Abstract
Objective
To investigate the expression of memory-related antioxidant genes and miRNAs under simulated weightlessness and the regulation of mechano growth factor (MGF) E domain, the peptide preventing nerve damage.
Results
Igf-iea and mgf mRNA levels, expression of antioxidant genes sod1 and sod2 and levels of miR-134 and miR-125b-3p increased in rat hippocampus after 14 days tail suspension to simulate weightlessness which was inhibited with intramuscular injection of E domain peptide. Therefore, administration of MGF E domain peptide could reverse increased expressions of memory-related igf-iea, mgf, sod1, sod2, miR-134 and miR-125b-3p in rat hippocampus under simulated weightlessness.
Conclusions
MGF may regulate the redox state and miRNA-targeted NR-CREB signaling, and intramuscular injection may be the alternative administration because of its safety, convenience and ability to pass through the blood brain barrier.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Space microgravity environment has lasting effects on the nervous system, which can lead to space motion sickness, space adaptation syndrome and cognitive disorders. The cognitive decline is also found under simulated microgravity such as head-down bed rest (HDBR) in humans and tail suspension in animals. The model of tail suspension induces cephalic fluid shift thus generating fluid redistribution and balance to simulate the fluid shift under microgravity condition (Frigeri et al. 2008). For example, the executive functions and the high-order aspects of cognition functions, were significantly diminished (Liu et al. 2012), with a detrimental effect on individual physiology and working memory (Liu et al. 2015) during HDBR. Furthermore, hippocampus-dependent spatial memory was depressed in mice (Wu et al. 2000) and in rats (Chen et al. 2013) after tail suspension. However, the mechanism remains unclear and there is no effective protective measure until now.
Antioxidases, such as glutathione peroxidase (GPX), superoxide dismutase (SOD) as well as catalase (CAT), can improve memory in mice with brain injury, Alzheimer’s disease or aging (Tsuru-Aoyagi et al. 2009; Massaad et al. 2009; Clausen et al. 2010; Lee et al. 2014). MiRNAs are small noncoding RNAs that regulate gene expression post-transcriptionally. Prior studies have shown that miRNAs played important role in memory formation-related synaptic plasticity (Aksoy-Aksel et al. 2014), neuronal activity (Sim et al. 2014) and brain development and function (Follert et al. 2014).
Insulin-like growth factor I (IGF-I), as a polypeptide, is a multipotent growth factor regulating cell proliferation, apoptosis (Yamahara et al. 2015), differentiation (Zhang et al. 2014), and so on. Igf-i gene has 6 exons. Splicing of exon 4 to exon 6 or exon 5–6 leads three mRNAs, igf-iea, igf-ieb and igf-iec, encoding the common mature IGF-I peptide, from the highly conserved exons 3 and 4, and the different E peptides Ea (in humans and rats), Eb (in humans) and Ec (in humans) whose counterpart is the Eb peptide in rodents. Rodent Eb or human Ec peptide is also called mechano growth factor (MGF). In humans, the three E peptides all have a common constitutive sequence of 16 amino acids (aa) from exon 4 and an active E domain, which of Ea, Eb and Ec contains 19 aa from exon 6, 61 aa from exon 5 and 24 aa from exons 5 and 8, respectively (Vassilakos et al. 2014).
The E domain of pro-IGF-I may have a role in regulating cell growth and differentiation in neuroblastoma cells (Vassilakos et al. 2014; Matheny et al. 2010). The carboxy terminal sequence of MGF with 24 aa, when given by intramuscular injection, improves muscle function (Vassilakos et al. 2014; Matheny et al. 2010), slows down age-related loss of muscle mass (Kandalla et al. 2011), and attenuates myocardial infarction (Peña et al. 2015). Furthermore, MGF is a neuroprotective agent and can protect neurons against brain ischemia in gerbils with carotid artery administration (Dluzniewska et al. 2005), prevent dopaminergic cells loss from 6-hydroxydopamine (6-OHDA) (Quesada et al. 2009) and protect SH-SY5Y cells against 6-OHDA-induced cell death (Quesada et al. 2009, 2011). This suggests that MGF may improve memory impairment because of its neuroprotective effects. Otherwise, both gene expression and protein expression of MGF were decreased with memory damage in tail-suspended rats in our previous study (Chen et al. 2013). However, it is unclear how MGF may regulate the expression of memory-related antioxidases and miRNAs. Intramuscular injection of a growth factor such as IGF-I could both ameliorate muscle function and prevent neuronal apoptosis induced by brain ischemia (Chang et al. 2010, 2013). Whether MGF, or another growth factor, could regulate the expressions of memory-related molecules by intramuscular injection remains unclear.
Therefore, this study is aimed to investigate the expressions of antioxidant genes related with memory and miRNAs targeting memory-related proteins under simulated weightlessness and the regulation effects of intramuscular injection of MGF, which can be helpful to understand the vital effect of MGF on the memory and provide a more safe and convenient mode of administration because of its ability to pass through the blood brain barrier.
Materials and methods
Reagents
A 24 aa peptide (YQPPSTNKNTKSQRRKGSTFEEHK), a modified human Ec peptide, containing at the position 23 a histidine (H) instead of the arginine (R) of the original human Ec peptide, corresponding to the unique C-terminal E domain region of the human MGF was synthesized and purified to >90 % by HPLC (Genescript Corp, NJ). The peptide was stabilized by amidating the C-terminus and switching the arginines at positions 14 and 15 to the D-stereoisomer. All other general chemicals made in China were of analytical grade.
Animals and treatment protocol
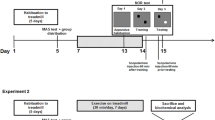
Male Sprague–Dawley rats (110–130 g) were housed at 23 ± 2 °C under 12-h light and dark cycles, were given a standard rodent unpurified diet (32 % protein, 14 % fat, and 54 % carbohydrate) and allowed access to food and water ad libitum. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of China Astronaut Research and Training Center. The protocol was approved by the Institutional Animal Care & Use Committee (IACUC) of China Astronaut Research and Training Center (Permit Number: ACC-IACUC-2012-003). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. Six groups (n = 8 in each group) of rats were used (Fig. 1). The hindlimbs of the tail-suspended group were elevated to a spinal orientation of 30° above horizontal while the forelimbs were free to ambulate around the entire range of the cage. Rats in the groups with tail suspension plus MGF E domain peptide treatment were injected with 0.1 ml peptide (1 μg/0.1 ml saline) in muscle membrane of gastrocnemius on the second day after tail suspension for 7 or 14 days. The control and tail-suspended groups were received same volume, but saline alone. All rats were anaesthetised and the hippocampus and cerebral cortex were isolated from head and immediately stored in liquid nitrogen (−196 °C) until RT-qPCR detection.
RNA extraction
Total RNA was extracted from the hippocampus and cerebral cortex with TRIzol. The amount of RNA was quantified from the A260/A280 ratio. RNA quality was also determined by electrophoresis on an ethidium bromide-stained 1.5 % agarose gel.
Reverse transcription and qPCR for gene expression
One μg DNase-treated (Takara) total RNA was subsequently reverse transcribed to cDNA using random hexamers (Takara) and reverse transcriptase (Takara). Primer pairs were optimized for annealing temperature and specificity was confirmed by melting curve analysis. QPCR was carried out using SYBR Green Fluorescein mix (Takara), with an initial activation step of 95 °C for 1 min followed by 45 cycles of denaturation (95 °C, 15 s), annealing (60 °C, 15 s) and extension (72 °C, 15 s), and final melting curve analysis. Expression levels were normalized against the endogenous gapdh control. The primers sequences used for qPCR (igf-iea, mgf, sod1, sod2, gpx1 and cat) were based on published sequences in GenBank Overview (http://www.ncbi.nlm.nih.gov/genbank/). They were designed using Primer premier 5 program version 5.0.0 and custom made by Invitrogen (Supplementary Table 1).
Reverse transcription and qPCR for miRNA expression
One μg DNase-treated total RNA was added to Poly(A) tail with Poly(A) Polymerase and subsequently reverse transcribed to cDNA with PrimeScript RTase using One Step PrimeScript miRNA cDNA Synthesis Kit (Takara). Primers were optimized for annealing temperature and specificity was confirmed by melting curve analysis. QPCR was carried out using SYBR Green Fluorescein premix Ex Taq TM II (Takara) and Uni-miR qPCR primer (Takara), with an initial activation step of 95 °C for 1 min followed by 45 cycles of denaturation (95 °C, 15 s), annealing (60 °C, 15 s) and extension (72 °C, 15 s), and final melting curve analysis. Expression levels were normalized against the endogenous control (5 s rRNA). The forward primers sequences used for qPCR (miR-124, miR-134, miR-132, miR-138, miR-125b-3p, miR-125b-5p and miR-145) were designed using Primer premier 5 program version 5.0.0 and custom made by Invitrogen (Supplementary Table 1).
Statistical analysis
Data are expressed as mean ± SEM. Differences in the means were tested using Student’ s t-test and analysis of variance. Bonferroni post hoc tests were used when the p value indicated a significant difference between the groups. A P-value of 0.05 was considered statistically significant.
Results
Mgf mRNA expression increases in rat hippocampus after 14-day tail suspension but is inhibited by treatment with E domain peptide
The expressions of igf-iea and mgf were detected under simulated microgravity and treatment with MGF E domain peptide. After tail suspension for 7 days, expression of igf-iea and mgf increased by 0.4-fold and 0.7-fold, respectively, in the hippocampus (P > 0.05) and there was no effect of peptide treatment on the expressions of these isoforms (Fig. 2a); expression of igf-iea or mgf had no change in cerebral cortex but the peptide significantly inhibited mgf expression by 50 % (P < 0.01) (Fig. 2b). After tail suspension for 14 days, expression of igf-iea and mgf increased by 1.7-fold (P < 0.01) and 0.4-fold (P < 0.05), respectively in the hippocampus but the peptide inhibited the high levels of igf-iea (P < 0.05) and mgf (P > 0.05) (Fig. 2c); no change of igf-iea mRNA but excessive mgf mRNA level by 0.9 fold (P < 0.05) in cerebral cortex and no effects of peptide treatment were found (Fig. 2d).
Mgf mRNA expression increases after 14-day tail suspension but is inhibited by E domain peptide treatment in rat hippocampus. Data are expressed as mean ± SEM, *P < 0.05, **P < 0.01. C7, control for 7 days; S7, tail suspension for 7 days; S7M, tail suspension with E domain peptide treatment for 7 days; C14, control for 14 days; S14, tail suspension for 14 days; S14M, tail suspension with E domain peptide treatment for 14 days
Expressions of sod1 and sod2 are slightly upregulated in rat hippocampus after 14-day tail suspension but recover after treatment with E domain peptide
The expressions of the antioxidant genes, sod1, sod2, gpx1 and cat, were measured. After tail suspension for 7 days, expressions of sod1 and sod2, in the hippocampus, had no change but, after E domain peptide treatment, reduced by 25 and 23 %, respectively (P < 0.05), but those of gpx1 or cat were not affected after tail suspension or peptide treatment (Fig. 3a). Expressions of all antioxidant genes had no change after either tail suspension or peptide treatment (Fig. 3b). Analogously, after tail suspension for 14 days, expressions of sod1 and sod2 were slightly upregulated in the hippocampus (P > 0.05) but recovered with peptide treatment (P < 0.05), but those of gpx1 or cat were not affected after tail suspension or peptide treatment (Fig. 3c). Expression of sod1 in the cerebral cortex, lessened by 66 % and then partly recovered with peptide administration (P < 0.05) but the expressions of other antioxidant genes did not change with tail suspension or peptide treatment (Fig. 3d).
Expression of sod1 and sod2 are slightly upregulated after 14-day tail suspension but recover with E domain peptide treatment in the rat hippocampus. Data are expressed as mean ± SEM, *P < 0.05. C7, control for 7 days; S7, tail suspension for 7 days; S7M, tail suspension with E domain peptide treatment for 7 days; C14, control for 14 days; S14, tail suspension for 14 days; S14M, tail suspension with E domain peptide treatment for 14 days
Screen of miRNAs and their targeted memory-related proteins
MiRNAs targeting memory-related proteins were also screened according to previous reports. Six miRNAs (miR-124, miR-134, miR-132, miR-138, miR-125b and miR-145) targeted memory-related proteins such as cAMP-response element binding protein (CREB), methyl CpG binding protein 2 (MeCP2), acyl protein thioesterase 1 (APT1), N-methyl-d-aspartate receptor subunit 2A (NR2A) and superoxide dismutase 2 (SOD2), and function in memory-related morphogenesis, plasticity and physiology of synapses and neuroprotection (Table 1).
Expression of miRNAs targeting memory-related proteins in rat hippocampus and cerebral cortex
Expression of these miRNAs were analyzed in the rat hippocampus and cerebral cortex by smiRNAdb, a database containing expression information for human, mouse, rat, zebrafish, worm and fruitfly small RNAs. Mature miR-125b has two types, miR-125b-3p and miR-125b-5p. Expressions of most miRNAs were all moderate although those of miR-145, miR-125b-3p and miR-134 were low in the hippocampus and cerebral cortex. In addition, expression of all miRNAs in hippocampus was higher than that in cerebral cortex (Fig. 4a). To verify these results, we measured the levels of the miRNAs by RT-qPCR. All miRNAs in hippocampus were richer compared with that in cerebral cortex (Fig. 4b).
Expression of miRNAs targeting memory-related proteins in rat hippocampus and cerebral cortex. The expression levels of miRNAs were analyzed by database in database in smiRNAdb (www.mirz.unibas.ch/cloningprofiles) (a) or RT-qPCR detection (b). The larger value in a or b represents the lower expression of miRNA
Levels of miR-134 and miR-125b-3p rise in the rat hippocampus after 14-day tail suspension but are reversed after treatment with E domain peptide
The levels of these miRNAs were measured after tail suspension and peptide treatment. After 7 days, there was no change in miRNA expression in the hippocampus or in the cerebral cortex but elevated miR-125b-5p in cerebral cortex was found after tail suspension or E domain peptide treatment (Fig. 5a, b). After 14 days, only expressions of miR-134 and miR-125b-3p rose in the rat hippocampus (P > 0.05) but were reversed with peptide treatment (P < 0.05) (Fig. 5c); no miRNA expression was changed with either tail suspension or peptide treatment in cerebral cortex, nevertheless, level of miR-134 rose after peptide treatment (P < 0.05) (Fig. 5d).
Levels of miR-134 and miR-125b-3p rise after 14-day tail suspension but are reversed with E domain peptide treatment in the rat hippocampus. Data are expressed as mean ± SEM, *P < 0.05, **P < 0.01. C7, control for 7 days; S7, tail suspension for 7 days; S7M, tail suspension with E domain peptide treatment for 7 days; C14, control for 14 days; S14, tail suspension for 14 days; S14M, tail suspension with E domain peptide treatment for 14 days
Discussion
Hippocampus and cerebral cortex are critical for spatial memory (Griffin, 2015) which declines under microgravity. Therefore, the expressions of memory-related molecules such as mgf, igf-iea, antioxidases, and miRNAs were examined in tail-suspended rat hippocampus and cerebral cortex. However, expressions of igf-iea, mgf, sod1, sod2, miR-134 and miR-125b-3p changed with tail suspension in the hippocampus not in the cerebral cortex. Changes occurred in structural proteins and proteins involved in metabolism in hippocampus in response to the microgravity environment (Sarkar et al. 2006). Expressions of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) were also changed in the hippocampus after space microgravity environment (Santucci et al. 2012). These results suggest that the hippocampus is relatively sensitive area in brain under microgravity.
Expressions of mgf, sod1, sod2, miR-134 targeting cAMP-response element binding protein (CREB) and miR-125b-3p targeting NMDA receptor (NR2A) were all upregulated in rat hippocampus after 14 days tail suspension. During neuron protection, MGF and SOD could protect neurons and prevent against oxidative stress, respectively. MGF may regulate the expression of SOD through activation of nuclear factor erythroid-2-related factor 2 (NRF2) (Quesada et al. 2011), an antioxidant protein and transcription factor, directly promoting the transcription of sod gene (Ruiz et al. 2013). During memory formation, NR2A-containing synaptic NMDA receptors leads to activation of CREB, long-term potentiation (LTP) and neuronal survival (Hardingham et al. 2002), which was inhibited by miR-134 and miR-125b-3p through post-transcriptional pathways. Moreover, no change of gpx1, cat, miR-124, miR-132, miR-138, miR-125b-5p or miR-145 was found in the hippocampus after 14 days. For those reasons, there may be two effects on hippocampus-dependent memory under simulated microgravity. The positive effect is MGF-NRF2-SOD mediated neuroprotective pathways and the negative is miR-134 and miR-125b-3p-mediated inhibition of NR2A-CREB pathway which were needed to be confirmed by the further research on the expressions of those proteins. Previously we had found a decrease in the expression of mRNA and protein for MGF in the hippocampus in the rat model of tail-suspension that was associated with a decrease in spatial memory. In the current work, however, as seen in Fig. 2c an increase in expression of IGF-IEa and MGF was seen in the hippocampus in the same model. The difference between these two studies may be related with that the decrease expression of MGF in previous work appeared after the training for spatial memory test.
Expression of igf-iea mRNA also increased in the rat hippocampus after 14 days tail suspension. MGF was more effective than insulin-like growth factor-I (IGF-I) Ea domain for protection of neurons (Aperghis et al. 2004). Different IGF-I isoforms Ea and MGF, probably indicate different and biological effects under various conditions (Philippou et al. 2014). There may be synergistic or complementary effects between IGF-I Ea and MGF under simulated microgravity which will be determined in the next study. Moreover, the responses to MGF E peptide treatments do not appear to be isoform (MGF)-specific, as might be expected.
No change of igf-iea, mgf, antioxidant genes or miRNAs was found in the rat hippocampus after 7 days tail suspension, which suggested that the expression of memory-related molecules was time specific in hippocampus under simulated microgravity. Expressions of other genes were unchanged except for increased mgf and decreased sod1 after 14 days and increased miR-125b-5p after 7 days in rat cerebral cortex. This suggests the different effect of simulated microgravity and the various responses on cerebral cortex from hippocampus.
Interestingly, peripheral administration of MGF E domain peptide recovered the expressions of igf-iea, mgf, sod1, sod2, miR-134 and miR-125b-3p in hippocampus after 14 days tail suspension, as well as those of mgf after 7 days and sod1 and miR-134 after 14 days in cerebral cortex. The positive and negative effects may play as adaptive responses for simulated microgravity, but, on the other hand, MGF may be able to maintain the steady regulation of memory process which is not excessive or inadequate because the effective and moderate neuroprotection of MGF (Dluzniewska et al. 2005) and there may also be negative feedback for MGF from periphery to central. Expressions of sod1 and sod2 were reversed in hippocampus with 7 days tail suspension plus peptide treatment which suggested an early effect of MGF on SOD level. These will be become clearer with further study.
The functional sequence of MGF, as well as its downstream signaling pathways remain unclear. Other research has shown that MGF may activate ERK through an IGF-IR-independent mechanism (Matheny et al. 2010).
Conclusion
Intramuscular injection of MGF E-domain could reverse the increased expression of memory-related sod1, sod2, miR-134 and miR-125b-3p in rat hippocampus under simulated weightlessness. This suggests that MGF could regulate the antioxidase SOD and miR-134-targeted and miR-125b-3p-targeted NR-CREB signaling involved in memory formation in the hippocampus under simulated microgravity and intramuscular injection may be the alternative administration because of its safety, convenience and ability to pass through the blood brain barrier. Further work will focus on whether the MGF E domain peptide plays a positive role in the memory process and expression of memory-related antioxidases and miRNAs.
References
Aksoy-Aksel A, Zampa F, Schratt G (2014) MicroRNAs and synaptic plasticity–a mutual relationship. Phil Trans R Soc Lond B 369(2013):0515
Aperghis M, Johnson IP, Cannon J, Yang SY, Goldspink G (2004) Different levels of neuroprotection by two insulin-like growth factor-I splice variants. Brain Res 1009:213–218
Chang HC, Yang YR, Wang PS, Kuo CH, Wang RY (2010) Effects of insulin-like growth factor 1 on muscle atrophy and motor function in rats with brain ischemia. Chin J Physiol 53:337–348
Chang HC, Yang YR, Wang PS, Kuo CH, Wang RY (2013) The neuroprotective effects of intramuscular insulin-like growth factor-I treatment in brain ischemic rats. PLoS One 8:e64015
Chen HL, Lv K, Qu LN, Zhang YL, Bi L, Zhong P, Ji GH, Cao HQ, Li YH (2013) Tail suspension disrupts cognition function and down-regulates memory-related proteins expression in rat hippocampus. Space Med Med Eng (Beijing) 26:426–432
Clausen A, Doctrow S, Baudry M (2010) Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging 31:425–433
Dharap A, Bowen K, Place R, Li LC, Vemuganti R (2009) Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 29:675–687
Dluzniewska J, Sarnowska A, Beresewicz M, Johnson I, Srai SK, Ramesh B, Goldspink G, Górecki DC, Zabłocka B (2005) A strong neuroprotective effect of the autonomous C-terminal peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB J 19:1896–1898
Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M (2010) RRegulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65:373–384
Follert P, Cremer H, Béclin C (2014) MicroRNAs in brain development and function: a matter of flexibility and stability. Front Mol Neurosci 7:5
Frigeri A, Iacobas DA, Iacobas S, Nicchia GP, Desaphy JF, Camerino DC, Svelto M, Spray DC (2008) Effect of microgravity on gene expression in mouse brain. Exp Brain Res 191:289–300
Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466:1105–1109
Griffin AL (2015) Role of the thalamic nucleus reuniens in mediating interactions between the hippocampus and medial prefrontal cortex during spatial working memory. Front Syst Neurosci 9:29
Hardingham GE, Fukunaga Y, Bading H (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5:405–414
Kandalla PK, Goldspink G, Butler-Browne G, Mouly V (2011) Mechano growth factor E peptide (MGF-E), derived from an isoform of IGF-1, activates human muscle progenitor cells and induces an increase in their fusion potential at different ages. Mech Ageing Dev 132:154–162
Lee WH, Kumar A, Rani A, Foster TC (2014) Role of antioxidant enzymes in redox regulation of N-methyl-d-aspartate receptor function and memory in middle-aged rats. Neurobiol Aging 35:1459–1468
Liu Q, Zhou R, Chen S, Tan C (2012) Effects of head-down bed rest on the executive functions and emotional response. PLoS One 7:e52160
Liu Q, Zhou R, Zhao X, Oei TP (2015) Effects of prolonged head-down bed rest on working memory. Neuropsychiatr Dis Treat 11:835–842
Lusardi TA, Farr CD, Faulkner CL, Pignataro G, Yang T, Lan J, Simon RP, Saugstad JA (2010) Ischemic preconditioning regulates expression of microRNAs and a predicted target, MeCP2, in mouse cortex. J Cereb Blood Flow Metab 30:744–756
Massaad CA, Washington TM, Pautler RG, Klann E (2009) Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 106:13576–13581
Matheny RW Jr, Nindl BC, Adamo ML (2010) Minireview: mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology 151:865–875
Peña JR, Pinney JR, Ayala P, Desai TA, Goldspink PH (2015) Localized delivery of mechano-growth factor E-domain peptide via polymeric microstructures improves cardiac function following myocardial infarction. Biomaterials 46:26–34
Philippou A, Maridaki M, Pneumaticos S, Koutsilieris M (2014) The complexity of the IGF1 gene splicing, posttranslational modification and bioactivity. Mol Med 20:202–214
Quesada A, Micevych P, Handforth A (2009) C-terminal mechano growth factor protects dopamine neurons: a novel peptide that induces heme oxygenase-1. Exp Neurol 220:255–266
Quesada A, Ogi J, Schultz J, Handforth A (2011) C-terminal mechano-growth factor induces heme oxygenase-1-mediated neuroprotection of SH-SY5Y cells via the protein kinase Cϵ/Nrf2 pathway. J Neurosci Res 89:394–405
Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ, Sander C, Tuschl T, Kandel E (2009) Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron 63:803–817
Ruiz S, Pergola PE, Zager RA, Vaziri ND (2013) Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83:1029–1041
Santucci D, Kawano F, Ohira T, Terada M, Nakai N, Francia N, Alleva E, Aloe L, Ochiai T, Cancedda R, Goto K, Ohira Y (2012) Evaluation of gene, protein and neurotrophin expression in the brain of mice exposed to space environment for 91 days. PLoS One 7:e40112
Sarkar P, Sarkar S, Ramesh V, Hayes BE, Thomas RL, Wilson BL, Kim H, Barnes S, Kulkarni A, Pellis N, Ramesh GT (2006) Proteomic analysis of mice hippocampus in simulated microgravity environment. J Proteome Res 5:548–553
Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, Khudayberdiev S, Leuschner PF, Busch CJ, Kane C, Hübel K, Dekker F, Hedberg C, Rengarajan B, Drepper C, Waldmann H, Kauppinen S, Greenberg ME, Draguhn A, Rehmsmeier M, Martinez J, Schratt GM (2009) A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol 11:705–716
Sim SE, Bakes J, Kaang BK (2014) Neuronal activity-dependent regulation of microRNAs. Mol Cells 37:511–517
Tsuru-Aoyagi K, Potts MB, Trivedi A, Pfankuch T, Raber J, Wendland M, Claus CP, Koh SE, Ferriero D, Noble-Haeusslein LJ (2009) Glutathione peroxidase activity modulates recovery in the injured immature brain. Ann Neurol 65:540–549
Vassilakos G, Philippou A, Tsakiroglou P, Koutsilieris M (2014) Biological activity of the e domain of the IGF-1Ec as addressed by synthetic peptides. Hormones (Athens) 13:182–196
Wu DW, Shen XY, Dong Q, Wang SP, Cheng ZH, Zhang SJ (2000) Effects of tail suspension on learning and memory function of mice. Space Med Med Eng (Beijing) 13:244–248
Yamahara K, Yamamoto N, Nakagawa T, Ito J (2015) Insulin-like growth factor 1: a novel treatment for the protection or regeneration of cochlear hair cells. Hear Res 330:2–9
Zhang X, Zhang L, Cheng X, Guo Y, Sun X, Chen G, Li H, Li P, Lu X, Tian M, Qin J, Zhou H, Jin G (2014) IGF-1 promotes Brn-4 expression and neuronal differentiation of neural stem cells via the PI3K/Akt pathway. PLoS One 9:e113801
Acknowledgments
This work was supported by the National instrumentation program of China (2013YQ190467, 2012YQ040140091), Shenzhen science & technology institute (JCYJ20150629164441050), Foundation of State Key Laboratory of Space Medicine Fundamentals and Application, China Astronaut Research and Training Center (SMFA15B01, SMFA13B02) and the Medicinal Science and Technology Research Project (BWS11J052).
Supporting information
Supplementary Table 1—sequences of qPCR primers for rats
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Lv, K., Dai, Z. et al. Intramuscular injection of mechano growth factor E domain peptide regulated expression of memory-related sod, miR-134 and miR-125b-3p in rat hippocampus under simulated weightlessness. Biotechnol Lett 38, 2071–2080 (2016). https://doi.org/10.1007/s10529-016-2210-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2210-4