Abstract
Neurally released nitric oxide (NO) functions as an inhibitory neurotransmitter of urethral but not detrusor smooth muscles while relaxing bladder vasculature and muscularis mucosae (MM). Here, the distribution of nitrergic nerves was examined in the mucosa of pig lower urinary tract using immunohistochemistry, and their vasodilatory functions were studied by measuring arteriolar diameter changes. Properties of smooth muscle cells in the lamina propria (SMC-LP) of urethra and trigone were also investigated using florescence Ca2+ imaging. In the bladder mucosa, neuronal nitric oxide synthase (nNOS)–immunoreactive nitrergic fibres projected to suburothelial arterioles and venules. Perivascular nitrergic nerves were intermingled with but distinct from tyrosine hydroxylase (TH)–immunoreactive sympathetic or calcitonin gene–related peptide (CGRP)–immunoreactive afferent nerves. MM receive a nitrergic but not sympathetic or afferent innervation. In the mucosa of urethra and trigone, nitrergic nerves were in close apposition with sympathetic or afferent nerves around suburothelial vasculature but did not project to SMC-LP. In suburothelial arterioles of bladder and urethra, N ω-nitro-L-arginine (L-NA, 100 μM), an NOS inhibitor, enhanced electrical field stimulation (EFS)–induced sympathetic vasoconstrictions, while tadalafil (10 nM), a phosphodiesterase type 5 (PDE5) inhibitor, suppressed the vasoconstrictions. SMC-LP developed asynchronous spontaneous Ca2+ transients without responding to EFS. The spontaneous Ca2+ transients were enhanced by acetylcholine (1 μM) and diminished by noradrenaline (1 μM) but not SIN-1 (10 μM), an NO donor. In the lower urinary tract mucosa, perivascular nitrergic nerves appear to counteract the sympathetic vasoconstriction to maintain the mucosal circulation. Bladder MM but not SMC-LP receive an inhibitory nitrergic innervation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the lower urinary tract, neuronal nitric oxide synthase (nNOS)–immunoreactive nitrergic nerve fibres are abundant in smooth muscle layers of the urethra and bladder trigone of several species including human (Persson et al. 1993; Smet et al. 1996). Consistent with the dense nitrergic innervation, neurally released nitric oxide (NO) plays a predominant role in the relaxation of smooth muscle in the bladder neck and urethra (Persson and Andersson 1992; Werkström et al. 1995; Bustamante et al. 2010), indicating their roles in the opening of bladder outlet preceding the initiation of micturition. Nitrergic nerves are also distributed in the detrusor smooth muscle (DSM) layer of the bladder, but neurally released NO has only a marginal role in the relaxation of DSM (Persson and Andersson 1992; Smet et al. 1996; Mitsui et al. 2020). DSM even respond poorly to exogenously applied NO (Persson and Andersson 1992; Filippi et al. 2007; Oger et al. 2010) as they do not express NO-sensitive guanylyl cyclase (Lies et al. 2013). Thus, the nitrergic nerves in the DSM layer may have roles other than a DSM relaxant. Nitrergic nerves projecting to smooth muscle layers in the lower urinary tract are considered to be originated from the major pelvic ganglia (Persson et al. 1998; Persson et al. 1998), while pudendal nerves may also include nitrergic fibres (Parlani et al. 1993).
Nitrergic nerves are distributed around the mucosal blood vessels of the lower urinary tract of several species including human (Keast and Kawatani 1994; Persson et al. 1995; Smet et al. 1996), although their projection to arterioles or venules has not been precisely defined. In the bladder mucosa of rats and mice, sympathetic vasoconstrictor fibres project to suburothelial arterioles and venules (Hashitani et al. 2011; Mitsui and Hashitani 2013; Shimizu et al. 2014; Mitsui et al. 2020), and therefore, it was envisaged that nitrergic nerves also innervate the blood vessels to counteract sympathetic vasoconstriction. This notion was supported by our recent study demonstrating the nitrergic vasodilatory transmission in suburothelial arterioles of rat bladder (Tanaka et al. 2021). In addition, nitrergic vasodilatory nerves functionally innervate the pre-capillary arterioles of the mouse bladder mucosa, where a sympathetic innervation is absent (Tanaka et al. 2021). Nevertheless, the distribution and function of nitrergic innervation in suburothelial microvasculature of the urethra or trigone remain to be further explored.
Despite the insignificant role of nitrergic nerves in regulating the contractility of bladder detrusor, electrical field stimulation (EFS) causes transient relaxations of muscularis mucosae (MM) of the pig bladder that are mediated by NO (Mitsui et al. 2020). MM are smooth muscle bundles that form a meshwork in the mucosa of human, pig and guinea pig bladders, but not mouse and rat (Dixon and Gosling 1983; Heppner et al. 2011; Mitsui et al. 2020) and develop spontaneous action potentials (Lee et al. 2018) and associated phasic contractions (Heppner et al. 2011; Isogai et al. 2016; Mitsui et al. 2020). Nevertheless, the nitrergic innervation to MM as a source of endogenous NO has not yet been morphologically demonstrated. In the urethral mucosa of rabbit or pig, sparsely distributed, longitudinally arranged smooth muscle cells (SMCs) have been described (Mattiasson et al. 1985; Brading 1999). We have also reported scatteredly distributed SMCs in the lamina propria (SMC-LP) of pig urethra and trigone (Mitsui et al. 2020). Because of the anatomical distribution of SMC-LP, they may be a cell population similar to bladder MM. However, it remains to be determined if the SMC-LP are spontaneously active and receives an inhibitory nitrergic innervation as the MM in the bladder.
Phosphodiesterase type 5 (PDE5) inhibitors, which enhance endogenous NO-cyclic guanosine monophosphate (cGMP) signalling, have been used for the treatment of lower urinary tract symptoms (LUTS) (Gacci et al. 2016), although their precise cellular target remains to be determined. We have recently reported that tadalafil, a PDE5 inhibitor, enhances the vasodilatory actions of neurally released NO in bladder arteries and arterioles of rats and mice (Tanaka et al. 2021), suggesting that perivascular nitrergic nerves can be a therapeutic target of PDE5 inhibitors. However, it remains to be determined if nitrergic fibres are similarly distributed and/or a target of PDE5 inhibitors in the bladder vasculature of larger animals such as pigs. Moreover, nitrergic innervations and their vasodilatory functions in the suburothelial vasculature of trigone or urethra need to be explored.
In the present study, the distribution of nitrergic nerves in the mucosa of pig bladder, trigone and urethra was compared using immunohistochemistry for nNOS. Co-staining of α-smooth muscle actin (α-SMA) was carried out to reveal nitrergic nerve fibres innervating suburothelial arterioles, venules or other smooth muscle elements, namely, MM in the bladder or SMC-LP in the trigone and urethra. Double-staining of sympathetic or primary afferent fibres with nitrergic fibres were also conducted to visualise their close apposition or co-localisation with nitrergic nerves. In suburothelial arterioles of the bladder and urethra as well as MM, the effects of the blockade of NOS or PDE5 with L-NA or with tadalafil, respectively, on EFS-evoked responses were also examined. In addition, the functional properties of SMC-LP and their innervation were investigated using intracellular Ca2+ imaging and immunohistochemistry.
Materials and methods
Ethical approval
Protocols used in the present study were approved by the animal experimentation ethics committee at Nagoya City University Graduate School of Medical Sciences.
Treatment of pig tissues
The bladder and proximal urethra of pigs of both sexes were obtained from a local abattoir. These tissues were immersed in physiological salt solution (PSS) at 4 °C and transported to the laboratory. The composition of PSS was as follows: 137.5 mM Na+, 5.9 mM K+, 2.6 mM Ca2+, 1.2 mM Mg2+, 15.5 mM HCO3−, 1.2 mM H2PO4−, 134.4 mM Cl− and 11.5 mM glucose. Small pieces of bladder and urethra were dissected under a dissection microscope. Tissues used were immersed in phosphate buffered saline (PBS) at 4 °C for immunohistochemistry and in PSS for functional experiments.
Immunohistochemistry
Whole mount preparations of the lamina propria of pig bladder or urethra were prepared by removing the smooth muscle layers and the urothelial tissue under the dissection microscope using small scissors and tweezers. Judging from the images of section of pig lower urinary tract in our previous study (Mitsui et al. 2020), the thickness of lamina propria whole mounts of bladder, trigone and urethra are about 1 mm, 500 μm and 500 μm, respectively. Whole mount preparations of DSM layer were also used. Preparation was pinned flat and immersed in Zamboni fixative for 5 min. After removing the pins, preparations were immersed in the same fixative for 4 h at 4 °C, immersed in DMSO to remove picric acid and washed in PBS.
For making cryosections of bladder or urethral smooth muscle layer, the tissues were fixed in a similar way as whole mounts and immersed in PBS containing 30% sucrose for 1 h at 4 °C. They were embedded in O.C.T. compound (Sakura Finetek, Tokyo, Japan) and stored in a freezer at − 80 °C. A cryostat was used for making 14 µm sections; they were mounted on MAS-coated glass slide (Matsunami Glass Industry, Osaka, Japan) and dried using a fan.
Immunohistochemical staining was conducted using a protocol described previously (Mitsui and Hashitani 2013). Briefly, whole mount preparations or sections of pig bladder and urethra were immersed in PBS containing 2% bovine serum albumin (BSA) for 10 min, immersed in Block Ace for 20 min and incubated with primary antibodies for 4 days at 4 °C. Tissue was incubated with biotinylated swine anti-rabbit IgG antibody (1:300, Dako, Glostrup, Denmark) for 30 min when the rabbit antibody was used. Tissue was then incubated with two secondary antibodies or a secondary antibody and fluorescence-labelled streptavidin as well as the nuclear staining reagent Hoechst 33,342 (10 µg/ml, Molecular Probes) for 2 h. All specimens were observed using a confocal laser scanning microscope (FV3000; Olympus, Tokyo, Japan). Specimens of at least 3 pigs were used for describing each immunohistochemical characteristic in the present study.
Primary antibodies used in the present study were as follows: goat antibody for neuronal nitric oxide synthase (nNOS, 1:300, Merk Millipore, Darmstadt, Germany), mouse monoclonal antibody for α-smooth muscle actin (α-SMA, 1:200, clone 1A4, Sigma, St. Louis, MO, USA), rabbit antibody for tyrosine hydroxylase (TH, 1:500, Abcam, Cambridge, UK) and rabbit antibody for calcitonin gene–related peptide (CGRP, 1:1000, ImmunoStar, Hudson, WI, USA).
Secondary antibody and the streptavidin used were as follows: Alexa488-conjugated donkey anti-goat IgG antibody (1:500, Molecular Probes, Eugene, OR, USA), TRITC-conjugated rabbit anti-mouse IgG antibody (1:100, Dako), Cy3-conjugated streptavidin (1:200, Jackson ImmunoResearch, West Grove, PA, USA), Cy3-conjugated goat anti-mouse IgG antibody (2.5 μg/ml, Merck-Chemicon, Darmstadt, Germany) and ALEXA488-conjugated streptavidin (10 μg/ml, Molecular Probes, Eugene, OR, USA). TRITC-conjugated rabbit anti-mouse IgG antibody was incubated with rat serum at 10:1 ratio before its dilution to reduce non-specific binding.
Measurement of vascular diameter changes
A flat lamina propria preparation (approximately 5-mm square) of pig bladder containing mucosal microvessels was pinned on the silicon-coated bottom of recording chamber (volume, approximately 2 ml). Preparation was superfused with warmed (36 °C) PSS at a constant flow rate of 1.3 ml/min. Changes in the diameter of mucosal arterioles were monitored with a video camera and analysed in real-time using the edge-tracking software Diamtrak. Electrical field stimulation (EFS; 100 µs duration, 20 Hz for 2 s) was applied to the preparation every 3 min using a pair of platinum plate electrodes.
Contractile studies in MM
Lamina propria preparations of pig bladders containing MM were prepared by removing the DSM layer and urothelium and cut into small strips (about 2–3 mm × 8–10 mm). Silk threads were tied around both ends of the strips, and one thread was fixed at the bottom of organ bath, while the other was connected to an isometric force transducer. Preparations were perfused with warmed (36 °C) PSS bubbled with 95% O2 and 5% CO2. Nerve-mediated responses were evoked by EFS (50 μs duration, 20 Hz for 1 s), and neural selectivity of these stimuli was confirmed by their sensitivity to tetrodotoxin (1 μM).
Fluorescence intracellular Ca2+ imaging in SMC-LP
The protocol of Ca2+ imaging used in the present study was modified from a protocol previously described (Hashitani et al. 2018). Briefly, preparations of the urethral or trigonal lamina propria were incubated with low Ca2+ (0.5 mM) PSS containing Cal-520 AM (10 μM, AAT Bioquest, Sunnyvale, CA, USA) and 0.01% cremophor EL for 50 min at 35 °C. Preparations were set into a recording chamber and superfused at 2 ml/min with PSS at 36 °C gassed with 5% CO2 in oxygen. Intracellular Ca2+dynamics of SMC-LP were viewed using a water immersion objective (UMPlanFL × 10 or × 20; Olympus) and captured by a CCD camera attached to an upright fluorescence microscope (BX51WI, Olympus) and a high-speed scanning polychromatic light source (C7773; Hamamatsu Photonics). Tissues were illuminated at 490 nm, fluorescence emissions above 515 nm were captured through a barrier filter, and images were obtained using a micro photoluminescence measurement system (AQUACOSMOS, Hamamatsu Photonics). The relative amplitude of Ca2+ transients was shown as ΔFt/F0 = (Ft – F0)/ F0 where Ft represents the fluorescence generated by an event, and F0 indicates the basal fluorescence.
Drugs
Tadalafil, N ω-nitro-L-arginine (L-NA), acetylcholine (ACh), noradrenaline, atropine, propranolol, SIN-1, nifedipine, CPA and U46619 were purchased from Sigma. Tetrodotoxin (TTX) was from Wako Pure Chemical Industries (Osaka, Japan). Tadalafil, SIN-1, CPA and U46619 were dissolved in DMSO, and nifedipine was in ethanol. Other drugs were dissolved in distilled water. Since plasma concentration of tadalafil is maintained above 10 nM even few days after the drug application (single 20 mg dose) in healthy male subjects (Forgue et al. 2006), 10–100 nM can be considered as clinically relevant concentration and was used in the present study.
Data analysis
Data in functional experiments are represented as mean ± SD. A normality test followed by two-tailed paired or unpaired Student’s t-test (for two groups) or one-way repeated measures ANOVA followed by Bonferroni post hoc test (for multiple groups) was used for statistical analysis. P < 0.05 is statistically significant. The number of pigs used for each experiment was indicated as n.
Results
Distribution of nitrergic nerves in the lamina propria of pig bladder
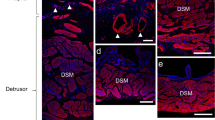
In whole mount preparations of lamina propria of pig bladder (non-trigonal region), bundles of α-smooth muscle actin (α-SMA)–immunoreactive smooth muscle cells (SMCs), namely, the muscularis mucosae (MM), were distributed as previously reported (Mitsui et al. 2020) (Fig. 1a). Suburothelial arterioles and venules were also identified in the same layer by their α-SMA immunoreactivity in vascular SMCs (Fig. 1a). Arterioles and venules could readily be distinguished by the different morphology of the α-SMA-immunoreactive mural cells (Fig. 1d). Thus, arteriolar SMCs were circumferentially oriented and tightly packed, while venular SMCs extended several processes in various directions and were more sparsely distributed (Mitsui and Hashitani 2013; Hashitani et al. 2018). Double immunostaining for α-SMA and neuronal nitric oxide synthase (nNOS) revealed that varicose nNOS-positive nitrergic fibres innervated MM (Fig. 1b, c). Perivascular nitrergic nerve fibres immunoreactive to nNOS also projected to suburothelial arterioles and venules (Fig. 1d, e). In the pig bladder mucosa, intrinsic nNOS-positive nitrergic neurons reported in the mouse bladder mucosa (Tanaka et al. 2021) were not detected.
Distribution of neuronal nitric oxide synthase (nNOS)–immunoreactive nitrergic nerve fibres in the mucosa of pig bladder. A low magnification micrograph of pig bladder mucosa immunostained using α-smooth muscle actin (α-SMA) antibody is shown (a). Various sizes of smooth muscle bundles called muscularis mucosae (MM) and mucosal blood vessels (asterisks) were detected. Double immunostaining for α-SMA (red) and nNOS (green) revealed that varicose nitrergic nerve fibres ran parallel to the long axis of smooth muscle cells of MM (b, c). An arteriole (A) and venule (V) revealed by α-SMA immunohistochemistry (red) were surrounded by nNOS-immunoreactive (green) nitrergic nerve fibres in the pig bladder mucosa (d, e). The scale bars indicate 200 µm (a), 100 µm (b) and 50 µm (d)
Nitrergic nerves intermingled with sympathetic and primary afferent nerves around bladder suburothelial vasculatures
In the bladder lamina propria double immunostaining for nNOS and TH, a sympathetic nerve marker, showed that perivascular nitrergic nerves were intermingled with sympathetic nerve fibres (Fig. 2a–d), while the neighbouring MM only received nitrergic nerve fibres (Fig. 2c). Varicose both nitrergic and sympathetic nerve fibres projected to the mucosal arterioles and venules (Fig. 2e–h). At higher magnification, the varicosities of perivascular nitrergic and sympathetic fibres were not colocalised (Fig. 2h inset), confirming that these nitrergic nerves are distinct from sympathetic fibres.
Comparison between projection patterns of nitrergic and sympathetic nerve fibres in the mucosa of pig bladder. A mucosal whole mount preparation of pig bladder that contained the muscularis mucosae (MM) and blood vessel (asterisk) was immunostained for nNOS (green) and TH (red), while Hoechst 33342 was used for nuclear staining (Hoechst, blue) (a). In this specimen, varicose nitrergic nerve fibres projected to both MM and vasculatures, while sympathetic fibres immunoreactive for TH only projected to the vasculatures but not MM (b–d). In another specimen, a mucosal arteriole (A) and venule (V) have nitrergic and sympathetic innervations (e–h). An enlarged image of these two types of perivascular nerves indicated by an arrow in h showed that their varicosities were not colocalised (h, inset). Thus, these nitrergic fibres were distinct from sympathetic fibres. The scale bars indicate 100 µm (a, e) and 25 µm (inset of h)
Double immunostaining for nNOS and calcitonin gene–related peptide (CGRP) showed that perivascular nitrergic nerves were intermingled with but distinct from CGRP-immunoreactive primary afferent nerve fibres (Fig. 3a–d). In contrast to MM in the guinea pig bladder, in which CGRP-containing nerve fibres are abundantly distributed (Lee et al. 2016), CGRP-immunoreactive afferent nerve fibres seldom projected to the MM in the pig bladder (Fig. 3c). Double immunostaining for α-SMA and CGRP showed that CGRP-immunoreactive afferent fibres were projected around both the mucosal arterioles and venules in the pig bladder (Fig. 3e, f).
Comparison between projection patterns of nitrergic and afferent nerve fibres in the mucosa of pig bladder. Double immunostaining for nNOS (green) and calcitonin gene–related peptide (CGRP, red) combined with nuclear staining (Hoechst, blue) was conducted using a mucosal whole mount of pig bladder (a–d). Nitrergic nerves projected to the muscularis mucosae (MM) and a blood vessel (asterisk), while CGRP-positive primary afferent nerve fibres only projected to the mucosal vasculature but not MM. Double immunostaining for α-SMA (red) and CGRP (green) showed that CGRP-positive afferent fibres (arrows) projected to both a mucosal arteriole (A) and venule (V) (e, f). Scale bars indicate 50 µm
Nitrergic nerve distribution in bladder DSM layer
The distribution of nitrergic nerves in the DSM layer was examined for a comparison. In whole mount preparations of DSM, nNOS-positive nitrergic nerve fibres including some varicose single fibres were distributed (Fig. 4a, b). In the sections of DSM, nitrergic nerves were found within the smooth muscle bundles as well as the septa (Fig. 4c, d).
Distribution of nitrergic nerve fibres in the detrusor of pig bladder. In a whole mount preparation of pig detrusor where smooth muscle cells were visualised by α-SMA immunoreactivity (red), nNOS-immunoreactive (green) nitrergic nerve fibres were detected (a, b). Some of them were varicose single nerve fibres (arrows). In a section of pig detrusor, nitrergic nerves were found within smooth muscle bundles as well as in space between the bundles (c, d). The scale bars indicate 100 µm (a) and 50 µm (c)
Nitrergic nerve distribution in the urethral wall
In whole mount preparations of lamina propria of pig urethra, α-SMA-immunoreactive SMC-LP were scattered and predominantly arranged in the longitudinal direction (Fig. 5a), but smooth muscle bundles comparable to the bladder MM were virtually absent. In contrast to the bladder MM, SMC-LP did not receive a nitrergic innervation, while varicose nitrergic fibres projected to neighbouring vascular SMCs of suburothelial arterioles and venules (Fig. 5b). Consistent with the functional nitrergic innervation to urethral SMCs (see Introduction), nitrergic nerves were distributed within the smooth muscle bundles as well as the septa in the sections of urethral musculature (Fig. 5c, d).
Distribution of nitrergic nerve fibres in the pig urethra. In a whole mount specimen of the mucosa of pig urethra immunostained for α-SMA, scattered smooth muscle cells (SMCs) and vascular SMCs of arteriole (A) and venule (V) were detected (a). In contrast to the bladder, the muscularis mucosae, i.e., large SMC bundles, were not found. An enlarged image of the same preparation with nNOS immunoreactivities (green) is presented (b). Nitrergic nerve fibres projected to the vascular SMCs of arteriole (A) and venule (V) but not the scattered mucosal SMCs. In a section of smooth muscle layer of pig urethra, nitrergic fibres were detected (c, d). The scale bars indicate 200 µm (a), 100 µm (b) and 50 µm (c)
Nitrergic nerves intermingled with sympathetic and primary afferent nerves in suburothelial vasculature of the urethra
Double immunostaining for nNOS and TH revealed that perivascular nitrergic nerves around suburothelial arterioles and venules of the urethra were intermingled with but distinct from varicose sympathetic fibres (Fig. 6a–d). Double immunostaining for nNOS and CGRP demonstrated that perivascular nitrergic nerves are in close proximity with CGRP-containing primary afferent nerves in both arterioles and venules of the urethra (Fig. 6e–h). Neither sympathetic nor afferent fibres projected to SMC-LP (not shown). Intrinsic nNOS-immunoreactive nitrergic neurons were not detected in the pig urethral mucosa.
Perivascular nitrergic nerves running parallel to sympathetic and afferent nerves in the pig urethral mucosa. In a whole mount preparation of pig urethral mucosa, nNOS-positive nitrergic nerves (green) and TH-positive sympathetic nerves (red) project to both the mucosal arteriole (A) and venule (V) (a–d). An area indicated by an arrow in d was enlarged to show that these two types of nerves are distinct from each other (d, inset). Perivascular calcitonin gene–related peptide (CGRP, red)–immunoreactive afferent fibres were intermingled with nitrergic fibres in the pig urethral mucosa (e–h). They are distinct types of nerves (h, inset). The scale bars indicate 50 µm (a, e), and 20 µm (insets of d and h)
Nitrergic nerve distribution in the trigonal wall
The distribution pattern of nitrergic nerve fibres in the lamina propria of pig trigone resembled that observed in the pig urethra. Perivascular nitrergic fibres of mucosal vasculature in the trigone were intermingled with but distinct from sympathetic fibres (Fig. 7a–d), while SMC-LP scattered in the mucosa of trigone did not receive a nitrergic innervation (Fig. 7e, f). In sections of the pig trigone, nitrergic fibres were distributed in the smooth muscle layer (Fig. 7g, h).
Distribution of nitrergic nerve fibres in the pig bladder trigone. In a mucosal whole mount of pig trigone, nNOS-positive nitrergic (green) and TH-positive sympathetic (red) nerves were detected around an arteriole (A) (a–d). In a different whole mount, scattered smooth muscle cells were detected by α-SMA immunoreactivity; these cells lacked the innervation of nNOS-immunoreactive fibres (e, f). Nitrergic nerve fibres were detected in the section of smooth muscle layer of pig trigone (g, h). The scale bars indicate 50 µm (a, g) and 100 µm (e)
Functional nitrergic innervation to suburothelial arterioles of the bladder and urethra
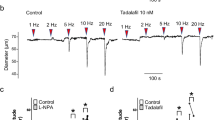
In suburothelial arterioles of the pig bladder mucosa, EFS (100 µs duration, 20 Hz for 2 s) often failed to evoke a vasoconstriction; any EFS-induced vasoconstrictions were transient. L-NA (100 μM) unmasked or enhanced the EFS-induced vasoconstrictions (n = 9) (Fig. 8a). EFS-induced arteriolar constrictions were abolished by tetrodotoxin (1 μM, n = 3) or sympathetic nerve depletion with guanethidine (10 μM, n = 3), indicating they are mediated by sympathetic nerves.
In 6 bladder mucosa preparations in which EFS evoked consistent arteriolar vasoconstrictions without L-NA (17.2 ± 8.9% of resting diameter, n = 6), 10 nM tadalafil attenuated EFS-induced vasoconstrictions (6.5 ± 7.4% of resting diameter, P < 0.05) (Fig. 8b) without changing the resting diameter (46.9 ± 24.7 μm, ranging from 15.5 μm to 79.4 μm, 46.5 ± 22.6 μm in tadalafil). In 3 preparations, subsequent application of L-NA (100 μM) enlarged the EFS-evoked constrictions from 6.7 ± 10.4% (n = 3) to 23.8 ± 18.8% of the resting diameter (Fig. 8b).
In suburothelial arterioles of the urethral mucosa where EFS failed to evoke sympathetic vasoconstrictions, L-NA unmasked their generation (n =3, Fig. 8c). In 3 other preparations in which EFS evoked arteriolar vasoconstriction in the absence of L-NA, tadalafil (10 nM) suppressed the vasoconstrictions in a manner sensitive to L-NA (Fig. 8d). EFS-induced constrictions in the urethral arteriole were abolished by tetrodotoxin (1 μM, n = 3) and largely suppressed (n = 3) by guanethidine (10 μM), indicating that the response is predominantly mediated by sympathetic nerves.
Effects of PDE5 inhibitor tadalafil on contractility of mucosal vasculatures in the pig bladder and urethra. Electrical field stimulation (EFS; 100 µs duration, 20 Hz, 2 s) indicated by red arrowheads did not distinctly changed the diameter of arteriole in a pig bladder mucosa preparation, while, in the presence of L-NA, it induced a diameter reduction (i.e., vasoconstriction) (a). L-NA reduced the resting diameter of arteriole. In a different bladder mucosa preparation, EFS-induced an arteriolar constriction which was inhibited by tadalafil (b). Subsequent L-NA enlarged EFS-induced constriction. In a mucosal preparation of pig urethra, EFS had no effect, while EFS-induced constriction was detected in the presence of L-NA (c). In a different urethral preparation, EFS evoked a constriction which was abolished by tadalafil (d). EFS-induced constriction was reappeared after subsequent L-NA application. Resting diameters were 35 μm (a), 78 μm (b), 62 μm (c) and 56 μm (d). Scale bars in d apply to all traces
Roles of endogenous NO in modulating nerve-evoked changes in MM contractility
In MM strips that had been pre-treated with U46619 (100 nM) and atropine (1 µM), EFS evoked an NO-mediated transient relaxation as reported previously (Mitsui et al. 2020). Tadalafil (100 nM) prolonged the half-width of EFS-induced relaxations without increasing their amplitude (n = 7, Fig. 9a–d).
Roles of nitrergic innervation in modulating nerve-evoked relaxation/contractions in MM. a In a MM strip that had been pre-constricted with U46619 (100 nM), a thromboxane A2 receptor agonist, and atropine (1 µM), EFS (20 Hz, 1 s every 3 min, red arrowheads) evoked transient relaxations. b Tadalafil (100 nM) increased the half-width (c) of EFS-induced relaxations without changing their amplitude (d). Two-tailed paired Student’s t-test, *P < 0.05. e In a different MM strip generating spontaneous phasic contractions, EFS evoked phasic contractions. f Tadalafil (100 nM) did not affect either the amplitude or half-width of the EFS-induced contractions. g Subsequent L-NA (10 μM) increased the amplitude and half-width of the EFS-induced contractions. Effects of tadalafil and subsequent L-NA on the amplitude (h) and half-width (i) of the EFS-induced contractions were summarised. One-way repeated measures ANOVA with Bonferroni post hoc analysis, *P < 0.05
In MM strips in which spontaneous phasic contractions were present, EFS evoked a phasic contraction that was predominantly mediated by neurally released ACh (see also Mitsui et al. 2020). Tadalafil (100 nM) did not affect either the amplitude or half-width of the EFS-induced contractions, while subsequent L-NA (10 μM) increased their amplitude and half-width (n = 6, Fig. 9e–i).
Properties of spontaneous Ca2+ transients in SMC-LP
SMC-LP in the urethra (Fig. 10a, supplementary video 1) and trigone (Fig. 10b, supplementary video 2) developed spontaneous Ca2+ transients. Spontaneous Ca2+ transients in individual SMC-LP were generated independently of each other, but displayed near synchronicity in two or three neighbouring SMC-LP. The mean amplitude, half-width or frequency of spontaneous Ca2+ transients in SMC-LP were not different between the urethra (n = 21) and trigone (n = 24) (Fig. 10c–e). In both urethra (n = 5) and trigone (n = 6), spontaneous Ca2+ transients in SMC-LP were abolished or largely suppressed by nifedipine (1 μM), an L-type voltage-dependent Ca2+ channel blocker. Residual spontaneous Ca2+ transients were abolished by CPA (10 μM), the blocker of sarco-endoplasmic reticulum Ca2+ ATPase, associated with a rise in the basal Ca2+ level (urethra, n = 4; trigone, n = 3). In both urethra and trigone, SMC-LP did not respond to EFS (10 or 20 Hz, 1 s), while arteriolar SMCs developed EFS-induced Ca2+ transients, and thus, SMC-LP appear to lack a functional innervation.
Spontaneous Ca2+ transients in smooth muscle cells of lamina propria (SMC-LP) in the pig urethra and trigone. a A micrograph of α-SMA-immunoreactive SMC-LP in the pig urethra (left) and Ca2+ florescence images of urethral SMC-LP (centre; basal condition, right; during Ca2+ transient). b Corresponding α-SMA micrograph and Ca2+ images of SMC-LP in the pig bladder trigon. Two-way arrows in a, b indicate the longitudinal axis of the urethra. The amplitude (c), half-width (d) and frequency (e) of spontaneous Ca2+ transients in urethral and trigonal SMC-LP are summarised for a comparison. Two-tailed unpaired Student’s t-test, *P < 0.05. f In three urethral SMC-LP developing asynchronous spontaneous Ca2+ transients, nifedipine (1 μM) largely suppressed their generation. g In three SMC-LP that had been exposed to nifedipine (1 μM), CPA (10 μM) abolished the residual spontaneous Ca2+ transients with a rise in the basal Ca2+ level
Effects of bath-applied neurotransmitters on spontaneous Ca2+ transients in urethral SMC-LP were examined. Bath-applied noradrenaline (1 μM) slowed and suppressed spontaneous Ca2+ transients or abolished their generation with a reduction in the basal Ca2+ level in a manner sensitive to propranolol, a β-adrenoceptor antagonist (1 μM, Fig. 11a). Effects of noradrenaline (1 μM) and subsequent propranolol (1 μM) on the amplitude and frequency of spontaneous Ca2+ transients were summarised (Fig. 11b, c). Bath-applied ACh (1 μM) transiently accelerated spontaneous Ca2+ transients and then ceased their generation with a sustained raise in the basal Ca2+ level in a manner sensitive to atropine, a muscarinic receptor antagonist (1 μM, Fig. 11d). Effects of ACh (1 μM) and subsequent atropine (1 μM) on the amplitude and frequency of spontaneous Ca2+ transients were summarised (Fig. 11e, f). Bath-applied SIN-1 (10 μM), an NO donor, failed to affect the amplitude (0.63 ± 0.15 in control, 0.63 ± 0.13 in tadalafil, P > 0.05, n = 7) or frequency (4.3 ± 0.91 in control, 4.3 ± 0.95 in tadalafil, P > 0.05, n = 7) of spontaneous Ca2+ transients.
Pharmacological profile of spontaneous Ca2+ transients in SMC-LP of the pig urethra. a In a SMC-LP of pig urethra exhibiting spontaneous Ca2+ transients, noradrenaline (NAd, 1 μM) slowed and diminished Ca2+ transients with a reduction in the basal Ca2+ level in a manner sensitive to propranolol (Prop, 1 μM) (a). Effects of NAd and subsequent Prop on the amplitude (b) and frequency (c) of spontaneous Ca2+ transients in SMC-LP were summarised. One-way repeated measures ANOVA with Bonferroni post hoc analysis, *P < 0.05. d In a different spontaneously active SMC-LP, acetylcholine (ACh, 1 μM) transiently accelerated spontaneous Ca2+ transients and then suppressed their generation with a rise in the basal Ca2+ level in a manner sensitive to atropine (Atr, 1 μM). Effects of ACh and subsequent Atr on the amplitude (e) and frequency (f) of spontaneous Ca2+ transients were summarised. One-way repeated measures ANOVA with Bonferroni post hoc analysis, *P < 0.05
Discussion
Nitrergic innervation to suburothelial vasculatures in pig bladder and urethra
In both bladder and urethra of pigs, varicose nitrergic nerve fibres were detected around the mucosal arterioles and venules (Fig. 12). These nNOS-positive perivascular nitrergic nerves were intermingled with but not colocalised with TH- or CGRP-positive perivascular nerves, indicating that nitrergic fibres are distinct from sympathetic or afferent fibres. This is consistent with a previous study demonstrating that chemical denervation of sympathetic nerves with 6-hydroxydopamine or primary afferent denervation with capsaicin has no effect on nerve-evoked, NO-mediated relaxations (Persson et al. 1997). Thus, it is unlikely that nNOS-containing nerves are in fact sympathetic or afferent nerves.
Comparison of nitrergic nerve distributions in the pig bladder and urethra. In the pig bladder mucosa, nitrergic nerve fibres (green) project to arterioles and venules and muscularis mucosae. The perivascular nitrergic nerve fibres are intermingled with sympathetic nerve fibres (orange), while the muscularis mucosae lacks sympathetic innervation. The activation of perivascular nitrergic fibres counteracts sympathetic constriction of vasculatures in the presence of PDE5 inhibitor, while nitrergic nerves relax the muscularis mucosae even in the absence of PDE5 inhibitor. Note that whether a single nitrergic neuron projects to both mucosal vasculatures and MM remains to be determined. Nitrergic fibres are present in the detrusor layer; their main targets may be interstitial cells rather than detrusor smooth muscle cells (SMCs). In the pig urethra and trigone, nitrergic and sympathetic nerves project to the mucosal vasculatures but not mucosal SMCs. Their smooth muscle layers have inhibitory nitrergic innervation (see Introduction)
In the rat major pelvic ganglion, NOS-immunoreactive cell bodies also display choline acetyltransferase immunoreactivity (Persson et al. 1998). Nitrergic relaxations induced by EFS in the rat urethral smooth muscles are abolished by bilateral cryoganglionectomy of the major pelvic ganglion (Persson et al. 1998), suggesting that nitrergic nerves projecting to the lower urinary tract are predominantly parasympathetic origin. Consistently, some NOS-immunoreactive nerves co-express acetylcholine esterase in the pig lower urinary tract (Persson et al. 1995). In the pig urethra, falls in the urethral pressure during voiding are accompanied by a rise in the mucosal blood flow (Greenland et al. 1996). Considering parasympathetic nerves dominant neural activity in the lower urinary tract during voiding phases, the increased blood flow likely results from arteriolar dilatation that is mediated by NO released from parasympathetic nerves. Neurally released NO would also reduce the arteriolar resistance in the bladder despite the extravascular compression by detrusor contractions, minimising the reduction in the bladder blood flow during voiding (Greenland and Brading 1996).
Functions of nitrergic transmission in suburothelial arterioles of bladder and urethra
Nitrergic nerve–mediated vasodilation responses in peripheral tissues are generally not clearly detectable (Toda and Okamura 2015), although perivascular nitrergic nerve–mediated dilation is evident in the cerebral artery (Toda and Okamura 1990). In suburothelial arterioles of the rat bladder, EFS induces guanethidine-sensitive sympathetic constrictions that are mediated by α-adrenoceptors (Hashitani et al. 2011). In suburothelial venules, sympathetic transmission accelerates spontaneous phasic constrictions via α-adrenoceptors activation, while exerting a β-adrenoceptor-mediated vasodilatory action that appears to involve endothelial NO release (Shimizu et al. 2014). Therefore, it was thought that the contractility of suburothelial arterioles and venules in the bladder are predominantly regulated by sympathetic nerves. However, our recent study demonstrated functional nitrergic transmission in suburothelial arterioles of rat and mouse bladder (Tanaka et al. 2021), highlighting the roles of neurally released NO in modulating vascular contractility.
In the present study, L-NA unmasked or enhanced EFS-induced suburothelial arteriolar constrictions of pig bladder or urethra and also reduced their basal diameter, indicating that NO is continuously released presumably from the endothelium. Time-dependent loss of EFS-induced vasoconstrictions seen in the present study is likely due to the increased production of endothelial NO with time under our in vitro experimental conditions. Consistent with the findings in rat bladder arterioles (Tanaka et al. 2021), tadalafil, a PDE5 inhibitor, diminished EFS-induced constrictions of suburothelial arterioles in the pig bladder and urethra without changing their basal diameter, suggesting that the blockade of PDE5 enhances the cGMP-dependent relaxation in arteriolar smooth muscle cells that was triggered by neurally released NO. Unfortunately, L-NPA, an nNOS-specific inhibitor, that enhances EFS-induced constrictions in rodent bladder arterioles without changing the basal diameter (Tanaka et al. 2021) had no effect in the pig vasculature, possibly due to the splicing variant in nNOS amongst different species. In the mucosal blood vessels of rat bladder expressing PDE5 (both in vascular smooth muscle cells and endothelial cells), the PDE5 inhibitor improves bladder hypoxia in spontaneously hypertensive rats (Morelli et al. 2010). Therefore, nitrergic nerve–mediated inhibition of the contractility in bladder microvessels (Tanaka et al. 2021, the present study) appears to be involved in the bladder protective actions of PDE5 inhibitors. Since the mucosa of lower urinary tract plays a fundamental role in sensing the tissue environment, adequate blood supply to this layer is crucial for maintaining the normal bladder functions. In this regard, a complex and counterbalancing control of arteriolar diameter has physiological relevance.
Arteries rather than arterioles play an important role in regulating blood flow control in the cheek pouch circulation of anaesthetised hamsters (Davis et al. 1986). However, both arterioles and arteries regulate peripheral resistance in the mesenteric circulation of conscious rats (Christensen and Mulvany 1993; Fenger-Gron et al. 1997). The distribution of blood flow within the bladder wall, e.g., between mucosal and muscle layers, is likely to be regulated by the arterioles investigated in the present study, while the upstream arteries may be also involved in the blood flow regulation. This notion appears to be consistent with observation that bladder feeding arteries (vesical arteries) are also regulated by nitrergic nerves (Tanaka et al. 2021).
The inhibition of sympathetic nerve–mediated vasoconstrictions by neurally released NO is likely due to its inhibitory actions on vascular SMCs. In addition, the proximity of nitrergic and sympathetic nerve fibres in the mucosal vasculatures of pig lower urinary tract may allow NO-induced presynaptic inhibition of sympathetic transmitter release. In the rat mesenteric artery where perivascular nitrergic and sympathetic nerves are in close proximity, an NOS inhibitor increases EFS-evoked neuronal noradrenaline release (Hatanaka et al. 2006).
Nitrergic innervation to MM in pig bladder
The present study demonstrates that varicose nNOS-immunoreactive nitrergic nerve fibres project to MM in the pig bladder (Fig. 12), indicating that EFS-induced, NOS inhibitor–sensitive relaxations of pig bladder MM (Mitsui et al. 2020) are mediated via NO released from nitrergic nerve fibres. The lack of sympathetic fibres in MM is consistent with the previous finding that the blockade of sympathetic transmission with guanethidine fails to affect MM EFS–induced contractions or relaxations (Mitsui et al. 2020). Considering that nitrergic nerves in the lower urinary tract are predominantly parasympathetic origin, NO could be co-released with acetylcholine from parasympathetic nerves. In the present study, the blockade of NOS with L-NA enlarged EFS-induced contractions of MM that are predominantly mediated by neurally released acetylcholine (Mitsui et al. 2020), and thus, neuronal NO may function as a self-limiting factor in parasympathetic nerve–mediated regulation of MM contractility. In contrast to MM, detrusor smooth muscles vigorously contract upon the excitation of parasympathetic nerves as they do not respond to NO.
Since non-voiding phasic contractions of detrusor smooth muscles stimulate mechanosensitive primary afferent nerves (Heppner et al. 2016), increased contractility of detrusor smooth muscles would trigger urinary urgency. In species that have MM in the bladder including human, spontaneous contractions in MM could also be a source of mechano-induced afferent stimulation (Heppner et al. 2011; Isogai et al. 2016; Mitsui et al. 2020). In the present study, CGRP-containing primary afferent nerves projected to suburothelial vasculature but not MM. Nevertheless, the close proximity of MM with the blood vessels would allow MM to mechanically stimulate perivascular afferents. Since tadalafil prolonged nitrergic relaxations of MM, MM could be a therapeutic target of PDE5 inhibitors for the treatment of urinary urgency seen in patients with overactive bladder (Gacci et al. 2016).
Properties of SMC-LP in pig urethra and trigone
SMC-LP scatteredly distributed in lamina propria of the urethra and trigone develop spontaneous Ca2+ transients predominantly arising from the opining of L-type voltage-dependent Ca2+ channels, suggesting that they fire action potentials as do MM in the bladder (Lee et al. 2018). Nevertheless, SMC-LP also generated nifedipine-resistant, residual Ca2+ transients that were blocked by the blockade of sarcoendoplasmic reticulum Ca2+ ATPase (SERCA) with CPA, and thus, spontaneous activity in SMC-LP may primarily depend on SR Ca2+ handling. Unlike MM that receive both nitrergic and cholinergic innervations, SMC-LP did not respond to EFS, suggesting their lack of a functional innervation. Nevertheless, bath-applied ACh raised Ca2+ level in SMC-LP via the activation of muscarinic receptors, while bath-applied noradrenaline caused β-adrenoceptor-mediated inhibition of spontaneous Ca2+ transients. These pharmacological characteristics of SMC-LP are similar to those of bladder MM (Moro et al. 2011, 2013). However, in contrast to MM in the bladder, SMC-LP did not have a nitrergic innervation (Fig. 12) and failed to respond to even bath-applied NO donor. A previous study demonstrated that rabbit urethral lamina propria is contacted upon the activation of α-adrenoceptors with EFS or bath-applied noradrenaline (Mattiasson et al. 1985). Moreover, pre-contracted rabbit urethral lamina propria is relaxed by neurally released NO or bath-applied ACh that trigger NO release (Zygmunt et al. 1993). Although it was not determined if the contractility of rabbit urethral lamina propria ascribed to vascular or non-vascular smooth muscle, SMC-LP in the pig urethra appears to have different properties from rabbit urethral lamina propria.
Conclusion
In the pig bladder and urethra, varicose nitrergic fibres were projected to suburothelial arterioles and venules, and neurally released NO exerts vasorelaxant effects that can be enhanced by clinically relevant concentration (10 nM) of tadalafil (Forgue et al. 2006). Thus, mucosal vasculatures in the lower urinary tract could be the site of action of PDE5 inhibitors for LUTS treatment. The varicose nitrergic nerve fibres also projected to MM of pig bladder where nitrergic relaxation has been reported (Mitsui et al. 2020). SMC-LP in the urethra developed spontaneous Ca2+ transients but did not receive a functional nitrergic innervation. Although SMC-LP responded to exogenously applied ACh or noradrenaline, their roles in regulating the function of urethral mucosa remain to be explored.
Abbreviations
- α-SMA:
-
α-Smooth muscle actin
- ACh:
-
Acetylcholine
- cGMP:
-
Cyclic guanosine monophosphate
- CGRP:
-
Calcitonin gene–related peptide
- DSM:
-
Detrusor smooth muscle
- EFS:
-
Electrical field stimulation
- LUTS:
-
Lower urinary tract symptoms
- L-NA:
-
N ω-nitro-L-arginine
- MM:
-
Muscularis mucosae
- nNOS:
-
Neuronal nitric oxide synthase
- NO:
-
Nitric oxide
- PDE5:
-
Phosphodiesterase type 5
- PSS:
-
Physiological salt solution
- SMC:
-
Smooth muscle cell
- SMC-LP:
-
Smooth muscle cell in the lamina propria
- TH:
-
Tyrosine hydroxylase
References
Brading AF (1999) The physiology of the mammalian urinary outflow tract. Exp Physiol 84:215–221
Bustamante S, Orensanz LM, Recio P, Carballido J, García-Sacristán A, Prieto D, Hernández M (2010) Functional evidence of nitrergic neurotransmission in the human urinary bladder neck. Neurosci Lett 477:91–94
Christensen KL, Mulvany MJ (1993) Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J Vasc Res 30:73–79
Davis MJ, Ferrer PN, Gore RW (1986) Vascular anatomy and hydrostatic pressure profile in the hamster cheek pouch. Am J Physiol 250:H291–H303
Dixon JS, Gosling JA (1983) Histology and fine structure of the muscularis mucosae of the human urinary bladder. J Anat 136:265–271
Fenger-Gron J, Mulvany MJ, Christensen KL (1997) Intestinal blood flow is controlled by both feed arteries and microcirculatory resistance vessels in freely moving rats. J Physiol 498:215–224
Filippi S, Morelli A, Sandner P, Fibbi B, Mancina R, Marini M, Gacci M, Vignozzi L, Vannelli GB, Carini M, Forti G, Maggi M (2007) Characterization and functional role of androgen-dependent PDE5 activity in the bladder. Endocrinology 148:1019–1029
Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, Wrishko RE, Mitchell MI (2006) Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol 61:280–288
Gacci M, Andersson KE, Chapple C, Maggi M, Mirone V, Oelke M, Porst H, Roehrborn C, Stief C, Giuliano F (2016) Latest evidence on the use of phosphodiesterase type 5 inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol 70:124–133
Greenland JE, Brading AF (1996) Urinary bladder blood flow changes during the micturition cycle in a conscious pig model. J Urol 156:1858–1861
Greenland JE, Dass N, Brading AF (1996) Intrinsic urethral closure mechanisms in the female pig. Scand J Urol Nephrol Suppl 179:75–80
Hashitani H, Mitsui R, Miwa-Nishimura K, Lam M (2018) Role of capillary pericytes in the integration of spontaneous Ca2+ transients in the suburothelial microvasculature in situ of the mouse bladder. J Physiol 596:3531–3552
Hashitani H, Takano H, Fujita K, Mitsui R, Suzuki H (2011) Functional properties of suburothelial microvessels in the rat bladder. J Urol 185:2382–2391
Hatanaka Y, Hobara N, Honghua J, Akiyama S, Nawa H, Kobayashi Y, Takayama F, Gomita Y, Kawasaki H (2006) Neuronal nitric-oxide synthase inhibition facilitates adrenergic neurotransmission in rat mesenteric resistance arteries. J Pharmacol Exp Ther 316:490–497
Heppner TJ, Layne JJ, Pearson JM, Sarkissian H, Nelson MT (2011) Unique properties of muscularis mucosae smooth muscle in guinea pig urinary bladder. Am J Physiol Regul Integr Comp Physiol 301:R351–R362
Heppner TJ, Tykocki NR, Hill-Eubanks D, Nelson MT (2016) Transient contractions of urinary bladder smooth muscle are drivers of afferent nerve activity during filling. J Gen Physiol 147:323–335
Isogai A, Lee K, Mitsui R, Hashitani H (2016) Functional coupling of TRPV4 channels and BK channels in regulating spontaneous contractions of the guinea pig urinary bladder. Pflugers Arch 468:1573–1585
Keast JR, Kawatani M (1994) Extensive distribution of NADPH diaphorase activity in the nerve supply of the cat lower urinary tract. J Auton Nerv Syst 50:161–169
Lee K, Isogai A, Antoh M, Kajioka S, Eto M, Hashitani H (2018) Role of K+ channels in regulating spontaneous activity in the muscularis mucosae of guinea pig bladder. Eur J Pharmacol 818:30–37
Lee K, Mitsui R, Kajioka S, Naito S, Hashitani H (2016) Role of PTHrP and Sensory Nerve peptides in regulating contractility of muscularis mucosae and detrusor smooth muscle in the guinea pig bladder. J Urol 196:1287–1294
Lies B, Groneberg D, Friebe A (2013) Correlation of cellular expression with function of NO-sensitive guanylyl cyclase in the murine lower urinary tract. J Physiol 591:5365–5375
Mattiasson A, Andersson KE, Sjögren C (1985) Contractant and relaxant properties of the female rabbit urethral submucosa. J Urol 133:304–310
Mitsui R, Hashitani H (2013) Immunohistochemical characteristics of suburothelial microvasculature in the mouse bladder. Histochem Cell Biol 140:189–200
Mitsui R, Lee K, Uchiyama A, Hayakawa S, Kinoshita F, Kajioka S, Eto M, Hashitani H (2020) Contractile elements and their sympathetic regulations in the pig urinary bladder: a species and regional comparative study. Cell Tissue Res 379:373–387
Morelli A, Filippi S, Comeglio P, Sarchielli E, Chavalmane AK, Vignozzi L, Fibbi B, Silvestrini E, Sandner P, Gacci M, Carini M, Vannelli GB, Maggi M (2010) Acute vardenafil administration improves bladder oxygenation in spontaneously hypertensive rats. J Sex Med 7:107–120
Moro C, Tajouri L, Chess-Williams R (2013) Adrenoceptor function and expression in bladder urothelium and lamina propria. Urology 81:211.e1–7
Moro C, Uchiyama J, Chess-Williams R (2011) Urothelial/lamina propria spontaneous activity and the role of M3 muscarinic receptors in mediating rate responses to stretch and carbachol. Urology 78:1442.e9–15
Oger S, Behr-Roussel D, Gorny D, Lebret T, Validire P, Cathelineau X, Alexandre L, Giuliano F (2010) Signalling pathways involved in sildenafil-induced relaxation of human bladder dome smooth muscle. Br J Pharmacol 160:1135–1143
Parlani M, Conte B, Manzini S (1993) Nonadrenergic, noncholinergic inhibitory control of the rat external urethral sphincter: involvement of nitric oxide. J Pharmacol Exp Ther 265:713–719
Persson K, Alm P, Johansson K, Larsson B, Andersson KE (1993) Nitric oxide synthase in pig lower urinary tract: immunohistochemistry, NADPH diaphorase histochemistry and functional effects. Br J Pharmacol 110:521–530
Persson K, Alm P, Johansson K, Larsson B, Andersson KE (1995) Co-existence of nitrergic, peptidergic and acetylcholine esterase-positive nerves in the pig lower urinary tract. J Auton Nerv Syst 52:225–236
Persson K, Alm P, Uvelius B, Andersson KE (1998) Nitrergic and cholinergic innervation of the rat lower urinary tract after pelvic ganglionectomy. Am J Physiol 274:R389–R397
Persson K, Andersson KE (1992) Nitric oxide and relaxation of pig lower urinary tract. Br J Pharmacol 106:416–422
Persson K, Johansson K, Alm P, Larsson B, Andersson KE (1997) Morphological and functional evidence against a sensory and sympathetic origin of nitric oxide synthase-containing nerves in the rat lower urinary tract. Neuroscience 77:271–281
Shimizu Y, Mochizuki S, Mitsui R, Hashitani H (2014) Neurohumoral regulation of spontaneous constrictions in suburothelial venules of the rat urinary bladder. Vascul Pharmacol 60:84–94
Smet PJ, Jonavicius J, Marshall VR, de Vente J (1996) Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience 71:337–348
Tanaka H, Mitsui R, Oishi M, Passlick S, Jabs R, Steinhäuser C, Tanaka KF, Hashitani H (2021) NO-mediated signal transmission in bladder vasculature as a therapeutic target of PDE5 inhibitors. Rodent Model Studies Br J Pharmacol 178:1073–1094
Toda N, Okamura T (1990) Mechanism underlying the response to vasodilator nerve stimulation in isolated dog and monkey cerebral arteries. Am J Physiol 259:H1511–H1517
Toda N, Okamura T (2015) Recent advances in research on nitrergic nerve-mediated vasodilatation. Pflugers Arch 467:1165–1178
Werkström V, Persson K, Ny L, Bridgewater M, Brading AF, Andersson KE (1995) Factors involved in the relaxation of female pig urethra evoked by electrical field stimulation. Br J Pharmacol 116:1599–1604
Zygmunt PK, Persson K, Alm P, Larsson B, Andersson KE (1993) The L-arginine/nitric oxide pathway in the rabbit urethral lamina propria. Acta Physiol Scand 148:431–439
Acknowledgements
The authors wish to thank Dr Richard Lang (Monash University) for critical reading of the manuscript.
Funding
The present study was partly supported by Grant-in-Aid for Scientific Research (C) from Japan Society for Promotion of the Science to R.M. (No. 19K08426) and H.H. (No. 17K11187, 20K09564).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The experimental protocols used in the present study were approved by the animal experimentation ethics committee at Nagoya City University Graduate School of Medical Sciences (No. H-30 M-44).
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Spontaneous Ca2+ transients in SMC-LP of the urethra corresponding to Ca2+ fluorescence images in Fig. 10a. (MP4 2561 KB)
Supplementary file2 Spontaneous Ca2+ transients in SMC-LP of the trigone corresponding to fluorescence images in Fig. 10b. (MP4 2200 KB)
Rights and permissions
About this article
Cite this article
Mitsui, R., Chikada, Y., Arai, K. et al. Functional nitrergic innervation of smooth muscle structures in the mucosa of pig lower urinary tract. Cell Tissue Res 386, 513–531 (2021). https://doi.org/10.1007/s00441-021-03521-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03521-9