Abstract
During their life cycle, plants are often exposed to phases of high salinity and dehydration stress. Extremophile plants have evolved mechanisms of stress tolerance allowing them to survive or recover from extremely adverse conditions such as water deficit stress and soil salinity. Plant adaptability environmental constraints are linked with deep modifications in proteomic profile, with relevance in abiotic tolerance. Research in extreme drought and high salinity tolerance in resurrection plants and halophytes, respectively, provided some insights into stress tolerance and stress recovery through dynamic changes in protein abundance. Identified proteins under drought and salinity conditions cover a wide range of biological functions: photosynthesis, energy metabolism, protein synthesis, protein folding and degradation and defence response. Proteins related to antioxidant metabolism and scavenging of oxygen radicals were found with higher abundance in halophytes and resurrection plants enabling them to cope with stressful conditions. Comprehensive data from recent proteomics studies confirming the relationship between stress tolerance and specific protein abundance are summarized in this paper.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Plants are frequently subjected to a variety of abiotic stresses which negatively affect plant performance and yield crop. Drought and salinity are major constraints limiting plant growth resulting in a huge restriction of world crop production (Barnabas et al. 2008; Athar and Ashraf 2009). Many studies revealed that plant responses to abiotic stresses through a network of regulatory mechanisms with specific characteristics for various species (Rodziewicz et al. 2014). According to Munns (2002), primary responses to water deficit stress and salinity have been considered typically similar. Indeed, these two constraints share dehydration that reaches, more or less intensely, all plant parts (Chaves et al. 2009). Nevertheless, under long-term salt stress, plants are responding not only to dehydration but also to ion toxicity (Chaves et al. 2009). Both soil salinity and water stress have been considered as transient conditions. Thus, fast and efficient recovery from these stresses may play a crucial role in plant stress adaptation (Chen et al. 2016). Nevertheless, previous investigations focused on plant stress response and ignored to evaluate plant recovery aspects after stress release. Recently, more attention has been devoted to stress release in plants (Perrone et al. 2012; Fang and Xiong 2015), which is considered as a major component of stress tolerance. The active management linking between different adaptive strategies at physiological and metabolic levels that result in dynamic changes in protein abundance can explain the ability of extremophile plants to alleviate and recover from the detrimental effects of a variety of biotic and abiotic stresses (Ghosh and Xu 2014; Kumari et al. 2015). These changes can be best explored using proteomic approach since proteins are the central players of an extensive range of cellular processes (Ghosh and Xu 2014). This powerful tool allows global investigation of plant proteomes at different levels and can be useful to compare and analyse proteome changes under stressful conditions (Fernandez-Garcia et al. 2011). The ability of extremophile plants to preserve healthy tissue and recover following stress relief (salt stress and water shortage) is a key feature for stress tolerance. Several model plants adapted to high salinity (halophytes) or prolonged period of water shortage (resurrection plants) have developed efficient adaptive strategies leading them to resist and continue their growth and development processes (Abreu et al. 2013). Halophytes and resurrection plants are of great importance for proteomic studies to further understanding the plant their tolerance to salt and drought stresses, respectively (Dinakar and Bartels 2013; Griffiths et al. 2014; Kumari et al. 2015). In this paper, we highlight at proteomic scale plant responses to salinity and drought that indicate a capacity for stress recovery and adaptability, resulting in improved stress tolerance.
7.2 Drought Stress
The drought has a major impact on plant growth and crop productivity mainly in arid and semiarid regions throughout the world (Gallé et al. 2007). According to the United Nations Food and Agriculture Organization (FAO), drought covers up to 26% of the earth’s land (Pitman and Lauchli 2002; Rehman et al. 2005). Water is the main constituent of all living organisms and required by all known life forms, as a medium for biochemical activities (Xiong and Zhu 2002; Moradi et al. 2018). Drought is a prolonged period of water scarcity, preventing normal plant growth and leading to extensive damage of crops. Most of higher plants are unable to survive under drought conditions (Dinakar and Bartels 2013). Their relative water content is around 85–100% under actively growing conditions and can withstand only moderate dehydration conditions 59–30%. Below 30% of water content, these plants cannot survive (Höfler et al. 1941). By contrast, a small group of vascular angiosperm plants has evolved unique mechanisms to preserve vital cellular components during severe dehydration and thus can tolerate severe water loss. These plants are known as resurrection plants which were used as model plants for studying desiccation tolerance due to their ability to adjust their water content with the relative humidity in the environment (Dinakar and Bartels 2013). The survival water deficit strategies include the rapid downregulation of growth process and the inhibition of water loss resulting in a quick and efficient photosynthetic capacity re-establishment following a rainfall event (Griffiths et al. 2014). The aptitude of resurrection plants to maintain healthy tissues and to restore plant growth through the strong reactivation of many metabolic pathways is a crucial trait of dehydration tolerance (Griffiths et al. 2014). Since desiccation tolerance is controlled by many mechanisms which can be measured at different levels, a combination between different approaches (physiology, proteomics, metabolomics and genomics) should be informative in order to elucidate mechanisms allowing plant adaptation to drought conditions. For its great importance and impact on drought response and adaption, more attention is paid in this paper on proteomics to examine the impact of drought on plant growth and survival.
7.3 Proteomic Analysis of Resurrection Plants
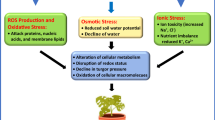
Here, we focused on specific protein families (proteins associated to photosynthetic process, energetic metabolism, stress and defence, protein folding and degradation, etc.) and protein modification that have been strongly linked and characterized as relevant to allow adaptation to limiting water conditions. In the last decades, proteomic approaches involved in the drought response in plants have been extensively studied. However, there are only few proteomic studies in resurrection plants which are limited to some species. Proteome changes upon drought stress confirm that the abundant proteins in the hydrated tissues are related to photosynthetic function and regulation and carbohydrate metabolism (Dinakar and Bartels 2013; Fig. 7.1). In fact, suppression of the photosynthetic electron transport chain is often observed as a consequence of the excess excitation energy related to the enhanced generation of reactive oxygen species (ROS) (Ghosh and Xu 2014). Different research findings on proteome field revealed that the impairment of photosynthetic process and subsequent recovery are key responses found during drought and after water deficit stress release, respectively (Ingle et al. 2007; Wang et al. 2010; Oliver et al. 2011). Water scarcity is associated with cell homeostasis and impairment of photosynthetic apparatus (Chaves et al. 2009). The main reason is CO2 diffusion reduction due to stomata closure (Cornic 2000; Chaves et al. 2009). Proteome changes during dehydration have been elucidated in a relatively drought-tolerant Populus euphratica by Bogeat-Triboulot et al. (2007). Enhanced abundance of proteins involved in photosynthesis and energy metabolisms such as ATP synthase subunit, ATPase subunit, RuBisCo activase and components of oxygen-evolving complex (OEC) was noticed under water deficit stress conditions. Moreover, an upregulation of proteins related to glycolysis such as GAPDH and PGK was noticed. By contrast, photosynthesis-related proteins (RuBisCo large subunit, chlorophyll a-/b-binding protein and oxygen-evolving complex protein) were downregulated in Selaginella tamariscina during dehydration (Wang et al. 2010). Ingle et al. (2007) related photosynthesis impairment in Xerophyta viscosa upon drought at 35% relative water content with decreased abundance of photosynthetic proteins such as the two components of luminal oxygen-evolving complex of PSII (PsbO, PsbP), the PSII stability factor HCF136, the α-subunit of the F-ATPase and the transketolase, a Calvin cycle-related enzyme. Similarly, several related proteins to chromatin structure and function, such as the SNF2P protein (an enzyme involved in ATP-dependent chromatin remodelling) in the model resurrection plant Sporobolus stapfianus, were found to be enhanced under water deficit stress (Oliver et al. 2011). According to Abreu et al. (2013), such modifications may play an important role on gene expression changes allowing drought adaptation. During dehydration, similar protein profile was shown by Jiang et al. (2007a) in the resurrection plant Boea hygrometrica , who related increased abundance of putative ATPase subunits matching a vacuolar H+-ATPase A subunit to preparation for rewatering. During dehydration, late embryogenesis abundant (LEA) proteins are excessively accumulated in resurrection plants indicating its key role in defence mechanism (Alamillo and Bartels 1996; Ndima et al. 2001). LEA proteins which are known to be synthesized and accumulated in embryo tissue upon desiccation process were identified for the first time in cotton seeds (Baker et al. 1988). It seems that LEA proteins have a great potential to improve crop tolerance to adverse environmental conditions (water deficit stress, salinity and cold). The crucial role of these proteins in cellular protection or mitigation effects of drought stress via ion sequestration and preserving minimum tissue water requirements was well documented (Chakrabortee et al. 2007). Different researches were in accordance to indicate the protective roles ensured by the high accumulation of LEA proteins inresurrection plants during water deficit stress (Michel et al. 1994; Ndima et al. 2001). Phosphorylation of LEA proteins was often described under water limitation, such as in Zea mays embryo LEA proteins (Goday et al. 1988) and in Craterostigma plantagineum (Röhrig et al. 2006).
Proteome analysis conducted in the resurrection plant Selaginella tamariscina revealed downregulation of proteins related to photosynthetic process, energy metabolism, defence- and stress-related proteins and cellular biogenesis under drought conditions (Wang et al. 2010). Antioxidant and energy metabolism-associated proteins were upregulated in B. hygrometrica indicating the activation of protective mechanisms in response to dehydration, leading to scavenging ROS, cell wall remodelling proteins, sucrose accumulation, etc. Hence, one can conclude that resurrection plants are able to cope with dehydration through the rapid and efficient accumulation of stress-protective proteins (Dinakar and Bartels 2013).
7.4 Salt Stress
Soil salinity has been considered a major threat affecting crop yield in drylands of the world (Munns 2002). It is estimated that 80 million ha of cultivated lands suffer from salinity problems (Zhang et al. 2012). However, the degree of salt damages depends on the plant age, the duration and the timing of stress (Atteya 2003; Lafitte et al. 2007; Ashraf et al. 2008). Besides, changes in soil salinity can occur in association with many factors: time and space (Epstein and Rains 1987), soil management, water properties, irrigation technique and climate change (de Lacerda et al. 2005). These fluctuations can affect crop adaptability to salt stress, by enhancing or reducing salinity impact on plant responses. The primary salinization of water naturally occurring increase salinity input by evapo-concentration, decrease freshwater input or increase freshwater extraction (Himabindu et al. 2016). By contrast, secondary salinization due to anthropic activities can generate more accented problems (Chaves et al. 2009; Himabindu et al. 2016). For their survival and growth re-establishment during stress period and subsequent recovery, respectively, plants respond by an adjustment in their metabolic pathways (Ghosh and Xu 2014). The mobilization of the metabolic machinery towards plant acclimation and survival occurs via a complex network based on the interaction of physiological, cellular and molecular events developing in the same time and rapidly (Chaves et al. 2009). In high-salt environments, in addition to osmotic stress imposed by water deficit, plants endure ion-specific stress (Blumwald et al. 2000; Shabala and Mackay 2011). Therefore, excessive soil salinity is found to cause dehydration, ion toxicity and nutrient deficiency, along with a suite of metabolic changes, affecting development of salt-sensitive plants and even some tolerant species (Wang et al. 2003; Flowers 2004; Sobhanian et al. 2011). Dehydration caused by salinity is associated with an impairment of photosynthesis, production of reactive oxygen species (ROS), solute accumulation and ion injury (Ashraf and Harris 2004). Understanding plant responses to individual or combined effects of drought and salt stress can play a determinant role in preserving plant productivity and phyto-resources grown under these conditions (Chaves et al. 2009). Adequate management techniques as proteomic approach have become a powerful tool allowing to better understand plant responses to adverse conditions and to improve resource use efficiency by plants (Rodziewicz et al. 2014).
7.5 Proteomic Analysis in Halophytes
Severe osmotic imbalance developed from excessive concentrations of salt leads to harmful modifications at various levels in cellular components (Vinocur and Altman 2005). To counter the adverse effects of salinity, halophytes respond by effective coordination between various adaptive mechanisms responsible for delivering salinity tolerance. To cope with salt stress, plants respond with increase or decrease the abundance of proteins which protects them and avoids the damaging effects of salt stress (Kumari et al. 2015). Hence, more attention has been given to halophytes which play a key role in proteomic research leading to elucidate their salt-adaptive mechanisms with the possibility of cloning genes and transferring the tissue tolerance trait to glycophytes (tobacco, rice, Arabidopsis) (Tang et al. 2011). Proteomic studies conducted in several halophytic species such as Suaeda aegyptiaca (Askari et al. 2006), Salicornia europaea (Fan et al. 2011), Cakile maritima (Debez et al. 2012), Aeluropus littoralis (Azri et al. 2016), Thellungiella halophila (Wang et al. 2013) and Halogeton glomeratus (Wang et al. 2015) have identified numerous salt-responsive proteins which fulfil a vast diversity of functions (Fig. 7.1): photosynthesis, carbohydrate and energy metabolism, cell growth and division, protein synthesis and folding, stress and defence, etc. (Zhang et al. 2012).
Photosynthesis-related proteins are differentially changed under saline conditions. RuBisCo a Calvin cycle enzyme, is implicated in the first major stage of carbon fixation process and the competing photorespiration pathway (Spreitzer and Salvucci 2002). Sengupta and Majumder (2009) found that RuBisCo (large subunit and small subunit) was upregulated in Porteresia coarctata leaves. However, RuBisCo LSU and RuBisCo SSU activity has slightly reduced in Aeluropus lagopoides (Sobhanian et al. 2010). According to Askari et al. (2006), plants need adequate photosynthetic rate to deal with salt stress. Increased CO2 assimilation was also observed in some halophytes such as Suaeda aegyptiaca and Suaeda salsa when grown under saline conditions, activating numerous photosynthetic enzymes. The preservation of adequate photosynthetic rate under such conditions in Suaeda aegyptiaca was strongly related to enhanced abundance of reaction centre proteins (D1 and D2). Impairment of energy metabolism during salt stress can be related to the high amount of needed energy by plants for their growth and development. This energy is mainly produced through glycolysis process, TCA cycle, ATP synthesis and electron transport chain (ETC) (Chitteti and Peng 2007; Du et al. 2010; Manaa et al. 2011). In a study conducted by Wang et al. (2009), several glycolytic and Krebs cycle enzymes and ATPase isoforms were found to be with higher abundance in Salicornia europaea subjected to salt. Other proteins involved in the glycolytic pathways (triosephosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase, PGK and enolase) showed enhanced relative abundance in Aeluropus lagopoides (Sobhanian et al. 2010). In addition, an increased accumulation of mitochondrial F1-ATPase beta subunit, ATP synthase CF1 alpha subunit and F1-ATPase, involved in ATP synthesis, has been reported by Wang et al. (2008a) in Physcomitrella patens in response to salt stress. According to Zhang et al. (2013), the control of ATP metabolism is an adaptive strategy to deal with high salinity.
Under salt stress, reactive oxygen species (ROS) are abundantly accumulated (Polle 2001), as a result of osmotic stress and nutrient imbalance (Munns and Tester 2008). An excessive generation of ROS may exceed the plant antioxidant defence system, resulting in oxidative stress that may disturb cell homeostasis and, consequently, may be implicated in programmed cell death activation (Dat et al. 2000). An efficient antioxidant defence machinery involving enzymatic and non-enzymatic systems has found to appear at a high level to scavenge ROS and to adapt to high salt levels. According to Xiong and Zhu (2002), the capacity to reduce oxidative damage seems to be an adaptative trait to enhance stress tolerance. Proteomic studies on several salt-responsive species (Solanum chilense, canola and S. europaea) showed a higher abundance of SOD in response to salt stress (Wang et al. 2009; Zhou et al. 2011), revealing its crucial role in defence response. Increased SOD levels were also noticed in Tangut nitraria (Cheng et al. 2015). However, its activity was induced in Puccinellia tenuiflora treated with 50 mM NaCl but significantly diminished when exposed to 150 mM NaCl (Yu et al. 2011). Suaeda aegyptiaca plants exposed to salt treatment, levels of antioxidant enzymes such as cytosolic isoform of Cu/Zn-SOD, GPX, quinone oxidoreductase, stromal isoform of APX as well as enzyme cyanase involved in degradation of cyanide ions were induced (Askari et al. 2006). Different studies have indicated that the salinity tolerance is strongly related to the antioxidative defence activity (Abogadallah 2010; Gupta and Huang 2014). Moreover, to avoid the risk of protein misfolding or unfolding which may result in non-functional proteins, cells produce proteins with chaperone functions such as chaperones, like heat-shock proteins (HSPs), as well as cytosolic, chloroplastic and mitochondrial chaperonins (Kosová et al. 2011; Kumari et al. 2015). The effect of salinity on HPS70 which are involved in various cellular processes was well discussed by Cheng et al. (2015) and Sobhanian et al. (2010). Changes in proteins involved in signal transduction were also observed under adverse conditions, including salinity (Zhang et al. 2013). These comprise receptors situated in the plasma membrane (PM) or in the cytoplasm, G protein, calcium-sensing proteins and phosphoproteins involving activation of kinase cascade (Ghosh and Xu 2014). Different families of Ca2+ signalling-related proteins were identified in plants, such as calmodulin (CaM) and calreticulin (CRT) (Cheng et al. 2009; Li et al. 2010) which were found to be upregulated by salt stress. The key role of calmodulins (CML) in the transduction of stress-response signals is reported in many studies. Increased tolerance to water shortage and salinity conditions was observed in the transgenic Arabidopsis expressing rice CML (OsMSR2) (Xu et al. 2011). The calreticulin (CRT), another Ca2+- binding protein, which is involved in calcium signaling in the endoplasmic reticulum (Qiu et al. 2012), is differentially accumulated in response to salt stress (Jiang et al. 2007b; Aghaei et al. 2008). Moreover, increased number of 14-3-3 proteins was often observed in plants exposed to high salinity, such as GF14a and GF14b in rice (Malakshah et al. 2007), 14-3-3-like protein A in wheat (Wang et al. 2008b) and 14-3-3 proteins in sugar beet (Yang et al. 2012). These proteins may play a key role in plant development (Roberts 2003) since they are known as positive regulators of the electrochemical gradient across the plasma membrane (Denison et al. 2011).
7.6 Recovery Aptitude in Extremophile Plants Dictates Survival
Recovery aptitude after salt/water stress may be strongly related to the severity and duration of applied stress. Under continuous drought conditions, yield loss is unavoidable (Chen et al. 2016). In their natural biotopes, crops are exposed to continuous cycles of drought and rehydration (Perrone et al. 2012). After stress release, plants require to resume rapidly their growth. The recovery phase is very complex that linked the readjustment of distinct processes to repair detrimental effects caused by water deficit stress leading to plant growth restoration (Chen et al. 2016) (Fig. 7.1). Generally, plants exposed to moderate stress quickly re-establish after water deficit stress release as compared to plants subjected to acute dehydration. In the latter, only 40–60% of the maximum of photosynthetic process is restored 1 day following stress release, and photosynthesis re-establishment endures few days, without always recovering maximum levels (Grzesiak et al. 2006; Gallé et al. 2007). Rewatering (for 10 days) of Populus euphratica, subjected previously to drought stress, was associated with increased abundance of some photosynthetic-related proteins, such as RuBisCo activase and proteins of the water-splitting complex (Bogeat-Triboulot et al. 2007). Hence, as soon as water shortage is relieved, plants start the stress-release cycle that can be distinguished by an active adjustment of proteome profile. For example, increased abundance of actin isoform B was found in different parts of soybean seedlings (leaf, hypocotyl and root) exposed to water deficit stress and following stress release (Mohammadi et al. 2012), indicating that actin may play a critical role in repairing injured membranes. Similarly, accumulation of proteins related to lignin biosynthesis, an important component of plant cell wall, was usually described under drought conditions. Modification in the cell wall is known to maintain cell osmotic balance and protective membrane integrity, which is of great importance to plant drought stress adaptability (Ghosh and Xu 2014). Among these proteins, caffeoyl-CoA 3-O-methyl-transferases and class III plant peroxidases showed enhanced abundance in roots of wild watermelon and maize plants (Yoshimura et al. 2008; Degenhardt and Gimmler 2000), during rewatering conditions.
Contrary to drought stress, few studies are trying to evaluate plant response to recovery phase after salt stress mitigation in the root environment, while the soil salinity has been considered a transient condition (Amzallag 1997; Pardossi et al. 1998). As for water limitation, modification in the cell wall was also observed following recovery from salt stress (Fig. 7.1).
Different cytoskeleton-associated proteins were commonly altered during water deficit stress and rewatering conditions, such as actin (Xu et al. 2010; Cao et al. 2017), profilin (Cao et al. 2017), tubulin (Peng et al. 2009; Pang et al. 2010) and other proteins associated with cytoskeleton dynamics. These alterations were found to be strongly related with other physiological responses, such as morphological response. In fact, the downregulation of profilin in Amygdalus mira roots resulted in a significant reduction of filamentous actin number leading to actin disorganization. These modifications were concomitant with the morphological aspect of Amygdalus mira roots which became shrivelled and brown (Cao et al. 2017).
7.7 Conclusion
Both salinity and drought are considered among the primary causes of plant loss worldwide. This review provides information of plant responses to salinity and water limitation at a proteomic level leading to elucidate adaptive mechanisms of salinity and drought tolerance in halophytes and resurrection plants. It seems that proteins may play a critical role in making these plants tolerant by minimizing ionic and osmotic effects. We can conclude that generally under abiotic stresses such as salinity and dehydration, proteins and metabolites related to photosynthesis are downregulated in tolerant species. Activation of various defence mechanisms together with upregulation of energy metabolism-related proteins and accumulation of high levels of osmoprotective compounds was often observed in these plants. At the recovery phase, rearrangement of proteome repertoire was found following salinity and drought stress, thus leading to repair injuries caused by drought and salinity. Future investigations on plant recovery aptitude are expected to improve our understanding of plasticity, enabling halophytes and resurrection plants to tolerate abiotic constraints to which they are exposed.
References
Abogadallah GM (2010) Insights into the significance of antioxidative defense under salt stress. Plant Signal Behav 5:369–374
Abreu IA, Farinha AP, Negrão S, Gonçalves N, Fonseca C, Rodrigues M, Batista R, Nelson JM, Saibo M, Oliveira MM (2013) Coping with abiotic stress: proteome changes for crop improvement. J Proteome 93:145–168
Aghaei K, Ehsanpour AA, Komatsu S (2008) Proteome analysis of potato under salt stress. J Proteome Res 7:4858–4868
Alamillo JM, Bartels D (1996) Light and stage of development influence the expression of desiccation-induced genes in the resurrection plant Craterostigma plantagineum. Plant Cell Environ 19:300–310
Amzallag GN (1997) Influence of periodic fluctuation in root environment on adaptation to salinity in Sorghum bicolor. Funct Plant Biol 24:579–586
Ashraf MPJC, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Ashraf M, Athar HR, Harris PJC, Kwon TR (2008) Some prospective strategies for improving crop salt tolerance. Adv Agron 97:45–110
Askari H, Edqvist J, Hajheidari M, Kafi M, Salekdeh GH (2006) Effects of salinity levels on proteome of Suaeda aegyptiaca leaves. Proteomics 6:2542–2554
Athar HR, Ashraf M (2009) Strategies for crop improvement against salinity and drought stress: an overview. In: Ashraf M, Öztürk M, Athar HR (eds) Salinity and water stress: improving crop efficiency. Springer, New York, pp 1–16
Atteya AM (2003) Alteration of water relations and yield of corn genotypes in response to drought stress. Bulg J Plant Physiol 29:63–76
Azri W, Barhoumi Z, Chibani F, Borji M, Bessrour M, Mliki A (2016) Proteomic responses in shoots of the facultative halophyte Aeluropus littoralis (Poaceae) under NaCl salt stress. Funct Plant Biol 43:1028–1047
Baker J, Steele C, Dure L (1988) Sequence and characterization of 6 Lea proteins and their genes from cotton. Plant Mol Biol 11:277–291
Barnabas B, Jagner K, Feher A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta Biomembr 1465(1–2):140–151
Bogeat-Triboulot MB, Brosché M, Renaut J, Jouve L, Le Thiec D, Fayyaz P, Vinocur B, Witters E, Laukens K, Teichmann T, Altman A, Hausman J-F, Polle A, Kangasjärvi J, Dreyer E (2007) Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol 143:876–892
Cao Y, Luo Q, Tian Y, Meng F (2017) Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yü et Lu roots. BMC Plant Biol 17(1)
Chakrabortee S, Boschetti C, Walton LJ, Sarkar S, Rubinsztein DC, Tunnacliffe A (2007) Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. Proc Natl Acad Sci 104:18073–18078
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot-Lond 103:551–560
Chen D, Wang S, Cao B, Cao D, Leng G, Li H, Yin L, Shan L, Deng X (2016) Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front Plant Sci 6:1241
Cheng Y, Qi Y, Zhu Q, Chen X, Wang N, Zhao X, Chen H, Cui X, Xu L, Zhang W (2009) New changes in the plasma-membrane-associated proteome of rice roots under salt stress. Proteomics 9:3100–3114
Cheng T, Chen J, Zhang J, Shi S, Zhou Y, Lu L, Wang P, Jiang Z, Yang J, Yang J, Zhang S, Shi J (2015) Physiological and proteomic analyses of leaves from the halophyte Tangut Nitraria reveals diverse response pathways critical for high salinity tolerance. Front Plant Sci 6:30
Chitteti BR, Peng Z (2007) Proteome and phosphoproteome differential expression under salinity stress in rice (Oryza sativa) roots. J Proteome Res 6:1718–1727
Cornic G (2000) Drought stress inhibits photosynthesis by decreasing stomatal aperture–not by affecting ATP synthesis. Trends Plant Sci 5:187–188
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
de Lacerda CF, Cambraia J, Oliva MA, Ruiz HA (2005) Changes in growth and in solute concentrations in sorghum leaves and roots during salt stress recovery. Environ Exp Bot 54:69–76
Debez A, Braun HP, Pich A, Taamalli W, Koyro HW, Abdelly C, Huchzermeyer B (2012) Proteomic and physiological responses of the halophyte Cakile maritima to moderate salinity at the germinative and vegetative stages. J Proteome 75:5667–5694
Degenhardt B, Gimmler H (2000) Cell wall adaptations to multiple environmental stresses in maize roots. J Exp Bot 51:595–603
Denison FC, Paul AL, Zupanska AK, Ferl RJ (2011) 14-3-3 proteins in plant physiology. Semin Cell Dev Biol 22:720–727
Dinakar C, Bartels D (2013) Desiccation tolerance in resurrection plants: new insights from transcriptome, proteome, and metabolome analysis. Front Plant Sci 4:482
Du CX, Fan HF, Guo SR, Tezuka T, Li J (2010) Proteomic analysis of cucumber seedling roots subjected to salt stress. Phytochemistry 71:1450–1459
Epstein E, Rains DW (1987) Advances in salt tolerance. In: Genetic aspects of plant mineral nutrition. Springer, Dordrecht, pp 113–125
Fan P, Feng J, Jiang P, Chen X, Bao H, Nie L, Jiang D, Lv S, Kuang T, Li Y (2011) Coordination of carbon fixation and nitrogen metabolism in Salicornia europaea under salinity: comparative proteomic analysis on chloroplast proteins. Proteomics 11:4346–4367
Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72:673–689
Fernandez-Garcia N, Hernandez M, Casado-Vela J, Bru R, Elortza F, Hedden P, Olmos E (2011) Changes to the proteome and targeted metabolites of xylem sap in Brassica oleracea in response to salt stress. Plant Cell Environ 34:821–836
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Gallé A, Haldimann P, Feller U (2007) Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol 174:799–810
Ghosh D, Xu J (2014) Abiotic stress responses in plant roots: a proteomics perspective. Front Plant Sci 5:6
Goday A, Sánchez-Martínez D, Gómez J, Puigdomènech P, Pagès M (1988) Gene expression in developing Zea mays embryos: regulation by abscisic acid of a highly phosphorylated 23-to 25-kD group of proteins. Plant Physiol 88:564–569
Griffiths CA, Gaff DF, Neale AD (2014) Drying without senescence in resurrection plants. Front Plant Sci 5:36
Grzesiak MT, Grzesiak S, Skoczowski A (2006) Changes of leaf water potential and gas exchange during and after drought in triticale and maize genotypes differing in drought tolerance. Photosynthetica 44:561–568
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 2014. Article ID 701596
Himabindu Y, Chakradhar T, Reddy MC, Kanygin A, Redding KE, Chandrasekhar T (2016) Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ Exp Bot 124:39–63
Höfler K, Migsch H, Rottenburg W (1941) Über die Austrocknungresistenz landwirtschaftlicher Kulturpflanzen. Forschungsdienst 12:50–61
Ingle R, Schmidt U, Farrant J, Thomson J, Mundree S (2007) Proteomic analysis of leaf proteins during dehydration of the resurrection plant Xerophyta viscosa. Plant Cell Environ 30:435–446
Jiang G, Wang Z, Shang H, Yang W, Hu Z, Phillips J, Deng X (2007a) Proteome analysis of leaves from the resurrection plant Boea hygrometrica in response to dehydration and rehydration. Planta 225:1405
Jiang Y, Yang B, Harris NS, Deyholos MK (2007b) Comparative proteomic analysis of NaCl stress responsive proteins in Arabidopsis roots. J Exp Bot 58:3591–3607
Kosová K, Vítámvás P, Prášil IT, Renaut J (2011) Plant proteome changes under abiotic stress–contribution of proteomics studies to understanding plant stress response. J Proteome 74:1301–1322
Kumari A, Das P, Parida AK, Agarwal PK (2015) Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front Plant Sci 6:537
Lafitte HR, Yongsheng G, Yan S, Li ZK (2007) Whole plant responses, key processes, and adaptation to drought stress: the case of rice. J Exp Bot 58:169–175
Li XJ, Yang MF, Chen H, Qu LQ, Chen F, Shen SH (2010) Abscisic acid pretreatment enhances salt tolerance of rice seedlings: proteomic evidence. Biochim Biophysi Acta (BBA)-Proteins Proteomics 1804:929–940
Malakshah SN, Rezaei MH, Heidari M, Salekdeh GH (2007) Proteomics reveals new salt responsive proteins associated with rice plasma membrane. Biosci Biotechnol Biochem 71:2144–2154
Manaa A, Ben Ahmed H, Valot B, Bouchet JP, Aschi-Smiti S, Causse M, Faurobert M (2011) Salt and genotype impact on plant physiology and root proteome variations in tomato. J Exp Bot 62:2797–2813
Michel D, Furini A, Salamini F, Bartels D (1994) Structure and regulation of an ABA- and desiccation-responsive gene from the resurrection plant Craterostigma plantagineum. Plant Mol Biol 24(4):549–560
Mohammadi PP, Moieni A, Hiraga S, Komatsu S (2012) Organ-specific proteomic analysis of drought-stressed soybean seedlings. J Proteome 75:1906–1923
Moradi P, Ford-Lloyd B, Pritchard J (2018) Metabolic responses of Thymus vulgaris to water deficit stress. Curr Metabolomics 6:64–74
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Ndima T, Farrant J, Thomson J, Mundree S (2001) Molecular characterization of XVT8, a stress-responsive gene from the resurrection plant Xerophyta viscosa Baker. Plant Growth Regul 35:137–145
Oliver MJ, Jain R, Balbuena TS, Agrawal G, Gasulla F, Thelen JJ (2011) Proteome analysis of leaves of the desiccation-tolerant grass, Sporobolus stapfianus, in response to dehydration. Phytochemistry 72:1273–1284
Pang Q, Chen S, Dai S, Chen Y, Wang Y, Yan X (2010) Comparative proteomics of salt tolerance in Arabidopsis thaliana and Thellungiella halophila. J Proteome Res 9:2584–2599
Pardossi A, Malorgio F, Oriolo D, Gucci R, Serra G, Tognoni F (1998) Water relations and osmotic adjustment in Apium graveolens during long-term NaCl stress and subsequent relief. Physiol Plant 102:369–376
Peng Z, Wang M, Li F, Lv H, Li C, Xia G (2009) A proteomic study of the response to salinity and drought stress in an introgression strain of bread wheat. Mol Cell Proteomics 8:2676–2686
Perrone I, Pagliarani C, Lovisolo C, Chitarra W, Roman F, Schubert A (2012) Recovery from water stress affects grape leaf petiole transcriptome. Planta 235:1383–1396
Pitman MG, Lauchli A (2002) Global impact of salinity and agricultural ecosystems. In: Lauchli A, Luttge U (eds) Salinity: environmental, plants, molecules. Springer, Dordrecht, pp 3–20
Polle A (2001) Dissecting the superoxide dismutase–ascorbate peroxidase–glutathione pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol 126:445–462
Qiu Y, Xi J, Du L, Poovaiah BW (2012) The function of calreticulin in plant immunity: new discoveries for an old protein. Plant Signal Behav 7:907–910
Rehman S, Harris PJC, Ashraf M (2005) Stress environments and their impact on crop production. In: Ashraf M, Harris PJC (eds) Abiotic stresses: plant resistance through breeding and molecular approaches. Haworth Press, New York, pp 3–18
Roberts MR (2003) 14-3-3 proteins find new partners in plant cell signalling. Trends Plant Sci 8:218–223
Rodziewicz P, Swarcewicz B, Chmielewska K, Wojakowska A, Stobiecki M (2014) Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol Plant 36:1–19
Röhrig H, Schmidt J, Colby T, Bräutigam A, Hufnagel P, Bartels D (2006) Desiccation of the resurrection plant Craterostigma plantagineum induces dynamic changes in protein phosphorylation. Plant Cell Environ 29:1606–1617
Sengupta S, Majumder AL (2009) Insight into the salt tolerance factors of a wild halophytic rice, Porteresia coarctata: a physiological and proteomic approach. Planta 229:911–929
Shabala SN, Mackay AS (2011) Ion transport in halophytes. Adv Bot Res 57:151–187
Sobhanian H, Motamed N, Jazii FR, Nakamura T, Komatsu S (2010) Salt stress induced differential proteome and metabolome response in the shoots of Aeluropus lagopoides (Poaceae), a halophyte C4 plant. J Proteome Res 9:2882–2897
Sobhanian H, Aghaei K, Komatsu S (2011) Changes in the plant proteome resulting from salt stress: toward the creation of salt-tolerant crops? J Proteome 74:1323–1337
Spreitzer RJ, Salvucci ME (2002) RUBISCO: structure, regulatory interactions and possibilities for a better enzyme. Annu Rev Plant Biol 53:449–475
Tang M, Liu X, Deng H, Shen S (2011) Over-expression of JcDREB, a putative AP2/EREBP domain-containing transcription factor gene in woody biodiesel plant Jatropha curcas, enhances salt and freezing tolerance in transgenic Arabidopsis thaliana. Plant Sci 181:623–631
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16:123–132
Wang WX, Barak T, Vinocur B, Shoseyov O, Altman A (2003) Abiotic resistance and chaperones: possible physiological role of SP1, a stable and stabilizing protein from Populus. In: Plant biotechnology 2002 and beyond. Springer, Dordrecht, pp 439–443
Wang X, Fan P, Song H, Chen X, Li X, Li Y (2009) Comparative proteomic analysis of differentially expressed proteins in shoots of Salicornia europaea under different salinity. J Proteome Res 8:3331–3345
Wang X, Chen S, Zhang H, Shi L, Cao F, Guo L, Xie Y, Wang T, Yan X, Dai S (2010) Desiccation tolerance mechanism in resurrection fern-ally Selaginella tamariscina revealed by physiological and proteomic analysis. J Proteome Res 9:6561–6577
Wang X, Chang L, Wang B, Wang D, Li P, Wang L, Yi X, Huang Q, Peng M, Guo A (2013) Comparative proteomics of Thellungiella halophila leaves from plants subjected to salinity reveals the importance of chloroplastic starch and soluble sugars in halophyte salt tolerance. Mol Cell Proteomics 12:2174–2195
Wang J, Meng Y, Li B, Ma X, Lai Y, Si E, Yang K, Xu X, Shang X, Wang H, Wang D (2015) Physiological and proteomic analyses of salt stress response in the halophyte Halogeton glomeratus. Plant Cell Environ 38:655–669
Wang X, Yang P, Gao Q, Liu X, Kuang T, Shen S, He Y (2008a) Proteomic analysis of the response to high-salinity stress in Physcomitrella Patens. Planta 228:167–177
Wang MC, Peng ZY, Li CL, Li F, Liu C, Xia GM (2008b) Proteomic analysis on a high salt tolerance introgression strain of Triticum aestivum/Thinopyrum ponticum. Proteomics 8:1470–1489
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25:131–139
Xu C, Sibicky T, Huang B (2010) Protein profile analysis of salt-responsive proteins in leaves and roots in two cultivars of creeping bentgrass differing in salinity tolerance. Plant Cell Rep 29:595–615
Xu GY, Rocha PSCF, Wang ML, Xu ML, Cui YC, Li LY, Zhu YX, Xia X (2011) A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 234:47–59
Yang L, Ma C, Wang L, Chen S, Li H (2012) Salt stress induced proteome and transcriptome changes in sugar beet monosomic addition line M14. J Plant Physiol 169:839–850
Yoshimura K, Masuda A, Kuwano M, Yokota A, Akashi K (2008) Programmed proteome response for drought avoidance/tolerance in the root of a C3 xerophyte (wild watermelon) under water deficits. Plant Cell Physiol 49:226–241
Yu J, Chen S, Zhao Q, Wang T, Yang C, Diaz C, Sun G, Dai S (2011) Physiological and proteomic analysis of salinity tolerance in Puccinellia tenuiflora. J Proteome Res 10:3852–3870
Zhang H, Han B, Wang T, Chen SX, Li HY (2012) Mechanisms of plant salt response: insights from proteomics. J Proteome Res 11:49–67
Zhang Y, Fonslow BR, Shan B, Baek MC, Yates IIIJR (2013) Protein analysis by shotgun/bottom-up proteomics. Chem Rev 113:2343–2394
Zhou S, Sauvé RJ, Liu Z, Reddy S, Bhatti S, Hucko SD, Fish T, Thannhauser TW (2011) Identification of salt-induced changes in leaf and root proteomes of the wild tomato, Solanum chilense. J Am Soc Hortic Sci 136:288–302
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Farhat, N., Debez, A. (2019). Molecular Mechanisms of Osmotic Stress Recovery in Extremophile Plants: What Can We Learn from Proteomics?. In: Hasanuzzaman, M., Nahar, K., Öztürk , M. (eds) Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes. Springer, Singapore. https://doi.org/10.1007/978-981-13-3762-8_7
Download citation
DOI: https://doi.org/10.1007/978-981-13-3762-8_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3761-1
Online ISBN: 978-981-13-3762-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)