Abstract
The urethra is a muscular tube that extends from the bladder neck and is composed of an inner layer of smooth muscle referred to as the internal urethral sphincter and an outer layer of striated muscle which forms the external urethral sphincter. The smooth muscle layer can be separated into an inner layer of longitudinally orientated smooth muscle and an outer, relatively thinner, layer of circular muscle. Tonic contraction of both the smooth and striated muscle components of the urethra generates a urethral closure pressure which exceeds intravesical pressure in the bladder to maintain urinary continence. It is likely that contraction of urethral smooth muscle is involved in the long-term maintenance of tone, since it can achieve this at relatively low energy cost, whereas the striated muscle contributes more to the rise in urethral tone that accompanies increases in bladder pressure secondary to coughing or other sudden increases in intra-abdominal pressure. The level of urethral smooth muscle tone is regulated by several autonomic neurotransmitters, including noradrenaline, acetylcholine, ATP and nitric oxide. However, it is also clear that urethral smooth muscle is capable of generating significant tone in the absence of neural input. In this chapter we will discuss the mechanisms responsible for contraction of urethral smooth muscle, with specific focus on the role of ion channels and Ca2+ handling proteins to this process. The mechanisms underlying spontaneous activity in urethral interstitial cells (UICs), putative pacemaker cells of the urethra, will also be examined along with the modulation of these mechanisms by key excitatory and inhibitory neurotransmitters.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Urinary continence is maintained by the concerted actions of the bladder, urethra, pelvic floor muscles, and surrounding connective tissues. The bladder and urethra work as a functional reciprocal unit under normal conditions, such that during the storage phase, the detrusor muscle of the bladder remains relaxed while the urethra is contracted to allow gradual filling of the bladder with urine and prevent leakage. In contrast, during voiding, the urethra relaxes and the detrusor contracts to facilitate emptying of the bladder [1, 2]. Therefore, the primary functions of the urethra are to (1) maintain urinary continence by generating a closure pressure that exceeds intravesical pressure and (2) facilitate voiding of urine at micturition. The urethra is composed of an inner layer of smooth muscle referred to as the internal urethral sphincter and an outer layer of striated muscle which forms the external urethral sphincter. Both muscle layers function together as a sphincter complex, albeit with distinct roles. The striated muscle is thought to be important in resisting rapid increases in abdominal pressure that occur during coughing or laughing. In contrast, contraction of the smooth muscle layer is important for generating and maintaining sufficient urethral closure pressure during bladder filling to prevent leakage [1, 3, 4]. Indeed, Jankowski et al. concluded that ‘urethral smooth muscle, in comparison with striated muscle, is capable of imparting a greater influence on the long-term functional responses of the urethra’ [5].
Stress urinary incontinence (SUI) arises when intravesical pressure exceeds urethral pressure, in response to sudden increases of intra-abdominal pressure and is common in women after vaginal delivery child birth [6]. Traditionally, this was primarily viewed as being due to loss of urethral support, referred to as urethral hypermobility. However, it has been reported that SUI in women was more likely to be associated with a reduction in urethral resistance, rather than anatomical changes in urethral support [7, 8]. There is also evidence that this may arise through an effect on urethral smooth muscle, as Prantil et al. showed that in a rat model of acute SUI, induced by simulated birth trauma, there was reduced urethral smooth muscle tone, indicating that ‘the basal smooth muscle tone acts as a prime coordinator to the continual maintenance of continence’ [9]. Urethral closure pressure also declines with age [10, 11], and this is correlated with a decrease in the density of circular smooth muscle in the urethra [12]. Therefore, it is clear that urethral smooth muscle tone makes an important contribution to urinary continence and its dysfunction is associated with urinary incontinence.
The smooth muscle of the urethra can be separated into two layers consisting of an internal longitudinally orientated layer and an outer, relatively thin, circular muscle layer. While it is clear that contraction of circularly orientated smooth muscle could maintain continence by occluding the lumen of the urethra, the precise role of the longitudinal layer is still unclear [1]. Urethral smooth muscle (USM) generates spontaneous tone [13], which can be modulated by several autonomic neurotransmitters including nitric oxide [14, 15], noradrenaline [16, 17], adenosine triphosphate [18,19,20,21] and acetylcholine [14, 22,23,24]. Although there are many unanswered questions regarding the mechanisms that underlie urethral tone, a clearer picture of the cellular mechanisms that contribute to spontaneous activity in USM is beginning to emerge. It is now recognised that several cell types within the smooth muscle layer of the urethra are spontaneously active, including smooth muscle cells and a population of cells, namely urethral interstitial cells (UICs). UICs are also referred to variously as interstitial cells [25], interstitial cells of Cajal (ICC, by analogy with similar cells that have been well described in the gastrointestinal tract [26]), ICC-like cells [27], and Cajal-like interstitial cells [28]. In this chapter we will describe the mechanisms proposed to underlie spontaneous activity in USM and examine how it is modulated by autonomic neurotransmitters.
2 Spontaneous Activity in Urethral Smooth Muscle
In comparison with the detrusor and gastrointestinal tract, studies on smooth muscle from the urethra are relatively sparse. The lack of research on this tissue has been attributed to the small size of the urethra in rats, guinea-pigs and mice [29], but it may also reflect an historical underestimation of the functional importance of urethral smooth muscle in maintaining continence. Nevertheless, early intracellular microelectrode recordings by Callahan and Creed on strips of guinea-pig USM, found that it generated spontaneous electrical activity which consisted of ‘bursts of spikes’ (once every 1–7 min) separated by quiescent periods [30]. There were 20–30 spikes observed within these bursts, which lasted for 7–10 s. The mean resting membrane potential in the quiescent periods was −42 mV. An interesting observation from this study was that the electrical activity in the USM was poorly coordinated and that there was ‘limited spread of activity’. For example, ‘if an electrode was withdrawn from one cell and inserted into another, less than 1 mm away, it was frequently found that the patterns of activity were out of phase’. They also reported that, in contrast to observations in the detrusor, it was not possible to record spikes evoked by extracellular current pulses in USM. They suggested that the limited spread of activity ‘probably prevents generalised, synchronous contraction’. Later studies by Callahan and Creed [31] on rabbit USM found that spontaneous electrical activity consisted of regular, single or (occasionally) compound spikes occurring at a frequency of 9–30 per min [31]. They also subsequently reported that rabbit USM developed action potentials and spontaneous depolarisations of ~16 mV in amplitude [24].
A seminal study by Hashitani et al. further elucidated the mechanisms underlying spontaneous electrical activity in the urethra [32]. They made intracellular microelectrode recordings from the circular smooth muscle layer of rabbit urethra and found that it produced large, regularly occurring depolarisations, termed slow waves (SWs) as well as smaller, irregularly occurring events, termed spontaneous transient depolarisations (STDs ) (Fig. 6.1). Both SWs and STDs were resistant to atropine, phentolamine, guanethidine and tetrodotoxin, indicating that they were not of neural origin. The L-type Ca2+ channel antagonist, nifedipine failed to block these events, however, they were inhibited by reducing external [Cl−] and by application of the Ca2+-activated chloride channel (CACC ) blockers niflumic acid and DIDS, suggesting an involvement of CACCs in their generation. Hashitani et al. also found that SWs and STDs were inhibited by the SERCA (sarcoplasmic /endoplasmic reticulum Ca2+ ATPase) inhibitor, cyclopiazonic acid (CPA ) as well as caffeine, indicating that Ca2+ released from intracellular stores was required for the generation of spontaneous electrical activity. These data indicated that the spontaneous electrical activity in USM was mediated by release of Ca2+ from intracellular stores which activated CACCs. Similar findings were also reported for guinea-pig USM, whereby STDs and slow waves were also abolished by niflumic acid, low chloride solution, CPA and caffeine [33].
Slow waves (SWs) and spontaneous transient depolarisations (STDs ) recorded from the rabbit urethra. (a, b) Records from the same cell on different time scales. Adapted from [32]
The finding that tonic contraction of USM was associated with phasic electrical activity was not intuitive and was somewhat at odds with the prevailing view that spontaneous myogenic tone depended on continuous entry of calcium through L-type Ca2+ channels [13]. However, given the poor coupling of electrical activity observed in the urethra [30], it is possible that asynchronous electrical events and their associated contractions, sum to form an overall tonic contraction. This idea was explored in more detail in imaging studies of intact USM strips [27, 34].
Hashitani and Suzuki examined the properties of spontaneous Ca2+ transients recorded from both smooth muscle and interstitial cells of the rabbit urethra in situ [27]. The topic of interstitial cells in USM will be addressed later in this chapter but, for now, we will focus on spontaneous activity that has been recorded in urethral smooth muscle cells (USMCs ). They reported that SMC in the rabbit urethra, loaded with fluorescent Ca2+ indicator, fluo-4 AM, generated spontaneous Ca2+ transients at a frequency of ~11 per min. These events occurred either as ‘non-propagated Ca2+ transients or intercellular Ca2+ waves within a muscle bundle’. However, in contrast to intercellular Ca2+ waves observed in detrusor smooth muscle bundles of the guinea-pig bladder [35], the Ca2+ waves originating from a single site in USM often failed to spread across muscle bundles. When changes in muscle tension were measured simultaneously with intracellular [Ca2+] it was found that ‘there was no correlation between muscle contractions and Ca2+ transients in any particular muscle bundle within the preparations, presumably arising from a low synchronicity between bundles’ [27].
Drumm et al. investigated this matter further by examining Ca2+ transients in USM strips dissected from SmMHC-Cre-GCaMP3 mice, which have a genetically encoded Ca2+ sensor, GCaMP3, selectively expressed in SMCs [34]. This study revealed that USMCs within smooth muscle bundles fired spontaneous intracellular Ca2+ transients. However, while distinct Ca2+ events could be imaged within individual USMC there was no evidence of intercellular propagation of Ca2+ events within USM bundles. Indeed, spontaneous Ca2+ events that occurred in adjacent SMCs were not synchronised and appeared to be independent of the activity occurring in their neighbouring cell. Furthermore, it was also apparent that the firing of intracellular Ca2+ waves in USMCs was associated with small contractions of individual USMC in muscle bundles. These contractions, like the intracellular Ca2+ waves, occurred asynchronously and failed to spread cell-to-cell across the muscle bundles.
Therefore, electrical and imaging studies of intact USM strips indicate that USM develops spontaneous depolarisations and Ca2+ transients, respectively, but that these events are poorly coupled, resulting in asynchronous contractions across the tissue that sum to give an overall tonic contraction. The cellular mechanisms underlying the spontaneous activity described above are discussed below.
3 Spontaneous Activity in Isolated Urethral Smooth Muscle Cells
Ca2+ waves recorded from rabbit USM, in situ, appear to rely on a combination of Ca2+ release from stores and Ca2+ influx via L-type Ca2+ channels, since they were inhibited by the SERCA inhibitor, CPA, the ryanodine receptor (RyR ) antagonist, ryanodine, the inositol trisphophate receptor (IP3R ) inhibitor, 2-APB and the L-type Ca2+ channel antagonist nifedipine [27]. The involvement of L-type Ca2+ channels was consistent with other reports showing that nifedipine inhibited spontaneous action potentials and spikes superimposed on slow spontaneous depolarisations in rabbit USM [36, 37] and that application of 60 mM [K+]o solution evoked robust Ca2+ transients and contractions in isolated rabbit USMCs [38]. Ca2+ waves in intact murine USM strips were also inhibited by Ca2+ store release modulators, including the SERCA inhibitor, thapsigargin, the RyR antagonist, tetracaine and the IP3R inhibitor, xestospongin C [34]. Surprisingly however, Ca2+ waves in murine USMCs were resistant to the L-type Ca2+ channel modulators nifedipine, nicardipine, isradipine and FPL, but were abolished by Ca2+-free bathing media, the store operated Ca2+ entry channel (SOCE) blocker SKF 96365 and the Orai channel antagonist, GSK-7975A [34]. Therefore, it appears that, in contrast to rabbit USMCs, Ca2+ waves in murine USMCs are not reliant on Ca2+ influx via L-type Ca2+ channels.
To date, only a few labs have made patch clamp recordings from freshly isolated USMCs. One of the main contributors to this field was Teramoto and colleagues in Alison Brading’s Lab in Oxford. They systematically characterised K+ATP channels in USM and showed that these channels have an important functional role in regulating membrane potential and relaxation of this tissue [39, 40]. The nature and molecular identity of K+ATP channels in USM has been comprehensively reviewed elsewhere [29, 41] and therefore will not be recapitulated here.
The intracellular microelectrode recordings by Hashitani et al. indicated that STDs and slow waves recorded from intact rabbit USM strips arose from activation of CACCs [32]. The first study to examine CACC currents (IClCa) in isolated USMCs was by Cotton et al. [42]. Using the perforated patch configuration of the whole cell voltage clamp technique, they found that freshly isolated SMC from the sheep urethra exhibited robust IClCa. Furthermore, they found that a proportion of these cells were spontaneously active, developing STDs and action potentials under current clamp that were sensitive to the CACC inhibitor anthracene-9-carboxylic acid (A-9-C , Fig. 6.2). These findings led the authors to propose that CACC in sheep USMCs may function as a pacemaker current. Later investigation of these cells found that ~10% of sheep USMCs developed spontaneous transient inward currents (STICs ) when maintained at −60 mV [43]. These events were inhibited by the CACC blockers niflumic acid and A-9-C, and reversed near ECl, confirming the importance of CACCs to spontaneous activity in USM. STICs in sheep USMCs were also inhibited by ryanodine and caffeine suggesting that Ca2+ release from intracellular stores was important for activation of these events. Sancho et al. found that Anoctamin 1 (Ano1 ) , also referred to as TMEM16A, and now recognised as the molecular correlate of CACCs [44,45,46], was expressed in the smooth muscle layer of the sheep urethra, consistent with idea that Ano1 encoded CACCs in sheep USMCs [47]. This study also reported Ano1 expression in USM of rats and mice, although Huang et al. (2009) failed to detect Ano1 in murine USM [48]. Precise reasons for the divergent findings in these studies is unclear, although differences in antibody specificity and experimental protocols have been suggested [47].
Effect of A-9-C on spontaneous electrical activity in sheep urethral SMC. (a) Top panel, shows recordings made in current clamp using a Cs+-filled pipette before addition of A-9-C (1 mM). The top two traces are a continuous recording. (b) events marked by * in a are compared on an expanded time scale, showing blockade of the action potential and reduced amplitude of the STD. Adapted from [42]
In order to further explore the cellular basis of the spontaneous activity described by Hashitani et al., in the rabbit urethra, Sergeant et al. undertook a patch clamp investigation to determine the properties of SMC freshly isolated from the rabbit urethra [25, 32]. Surprisingly however, despite having robust L-type Ca2+ currents, evidence for IClCa in these cells was weak. The large slowly developing inward currents and tail currents, typical of IClCa, that were evident in sheep USMCs, were not readily resolvable in rabbit USMCs. Current-voltage (IV) data averaged from 21 SMCs demonstrated that these cells had only small, sustained, inward currents at −30 mV and which reversed close to ECl. Furthermore, only ~3% of the rabbit SMC developed spontaneous electrical activity, even though they could respond to depolarising current injection (40 ms) by producing action potentials [25]. A later study by Hollywood et al. examined inward currents in SMCs isolated from the human proximal urethra using the whole cell patch clamp technique, and while L and T-type Ca2+ currents were reported, there was no evidence of IClCa [49]. However, this subject warrants further investigation, using the perforated patch configuration of the whole cell patch clamp technique to optimise the conditions required to detect IClCa. Therefore, at the present time, there is still a lack of consensus regarding the contribution of CACCs in USMCs to spontaneous activity in intact USM strips. However, an interesting observation by Sergeant et al. was that although the evidence for IClCa in rabbit USMCs was weak, there was another cell type, termed ‘interstitial cells’ (referred to in this review as urethral interstitial cells, UICs) which had a robust IClCa and were spontaneously active [25].
4 Spontaneous Activity in Urethral Interstitial Cells
The first evidence that the urethra may contain a novel population of interstitial cells came from a study by Smet et al., which described a population of interstitial cells in human urethra preparations that were immuno-positive for vimentin and cGMP and bore a striking resemblance to the ICC described in the GI tract [50]. Smet et al. suggested that the UIC could be the targets for neuronally released nitric oxide and, by analogy, act in similar fashion to ICC in the GI tract which are also known to serve as mediators of neurotransmission [51,52,53,54]. However, Smet et al. did not propose a role for these cells in the generation of spontaneous electrical activity in USM. Interestingly however, Hashitani et al. did allude to the possibility that ICC could be involved in the development of SWs in USM by noting that interstitial cells of Cajal were mandatory for the development of SWs, in large tissues of the GI tract, but that such cells had not been discovered in USM [32].
Enzymatic dispersal of rabbit USM not only yielded SMCs, as noted above, but also a small population of branched, non-contractile cells that were darker and thinner than the SMCs [25], and were similar in appearance to freshly isolated ICC from the canine proximal colon [55]. These cells were immuno-positive for the intermediate filament vimentin and possessed several ultrastructural characteristics of GI ICC, including an incomplete basal lamina, abundant caveolae, abundant mitochondria, a well-developed smooth endoplasmic reticulum and a sparse rough endoplasmic reticulum. More than 80% of UICs were spontaneously active, generating STDs under current clamp and STICs when voltage clamped at −60 mV. These events reversed close to ECl and were blocked by the traditional CACC inhibitors, niflumic acid and A-9-C [25, 26]. More recently, Fedigan et al. [56] demonstrated that STICs and STDs in UICs were also sensitive to the TMEM16A channel inhibitors, T16Ainh-A01 and CACCinh-A01, indicating that spontaneous electrical activity in UICs is likely to result from activation of TMEM16A channels (Fig. 6.3). Fedigan et al. also showed that the TMEM16A inhibitors reduced the level of spontaneous tone and the amplitude of nerve-evoked contractions of rabbit USM strips, indicative of an important functional role for these channels in urethral contraction [56].
Effect of CACC/TMEM16A inhibitors, T16Ainh-A01 and CACCinh-A01, on STICs (a, c, respectively) and STDs (b, d, respectively) recorded from freshly isolated rabbit UICs. Adapted from [56]
Spontaneous activity in UIC is also dependent on Ca2+ release from intracellular stores as the STICs were abolished by the SERCA inhibitor CPA and were also sensitive to the RyR inhibitor, ryanodine, the phospholipase C (PLC) inhibitor, NCDC and the IP3R inhibitor, 2-APB [57]. When UIC were held under voltage clamp at ~−30 mV and studied with K+-rich pipettes, these cells also developed spontaneous transient outward current (STOCs ) . These STOCs could be categorised into two broad groups, based on their duration; (i) ‘slow’ STOCs (~1 s duration) that were coupled with STICs and (ii) ‘fast’ STOCs (<100 ms duration) that occurred independently of slow STOCs and STICs. Interestingly, while all events were abolished when RyRs were inhibited, only the slow STOCs and STICs were sensitive to 2-APB (Fig. 6.4). This observation led the authors to hypothesise that 2-APB-sensitive STICs and ‘slow’ STOCs arose from global Ca2+ events involving IP3Rs, such as propagating Ca2+ waves, whereas the fast STOCs arose from RyR-dependent, localised Ca2+ events, such as Ca2+ sparks [57]. This idea was largely corroborated by Johnston et al. who, using a Nipkow spinning disc confocal microscope, reported that UIC loaded with Fluo4-AM, exhibited spontaneous Ca2+ waves [58]. Simultaneous voltage clamp and Ca2+ imaging experiments revealed that STICs were associated with Ca2+ waves (Figs. 6.5 and 6.6). Application of 2-APB abolished STICs, but only reduced the spatial spread of Ca2+ waves [58]. In contrast, inhibition of RyRs with tetracaine, or ryanodine, abolished all spontaneous Ca2+ events. These findings prompted the idea that propagation of Ca2+ waves, and subsequent activation of STICs, required Ca2+ release from IP3Rs, but that the initiation of these events was dependent on Ca2+ release via RyRs. In this model, Ca2+ release from RyRs was proposed as the ‘prime oscillator’.
The IP3R inhibitor 2APB (100 μM) inhibits STICs and ‘slow’ STOCs, but not ‘fast’ STOCs in freshly isolated rabbit UIC voltage clamped at −30 mV. Adapted from [57]
STICs in freshly isolated rabbit UICs are associated with spontaneous Ca2+ waves . (a.i, a.ii) Show a pseudo linescan image and corresponding intensity profile plot, of spontaneous Ca2+ waves in a rabbit UIC loaded with fluo4-AM. (b), is a simultaneous voltage clamp recording at −60 mV, showing that STICs are associated with the spontaneous Ca2+ waves. (c) Shows the Ca2+ wave and STIC depicted in the highlighted area in a.i, a.ii and b on an expanded time scale
Simultaneous recording showing that STICs and spontaneous Ca2+ waves in freshly isolated rabbit UICs are abolished by removal of extracellular Ca2+. (a.i, a.ii) Show a pseudo linescan image and corresponding intensity profile plot, of spontaneous Ca2+ waves in a rabbit UIC loaded with fluo4-AM. (b) Simultaneous voltage clamp recording at −60 mV, showing that STICs are abolished by removal of extracellular Ca2+
In addition to Ca2+ release from intracellular stores, it is clear that spontaneous activity in UIC is also dependent on Ca2+ influx across the plasma membrane [25, 27, 58]. STDs and spontaneous Ca2+ waves in UIC were abolished by removal of external Ca2+ and the frequency of Ca2+ waves was correlated with the external Ca2+ concentration ([Ca2+]o) as a reduction in [Ca2+]o from 1.8 to 0.9 mM decreased Ca2+ wave frequency by ~40%, whereas an increase in [Ca2+]o to 3.6 mM enhanced their frequency [58]. Similar findings were reported for UIC in situ [27]. Figure 6.6 is a representative trace showing the effect of Ca2+ removal on STICs and spontaneous Ca2+ waves, recorded simultaneously, in an isolated UIC. However, the mechanisms underlying Ca2+ influx in UIC and their interaction with Ca2+ release mechanisms has only recently been clarified.
Isolated UIC displayed robust L-type Ca2+ currents in response to step depolarisation [25]; however, STICs recorded at −60 mV in the same cell type were resistant to L-type Ca2+ channel blockade with nifedipine [57] or D-cis diltiazem [59], demonstrating that spontaneous activity in rabbit UICs is not reliant on Ca2+ influx via L-type Ca2+ channels. Similarly, spontaneous Ca2+ waves in non-voltage-clamped UICs were also unaffected by nifedipine [27, 58], consistent with this idea. Johnston et al. also noted that although spontaneous Ca2+ waves were abolished in Ca2+-free media, caffeine-induced Ca2+ transients remained intact, suggesting that the abolition of spontaneous activity by removal of external Ca2+, was not a function of Ca2+ store depletion. Consistent with this idea were the findings of Bradley et al. who found that although UICs exhibited a robust capacitative Ca2+ entry signal upon Ca2+ store depletion, blockers of this pathway, including Gd3+ (10 μM) and La3+ (10 μM), did not abolish spontaneous activity [60].
It now appears that one of the key Ca2+ influx pathways that drives spontaneous activity in UICs is via reverse mode-sodium calcium exchange (NCX ) . The selective reverse NCX inhibitors KB-R7943 and SEA0400 [61,62,63,64] significantly reduced both the frequency of Ca2+ oscillations and STICs in isolated rabbit UICs, and the level of spontaneous tone in intact USM strips [65]. Furthermore, a reduction in extracellular Na+ levels (to promote reverse mode NCX) increased the frequency of Ca2+ waves in UICs. Drumm et al. [66] investigated this issue further by examining the effect of the NCX inhibitors, as well as application of Ca2+-free solution, on Ca2+ waves recorded using faster acquisition rates (50–97 FPS) than those employed in earlier studies [58, 65]. In contrast to these studies, Drumm et al. [66] found that application of Ca2+-free solution, KB-R7943 and SEA0400 (Fig. 6.7) abolished propagating Ca2+ waves, but unmasked brief, localised Ca2+ sparks that were not detected in the earlier studies [58, 65]. These effects were very similar to those induced by blockade of IP3Rs with 2-APB (Fig. 6.7) suggesting that the role of Ca2+ influx via reverse NCX was to sensitise IP3Rs to Ca2+, which allows localised Ca2+ release events from RyRs to be converted into propagating Ca2+ waves.
Representative pseudo linescan images showing effects of application of Ca2+-free external solution (a), the reverse NCX blockers KB-R 7943 (b) and SEA-0400 (c) and the IP3R inhibitor 2-APB (d) on Ca2+ events, recorded at fast acquisition rates (50–97 FPS), in freshly isolated rabbit UICs. (e) Shows the Ca2+ events highlighted by the white boxes in d (before and during the presence of 2-APB) on an expanded time scale. Adapted from [67]
Another Ca2+ influx pathway that has been proposed to regulate spontaneous activity in UICs is via cyclic nucleotide-gated (CNG) channels [59]. CNG channels are permeable to Na+ and Ca2+ and their activation can induce local changes in cytosolic Ca2+ levels in response to a rise in cAMP or cGMP. CNGA1 and CNGB1 subunits, which form functional CNGA1, or rod retinal-like CNG channels, were strongly expressed in a subpopulation of vimentin-positive ICC of the rat urethra, whereas only weak and diffuse CNG1A-immunoreactivity was evident in rat USMCs [67]. Furthermore, inhibition of CNG channels, with L-cis diltiazem, reduced the frequency of STICs and Ca2+ waves in rabbit UIC [59], however, the precise contribution of CNG channels to spontaneous activity in UIC is still unclear and requires further investigation.
Ward et al. suggested that mitochondrial Ca2+ handling may regulate the frequency of pacemaker activity in GI muscles [68]. This was based on the observations that inhibition of the electrochemical gradient across the inner mitochondrial membrane with the mitochondrial uncouplers FCCP and CCCP or the respiratory chain (complex III) inhibitor antimycin, inhibited pacemaker currents in cultured ICC and blocked slow wave activity in intact GI muscles from mouse, dog and guinea-pig. Sergeant et al. reported similar findings on Ca2+ waves and STICs recorded from freshly isolated rabbit UICs [69] and Hashitani et al. also reported that CCCP could inhibit spontaneous Ca2+ waves in rabbit UICs [70]. These data indicated that spontaneous activity in UICs may also be dependent on Ca2+ handling by mitochondria, as well as on Ca2+ release from the sarcoplasmic reticulum and Ca2+ influx across the plasma membrane. However, a recent study showed that antimycin blocked TMEM16A currents and that CCCP inhibited CaV3.2 currents [71]. Therefore, firm conclusions on the role of mitochondrial Ca2+ handling, based on use of these agents, should be treated with caution and further investigation is required to determine the contribution of mitochondrial Ca2+ handling to spontaneous activity in UIC.
Overall, it is clear that the smooth muscle layer of the rabbit urethra possesses a population of interstitial cells (UIC s), which resemble ICC pacemaker cells in the GI tract, and which exhibit a pattern of spontaneous activity that is similar in nature to that recorded from intact strips of rabbit USM [25, 26, 72]. These observations prompted suggestions that UIC may function as pacemaker cells in the urethra. However, this hypothesis still requires further investigation. To date, there has only been one study that has simultaneously measured spontaneous activity in UICs and smooth muscle cells in USM. Hashitani and Suzuki were able to record Ca2+ transients in UICs and USMCs in tissue strips loaded with Fluo4-AM [27]. They found that Ca2+ transients recorded from rabbit UICs in situ were similar in nature to those recorded in isolated UICs [58]. Thus, they were abolished by application of CPA, ryanodine, caffeine and 2-APB and by removal of extracellular Ca2+, but were resistant to application of nifedipine. On some occasions (21 preparations) spontaneous Ca2+ transients in USMCs were observed simultaneously with those of UICs within a field of view. Interestingly, in five of these preparations, UICs and USMCs generated synchronous Ca2+ transients with close temporal correlation between the signals in the two cell types, indicative of a degree of synchronicity between these cells (Fig. 6.8). In the remaining 16 preparations, USMCs generated Ca2+ transients independently from UICs, albeit at a lower frequency than those in the UICs. In contrast, when pairs of UICs were visualised in the same field of view, synchronous Ca2+ transients were observed in 17 out of 22 preparations, indicative a high degree of coupling between UICs. These authors concluded that ‘UIC s may act as a primary pacemaker in generating spontaneous contractions of USM. However, signal transmission from UIC s to USMCs may be much less extensive than that between ICC and smooth muscle cells in the GI tract, and thus electrical pacemaking signals generated by UICs may be less securely transmitted’ to smooth muscles’ [27]. Thornbury et al. reached similar conclusions, noting that ‘there are multiple pacemakers within the urethra, but unlike the GI tract, these are not well networked’ [73]. Therefore, it is apparent that both USMCs and UICs are capable of generating spontaneous activity but, in the rabbit urethra, at least, it appears that spontaneous activity originating from UICs is dominant and forms the basis of pacemaker in the intact tissue. Definitive determination of UIC function in the future will be dependent upon determining the identity of these cells by the presence of selective markers and examining tissue function when these cells are lesioned.
Analysis of the temporal relationship of Ca2+ transients between UICs and USMCs. (a) A series of frames at intervals of 0.2 s demonstrating USMC Ca2+ transients originating from an UIC. (b) In the same preparation, synchronous Ca2+ transients were generated by an UIC (1) and a USMC (2). (c) A cross-correlogram for UICs and USMCs showed a peak near lag period zero. Adapted from [27]
5 Modulation of Spontaneous Activity in UIC by Autonomic Neurotransmitters
5.1 Nitric Oxide
The principal inhibitory neurotransmitter in the urethra is Nitric Oxide (NO [14, 15]). NO is thought to exert its effects by activating the cGMP/protein kinase G (PKG) pathway, since electrical field stimulation (EFS ) of inhibitory nerves or exogenous application of NO can elevate cGMP levels in this tissue [74,75,76], and neurogenic urethral relaxations are significantly attenuated in mice lacking cGMP-dependent protein kinase G1 [77]. The NO donor, sodium nitroprusside (SNP), reduced the frequency of slow waves in rabbit USM [32] indicating that NO exerts its inhibitory effects in USM by inhibiting spontaneous activity. There is evidence that spontaneous activity in both UICs and USMCs is affected by NO, however, the degree to which these effects account for neurogenic relaxations of USM is still under debate. Application of the NO donor, DEA-NONOate, dramatically reduced the frequency of Ca2+ transients in USMCs, in situ [34] and relaxations induced by DEA-NONOate were absent in smooth muscle guanylyl cyclase knockout (SM-GCKO) mice USM [78]. These findings prompted the authors to conclude that ‘NO-GC found in SMCs of the urethral sphincter mediates NO-induced relaxation’. Unfortunately, this study did not examine nerve-mediated NO responses, as responses to exogenous application of agonists and NO donors can often be quite different to those induced by stimulation of intrinsic nerves. For example, intramuscular ICC (IC-IM) in the murine fundus are known to mediate neural responses to NO, but W/Wv mice, which lack IC-IM can still relax in response to the NO-donor SNP [51]. Lies et al. also showed that DEA-NONOate-induced relaxations were maintained in mice lacking NO-guanylyl cyclase (NO-GC) in c-kit-positive ICC [78]. Therefore, if UICs are involved in mediating NO responses in the murine urethra, they are not c-kit positive.
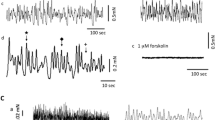
Several lines of evidence indicate that UICs could be involved in NO signalling in the urethra. Treatment of USM with SNP led to increased levels of cGMP fluorescence in interstitial cells [50, 79] and stimulation of nitrergic nerves in USM, led to increased cGMP production in both UICs and SMCs [80]. Rabbit UICs (identified by c-kit immunoreactivity) had frequent points of contact with neuronal nitric oxide synthase containing nerves, consistent with the idea that they play a role in the inhibitory nitrergic neurotransmission of USM [81]. Sergeant et al. provided direct evidence that spontaneous activity in UICs was modulated by NO, as STDs, recorded under current clamp, and STICs recorded under voltage clamp in freshly isolated rabbit UICs were inhibited by the NO donor, DEA-NO, the soluble guanylate cyclase activator, YC-1, or the cGMP analogue, 8-Br-cGMP [82]. Finally, spontaneous Ca2+ transients recorded in situ from UICs of the rabbit urethra, were inhibited by the NO donor, SIN-1 [27]. It appears that inhibitory effects of NO in UICs are mediated by activation of PKG, and not protein kinase A (PKA), as the PKG activator SP-8-br-cGMP reduced the frequency of STDs and Ca2+ waves, whereas application of the adenylate cyclase activator, forskolin or the membrane permeable cyclic AMP analogue, 8-Br-cAMP did not affect spontaneous Ca2+ waves in rabbit UIC [83].
5.2 Adenosine Triphosphate
Nitric Oxide is not the only inhibitory neurotransmitter in the urethra. Several studies have demonstrated that adenosine triphosphate (ATP) or its derivatives, adenosine and adenosine diphosphate (ADP), may also be potent inhibitory neurotransmitters in USM, since EFS of USM strips induced relaxations that were inhibited by purinergic receptor antagonists and mimicked by exogenous application of ATP [18,19,20,21]. However, it has also been noted that ATP, or related compounds, can produce excitatory effects in USM [21, 30, 84,85,86]. One explanation that could account for these opposite effects is the level of tone on the tissue prior to ATP application. For example, relaxant effects were observed in preparations which had been ‘pre-contracted’ with agonists such as arginine vasopressin or noradrenaline, whereas contractions were observed if ATP was applied at resting tone. Bradley et al. showed that the contractile effects induced by application of ATP to the rabbit urethra were mimicked by the P2Y receptor agonist, 2-MeSADP and were inhibited by the selective P2Y1 receptor antagonist, MRS2500 suggesting that P2Y receptors were involved in the excitatory effects of ATP in rabbit USM [85]. Involvement of UICs in this response was inferred from experiments which showed that ATP and P2Y receptor agonists increased the frequency of spontaneous Ca2+ waves and STICs in freshly isolated UICs (Fig. 6.9). Interestingly however, stimulation of purinergic nerves in strips of rabbit USM yielded contractions that were inhibited by desensitisation of P2X receptors using α,β-methylene ATP but were unaffected by the P2Y receptor antagonist MRS2500 [86]. This suggested that, in USM, neurally released ATP targeted P2X receptors and not P2Y receptors. Further investigation revealed that isolated rabbit USMCs had robust P2X receptor currents that could be inhibited by α,β-methylene ATP. Therefore, it appears that P2Y receptor-dependent responses in UICs are unlikely to be involved in purinergic nerve-mediated responses in rabbit USM.
Representative pseudo linescan images showing effects of ATP (a) and the P2Y1 receptor agonist 2-MeSADP (b) on Ca2+ waves in isolated rabbit UICs. Adapted from [85]
5.3 Noradrenaline
Noradrenaline (NA) is the principal excitatory neurotransmitter in the urethra [16, 17] and augments the level of urethral tone via activation of post-junctional α1-adrenoceptors [13, 17]. Hashitani et al. indicated that α1-adrenoceptor-mediated increases in tone resulted from up-regulation of the endogenous pacemaker mechanism, since exogenous application of NA increased the frequency of slow waves in rabbit USM [32]. Sergeant et al. demonstrated that spontaneous activity in freshly isolated UICs was also enhanced by NA suggesting that these cells could be involved in this response [25, 87]. The effects of NA were attenuated by CPA and 2-APB as well as niflumic acid and A-9-C, suggesting that activation of α1-adrenoceptors led to release of Ca2+ from IP3-sensitive stores, which in turn stimulated CACC. Similarly, bath application of the α1-adrenoceptor agonist, phenylephrine increased the frequency of Ca2+ transients in UICs in situ and elevated intracellular Ca2+ levels [27]. Sergeant et al. [87] and Fedigan et al. [56] demonstrated that EFS-induced contractions of rabbit USM were inhibited by the CACC inhibitors, niflumic acid, A-9-C and T16Ainh-A01, respectively. Since CACC are more prominent in rabbit urethral UICs, compared to USMCs, it is tempting to speculate on a role for UIC in mediating neural responses in USM, as is the case for IC-IM in the gastric fundus and ICC in the deep muscular plexus (IC-DMP) of the small intestine [88]. However, a comparison of neural responses in USM lacking UICs has yet to be made and therefore this idea remains speculative. Furthermore, Ca2+ waves in murine SMC were also accelerated by application of phenylephrine indicating that USMCs can be directly modulated by NA [34]. Kyle et al. also showed that phenylephrine reduced depolarisation-evoked large conductance Ca2+-activated K+ (BK) currents in rabbit USMCs and suggested that the reduction in this current altered compound action potentials to promote excitability of USM [89].
Walsh et al. [90] demonstrated that contractile responses of rabbit USM in response to EFS and exogenous application of phenylephrine were all potently inhibited by the Rho-associated kinase (ROK) inhibitors, Y27632 and H1152 [90]. Surprisingly, however, ROK inhibition had no effect on the phosphorylation of the known ROK substrates, myosin regulatory light chains (LC20) at S19 or the myosin-targeting subunit of myosin light chain phosphatase (MYPT1), in either the absence or presence of contractile stimuli. Therefore ROK plays an important role in USM contraction induced by NA; however, the mechanisms underlying this response require further investigation.
5.4 Acetylcholine
Although the bladder neck and proximal urethra receive a rich cholinergic innervation [84], the functional role of acetylcholine (ACh) in the urethra is still unclear. Studies have indicated that inhibition of muscarinic receptors has little effect on urethral tone in vivo [91] or in vitro [14, 87]. However, stimulation of cholinergic nerves in vitro has been demonstrated to contract the urethra of sheep, pigs, dogs and rabbits [14, 22,23,24] and these effects appear to be mediated post-junctionally via activation of M2 and/or M3 muscarinic receptors [92, 93]. In the isolated sheep urethra, a significant component of the neurogenic contraction is sensitive to atropine [14] consistent with the idea that muscarinic stimulation can augment urethral tone. Similarly, application of cholinergic agonists to porcine urethra in vitro contracts the longitudinal and circular muscle layers equally. However, in the rabbit urethra, although an atropine-sensitive component of excitatory junction potentials has been demonstrated [24, 94], it was small compared to the α1-adrenoceptor-mediated component, suggesting that muscarinic receptors contributed minimally to the electrical response to nerve stimulation in this preparation. In humans, there is little evidence that muscarinic receptor activation has any significant effect on intraurethral pressure in vivo [95].
In addition to the species variability noted above, a number of observations suggest that the response to muscarinic stimulation varies along the length of the urethra. Thus, Nagahama et al. demonstrated that application of carbachol produced robust contractions in the male proximal urethra but had little effect on the distal urethra [96]. Interestingly, application of carbachol to strips of distal rabbit urethra pre-contracted with noradrenaline, resulted in relaxations of tone. These effects were abolished when NO synthase was inhibited, suggesting that muscarinic stimulation may also induce release of NO from nitrergic nerves.
The role, if any, of interstitial cells in cholinergic innervation of the urethra has not yet been ascertained. However, the cholinergic agonist carbachol evoked a series of oscillatory inward currents when applied to single UIC voltage clamped at −60 mV [26] and these events were inhibited by the CACC inhibitor A-9-C, suggesting a common activation pathway to that described by noradrenaline above. In contrast, Kyle et al. were unable to detect any inward currents elicited by muscarinic agonists in freshly dispersed rabbit USMCs [89]. However, they demonstrated that carbachol altered compound action potential characteristics, elicited by 1 s current injections. Therefore, it is apparent that electrical activity in both UICs and USMCs can be directly modulated by carbachol, but more experiments are required to elucidate the mechanisms involved in cholinergic responses at the whole tissue level.
6 Summary
Urethral smooth muscle generates spontaneous tone that makes a contribution to the maintenance of urinary continence by generating a urethral closure pressure that exceeds intravesical bladder pressure. Stress urinary incontinence (SUI) is common in women after vaginal delivery child birth [6] and is typically thought of as being due to reduced urethral support, referred to as urethral hypermobility. However, urethral hypermobility is not predictive of SUI and it is also recognised that it can coexist with a defective closure mechanism, known as ‘intrinsic sphincter deficiency’ [8, 97]. There are currently no FDA-approved pharmacological treatments for SUI, therefore it is crucial that the mechanisms responsible for the maintenance of urethral tone are elucidated, in order to identify novel therapeutic targets. This chapter provides a summary of the mechanisms responsible for spontaneous activity in urethral smooth muscle.
The urethra is regarded as a ‘tonic’ smooth muscle but, counterintuitively, there is now consensus that urethral smooth muscle tone is achieved by the averaging effect of numerous small asynchronous ‘phasic’ contractions in the tissue [27, 34, 73]. The phasic contractions of urethral smooth muscle appear to arise from activation of CACC/Ano1 channels and may originate in specialised cells referred to as urethral interstitial cells. This requires further investigation. Spontaneous activity in both USMCs and UICs involves Ca2+ release from intracellular stores and Ca2+ influx across the plasma membrane and is regulated by several autonomic neurotransmitters, including noradrenaline, acetylcholine, nitric oxide and adenosine triphosphate. Much of the work described in this chapter is based on animal studies, however it is apparent that there are species differences regarding the role of Ca2+ influx via L-type Ca2+ channels and on the cellular basis of Ano1 expression and activity. Therefore, it is imperative that future studies should examine the mechanisms responsible for spontaneous activity in human USM samples and examine if this activity, or its modulation by neurotransmitters, is altered in conditions that are associated with USM dysfunction, such as stress urinary incontinence.
References
Brading AF. The physiology of the mammalian urinary outflow tract. Exp Physiol. 1999;84(1):215–21.
de Groat WC, Yoshimura N. Anatomy and physiology of the lower urinary tract. Handb Clin Neurol. 2015;130:61–108.
Rother P, Löffler S, Dorschner W, Reibiger I, Bengs T. Anatomic basis of micturition and urinary continence. Muscle systems in urinary bladder neck during ageing. Surg Radiol Anat. 1996;18(3):173–7.
Greenland JE, Dass N, Brading AF. Intrinsic urethral closure mechanisms in the female pig. Scand J Urol Nephrol Suppl. 1996;179:75–80.
Jankowski RJ, Prantil RL, Chancellor MB, de Groat WC, Huard J, Vorp DA. Biomechanical characterization of the urethral musculature. Am J Physiol Ren Physiol. 2006;290(5):F1127–34.
Norton P, Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67.12.
DeLancey JO, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, Weadock WJ, Ashton-Miller JA. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008;179:2286–90.13.
DeLancey JO. Why do women have stress urinary incontinence? Neurourol Urodyn. 2010;29(Suppl 1):S13–7.
Prantil RL, Jankowski RJ, Kaiho Y, de Groat WC, Chancellor MB, Yoshimura N, Vorp DA. Ex vivo biomechanical properties of the female urethra in a rat model of birth trauma. Am J Physiol Ren Physiol. 2007;292(4):F1229–37.
Rud T. Urethral pressure profile in continent women from childhood to old age. Acta Obstet Gynecol Scand. 1980;59(4):331–5.
Trowbridge ER, Wei JT, Fenner DE, Ashton-Miller JA, DeLancey JO. Effects of aging on lower urinary tract and pelvic floor function in nulliparous women. Obstet Gynecol. 2007;109(3):715–20.
Clobes A, DeLancey JO, Morgan DM. Urethral circular smooth muscle in young and old women. Am J Obstet Gynecol. 2008;198(5):587.e1–5.
Bridgewater M, MacNeil HF, Brading AF. Regulation of tone in pig urethral smooth muscle. J Urol. 1993;150:223–8.
Thornbury KD, Hollywood MA, McHale NG. Mediation by nitric oxide of neurogenic relaxation of the urinary bladder neck muscle in sheep. J Physiol. 1992;451:133–44.
Andersson KE, Garcia Pascual A, Persson K, Forman A, Tøttrup A. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. J Urol. 1992;147(1):253–9.
Andersson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993;45:253–307.
Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56(4):581–631.
Ohnishi N, Park YC, Kurita T. Role of ATP and related purine compounds on urethral relaxation in male rabbits. Int J Urol. 1997;4(2):191–7.
Pinna C, Puglisi L, Burnstock G. ATP and vasoactive intestinal polypeptide relaxant responses in hamster isolated proximal urethra. Br J Pharmacol. 1998;124(6):1069–74.
Pinna C, Glass R, Knight GE. Purine- and pyrimidine-induced responses and P2Y receptor characterization in the hamster proximal urethra. Br J Pharmacol. 2005;144(4):510–8.
Hernandez M, Knight GE, Wildman SS, Burnstock G. Role of ATP and related purines in inhibitory neurotransmission to the pig urinary bladder neck. Br J Pharmacol. 2009;157(8):1463–73.
Noda K, Takebe M, Oka M, Hirouchi M, Ukai Y, Toda N. Functional role of inhibitory and excitatory nerves in the porcine lower urinary tract. Eur J Pharmacol. 2002;456(1–3):81–90.
Van der Werf BA, Creed KE. Mechanical properties and innervation of the smooth muscle layers of the urethra of greyhounds. BJU Int. 2002;90(6):588–95.
Creed KE, Oike M, Ito Y. The electrical properties and responses to nerve stimulation of the proximal urethra of the male rabbit. Br J Urol. 1997;79(4):543–53.
Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526(2):359–66.
Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Interstitial cells of Cajal in the urethra. J Cell Mol Med. 2006;10(2):280–91.
Hashitani H, Suzuki H. Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal-like cells of the rabbit urethra in situ. J Physiol. 2007;583(Pt 2):505–19.
Drumm BT, Koh SD, Andersson KE, Ward SM. Calcium signalling in Cajal-like interstitial cells of the lower urinary tract. Nat Rev Urol. 2014;11(10):555–64.
Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol. 2006;570(1):13–22.
Callahan SM, Creed KE. Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br J Pharmacol. 1981;74(2):353–8.
Callahan SM, Creed KE. The effects of oestrogens on spontaneous activity and responses to phenylephrine of the mammalian urethra. J Physiol. 1985;358:35–46.
Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996;118:1627–32.
Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol. 1999;514(Pt 2):459–70.
Drumm BT, Rembetski BE, Cobine CA, Baker SA, Sergeant GP, Hollywood MA, Thornbury KD, Sanders KM. Ca2+ signalling in mouse urethral smooth muscle in situ: role of Ca2+ stores and Ca2+ influx mechanisms. J Physiol. 2018;596:1433–66.
Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol. 2001;530(Pt 2):273–86.
Hashitani H, Yanai Y, Kohri K, Suzuki H. Heterogeneous CPA sensitivity of spontaneous excitation in smooth muscle of the rabbit urethra. Br J Pharmacol. 2006;148(3):340–9.
Bradley JE, Anderson UA, Woolsey SM, Thornbury KD, McHale NG, Hollywood MA. Characterization of T-type calcium current and its contribution to electrical activity in rabbit urethra. Am J Phys Cell Physiol. 2004;286(5):C1078–88.
Drumm BT, Sergeant GP, Hollywood MA, Thornbury KT, Matsuda TT, Baba A, Harvey BJ, McHale NG. The effect of high [K(+)]o on spontaneous Ca(2+) waves in freshly isolated interstitial cells of Cajal from the rabbit urethra. Phys Rep. 2014;2(1):e00203.
Teramoto N, Brading AF. Activation by levcromakalim and metabolic inhibition of glibenclamide-sensitive K channels in smooth muscle cells of pig proximal urethra. Br J Pharmacol. 1996;118(3):635–42.
Teramoto N, McMurray G, Brading AF. Effects of levcromakalim and nucleoside diphosphates on glibenclamide-sensitive K+ channels in pig urethral myocytes. Br J Pharmacol. 1997;120(7):1229–40.
Kyle BD. Ion channels of the mammalian urethra. Channels (Austin). 2014;8(5):393–401.
Cotton KD, Hollywood MA, McHale NG, Thornbury KD. Ca2+ current and Ca(2+)-activated chloride current in isolated smooth muscle cells of the sheep urethra. J Physiol. 1997;505(Pt 1):121–31.
Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Spontaneous Ca2+ activated Cl− currents in isolated urethral smooth muscle cells. J Urol. 2001;166(3):1161–6.
Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–4.
Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134(6):1019–29.
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455(7217):1210–5.
Sancho M, García-Pascual A, Triguero D. Presence of the Ca2+-activated chloride channel anoctamin 1 in the urethra and its role in excitatory neurotransmission. Am J Physiol Ren Physiol. 2012;302(3):F390–400.
Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci U S A. 2009 Dec 15;106(50):21413–8.
Hollywood MA, Woolsey S, Walsh IK, Keane PF, McHale NG, Thornbury KD. T- and L- type Ca2+ currents in freshly dispersed smooth muscle cells from the human proximal urethra. J Physiol. 2003;550(Pt 3):753–64.
Smet PJ, Jonavicius J, Marshall VR, de Vente J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71(2):337–48.
Burns AJ, Lomax AEJ, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–13.
Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515.
Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–29.
Ward SM, Beckett EAH, Wang XY, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20(4):1393–403.
Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci U S A. 1989;86:7280–4.
Fedigan S, Bradley E, Webb T, Large RJ, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP. Effects of new-generation TMEM16A inhibitors on calcium-activated chloride currents in rabbit urethral interstitial cells of Cajal. Pflugers Arch. 2017;469:1443–55.
Sergeant GP, Hollywood MA, McCloskey KD, McHale NG, Thornbury KD. Role of IP(3) in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Phys Cell Physiol. 2001;280(5):C1349–56.
Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565(Pt 2):449–61.
Sancho M, Bradley E, Garcia-Pascual A, Triguero D, Thornbury KD, Hollywood MA, Sergeant GP. Involvement of cyclic nucleotide-gated channels in spontaneous activity generated in isolated interstitial cells of Cajal from the rabbit urethra. Eur J Pharmacol. 2017;814:216–25.
Bradley E, Hollywood MA, McHale NG, Thornbury KD, Sergeant GP. Pacemaker activity in urethral interstitial cells is not dependent on capacitative calcium entry. Am J Phys Cell Physiol. 2005;289(3):C625–32.
Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996;271:22391–7.
Watano T, Kimura J, Morita T, Nakanishi H. A novel antagonist, KB-R7943, of the Na+/Ca2+ exchange current in guinea-pig cardiac ventricular cells. Br J Pharmacol. 1996;119:555–63.
Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, et al. SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther. 2001;298:249–56.
Lee C, Visen NS, Dhalla NS, Le HD, Isaac M, Choptiany P, et al. Inhibitory profile of SEA0400 [2-[4-[(2,5-difluorophenyl) methoxy]phenoxy]-5-ethoxyaniline] assessed on the cardiac Na+-Ca2+ exchanger, NCX1.1. J Pharmacol Exp Ther. 2004;311:748–57.
Bradley E, Hollywood MA, Johnston L, Large RJ, Matsuda T, Baba A, McHale NG, Thornbury KD, Sergeant GP. Contribution of reverse Na+/Ca2+ exchange to spontaneous activity in interstitial cells of Cajal in the rabbit urethra. J Physiol. 2006;574(Pt 3):651–61.
Drumm BT, Large RJ, Hollywood MA, Thornbury KD, Baker SA, Harvey BJ, McHale NG, Sergeant GP. The role of Ca(2+) influx in spontaneous Ca(2+) wave propagation in interstitial cells of Cajal from the rabbit urethra. J Physiol. 2015;593(15):3333–50.
Triguero D, Sancho M, Garcia-Flores M, Garcia-Pascual A. Presence of cyclic nucleotide-gated channels in the rat urethra and their involvement in nerve-mediated nitrergic relaxation. Am J Physiol Ren Physiol. 2009;297:F1353–60.
Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525(Pt 2):355–61.
Sergeant GP, Bradley E, Thornbury KD, McHale NG, Hollywood MA. Role of mitochondria in modulation of spontaneous Ca2+ waves in freshly dispersed interstitial cells of Cajal from the rabbit urethra. J Physiol. 2008;586(19):4631–42.
Hashitani H, Lang RJ, Suzuki H. Role of perinuclear mitochondria in the spatiotemporal dynamics of spontaneous Ca2+ waves in interstitial cells of Cajal-like cells of the rabbit urethra. Br J Pharmacol. 2010;161(3):680–94.
Drumm BT, Sung TS, Zheng H, Baker SA, Koh SD, Sanders KM. The effects of mitochondrial inhibitors on Ca2+ signalling and electrical conductances required for pacemaking in interstitial cells of Cajal in the mouse small intestine. Cell Calcium. 2018;72:1–17.
McHale NG, Hollywood MA, Sergeant GP, Shafei M, Thornbury KT, Ward SM. Organization and function of ICC in the urinary tract. J Physiol. 2006;576(Pt 3):689–94.
Thornbury KD, Hollywood MA, McHale NG, Sergeant GP. Cajal beyond the gut: interstitial cells in the urinary system—towards general regulatory mechanisms of smooth muscle contractility? Acta Gastroenterol Belg. 2011;74(4):536–42.
Morita T, Tsujii T, Dokita S. Regional difference in functional roles of cAMP and cGMP in lower urinary tract smooth muscle contractility. Urol Int. 1992;49(4):191–5.
Dokita S, Smith SD, Nishimoto T, Wheeler MA, Weiss RM. Involvement of nitric oxide and cyclic GMP in rabbit urethral relaxation. Eur J Pharmacol. 1994;266(3):269–75.
Persson K, Andersson KE. Non-adrenergic, non-cholinergic relaxation and levels of cyclic nucleotides in rabbit lower urinary tract. Eur J Pharmacol. 1994;268(2):159–67.
Persson K, Pandita RK, Aszòdi A, Ahmad M, Pfeifer A, Fässler R, Andersson KE. Functional characteristics of urinary tract smooth muscles in mice lacking cGMP protein kinase type I. Am J Phys Regul Integr Comp Phys. 2000;279(3):R1112–20.
Lies B, Groneberg D, Friebe A. Correlation of cellular expression with function of NO-sensitive guanylyl cyclase in the murine lower urinary tract. J Physiol. 2013;591(21):5365–75. https://doi.org/10.1113/jphysiol.2013.262410.
Waldeck K, Ny L, Persson K, Andersson KE. Mediators and mechanisms of relaxation in rabbit urethral smooth muscle. Br J Pharmacol. 1998;123(4):617–24.
García-Pascual A, Sancho M, Costa G, Triguero D. Interstitial cells of Cajal in the urethra are cGMP-mediated targets of nitrergic neurotransmission. Am J Physiol Ren Physiol. 2008;295(4):F971–83.
Lyons AD, Gardiner TA, McCloskey KD. Kit-positive interstitial cells in the rabbit urethra: structural relationships with nerves and smooth muscle. BJU Int. 2007;99(3):687–94.
Sergeant GP, Johnston L, McHale NG, Thornbury KD, Hollywood MA. Activation of the cGMP/PKG pathway inhibits electrical activity in rabbit urethral interstitial cells of Cajal by reducing the spatial spread of Ca2+ waves. J Physiol. 2006;574(Pt1):167–81.
Drumm BT, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG, Harvey BJ. The role of cAMP dependent protein kinase in modulating spontaneous intracellular Ca2+ waves in interstitial cells of Cajal from the rabbit urethra. Cell Calcium. 2014;56(3):181–7.
Deplanne V, Palea S, Angel I. The adrenergic, cholinergic and NANC nerve-mediated contractions of the female rabbit bladder neck and proximal, medial and distal urethra. Br J Pharmacol. 1998;123(8):1517–24.
Bradley E, Kadima S, Drumm B, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP. Novel excitatory effects of adenosine triphosphate on contractile and pacemaker activity in rabbit urethral smooth muscle. J Urol. 2010;183(2):801–11.
Bradley E, Kadima S, Kyle B, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP. P2X receptor currents in smooth muscle cells contribute to nerve mediated contractions of rabbit urethral smooth muscle. J Urol. 2011;186(2):745–52.
Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Phys Cell Physiol. 2002;283(3):C885–94.
Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576(Pt 3):675–82.
Kyle BD, Bradley E, Large R, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA. Mechanisms underlying activation of transient BK current in rabbit urethral smooth muscle cells and its modulation by IP3-generating agonists. Am J Phys Cell Physiol. 2013;305(6):C609–22.
Walsh MP, Thornbury K, Cole WC, Sergeant G, Hollywood M, McHale N. Rho-associated kinase plays a role in rabbit urethral smooth muscle contraction, but not via enhanced myosin light chain phosphorylation. Am J Physiol Ren Physiol. 2011;300(1):F73–85.
Thind P, Lose G, Colstrup H, Andersson KE. The influence of beta-adrenoceptor and muscarinic receptor agonists and antagonists on the static urethral closure function in healthy females. Scand J Urol Nephrol. 1993;27(1):31–8.
Yamanishi T, Chapple CR, Yasuda K, Yoshida K, Chess-Williams R. The role of M2 muscarinic receptor subtypes mediating contraction of the circular and longitudinal smooth muscle of the pig proximal urethra. J Urol. 2002;168(1):308–14.
Mutoh S, Latifpour J, Saito M, Weiss RM. Evidence for the presence of regional differences in the subtype specificity of muscarinic receptors in rabbit lower urinary tract. J Urol. 1997;157(2):717–21.
Ito Y, Kimoto Y. The neural and non-neural mechanisms involved in urethral activity in rabbits. J Physiol. 1985;367:57–72.
Thind P, Lose G, Colstrup H, Andersson KE. The urethral resistance to rapid dilation: an analysis of the effect of autonomic receptor stimulation and blockade and of pudendal nerve blockade in healthy females. Scand J Urol Nephrol. 1995;29(1):83–91.
Nagahama K, Tsujii T, Morita T, Azuma H, Oshima H. Differences between proximal and distal portions of the male rabbit posterior urethra in the physiological role of muscarinic cholinergic receptors. Br J Pharmacol. 1998;124(6):1175–80.
Fleischmann N, Flisser AJ, Blaivas JG, Panagopoulos G. Sphincteric urinary incontinence: relationship of vesical leak point pressure, urethral mobility and severity of incontinence. J Urol. 2003;169(3):999–1002.
Acknowledgments
The authors are grateful for grant support from the Wellcome Trust (064212), NIH (RO1 DK68565) and the Health Research Board (PD/2005/4 & RP/2006/127) and for technical support from Ms. Billie McIlveen.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sergeant, G.P., Hollywood, M.A., Thornbury, K.D. (2019). Spontaneous Activity in Urethral Smooth Muscle. In: Hashitani, H., Lang, R. (eds) Smooth Muscle Spontaneous Activity. Advances in Experimental Medicine and Biology, vol 1124. Springer, Singapore. https://doi.org/10.1007/978-981-13-5895-1_6
Download citation
DOI: https://doi.org/10.1007/978-981-13-5895-1_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5894-4
Online ISBN: 978-981-13-5895-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)