Abstract
As of 2017, about 200 complex organic molecules have been detected in interstellar molecular clouds. It was 1969 when the first organic molecule in space, H2CO, was discovered. Since then many organic molecules were discovered by using the NRAO 11 m (upgraded later to 12 m), Nobeyama 45 m, IRAM 30 m, and other highly sensitive radio telescopes as a result of close collaboration between radio astronomers and microwave spectroscopists. It is noteworthy that many well-known organic molecules such as CH3OH, C2H5OH, (CH3)2O, and CH3NH2 were detected in the 1970s. It is thought that organic molecules are formed on surfaces of cold dust particles in a molecular cloud and then are evaporated by the UV photons emitted from a star inside the molecular cloud.
Organic molecules are known to exist in star-forming regions and in protoplanetary disks where planets are formed. Therefore it was a natural consequence that astronomers considered a relationship between organic molecules in space and the origin of life. Several astronomers challenged to detect glycine and other prebiotic molecules without success. ALMA is expected to detect such important materials to further examine the “exogenous delivery” hypothesis of organic molecules.
In this chapter I summarize the history of the searches for complex organic molecules in space together with difficulties in observing very weak signals from larger molecular species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
It is well-known that our solar system was formed from its natal molecular cloud 4.6 billion years ago. After the formation of Earth and other planets in our solar system, these planets underwent frequent impacts by smaller bodies for several hundred million years (so-called late heavy bombardment). Though life on Earth may have emerged during or shortly after or before this heavy bombardment phase, as early as about 3.9 billion years ago, discussions are continuing on its exact timing. In 2017 a paper was published that claims a discovery of early trace of life in sedimentary rocks at 3.95 billion years ago (Tashiro et al. 2017; see also Chap. 15). It means that life on Earth existed about 4 billion years ago, i.e., only 600 million years after the formation of Earth.
It is generally accepted that origin of life was the result of chemical evolution of organic molecules on the primordial Earth. Thus, an important issue in astrobiology is where prebiotic organic molecules are formed, terrestrial or extraterrestrial. After the famous Urey-Miller’s experiment, many researchers believed that the primary formation site of prebiotic organic molecules was the surface of Earth under reducing atmosphere (see Chap. 4). Recent modelling of the Earth’s early atmosphere suggests more neutral conditions which preclude the formation of significant amount of prebiotic organic molecules. The situation, in turn, lead people to consider another possibility: delivery of extraterrestrial prebiotic organic molecules through comets, asteroids, meteorites, and interplanetary dust particles (the exogenous delivery hypothesis). One research suggested that extraterrestrial organic compounds may be more abundant by three orders of magnitude than their terrestrial formation (Ehrenfreund et al. 2002). It is likely that a combination of these sources contributed to the building blocks of life on the early Earth (see Fig. 2.1). What is certain is that once life emerged on the primordial Earth, it was capable to adapt quickly to the surrounding environment for its survival through finding shelter from the UV photons and energy source. This continuing process led to complex metabolic life and even our own existence.
A schematic diagram on the origin of prebiotic organic molecules. (Courtesy by Dr. Amie Elsila (Deamer et al. 2002))
In this chapter, I will review prebiotic organic molecules in space, which may be brought to the early Earth. If the exogenous delivery hypothesis works, similar process would occur even in other extrasolar Earth-like planets.
2 Molecules in Space
2.1 Molecules Observed in Space
Molecules exist in a variety of physical conditions in the Universe. It was in 1940 when unidentified ultraviolet (UV) absorption lines were attributed to CH and CN in diffuse interstellar clouds (McKellar 1940). This was the first report of molecules in space.
In the early 1930s, radio astronomy opened new eyes to the universe. In 1963 the OH molecule was discovered by a radio telescope (Weinreb et al. 1963). As of December 2017, some 200 molecules were detected or reported, primarily by means of radio astronomical observations, in interstellar clouds, circumstellar envelopes, and even external galaxies. The smallest and the lightest molecule is H2. A list of molecules in space can be found in, e.g., the Cologne Database for Molecular Spectroscopy (2001).
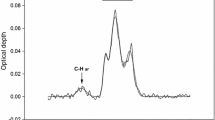
1969 was the year when the first organic polyatomic molecule, H2CO, was detected toward many galactic and extragalactic sources. Prior to the detection of H2CO, the first “large” millimeter-wave telescope, the 11 m radio telescope of the National Radio Astronomy Observatory (NRAO) in the USA, started its operation in 1968. The NRAO 11 m radio telescope was one of pioneering telescopes (later, this telescope was upgraded to 12 m); it discovered CO, CH3CN, HCN, HNCO, (CH3)2O, C2H, C2H5OH, NH2CN, C2H5CN, H2CCO, C3N, and HNCS in the 1970s. It was in the 1970s when other countries joined the “molecular hunting.” Australia used the Parkes 64 m radio telescope and detected H2CS, CH2NH, CH2CHCN, and HCOOCH3. Japan constructed the 6 m millimeter-wave telescope and succeeded in discovering CH3NH2. Detailed detection history can be found in the reference (Ohishi 2016). Spectral line surveys are powerful means in detecting new molecules in space. Figure 2.2 shows an example of a spectral line survey observation toward a cold, dark molecular cloud, Taurus Molecular Cloud 1 (TMC-1) conducted by using the 45 m radio telescope at the Nobeyama Radio Observatory, the National Astronomical Observatory of Japan (Kaifu et al. 2004). We are able to see clear spectral patterns by linear molecules (with equal frequency intervals) such as HC3N, HC5N, and HC7N, in Fig. 2.2. The NAOJ team succeeded in detecting 17 new molecules in space through the spectral line survey observations.
Observed spectrum from 8.8 to 50.0 GHz toward TMC-1 by the Nobeyama 45 m radio telescope. The ordinate, the antenna temperature, is usually used in radio astronomy for expressing energy of received radio waves; it is known that the Boltzmann constant (k) times temperature (T) is equal to energy. The frequency resolution was 37 kHz, and the total number of data bins is 2 million. (This figure is modified from Fig. 1 in Ohishi (2016)). A part of identified molecular species are shown with their chemical formulas. The horizontal bars with the quantum number J, which denotes the total angular momentum of the molecular rotations, show spectral patterns by linear molecules (equal frequency intervals) such as HC3N, HC5N, and HC7N
It should be stressed that many organic molecules were already detected in the 1970s. It is well-known that CH3OH is widespread in a variety of sources; CH3OH masers are commonly observed toward young star-forming regions. Large organic molecules, such as CH3CN, CH3CHO, (CH3)2O, and NH2CHO, are usually observed toward dense and hot regions with the number density of molecular hydrogen (n(H2)) >107–8 cm−3 and the gas kinetic temperature >200 K.
2.2 Classification of Molecules in Space
Molecules in space can be classified into several categories: simple molecules, molecular ions, radicals, ring molecules, and stable molecules. Simple molecules can be seen in a variety of sources. Among them, the most abundant molecule is H2. Indeed, around 99.99% of molecules in space are H2. The next abundant molecules are H2O and CO whose relative abundances to H2 are about 10−4. H2O, CO, and CO2 are ubiquitous molecules in the solid phase (Herbst and van Dishoeck 2009); these molecules in dust mantles are often observed in absorption toward bright infrared (IR) sources. Molecular ions and radicals are characteristic for molecules in space (Herbst and van Dishoeck 2009). Under the terrestrial physical conditions where the mean free time of these molecules is the order of milliseconds, such molecular ions or radicals react with other atom or molecule and are converted into other species immediately after they are formed. Thus, they are often called as “short-lifetime” molecules. However, under the interstellar and circumstellar conditions where the mean free time is typically of the order of a year, such “short-lifetime” molecules can survive for a long time. It is now known that molecular ions play an important role in the gas phase where “ion-molecule” reactions can successfully explain the formation of many major molecules in space (Herbst and van Dishoeck 2009).
Large “complex organic molecules” with six or more atoms (hereafter COMs; Herbst and van Dishoeck 2009), including prebiotic molecules, are the primary topic of this chapter; they belong to “stable molecules.” Many of large COMs are closed-shell molecules, such as CH3OH and C2H5OH, which exist stably under the standard terrestrial conditions. Past studies showed that many of large organic species are formed on cold (10–15 K) dust grains through the hydrogenation (addition of hydrogen atoms) to small molecules which are adsorbed from the gas phase to the surface of the dust grains (Herbst and van Dishoeck 2009). For example, gas-phase CO is adsorbed onto dust grains. Since the hydrogen atoms on the dust surface can move quickly through the “tunneling effect,” CO is converted to H2CO and ultimately to CH3OH (Herbst and van Dishoeck 2009). When a star is formed in the center of a molecular cloud core, the solid-phase CH3OH molecules may be heated by the UV photons from the central star and evaporated back to the gas phase; such gas-phase large COMs can be observed by telescopes. Since prebiotic molecules are categorized into COMs, a large number of prebiotic molecules are thought to be formed on the surfaces of dust particles (Herbst and van Dishoeck 2009).
3 Prebiotic Organic Molecules in Space
3.1 Why Prebiotic Organic Molecules Are Made of H, C, N, and O?
In Sect. 2.2.1, I described that many organic molecules in space have been known since 1970s, and there are a variety of molecules which can be related to “life.” We know that the building blocks of life contain primarily H, C, N, and O. There would be a natural reasoning why molecules containing these four elements are abundant in space.
The cosmic elemental abundances are well-known in astronomy (see Table 2.1). It is clear that H, C, N, and O are the four most abundant elements in the Universe, except for He. This is the primary reason why water (H2O) in space is the second most abundant molecule next to H2 (see Sect. 2.2.2). With a similar consideration, it would be easily understood why carbon-bearing species, i.e., organic molecules, can be abundant in space. Helium is not contained in building blocks of life, which is chemically inactive.
3.2 Prebiotic Organic Molecules of the Greatest Importance in Space
In this subchapter, I will show a few prebiotic molecules discovered in space, which would have the greatest importance to astrobiology. Other prebiotic molecules are also important.
Glycolaldehyde (CH2OHCHO)
An epoch-making event in astrobiology was the discovery of glycolaldehyde in 2000 toward Sagittarius B2 (Sgr B2), a giant molecular cloud in the central region of the Milky Way galaxy (Hollis et al. 2000). The paper was titled “Interstellar Glycolaldehyde: The First Sugar.” In general sugar is an important constituent of life: ribose (C5H10O5) is a pentose class sugar monomer, and its modified form, deoxyribose, is known to be the backbone of the DNA. The first detection report was based on observed data by using the NRAO 12 m, with relatively poor signal-to-noise ratios. In 2004 a confirmation paper was published by using a new 100 m radio telescope, the Green Bank Telescope (GBT) (Hollis et al. 2004). Glycolaldehyde was detected around a solar-type young star, IRAS16293-2422, through Atacama Large Millimeter Array (ALMA) observations (Jørgensen et al. 2012). It was suggested that UV photochemistry of a CH3OH-CO mixed ice on dust surfaces, which has undergone mild heating, would form glycolaldehyde. Followed by these detections, glycolaldehyde has been reported detected in other massive star-forming regions and solar-type star-forming regions.
According to the detection report (Hollis et al. 2000), the generic form of sugar can be expressed as (H2CO)n (n > 2). Because glycolaldehyde has the form of (H2CO)2, it was regarded as the simplest sugar in space. This discovery had led astronomers to seriously search for other “prebiotic molecules” in space. It was unfortunate that the correct generic form of sugar is (H2CO)n (n > 3), not 2!, i.e., glycolaldehyde is not a sugar.
n- and i-Propyl Cyanide (n- and i-C3H7CN)
It is known that meteorites found on Earth contain a variety of COMs including more than 80 amino acids (Elsila et al. 2007). Since meteorites are formed in protoplanetary nebula which are formed in interstellar medium, the amino acids and their precursors would have an interstellar origin. In this regard, it is crucial for astrobiology to understand how complex organic species can be formed in the interstellar medium.
In 2009 the first detection of normal-propyl cyanide (n-C3H7CN), the largest organic molecule in this source, was reported toward Sgr B2 by using the 30 m radio telescope operated by the Institut de Radioastronomie Millimétrique (IRAM) (Belloche et al. 2009). Propyl cyanide is the smallest alkyl cyanide, which has a few isomers: a straight-chain isomer, normal-propyl cyanide (also known as butyronitrile or 1-cyanopropane), and a branched-chain isomer, iso-propyl cyanide (i-C3H7CN, also known as iso-butyronitrile or 2-cyanopropane). Five years after the detection of n-C3H7CN, i-C3H7CN was detected for the first time toward SgrB2 by using ALMA (Belloche et al. 2014). Amazingly the abundance of i-C3H7CN was 40% of n-C3H7CN, suggesting that branched carbon-chain molecules may be generally abundant in the interstellar medium. The detection of i-C3H7CN further suggests the presence of amino acids in the interstellar medium, for which such branched-chain structure is a key characteristic.
Propylene Oxide (CH3CHCH2O)
The origin of homochirality in biological molecules, especially the use of only the L-amino acids and D-sugars, is a long-standing question to be solved in astrobiology. A model for explaining the origin of homochirality involving extraterrestrial sources of circularly polarized light (CPL) was first proposed by Rubenstein et al. (1983). The proposed scenario is as follows: (1) circularly polarized UV photons from nearby main-sequence star(s) penetrated into the natal molecular cloud that formed our solar system, (2) the photons acted on chiral molecules in the natal cloud, and resulted in an excess of one enantiomer, and (3) the excessing enantiomer was amplified until the other enantiomer had been disappeared. Since it is well-known that CPL is actually observed in the interstellar space and it has been observed in laboratories that the CPL causes an excess of one enantiomer, the scenario may work if there are chiral molecules in the interstellar molecular clouds. See Bailey (2001) for further details.
In 2016 the first report was made on the detection of a chiral molecule in space (McGuire et al. 2016). Propylene oxide was detected in the gas phase in a cold, extended molecular shell around the embedded, massive proto-stellar clusters in the Sagittarius B2 star-forming region. The fact that propylene oxide is extended suggested that similar chiral molecules may exist in a variety of astronomical sources in our Milky Way galaxy. It should be noted that chiral molecular structures of propylene oxide, S-propylene oxide and R-propylene oxide, cannot be distinguished spectroscopically.
4 Challenges in Searching for Amino Acids and Nucleobases in Space
4.1 Amino Acids
Since the first trial in detecting interstellar glycine (Brown et al. 1979), many trials to detect the simplest amino acid, glycine (NH2CH2COOH), were made toward Sgr B2 and other high-mass forming regions. None of them were successful. Astronomers have been searching for glycine, because if amino acids are formed in interstellar molecular clouds, significant amount of them may be delivered to planets, which would have been used as the “seed” of life on Earth and other extrasolar planets. Thus, detection of amino acids would accelerate the discussion concerning the universality of “life.”
However, the past unsuccessful searches for glycine demanded us to reconsider the search strategy. One idea to overcome this situation is to search for sources rich in precursors to glycine prior to searching for glycine. Although the chemical evolution of interstellar N-bearing complex organic molecules is not well known, methylamine (CH3NH2) has been proposed as a promising precursor to glycine. CH3NH2 can be formed from abundant molecular species, CH4 and NH3, on icy dust surface, when the dust temperature is high (Holtom et al. 2005; Kim and Kaiser 2011). Another possible route to form CH3NH2 is hydrogenation (addition of hydrogen) to HCN on dust surface (Kim and Kaiser 2011; Dickens et al. 1997; Theule et al. 2011): HCN → CH2NH → CH3NH2. According to the laboratory study (Theule et al. 2011), the hydrogenation to HCN can form CH3NH2 even at the dust temperature 15 K. Figure 2.3 shows possible formation paths to CH3NH2, starting from well-known abundant interstellar molecular species, HCN, CH4, and NH3. Once CH3NH2 is formed on the dust surface, CH3NH2 and another abundant molecule, CO2, can form glycine under UV irradiation (Kim and Kaiser 2011). Recent sensitive observations revealed sources rich in CH2NH (Suzuki et al. 2016); some of these sources show very high abundance of CH3NH2 (Ohishi et al. 2018), which in turn may be suitable for searching for glycine in space.
One of the scientific purposes of ALMA is the detection of prebiotic molecules in molecular clouds where stars and planets are formed. Several projects have been going on toward the detection of glycine and other amino acids.
4.2 Nucleobases
A sequence of a DNA determines the corresponding sequence of amino acids forming a protein, including an enzyme; an enzyme plays an essential role to form and repair a DNA. In other words, both amino acids and nucleic acids (adenine, thymine, cytosine, guanine, and uracil) are the most important building blocks of life. Although the millimeter- and submillimeter-wave spectral lines of these nucleic acids are not known, the spectra of pyrimidine (c-C4H4N2) have already been measured in laboratories. Pyrimidine is a six-membered ring molecule, which is known to be a direct precursor to three of the DNA and RNA bases: thymine, cytosine, and uracil.
Extraterrestrial pyrimidine was reported detected in meteoritic organic matter (Stoks and Schwartz 1981) and possibly in the carbonaceous dust of Comet Halley (Krueger et al. 1991). In the past, there were two unsuccessful searches for interstellar pyrimidine. The first search was made in 1973 by the NRAO 11 m telescope toward Sgr B2 and Orion KL (Simon and Simon 1973); however, no upper limit to its column density was reported. The second search was made in 2003 by the James Clerk Maxwell Telescope in the 329–363 GHz range, with upper limits to its column density of a few × 1014 cm−2 toward Sgr B2(N), Orion KL, and W51 (Kuan et al. 2003). More sensitive searches by, e.g., ALMA will reveal the existence of precursors to nucleic acids in space in the future.
5 Conclusion
In astronomy, it is well-known that organic molecules are ubiquitous in star-forming regions where planets are formed. Most of organic molecules are thought to be formed on surfaces of dust particles which will be incorporated into planets, meteorites, comets, and other small bodies. It is known that amino acids and nucleic acids are found in a few meteorites (Stoks and Schwartz 1981). The fact that the simplest amino acid, glycine, was confirmed in the coma of comet 67P/Churyumov-Gerasimenko (Altwegg et al. 2016) would make the exogenous delivery hypothesis the most plausible scenario on the origin of prebiotic organic molecules.
New and powerful radio telescope ALMA has started its full operation. The telescope is expected to reveal the existence of amino acids and other prebiotic organic molecules in star- and planet-forming regions, which are directly related to the initial compounds of chemical evolution toward the origin of life.
References
Altwegg K, Balsiger H, Bar-Nun A et al (2016) Prebiotic chemicals – amino acid and phosphorus – in the coma of comet 67P/Churyumov-Gerasimenko. Sci Adv 2:e1600285
Bailey J (2001) Astronomical sources of circularly polarized light and the origin of homochirality. Orig Life Evol Biosph 31:167–183
Belloche A et al (2009) Increased complexity in interstellar chemistry: detection and chemical modeling of ethyl formate and n-propyl cyanide in Sagittarius B2(N). Astron Astrophys 499:215–232
Belloche A, Garrod RT, Müller HSP, Menten KM (2014) Detection of a branched alkyl molecule in the interstellar medium: iso-propyl cyanide. Science 345:1584–1587
Brown RD et al (1979) A search for interstellar glycine. Mon Not R Astron Soc 186:5P–8P
CDMS (The Cologne Database for Molecular Spectroscopy) (2001) http://www.astro.uni-koeln.de/cdms/molecules. Accessed 18 Dec 2017
Chronological Scientific Tables (Rikanenpyou) (2017) National astronomical Observatory of Japan and Maruzen
Deamer DW, Dworkin JP, Sandford SA, Bernstein MP, Allamandola LJ (2002) The first cell membranes. Astrobiology 2:371–381
Dickens JE et al (1997) Hydrogenation of interstellar molecules: a survey for methylenimine (CH2NH). Astrophys J 479:307–312
Ehrenfreund P et al (2002) Astrophysical and astrochemical insights into the origin of life. Rep Prog Phys 65:1427–1487
Elsila JE, Dworkin JP, Bernstein MP, Martin MP, Sandford SA (2007) Mechanisms of amino acid formation in interstellar ice analogs. Astrophys J 660:911–918
Herbst E, van Dishoeck E (2009) Complex organic interstellar molecules. Annu Rev Astron Astrophys 47:427–480
Hollis JM, Lovas FJ, Jewell PR (2000) Interstellar glycolaldehyde: the first sugar. Astrophys J 540:L107–L110
Hollis JM, Jewell PR, Lovas FJ, Remijan A (2004) Green bank telescope observations of interstellar glycolaldehyde: low-temperature sugar. Astrophys J 613:L45–L48
Holtom PD et al (2005) A combined experimental and theoretical study on the formation of the amino acid glycine (NH2CH2COOH) and its isomer (CH3NHCOOH) in extraterrestrial ices. Astrophys J 626:940–952
Jørgensen JK et al (2012) Detection of the simplest sugar, Glycolaldehyde, in a solar-type protostar with ALMA. Astrophys J 757:L4 (6 pp.)
Kaifu N et al (2004) A 8.8–50GHz complete spectral line survey toward TMC-1 I. Survey data. Publ Astron Soc Jpn 56:69–173
Kim YS, Kaiser RI (2011) On the formation of amines (RNH2) and the cyanide anion (CN−) in electron-irradiated ammonia-hydrocarbon interstellar model ices. Astrophys J 729:68
Krueger FR, Korth A, Kissel J (1991) The organic matter of Comet Halley as inferred by joint gas phase and solid phase analyses. Space Sci Rev 56:167
Kuan YJ et al (2003) A search for interstellar pyrimidine. Mon Not R Astron Soc 345:650–656
McGuire BA et al (2016) Discovery of the interstellar chiral molecule propylene oxide (CH3CHCH2O). Science 352:1449–1452
McKellar A (1940) Evidence for the molecular origin of some Hitherto unidentified interstellar lines. Pub Astron Soc Pac 52:187–192
Ohishi M (2016) Search for complex organic molecules in space. J Phys Conf Ser 728:052002
Ohishi M et al (2018) Detection of new methylamine (CH3NH2) sources: candidates for future glycine surveys. Submitted to Publ Astr Soc Japan
Rubenstein E, Bonner WA, Noyes HP, Brown GS (1983) Supernovae and life. Nature 306:118
Simon MN, Simon M (1973) Search for interstellar acrylonitrile, pyrimidine, and pyridine. Astrophys J 184:757–761
Stoks PG, Schwartz AW (1981) Nitrogen-heterocyclic compounds in meteorites – significance and mechanisms of formation. Geochim Cosmochim Acta 45:563
Suzuki T et al (2016) Survey observations of a possible glycine precursor, methanimine (CH2NH). Astrophys J 825:79
Tashiro T et al (2017) Early trace of life from 3.95 Ga sedimentary rocks in Labrador, Canada. Nature 549:516–518
Theule P et al (2011) Hydrogenation of solid hydrogen cyanide HCN and methanimine CH2NH at low temperature. Astron Astrophys 534:A64
Weinreb S, Barrett AH, Meeks ML, Henry JC (1963) Radio observations of OH in the interstellar medium. Nature 200:829–831
Acknowledgments
I would like to thank Dr. Amie Elsila for permitting the use of Fig. 2.1. This work was supported by the JSPS Kakenhi Grant Number JP15H03646. We utilized the Japanese Virtual Observatory (JVO; http://jvo.nao.ac.jp/) in finding relevant reference papers. This work has made use of NASA’s Astrophysics Data System.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ohishi, M. (2019). Prebiotic Complex Organic Molecules in Space. In: Yamagishi, A., Kakegawa, T., Usui, T. (eds) Astrobiology. Springer, Singapore. https://doi.org/10.1007/978-981-13-3639-3_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-3639-3_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3638-6
Online ISBN: 978-981-13-3639-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)