PCR.
4.3.3 RNA-Sequencing Assay
Adaptor ligation and PCR amplification were performed using a TruSeq RNA sample prep kit (Illumina, San Diego, CA, USA). SPRI beads (Ampure XP, Beckman, Brea, CA, USA) were used in each purification step after RNA fragmentation for size selection. All libraries were analyzed for quality and concentration using a TBS380 PicoGreen (Invitrogen). Sequencing was performed using a HiSeq 4000 SBS Kit (Illumina). RNA-seq reads were mapped to the human reference genome using TopHat2. Gene expression analysis and measurement was performed with FeatureCount software and FPKM. Genes with FDR < 0.05 and |log2 (fold)| ≥ 1 were marked as significant.
4.3.4 Whole-Cell Protein Extraction and Western Blot Analysis
Cells osteogenically induced under normal gravity or space microgravity for 2 days were used for analysis of activity of proteins. Lysates were prepared by sonication of the scaffold in lysis buffer containing 1 mM NaF, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM Na3VO4, 1 mM EDTA, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), and a phosphatase inhibitor. Protein concentration was determined using the BSA protein assay kit. The expression levels of Runx2 or p-Runx2, were determined by western blotting. Proteins from each sample were subjected to SDS-PAGE in a 10% gel, and then transferred to a 4.5-μm PVDF membrane. The membrane was blocked with a BLOTTO solution, and then incubated with anti-Runx2, anti-tyrosine antibody or with anti-GAPDH antibody at 4 °C overnight. Afterwards, the membrane was reprobed with appropriate secondary antibodies (conjugated with horseradish peroxidase) for 1 h. Next, the immunoreactive proteins were visualized using ECL Western blotting detection reagents, and light emission was quantified with a Tanon 6600 Luminescence Imaging Workstation.
4.3.5 Histochemical and Immunohistochemical Analyses
Cells osteogenically induced under normal gravity and space microgravity for 7 days and fixed with 4% formaldehyde were embedded in paraffin and then sectioned at 3-μm thickness. Alkaline phosphatase activity was visualized using the ALP Gomori Staining Kit.
To determine the expression levels of ALP, COL1A1 and Runx2 in cells osteogenically induced under normal gravity or space microgravity for 7 days, paraffin-embedded slices were incubated with anti-ALP, anti-COL1A1 and anti-Runx2 antibodies at a dilution of 1:100 overnight at 4 °C. Slides were washed with PBS three times and then incubated in the REAL EnVision Detection System.
4.3.6 Scanning Electronic Microscope (SEM) Assay
To examine the effect of microgravity on the morphology of cells in the scaffold, the sample fixed with 4% formaldehyde was used for the SEM assay. After the 4% formaldehyde was discarded, the sample was fixed in 2.5% glutaraldehyde (Sigma-Aldrich, Shanghai, China) in PBS at 4 °C overnight. The sample was then washed with PBS for 15 min and post-fixed in 1% OsO4 in PBS for 2 h. The sample was washed with PBS again for 15 min and then dehydrated with a graded ethanol series for 15 min. After being dehydrated two times in 100% ethanol, the sample was transferred into isoamyl acetate for 1 h, dehydrated with liquid CO2 in a Hitachi HCP-2 critical point dryer, coated with gold-palladium in a Hitachi E-1010 ion sputter for 5 min, and examined under a Hitachi SU8010 SEM. The cells were randomly selected in five fields of the same magnification, and the length of bipolar cells in the microgravity group and the gravity group was compared.
4.3.7 Statistical Analysis
The images of western blots were semi-quantitatively analyzed in the ImageJ software (NIH, USA). Statistical analyses involved Student’s t test and significance was assumed at P values < 0.05.
4.4 The Effects of Space Microgravity on the Transcriptome Expression of hMSCs
4.4.1 Global RNA-Seq Data Analysis and Gene Ontology of Genes and Genomes Enrichment Analysis of Differentially Expressed Genes
hMSCs under osteogenic induction for 2 days both in space and on the ground was fixed with RNAlater and saved for 10 days at 4 °C. Then we prepared RNA samples and quantified expression level of transcriptome of space flight and ground cells by RNA-sequence technology. Using a moderately stringent cut-off (log2 (fold change) ≥ 1 and FDR < 0.05) 548 genes (302 genes upregulated and 246 genes downregulated) significantly change their expression levels. Using a higher stringency cut-off (log2 (fold change) ≥ 1 and FDR < 0.01) only 162 of the quantified genes were considered significantly altered (Fig. 9a).
4.4.2 Gene Ontology of Genes and Genomes Enrichment Analysis of Differentially Expressed Genes
Although at a different level, genes with many biological processes displayed significant altered expression. Using GO significant enrichment analysis, it was found that these altered genes were mainly involved in response to stress, cell proliferation, regulation of cell proliferation, inflammatory reaction and so on. Top10 of GO term enrichment list was shown in Fig. 9b.
4.4.2.1 Stress Response-Related Genes
After a two-day space flight, stress response-related genes are the largest number group of the changed genes. Of 548 changed genes, 127 genes were associated with stress response. Among them, genes involved in endoplasmic reticulum stress response, DNA-damage response and oxidative stress response occupy the most, and some other stress response genes also changed under SF environment.
The stress response GADD45 family of genes are rapidly induced by a wide variety of endogenous and exogenous stress stimuli (Yang et al. 2009) and they are implicated in cell cycle arrest, DNA demethylation and repair, apoptosis, cell survival, genomic stability, inflammation, and in response to physiological (Jung et al. 2007; Smith et al. 2000; Barreto et al. 2007). In the present study, two isoforms of GADD45, GADD45B and GADD45G, exhibited apparently up-regulated expression after exposure to 2-days space flight, which suggested space flight may cause DNA damage and cycle arrest of osteogenic-induced hMSCs. MMP1, also induced by DNA damage, is significantly increased under space flight (Leiros et al. 2017). CHAF1A, which participate into DNA replication and repair (Rodriges et al. 2016), was also increased. Pyruvate dehydrogenase kinase 4 (PDK4), encodes a mitochondrial protein and regulates of glucose metabolism by inhibiting the pyruvate dehydrogenase complex, was also increased by space flight. In addition, a large number of altered genes response to oxidative stress, involving SOD2, PARP1, TXNIP, HDAC2, HFE, HSPA6, HMGA1 and BIRC3 all increased under space flight environment.
Space flight also inhibited the expression of some stress-stress related genes. RNF5, one E3 ubiquitin ligase that has been implicated in motility and cellular stress response (Didier et al. 2003), was inhibited under SF. In addition, the most obvious reduction genes class was associated to endoplasmic reticulum stress. Some proteins associated with protein folding and transport in the endoplasmic reticulum were significantly decreased, such as HYOU1, at the same time, some endoplasmic reticulum stress induced genes increased, involving HSPA6, ATF3 and YOD1. The TK1 and TK2, which were involved in DNA synthesis, was also significantly reduced after space flight.
4.4.2.2 Space Flight Promoted Inflammation Response
Space flight was associated with significant shifts in expression of mRNAs involved with the inflammation response. Levels of mRNA of the chemokine family members CCL3 (3.76-log fold), CCL20 (3.63-log fold), CXCL2 (3.50-log fold), CXCL3 (2.70-log fold), CXCL5 (1.77-log fold), CXCL6 (1.70-log fold); inflammatory cytokines IL1A (2.64-log fold), IL1B (3.23-log fold) genes were significantly increased in space flight compared with ground control. And the expression of BCL6, a factor known to be important in resolving inflammatory responses, was also increased 2.56 log fold. GNAI (1.88-log fold), which expression is promoted by chemokine, was also increased after space flight. Conversely, GNG2, which expression is inhibited by GNAI, mRNA level was significantly decreased 2.62-log fold) after space flight.
4.4.2.3 Space Flight Inhibited Cell Cycle
Biological process enriches analyze showed that of expression changed 528 genes, 68 genes was relative to cell cycle. RNA-sequence revealed the down regulation of CDC6, CCNB1 and CDK1 genes at 2.75, 2.29 and 1.85 log fold respectively when hMSCs were osteogenic induced after space flight. Similarly, CDC25B, which activates complex of CDK1/Cyclin B and is required for entry into mitosis (Forrest et al. 1999; Lincoln et al. 2002; Nilsson and Hoffmann 2000), was decreased 2.07 log fold. CDCA8 (2.62-log fold), an essential regulator of mitosis and cell division, was also inhibited by space flight. On the other hand, CDKN1C, which is capable of inhibiting several CDK with demonstrated roles in the G1/S-phase transition (Lopez-Abad et al. 2016), the expression of which was increased at 2.58-log fold by SF. GADD45B and GADD45G, whose transcript level is increased following stressful growth arrest conditions, was also increased 2.22-log fold under space environment. All the changed genes revealed that space flight arrest cell cycle and inhibited mitosis of hMSCs under osteogenic differentiation.
4.4.2.4 hMSCs Differentiation Potential
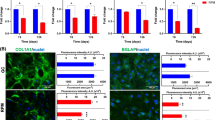
According to the RNA-Seq assay, we found a significant difference in the expression of 21 osteogenesis-related genes in space samples compared to ground samples (Table 7). The expression of seven members of the collagen family was significantly reduced, including that of COL1A1, COL1A2, COL3A1, COL4A1, COL5A1, and COL18A1. In addition, the expression of ALP, a marker of early osteogenesis, and Runx2, a key transcription factor in early osteoblasts, was also significantly decreased under the microgravity environment. Teal-time PCR results confirmed that the expression levels of Runx2, ALP and COL1A1 mRNA in the cells osteogenically induced under space microgravity declined remarkably as compared to those in cells osteogenically induced under normal gravity, especially COL1A1, a highly expressed bone-specific gene in early osteoblasts (Fig. 10a) We also measured the activation of the Runx2 protein in cells osteogenically induced for 2 days. The results showed that space microgravity inhibited both mRNA expression and activity of the Runx2 protein (Fig. 10b); these data indicated that space microgravity inhibited early genes specific for osteogenesis of hMSCs by decreasing the expression and activity of Runx2.
Effects of space microgravity on the expression of mRNA and proteins specific for osteogenesis. a The mRNA expression of ALP, COL1A1, and Runx2 in cells osteogenically induced for 2 days. The quantitative data were obtained by real-time PCR (n = 3). b The expression and activity of the Runx2 protein in cells osteogenically induced for 2 days (n = 3). The band intensity was quantified in triplicate by means of the ImageJ software for each protein, and the data were normalized to a corresponding GAPDH value. c The histochemical detection of ALP in cells osteogenically induced for 7 days. d Immunohistochemical detection of ALP, COL1A1, and Runx2 in cells osteogenically induced for 7 days. *P < 0.05, ** P < 0.01, ***P < 0.005. G: normal gravity, MG: space microgravity (Reprinted from Zhang et al. 2018)
Analysis of ALP, COL1A1, and Runx2 expression in cells osteogenically induced for 2 days revealed that space microgravity decreased the expression of genes specific for osteogenesis. Therefore, we inferred that space microgravity could inhibit the osteogenic potential of hMSCs. By SEM assay of cells osteogenically induced for 7 days, we found that space microgravity resulted in obvious changes of cell morphology compared with normal gravity, including polygonal retraction and shorter cell spindle (Fig. 11a). In addition, H&E staining indicated that the cell shape became round, as opposed to the triangular or fusiform shape of cells induced in normal gravity (Fig. 11b). Histochemical staining of ALP in cells osteogenically induced for 7 days confirmed this hypothesis. hMSCs could be induced to differentiate into osteoblasts with positive ALP staining under normal gravity (Fig. 11c). In contrast, the positive staining decreased in cells osteogenically induced for 7 days under space microgravity. Immunohistochemical analysis was also used to detect the protein expression of COL1A1 and Runx2 in the cells osteogenically induced for 7 days. In agreement with our hypothesis, few cells with positive staining for COL1A1 and Runx2 were found among the cells osteogenically induced under space microgravity (Fig. 11d).
Effects of space microgravity on hMSCs morphology. a Morphology of cells osteogenically induced for 7 days under microgravity and normal gravity was analyzed by SEM. Bipolar cell length was measured, and the actual length was calculated according to scale. b H&E staining of cells osteogenically induced for 7 days under microgravity and normal gravity. H&E staining was performed to locate the cells and observe the cells’ morphology on the scaffold. G: normal gravity, MG: space microgravity (Reprinted from Zhang et al. 2018.)
It has been demonstrated that simulated microgravity increases adipogenesis of hMSCs, and activation of transcription factor PPARγ2 plays a crucial role in the expression of marker genes specific for adipogenesis (Zayzafoon et al. 2004). To identify the effects of space microgravity on the adipogenesis of hMSCs, we analyzed the expression of genes specific for adipogenesis. Figure 12a shows that expression of adipocyte markers, such as adipsin, leptin, and glucose transporter 4 (Glut4), was increased in cells osteogenically induced for 2 days under space microgravity. Meanwhile, space microgravity promoted expression of PPARγ2 (Fig. 12b), suggesting that space microgravity stimulates adipogenic differentiation even under osteogenic induction conditions.
Effects of space microgravity on the mRNA expression of genes specific for adipogenesis. a The mRNA expression of adipsin, glut4, and leptin in cells osteogenically induced for 2 days. The quantitative data were obtained by real-time PCR (n = 3). b The mRNA expression of PPARγ, CEBPA, CEBPB, and TRIB3 in cells osteogenically induced for 2 days. The quantitative data were obtained by real-time PCR (n = 3). The data were normalized to the corresponding GAPDH value. *P < 0.05, ** P < 0.01, ***P < 0.005. G: normal gravity, MG: space microgravity (Reprinted from Zhang et al. 2018)
CEBPA and CEBPB are key early regulators of adipogenesis. CEBPB directly induces the expression of PPARγ2, and PPARγ2 in turn activates the transcription of CEBPA (Barlier-Mur et al. 2003). Therefore, we analyzed the expression of CEBPA and CEBPB as shown in Fig. 12b. The results revealed that mRNA and protein levels of CEBPB increased in cells osteogenically induced for 2 days under space microgravity as compared with the cells osteogenically induced under normal gravity. In contrast, the level of CEBPA expression was not significantly different between cells osteogenically induced under normal gravity and space microgravity. One of the reasons may be that osteogenic induction for only 2 days is not enough to cause PPARγ2 to switch to activation of the CEBPA expression.
4.4.2.5 Dysregulation of Genes Involved with Signaling Pathway
TNF-α/NF-KB is involved in a variety of cellular physiological responses, such as cell growth, differentiation, apoptosis, immune, inflammation, stress response, and many other physiologic processes (Barnes and Karin 1997; Karin and Greten 2005). As one of inflammatory response regulation pathway, we selected genes involved in the TNF-α/NF-KB signaling system. The TNF-α downstream target TNF-α-induced protein 6 (TNFAIP6) mRNA was significantly increased in SF hMSCs (3.27-log fold). REL, a subunit of NF-KB family, was also increased under SF (1.91-log fold). However, TNFRSF1A associated via death domain (TRADD) mRNA decreased (3.02-log fold). P53 pathway was also changed significantly by KEGG pathway. GADD45B, GADD45G, CCND2 were all involved in P53 pathway and related to gene damage, and other gene involved in P53 pathway was CASP3, an apoptosis-related gene.
4.4.2.6 Validation of Gene Expression by qRT-PCR
Quantitative real-time PCR (qRT-PCR) was performed to validate the differentially expressed genes involved in the RNA-seq data (Fig. 13). The results showed that the expression patterns of these thirteen genes were highly in agreement with the RNA-seq results.
Quantitative PCR was used to verify gene expression. a q-PCR detection of genes associated with stress response GADD45B, PDK4, RNF5, TK1 and TK2; b The expression of inflammation-related genes IL1A and GANI1; c Expression of cell cycle related genes: CDC6, CDC25B and CDKN1C; D: Expression of NFKB2 and CASP3, TNFAIP1 in the signaling pathway.. *P < 0.05, ** P < 0.01. G: normal gravity; MG: space microgravity
4.4.3 Discussion
Expose to the space stresses, including microgravity, radiation, hypoxia and other factors, the physical condition of astronauts changed greatly, including bone loss, muscular atrophy and impaired immune system function and cardiovascular deconditioning (Atkov 1992; Basso et al. 2005; Bucaro et al. 2004; Heer et al. 1999; Leach 1992). The changes of physiological function were ultimately caused by changes in the biological process of cells. The effect of space environment on different types of cells is complex and varied. Space flight has been shown to result in suppression of antigen-specific T cell function, inducing a sustained vascular endothelial cell dysfunction, promoting human fibroblast cells proliferating (Fritsch-Yelle et al. 1996; Leach 1992; Mehta et al. 2000; Zhang et al. 2016). The main purpose of this article was to explore mRNA expression change of osteogenic induced hMSCs in response to microgravity in space. By RNA-sequence assay, we found that a series of biological processes mRNA expression level significantly changed, including response to stress, cell cycle and inflammation response. Moreover, space flight may affect TNF-α/NF-KB signaling system of osteogenic-induced hMSCs.
When stimulated by internal and external factors, cells will produce overmuch ROS and other free radicals and then result in cell oxidative stress through destructing the balance of oxidation and antioxidant system (Reuter et al. 2010; Pi et al. 2010). Overexpression of ROS can regulate many oxidizing substances and antioxidant-related gene expression by activating NRF2, NF-KB and MAPK pathways (Kanninen et al. 2011; Sykiotis et al. 2011; Pantano et al. 2006). It has been reported that space flight can induce oxidative stress response (Mao et al. 2014; Gambara et al. 2017). In this study, we also found that space flight increased the expression of a mass of genes related to oxidative stress. TXNIP, as an oxidative stress biomarker, inhibits the antioxidative function of thioredoxin resulting in the accumulation of reactive oxygen species and cellular stress. Hypoxia, availability of glucose or radiation can induce TXNIP expression and it has also been reported that space environment can induce TXNIP expression (Yu et al. 2010; Li et al. 2015; Hoshikawa et al. 2011; Versari et al. 2013). In the present study, space flight promoted TXNIP expression. SOD2 is one of the markers of oxidative stress response and it has been reported that simulated microgravity or radiation increased SDO2 gene expression and increased expression of SOD2 can reduce cellular ROS (Chen et al. 2016; Zhang et al. 2017; Oscarsson et al. 2017; Qiu et al. 2010). HSPA4, which is often called Hsp70 and known as the major heat shock protein that prevents stress-induced apoptosis, was upregulation under space flight environment in many kind of cells (Gritsyna et al. 2015; Cui et al. 2010), while some others considered that space flight inhibited Hsp70 expression (Cubano and Lewis 2001; Amann et al. 1992; Ishihara et al. 2008). In the present study, HSPA6, a member of Hsp70 family, was promoted by space flight. Survival related gene, HMGA1(Mahajan et al. 2013) and anti-apoptotic gene, BIRC3(Gressot et al. 2017), expression was also promoted by space flight. Together these data support the hypothesis that space flight cause cell oxidative stress and the cells adapted to this stress response by changing the expression of related genes.
Exogenous high temperature and pressure, ultraviolet, strong acid and alkali and other physical and chemical factors and endogenous oxidative stress, DNA replication and transmission errors can cause DNA damage. Cells can maintain genome integrity through the activation effector proteins of DNA repair, apoptosis and cell cycle arrest (Girardi et al. 2012; Hoshino et al. 2007; Lu et al. 2017a, b). We found that SF decreased the TK1, TK2 expression. TK genes catalyze cytosolic and mitochondrial deoxy thymidine phosphorylation and all the three synthases are necessary for DNA replication (Munch-Petersen 2010). The TK1 and TK2 deficiency could prevent DNA replication process and then increase cellular sensitivity to DNA damage. In addition, under SF environment, the DNA repair related genes GADD45B, GADD45G, PARP1, and HDAC2 (Lu et al. Lu et al. 2017a, b; Huang et al. 2016; Liu et al. 2009) was increased. SF also inhibited some cell cycle essential genes expression. CCNB/CDK1 protein complex form the key substrate phosphorylation and translate the cell cycle from the G2 to M period (Sherr 1996). It is necessary to start the cell cycle factor. CDC6, CDC25B and CDCA8 also play a key role in the smooth progress of the cell cycle. The inhibition effect of SF on these cell cycle related genes could cause cell cycle arrest. Apoptosis is essential for the development and survival of most multicellular animals by preventing growth of cells mutation due to DNA damage. CASP3, as a biomarker of apoptosis, was increased by SF. The high expression of CASP3 was also indicated by other space flight (Novoselova et al. 2015). However, besides to CASP3, the expression of other members of the CASP family did not change significantly. CCND2, CASP3, GGDD45B, and GGAD45G are all involved in P53 pathway and P53 pathway was significantly change by KEGG pathway analyze. BCL2L11 belongs to BCL2 family and acts as an apoptotic activator by interacting with other members of the BCL-2 protein family (Concannon et al. 2010; Fischer et al. 2007). SF also promoted the expression of BCL2L11 in our study. The above results show that SF may inhibit DNA replication by decreasing the synthesis of thymine and then result in DNA damage and the DNA damage would induce DNA repair-related genes expression, cycle arrest and cell apoptosis, and P53 pathway played an important role in it.
Some studies have shown that simulated microgravity inhibits proliferation and osteogenesis of hMSCs and increases their adipogenesis (Orlic et al. 2001; Zayzafoon et al. 2004; Dai et al. 2007). On the other hand, these simulation methods involving clinostats, random positioning machine (RPM) and rotating wall vessel (RWV) bioreactors, cannot actually remove the force of gravity. The constant rotation of a sample may result in the g-vector being time-averaged to near-zero (Klaus 2001) and the controversy still exists regarding these experiments. Thus, the effects of real space microgravity on the osteogenic differentiation of hMSCs should be examined in a real space environment. In this study, we analyzed the effects of space microgravity on the osteogenic differentiation of hMSCs exposed to space microgravity for 2 days and 7 days by detecting the expression of ALP, a marker of early osteogenesis. We demonstrated that space microgravity reduced the osteogenic potential of hMSCs by inhibiting the expression of ALP. This finding is consistent with other data indicating downregulation of the markers of osteoblastic function and differentiation under simulated microgravity (Nakamura et al. 2003). We next analyzed another protein osteoblastic marker, COL1A1, a major ECM component secreted by osteoblasts during differentiation (Ontiveros and McCabe 2003; Owen et al. 1990). We detected reduced expression of COL1A1, which confirmed that space microgravity inhibits osteogenesis of hMSCs. In addition, space microgravity increased the expression of marker genes specific for adipogenesis, such as leptin, adispin, and GLUT4 mRNA, even under osteogenic induction conditions. In agreement with these data, the expression of PPARγ2, a key transcription factor involved in adipogenic differentiation (Tontonoz et al. 1994), was increased under space microgravity, and the increased expression of PPARγ2 resulted from increased CEBPB expression. Therefore, we concluded that gene expression in hMSCs shifts toward adipogenesis instead of osteogenesis under space microgravity.
Many articles have reported that space flight can cause the animal or cell immune dysfunction and inflammatory response (Chang et al. 2015; Strewe et al. 2015; Crucian et al. 2014; Kizilay et al. 2017). By RNA-sequence results and real-time PCR validation, we found that space flight induced the expression of some chemokine family members and inflammatory cytokine family members in osteogenic-induced hMSCs. The ground simulated microgravity experiments also showed that simulated microgravity contributed to intracellular inflammatory responses of hMSCs under osteogenic differentiation (Gershovich et al. 2013). It has also been reported that hMSCs isolated from elder patients secreted high levels of inflammatory cytokines, therefore, we speculate that cell cycle arrest and apoptosis caused by space flight may lead to elevated expression of inflammatory factors. Inflammation stress may hinder the normal physiological function of hMSCs, such as differentiation potential.
Abbreviations
- ALP:
-
Alkaline phosphatase
- BMP2:
-
Bone morphogenetic protein-2
- ECM:
-
Extracellular matrix
- FBS:
-
Heat-inactivated fetal bovine serum
- GADD45:
-
Growth arrest and DNA damage inducible alpha
- GO:
-
Gene ontology
- hBMSCs:
-
Human bone marrow mesenchymal stem cells
- LSM:
-
Lymphocyte separation medium
- MMP1:
-
Matrix metallopeptidase 1
- MSCs:
-
Mesenchymal stem cells
- NG:
-
Normal ground gravity
- PBS:
-
Phosphate buffer saline
- PLGA:
-
Synthetic poly (D, L-lactide-co-glycolide)
- qRT-PCR:
-
Quantitative real-time PCR
- RNA-SEQ:
-
RNA sequence
- RPM:
-
Random positioning machine
- SEM:
-
Scanning electronic microscope
- SMG:
-
Simulated microgravity
- α-MEM:
-
Α-minimum essential medium
References
Amann RP, Deaver DR, Zirkin BR et al (1992) Effects of microgravity or simulated launch on testicular function in rats. J Appl Physiol (Bethesda, Md.: 1985) 73(2 Suppl):174s–185s
Atkov O (1992) Some medical aspects of an 8-month’s space flight. Adv Space Res Off J Comm Space Res (COSPAR) 12(1):343–345
Barlier-Mur AM, Chailley-Heu B, Pinteur C et al (2003) Maturational factors modulate transcription factors CCAAT/enhancer-binding proteins alpha, beta, delta, and peroxisome proliferator-activated receptor-gamma in fetal rat lung epithelial cells. Am J Respir Cell Mol Biol 29(5):620–626
Barnes PJ, Karin M (1997) Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336(15):1066–1071
Barreto G, Schafer A, Marhold J et al (2007) Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 445(7128):671–675
Basso N, Bellows CG, Heersche JN (2005) Effect of simulated weightlessness on osteoprogenitor cell number and proliferation in young and adult rats. Bone 36(1):173–183
Bucaro MA, Fertala J, Adams CS et al (2004) Bone cell survival in microgravity: evidence that modeled microgravity increases osteoblast sensitivity to apoptogens. Ann N Y Acad Sci 1027:64–73
Carmeliet G, Nys G, Bouillon R (1997) Microgravity reduces the differentiation of human osteoblastic MG-63 cells. J Bone Miner Res Off J Am Soc Bone Miner Res 12(5):786–794
Cedar SH (2006) The function of stem cells and their future roles in healthcare. Br J Nurs (Mark Allen Publishing) 15(2):104–107
Chang TT, Spurlock SM, Candelario TL et al (2015) Spaceflight impairs antigen-specific tolerance induction in vivo and increases inflammatory cytokines. FASEB J Off Publ Fed Am Soc Exp Biol 29(10):4122–4132
Chen XJ, Lin F, Qiao C (2004) Isolation, culture and osteogenic potential of human adipose stromal cells and [J]. J Pract Stomatol 20:12–15
Chen H, Lv K, Dai Z et al (2016) Intramuscular injection of mechano growth factor E domain peptide regulated expression of memory-related sod, miR-134 and miR-125b-3p in rat hippocampus under simulated weightlessness. Biotech Lett 38(12):2071–2080
Claassen DE, Spooner BS (1994) Impact of altered gravity on aspects of cell biology. Int Rev Cytol 156:301–373
Claassen DE, van Twest JS, Spooner BS (1994) Formation and vesiculation of biomembranes during spaceflight. Adv Space Res Off J Comm Space Res (COSPAR) 14(8):111–114
Concannon CG, Tuffy LP, Weisova P et al (2010) AMP kinase-mediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J Cell Biol 189(1):83–94
Crucian BE, Zwart SR, Mehta S et al (2014) Plasma cytokine concentrations indicate that in vivo hormonal regulation of immunity is altered during long-duration spaceflight. J Interf Cytokine Res Off J Int Soc Interf Cytokine Res 34(10):778–786
Cubano LA, Lewis ML (2001) Effect of vibrational stress and spaceflight on regulation of heat shock proteins hsp70 and hsp27 in human lymphocytes (Jurkat). J Leukoc Biol 69(5):755–761
Cui Y, Zhou J, Li C et al (2010) Effects of simulated weightlessness on liver Hsp70 and Hsp70mRNA expression in rats. Int J Clin Exp Med 3(1):48–54
Dai ZQ, Wang R, Ling SK et al (2007) Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif 40(5):671–684
Dai Z, Wu F, Chen J et al (2013) Actin microfilament mediates osteoblast Cbfa1 responsiveness to BMP2 under simulated microgravity. PLoS ONE 8(5):e63661
Dai Z, Guo F, Wu F et al (2014) Integrin alphavbeta3 mediates the synergetic regulation of core-binding factor alpha1 transcriptional activity by gravity and insulin-like growth factor-1 through phosphoinositide 3-kinase signaling. Bone 69:126–132
Didier C, Broday L, Bhoumik A et al (2003) RNF5, a RING finger protein that regulates cell motility by targeting paxillin ubiquitination and altered localization. Mol Cell Biol 23(15):5331–5345
Fischer SF, Bouillet P, O’Donnell K et al (2007) Proapoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody-forming cells. Blood 110(12):3978–3984
Forrest AR, McCormack AK, DeSouza CP et al (1999) Multiple splicing variants of cdc25B regulate G2/M progression. Biochem Biophys Res Commun 260(2):510–515
Fritsch-Yelle JM, Charles JB, Jones MM et al (1996) Microgravity decreases heart rate and arterial pressure in humans. J Appl Physiol (Bethesda, Md.: 1985) 80(3):910–914
Fu Y, Wang T, Liang WW (2006) Isolation, culture and differentiation of rat bone marrow-derived mesenchymal stem cells into neuron-like cells. Lingnan J Emerg Med 11:1–3
Gambara G, Salanova M, Ciciliot S et al (2017) Gene expression profiling in slow-type calf soleus muscle of 30 days space-flown mice. PLoS ONE 12(1):e0169314
Gershovich PM, Gershovich IuG, Buravkova LB (2013) The effects of simulated microgravity on the pattern of gene expression in human bone marrow mesenchymal stem cells under osteogenic differentiation. Fiziol Cheloveka 39(5):105–111
Girardi C, De Pitta C, Casara S et al (2012) Analysis of miRNA and mRNA expression profiles highlights alterations in ionizing radiation response of human lymphocytes under modeled microgravity. PLoS ONE 7(2):e31293
Gressot LV, Doucette T, Yang Y et al (2017) Analysis of the inhibitors of apoptosis identifies BIRC3 as a facilitator of malignant progression in glioma. Oncotarget 8(8):12695–12704
Gritsyna YV, Abdusalamova ZR, Vikhlyantsev IM et al (2015) Changes in gene expression and content of Hsp70 and Hsp90 in striated muscles of mice after 30-day space flight on the biosatellite Bion-M1. Dokl Biochem Biophys 463:199–202
Gurkin LW (1992) The NASA sounding rocket program and space sciences. ASGSB Bull Publ Am Soc Gravit Space Biol 6(1):113–120
Heer M, Kamps N, Biener C et al (1999) Calcium metabolism in microgravity. Eur J Med Res 4(9):357–360
Hill PA (1998) Bone remodelling. Br J Orthod 25(2):101–107
Hoshikawa H, Indo K, Mori T, Mori N (2011) Enhancement of the radiation effects by D-allose in head and neck cancer cells. Cancer Lett 306(1):60–66
Hoshino T, Nakaya T, Araki W et al (2007) Endoplasmic reticulum chaperones inhibit the production of amyloid-beta peptides. Biochem J 402(3):581–589
Hoson T, Kamisaka S, Masuda Y et al (1997) Evaluation of the three-dimensional clinostat as a simulator of weightlessness. Planta 203(Suppl):S187–197
Huang Y, Dai ZQ, Ling SK et al (2009) Gravity, a regulation factor in the differentiation of rat bone marrow mesenchymal stem cells. J Biomed Sci 16:87
Huang R, Langdon SP, Tse M et al (2016) The role of HDAC2 in chromatin remodelling and response to chemotherapy in ovarian cancer. Oncotarget 7(4):4695–4711
Hughes-Fulford M, Tjandrawinata R, Fitzgerald J et al (1998) Effects of microgravity on osteoblast growth. Gravit Space Biol Bull Publ Am Soc Gravit Space Biol 11(2):51–60
In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C et al (2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells (Dayton, Ohio) 22(7):1338–1345
Ishihara A, Fujino H, Nagatomo F et al (2008) Gene expression levels of heat shock proteins in the soleus and plantaris muscles of rats after hindlimb suspension or spaceflight. J Physiol Sci JPS 58(6):413–417
Jung HJ, Kim EH, Mun JY et al (2007) Base excision DNA repair defect in Gadd45a-deficient cells. Oncogene 26(54):7517–7525
Kanninen K et al (2011) Targeting glycogen synthase kinase-3 for therapeutic benefit against oxidative stress in Alzheimer’S disease: involvement of the Nrf2-ARE pathway. Intem J Alzheimer’s Dis 15:56–64
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5(10):749–759
Katembe WJ, Edelmann RE, Brinckmann E et al (1998a) Development of spaceflight experiments with Arabidopsis as a model system in gravitropism studies. J Plant Res 111:463–470
Katembe WJ, Edelmann RE, Brinckmann E, Kiss JZ (1998b) The development of spaceflight experiments with Arabidopsis as a model system in gravitropism studies. J Plant Res 111(1103):463–470
Kizilay Mancini O, Lora M, Shum-Tim D et al (2017) A proinflammatory secretome mediates the impaired immunopotency of human mesenchymal stromal cells in elderly patients with atherosclerosis. Stem Cells Transl Med 6(4):1132–1140
Klaus DM (2001) Clinostats and bioreactors. Gravit Space Biol Bull: Publ Am Soc Gravit Space Biol 14(2):55–64
Krikorian AD, Levine HG, Kann RP et al (1992) Effects of spaceflight on growth and cell division in higher plants. Adv Space Biol Med 2:181–209
Lang TF, Leblanc AD, Evans HJ et al (2006) Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res Off J Am Soc Bone Miner Res 21(8):1224–1230
Lazarenko OP, Rzonca SO, Suva LJ et al (2006) Netoglitazone is a PPAR-gamma ligand with selective effects on bone and fat. Bone 38(1):74–84
Leach CS (1992) Biochemical and hematologic changes after short-term space flight. Microgravity Q MGQ 2(2):69–75
Lee OK, Kuo TK, Chen WM et al (2004) Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103(5):1669–1675
Leiros GJ, Kusinsky AG, Balana ME et al (2017) Triolein reduces MMP-1 upregulation in dermal fibroblasts generated by ROS production in UVB-irradiated keratinocytes. J Dermatol Sci 85(2):124–130
Li Y, Miao LY, Xiao YL et al (2015) Hypoxia induced high expression of thioredoxin interacting protein (TXNIP) in non-small cell lung cancer and its prognostic effect. Asian Pac J Cancer Prev APJCP 16(7):2953–2958
Lincoln AJ, Wickramasinghe D, Stein P et al (2002) Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet 30(4):446–449
Liu B, Suyeoka G, Papa S, Franzoso G et al (2009) Growth arrest and DNA damage protein 45b (Gadd45b) protects retinal ganglion cells from injuries. Neurobiol Dis 33(1):104–110
Lopez-Abad M, Iglesias-Platas I, Monk D (2016) Epigenetic characterization of CDKN1C in placenta samples from non-syndromic intrauterine growth restriction. Front Genet 7:62
Lu T, Zhang Y, Wong M et al (2017a) Detection of DNA damage by space radiation in human fibroblasts flown on the International Space Station. Life Sci Space Res 12:24–31
Lu Y, Kwintkiewicz J, Liu Y et al (2017b) Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Can Res 77(7):1709–1718
Mahajan L, Pandit H, Madan T et al (2013) Human surfactant protein D alters oxidative stress and HMGA1 expression to induce p53 apoptotic pathway in eosinophil leukemic cell line. PLoS ONE 8(12):e85046
Mao XW, Pecaut MJ, Stodieck LS et al (2014) Biological and metabolic response in STS-135 space-flown mouse skin. Free Radical Res 48(8):890–897
Marie PJ, Kaabeche K (2006) PPAR gamma activity and control of bone mass in skeletal unloading. PPAR Res 2006:64807
Mehta SK, Stowe RP, Feiveson AH et al (2000) Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis 182(6):1761–1764
Mesland DA, Anton AH, Willemsen H et al (1996) The Free Fall Machine—a ground-based facility for microgravity research in life sciences. Microgravity Sci Technol 9(1):10–14
Munch-Petersen B (2010) Enzymatic regulation of cytosolic thymidine kinase 1 and mitochondrial thymidine kinase 2: a mini review. Nucleosides Nucleotides Nucleic Acids 29(4–6):363–369
Nakamura H, Kumei Y, Morita S et al (2003) Suppression of osteoblastic phenotypes and modulation of pro- and anti-apoptotic features in normal human osteoblastic cells under a vector-averaged gravity condition. J Med Dent Sci 50(2):167–176
Nasef A, Ashammakhi N, Fouillard L (2008) Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med 3(4):531–546
Nilsson I, Hoffmann I (2000) Cell cycle regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res 4:107–114
Novoselova EG, Lunin SM, Khrenov MO et al (2015) Changes in immune cell signalling, apoptosis and stress response functions in mice returned from the BION-M1 mission in space. Immunobiology 220(4):500–509
O’Donoghue K, Choolani M, Chan J et al (2003) Identification of fetal mesenchymal stem cells in maternal blood: implications for non-invasive prenatal diagnosis. Mol Hum Reprod 9(8):497–502
Ontiveros C, McCabe LR (2003) Simulated microgravity suppresses osteoblast phenotype, Runx2 levels and AP-1 transactivation. J Cell Biochem 88(3):427–437
Orlic D, Kajstura J, Chimenti S et al (2001) Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA 98(18):10344–10349
Oscarsson N, Ny L, Molne J et al (2017) Hyperbaric oxygen treatment reverses radiation induced pro-fibrotic and oxidative stress responses in a rat model. Free Radic Biol Med 103:248–255
Owen TA, Aronow M, Shalhoub V et al (1990) Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143(3):420–430
Pan Z, Yang J, Guo C et al (2008) Effects of hindlimb unloading on ex vivo growth and osteogenic/adipogenic potentials of bone marrow-derived mesenchymal stem cells in rats. Stem Cells Dev 17(4):795–804
Pantano C, Reynaert NL, van der Vliet A et al (2006) Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal 8(9–10):1791–1806
Pereira RF, Halford KW, O’Hara MD et al (1995) Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci USA 92(11):4857–4861
Pi J, Zhang Q, Fu J et al (2010) ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol 244(1):77–83
Platt ID, El-Sohemy A (2009) Regulation of osteoblast and adipocyte differentiation from human mesenchymal stem cells by conjugated linoleic acid. J Nutr Biochem 20(12):956–964
Qiu X, Brown K, Hirschey MD et al (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12(6):662–667
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49(11):1603–1616
Rodriges Blanko E, Kadyrova LY et al (2016) DNA mismatch repair interacts with CAF-1- and ASF1A-H3-H4-dependent Histone (H3-H4)2 tetramer deposition. J Biol Chem 291(17):9203–9217
Rucci N, Rufo A, Alamanou M et al (2007) Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J Cell Biochem 100(2):464–473
Saxena R, Pan G, Dohm ED, McDonald JM (2011) Modeled microgravity and hindlimb unloading sensitize osteoclast precursors to RANKL-mediated osteoclastogenesis. J Bone Miner Metab 29(1):111–122
Schneider V, Oganov V, LeBlanc A et al (1995) Bone and body mass changes during space flight. Acta Astronaut 36(8–12):463–466
Sherr CJ (1996) Cancer cell cycles. Science (New York, N.Y.) 274(5293):1672–1677
Shi D, Meng R, Deng W et al (2010) Effects of microgravity modeled by large gradient high magnetic field on the osteogenic initiation of human mesenchymal stem cells. Stem Cell Rev 6(4):567–578
Smith ML, Ford JM, Hollander MC et al (2000) p53-mediated DNA repair responses to UV radiation: studies of mouse cells lacking p53, p21, and/or gadd45 genes. Mol Cell Biol 20(10):3705–3714
Sotiropoulou PA, Papamichail M (2007) Immune properties of mesenchymal stem cells. Methods Mol Biol 407:225–243
Strewe C, Crucian BE, Sams CF et al (2015) Hyperbaric hyperoxia alters innate immune functional properties during NASA Extreme Environment Mission Operation (NEEMO). Brain Behav Immun 50:52–57
Sun Z, Cao X, Hu Z et al (2015a) MiR-103 inhibits osteoblast proliferation mainly through suppressing Cav1.2 expression in simulated microgravity. Bone 76:121–128
Sun Z, Cao X, Zhang Z et al (2015b) Simulated microgravity inhibits L-type calcium channel currents partially by the up-regulation of miR-103 in MC3T3-E1 osteoblasts. Sci Rep 5:8077
Sykiotis GP, Habeos IG, Samuelson AV et al (2011) The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care 14(1):41–48
Tamma R, Colaianni G, Camerino C et al (2009) Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J Off Publ Fed Am Soc Exp Biol 23(8):2549–2554
Tilton FE, Degioanni JJ, Schneider VS (1980) Long-term follow-up of Skylab bone demineralization. Aviat Space Environ Med 51(11):1209–1213
Tontonoz P, Hu E, Spiegelman BM (1994) Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 79(7):1147–1156
Tuan RS, Boland G, Tuli R (2003) Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther 5(1):32–45
Versari S, Longinotti G, Barenghi L et al (2013) The challenging environment on board the International Space Station affects endothelial cell function by triggering oxidative stress through thioredoxin interacting protein overexpression: the ESA-SPHINX experiment. FASEB J Off Publ Fed Am Soc Exp Biol 27(11):4466–4475
Wang T (2008) 2007 annual meeting of American Heart Association and the latest results of cardiac resuscitation. Chin Emerg Med 28:112–113
Wang T, Fu Y, Fang XS (2007) Differentiation and identification of bone marrow mesenchymal stem cells into cardiomyocytes in vitro. J Sun Yat-Sen Univ 28:S9–11
Wang X, Guo B, Li Q et al (2013) miR-214 targets ATF4 to inhibit bone formation. Nat Med 19(1):93–100
Wang T, Sun Q, Xu W et al (2015) Modulation of modeled microgravity on radiation-induced bystander effects in Arabidopsis thaliana. Mutat Res 773:27–36
Warejcka DJ, Harvey R, Taylor BJ et al (1996) A population of cells isolated from rat heart capable of differentiating into several mesodermal phenotypes. J Surg Res 62(2):233–242
Yang Z, Song L, Huang C (2009) Gadd45 proteins as critical signal transducers linking NF-kappaB to MAPK cascades. Curr Cancer Drug Targets 9(8):915–930
Yu FX, Chai TF, He H, Hagen T, Luo Y (2010) Thioredoxin-interacting protein (Txnip) gene expression: sensing oxidative phosphorylation status and glycolytic rate. J Biol Chem 285(33):25822–25830
Zayzafoon M, Gathings WE, McDonald JM (2004) Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 145(5):2421–2432
Zhang Y, Lu T, Wong M et al (2016) Transient gene and microRNA expression profile changes of confluent human fibroblast cells in spaceflight. FASEB J Off Publ Fed Am Soc Exp Biol 30(6):2211–2224
Zhang Z, Lang J, Cao Z et al (2017) Radiation-induced SOD2 overexpression sensitizes colorectal cancer to radiation while protecting normal tissue. Oncotarget 8(5):7791–7800
Zhang C, Li L, Jiang Y et al (2018) Space microgravity drives transdifferentiation of human bone marrow-derived mesenchymal stem cells from osteogenesis to adipogenesis. FASEB J Off Publ Fed Am Soc Exp Biol 32(8):4444–4458
Zong C, Xue D, Yuan W et al (2010) Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur Cells Mater 20:109–120
Zuo B, Zhu J, Li J et al (2015) microRNA-103a functions as a mechanosensitive microRNA to inhibit bone formation through targeting Runx2. J Bone Miner Res Off J Am Soc Bone Miner Res 30(2):330–345
Acknowledgements
We much thank the opportunity of space experiment supplied by SJ-10 Recoverable Scientific Satellite. This study was supported by the grants from Strategically Guiding Scientific Special Project from Chinese Academy of Sciences (XDA04020202-23), Chinese National Nature Science Foundation (U1738102, 81570932).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Science Press and Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zhang, C., Li, L., Wang, J. (2019). Effects of Space Microgravity on the Trans-differentiation Between Osteogenesis and Adipogenesis of Human Marrow-Derived Mesenchymal Stem Cells. In: Duan, E., Long, M. (eds) Life Science in Space: Experiments on Board the SJ-10 Recoverable Satellite. Research for Development. Springer, Singapore. https://doi.org/10.1007/978-981-13-6325-2_12
Download citation
DOI: https://doi.org/10.1007/978-981-13-6325-2_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6324-5
Online ISBN: 978-981-13-6325-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)