Abstract
The incorporation of the innate immune system into humans is essential for survival and health due to the rapid replication of invading microbes and the delayed action of the adaptive immune system. Antimicrobial peptides are important components of human innate immunity. Over 100 such peptides have been identified in various human tissues. Human cathelicidin LL-37 is best studied, and there has been a growing interest in designing new peptides based on LL-37. This chapter describes the alternative processing of the human cathelicidin precursor, protease digestion, and lab cutting of LL-37. Both a synthetic peptide library and structure-based design are utilized to identify the active regions. Although challenging, the determination of the 3D structure of LL-37 enabled the identification of the core antimicrobial region. The minimal region of LL-37 can be function-dependent. We discuss the design and potential applications of LL-37 into antibacterial, antibiofilm, antiviral, antifungal, immune modulating, and anticancer peptides. LL-37 has been engineered into 17BIPHE2, a stable, selective, and potent antimicrobial, antibiofilm, and anticancer peptide. Both 17BIPHE2 and SAAP-148 can eliminate the ESKAPE pathogens and show topical in vivo antibiofilm efficacy. Also discussed are other application strategies, including peptide formulation, antimicrobial implants, and peptide-inducing factors such as vitamin D and sunlight. Finally, we summarize what we learned from peptide design based on human LL-37.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The discovery of penicillin in 1928 is one of the greatest achievements in human history. However, overreliance on antimicrobial drugs against infectious diseases led to a rapid development of resistance in bacteria. Antimicrobial resistance posts a great risk to the human health-care system. To counteract the impacts of emerging multidrug resistance, alternatives are in urgent need. Antimicrobial peptides (AMPs) have been recognized as one such alternative because bacterial resistance development is rare or not yet observed (Zasloff 2002; Boman 2003; Jayaram and Chen 2015; Wang et al. 2015; Mishra et al. 2017b). They are ancient molecules optimized through their coevolution with bacteria over millions of years (Peschel and Sahl 2006; Spencer et al. 2014).
According to the antimicrobial peptide database (APD; http://aps.unmc.edu/AP), naturally occurring AMPs (<100 amino acids) have been discovered in the six life kingdoms, including bacteria, archaea, protists, fungi, plants, and animals (Wang and Wang 2004; Wang et al. 2009, 2016). In invertebrates, innate immune systems are the only defense weapon against microbial infection. The discovery of cecropins, magainins, and defensins in the 1980s laid the foundation for deciphering the pathogen-specific innate immune response pathways (Boman 2003; Lehrer and Lu 2012; Meister et al. 1997; Zasloff 1987). In mammals, several defense mechanisms guard against the threat of infection, ranging from the innate to adaptive immune systems, including skin barriers and physical factors such as urine flow, pH, and ionic composition. In humans, 124 AMPs were identified in various tissues as of June 2018 (Wang et al. 2016). Typical examples include lysozyme, defensins, histatins, cathelicidins, lactoferricin, kinocidins, ribonuclease, and dermcidin (reviewed in Wang 2014). On average, these molecules have a chain length of 44.6 amino acids with a net charge of +4.8. Such properties allow them to adopt unique structures for host defense.
Unlike other animals, there is only one cathelicidin gene in humans encoded on chromosome 3p21.3 (Frohm et al. 1997). Cathelicidins are synthesized as preproproteins with a highly conserved N-terminal domain and a highly variable C-terminal antimicrobial domain. The N-terminal domain usually consists of 94–114 amino acids and shares sequence homology with cathelin, a cysteine protease inhibitor derived from porcine neutrophils, hence the name cathelin-like domain (CLD). At present, there are 113 mature cathelicidin peptides in the APD, ranging from hagfish to humans (Wang et al. 2016). Human cathelicidin LL-37 is one of the best-studied AMPs. It is widely distributed in the human saliva, sperm, skin, gastrointestine, urinary tract, and respiratory airways. LL-37 is expressed by a number of cells, including monocytes, neutrophils, mast cells, stem cells, NK cells, and B and T cells. As an innate immune peptide, it is upregulated upon pathogen invasion or by immune stimuli such as vitamin D and sunlight. LL-37 has a broad-spectrum activity at a micromolar concentration against bacteria, fungi, viruses, and fungi at least in vitro (Durr et al. 2006; Vandamme et al. 2012). The observation that the concentration of LL-37 in certain human tissues is below the minimal inhibitory concentration (MIC) required to kill pathogens, however, led to an emphasis on immune modulation (Scott et al. 2002). It is now accepted that human cathelicidin LL-37 is a moonlighting peptide with multiple functional roles, ranging from antimicrobial to immune regulation. The moonlighting properties of LL-37 provide a basis for its involvement in a variety of human diseases such as infection, diabetes, cancer metastasis, and atherosclerosis (Scott et al. 2002; Vandamme et al. 2012).
This chapter summarizes the discovery, design, and potential applications of new AMPs based on the LL-37 template. Both structure-based design and library screening are covered. It is useful to mention the challenges in structural determination of LL-37 by NMR spectroscopy because of the importance of this structure for a better understanding of the antimicrobial activity of the peptide as well as for structure-based peptide discovery. To facilitate a connection between LL-37 and its fragments, we use the nomenclature and numbering of LL-37 throughout the text. For example, KR-12 (Table 12.1) means a 12-residue peptide corresponding to residues 18–29 of LL-37; it starts with amino acids K18R19 at the N-terminus and ends with R29 at the C-terminus. Subsequent sections are devoted to the potential applications of these peptides in antibiofilm, antiviral, antifungal, anticancer, surface immobilization, and immune modulation studies with a focus on the relationship between intact LL-37 and its fragments. It seems a slightly different region can be utilized for peptide design depending on the targeted pathogen or disease, including laboratory preference. Some LL-37 designer peptides have been shown to have antibiofilm efficacy in animal models.
2 Processing of the Human Cathelicidin Gene Product
2.1 Mature Peptide LL-37
Human cathelicidin is expressed as an 18 kDa precursor protein (hCAP-18). It was discovered in 1995 by three laboratories based on the highly conserved “cathelin” domain (Cowland et al. 1995; Larrick et al. 1995; Agerberth et al. 1995). One of the groups predicted the mature form as FALL-39 by comparison with the pig cathelicidin PR-39, a 39-residue peptide rich in amino acids P and R (Agerberth et al., 1995). The mature form was established as a 37-residue peptide LL-37 after its isolation from granulocytes (Gudmundsson et al. 1996). In neutrophils, hCAP-18 is processed to the antimicrobial peptide LL-37 (a 37-residue peptide starting with two leucines) by extracellular cleavage with proteinase 3 (Sorensen et al. 2001). Thus, the LL-37 pathway is composed of the precursor hCAP-18, the cathelin-like domain, LL-37, and its further cleaved fragments under natural conditions. Interestingly, the precursor of human LL-37 can also be cleaved into alternative forms below.
2.2 ALL-38 from the Human Reproductive System
AMPs appear to play a role in sperm fertilization. During sexual intercourse, hCAP-18, along with sperm, is injected into the vagina where it is cleaved into ALL-38 under acidic conditions by gastricsin. ALL-38, with one more alanine at the N-terminus than LL-37, showed similar antibacterial activity (Sorensen et al. 2003).
2.3 An Uncharacterized Alternative Form from Fat Cells
A recent exciting discovery is that fat cells also participate in host defense. Adipocytes can release a mature peptide longer than LL-37 to protect against the S. aureus infection (Zhang et al. 2015). However, the exact peptide sequence has not been elucidated.
2.4 TLN-58 from a Diseased Skin State
It is fascinating that hCAP-18 can also be cleaved upstream of the LL-37 sequence into another longer mature peptide with 58 amino acids (Murakami et al. 2017). TLN-58 is isolated from the lesion vesicle of palmoplantar pustulosis, a diseased skin condition on the palms and soles. Similar to human LL-37, this peptide form can upregulate IL-17C, IL-8, IL-23, IL-1α, and IL-1β mRNA and protein expression in normal human keratinocytes.
These findings provide additional evidence for the idea of one cathelicidin gene and multiple peptides (Wang 2014). It is likely that the single cathelicidin gene can be translated and processed in other manners yet to be discovered. Once a link is established between the cathelicidin form and disease, our detection of a particular form of human cathelicidin may serve as a biomarker for that disease.
3 Physical and Structural Basis of LL-37 in Targeting Bacterial Membranes
3.1 Bacterial Recognition via Electrostatic Interactions
Cationic LL-37 is active against a broad range of Gram-positive and Gram-negative pathogens (updated in Fig. 12.1). It is proposed that this linear peptide, like other cationic AMPs, targets anionic bacterial membranes via the carpet or toroidal pore model (Oren et al. 1999; Henzler et al. 2003; Lee et al. 2011). The membrane targeting of LL-37 is determined by its sequence property. According to the APD (Wang and Wang 2004), LL-37 (M. Wt. 4493.312 and molecular formula C205H341N59O53) has a net charge of +6 (i.e., sum of 6 Lys, 5 Arg, 2 Asp, and 3 Glu), allowing it to recognize the negative charge on bacterial surfaces, rather than mammalian cells. While bacterial membranes are rich in anionic PGs, mammalian cell membranes contain mainly zwitterionic phosphorylcholines (PCs) (Wang et al. 2014a, b). This preference of bacterial membranes is supported by in vitro studies using model membranes (Sevcsik et al. 2008).
Antibacterial activity of human antimicrobial peptide LL-37. Updated based on Vandamme et al. (2012) and Durr et al. (2006), the antimicrobial peptide database (http://aps.unmc.edu/AP) and the PubMed
The negative charge on the bacterial surface originates from a different basis: an outer membrane for Gram-negative bacteria but a cell wall for Gram-positive bacteria. In the outer membranes of Gram-negative bacteria, anionic lipopolysaccharides (LPS) are dominant. They are composed of a polysaccharide moiety and a lipid A. The cell wall of Gram-positive bacteria comprises peptidoglycan and lipoteichoic acids (LTA) that maintain cell integrity. These outer layers confer negative charges to the surface and are important targets for cationic AMPs. Such an electrostatic attraction is the first step of peptide-bacteria interaction. This is because bacterial surface modification to decrease negative charge can reduce peptide activity. For instance, modification of lipid A with glucosamine confers resistance to LL-37 (Shah et al. 2014). In the case of S. aureus, knockout of the gene for alanylation of teichoic acid of the cell wall or lysylation of phosphatidylglycerols (PG) in the inner membrane makes the bacterium more susceptible to cationic LL-37 (Saar-Dover et al. 2012; Peschel and Sahl 2006). Likewise, citrullination of LL-37 can make it less positively charged and reduces its LPS neutralization (Koziel et al. 2014). Host Toll-like receptors (TLRs) play an important role in recognizing these bacterial components. TLR2 is required to respond to cell wall preparations of Gram-positive pathogens, whereas TLR4 is involved in recognition of LPS (Takeuchi et al. 1999).
3.2 Challenges for Structural Studies of LL-37
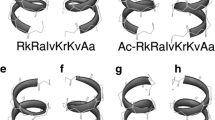
Positive charge alone, however, may not be sufficient for membrane binding of AMPs. The membrane targeting of LL-37 is determined by its three-dimensional structure. Circular dichroism (CD) and FT-IR analysis indicate a helical conformation, leading to an immediate classification of this human peptide into the helical family. In particular, an amphipathic helix of this cationic peptide, with distinct hydrophobic and hydrophilic surfaces, facilitates its interaction with anionic bacterial membranes (Oren et al. 1999). However, it has to wait for the solution nuclear magnetic resonance (NMR) spectroscopy to determine the atomic structure of LL-37 that informs us where the helix starts and ends and whether there is a helix break upon membrane binding. It took years of work to complete the 3D structure of LL-37 due to multiple challenges. The first challenge was the complex nature of bacterial membranes. Consequently, solution NMR studies were conducted in the presence of membrane-mimetic micelles such as anionic sodium dodecyl sulfate (SDS). The deuteration of micelles simplified the NMR spectra and allowed us to focus only on the peptide signals. As SDS has a head group different from that of the anionic lipid in bacteria, we also explored the possible use of a series of short-chain PGs for structural studies of AMPs (Wang et al. 2004; Keifer et al. 2004; Wang 2006, 2007, 2008). The second challenge was spectral resolution. The first solution NMR studies revealed the need of 3D NMR for structural determination of LL-37 bound to SDS micelles (Li et al. 2006a). The separation of the overlapped cross peaks onto numerous 2D planes along the 15N or 13C dimension of 3D NMR spectra yielded the needed spectral resolution for a complete assignment of the LL-37 signals and subsequent NOE assignments. The third challenge was the requirement of establishing a bacterial expression system to produce stable isotope-labeled LL-37 required for 3D NMR studies. The toxicity of LL-37 to the expression host made it necessary to express the peptide as a fusion protein. Another challenge was the difficulty to release the recombinant peptide from the fusion protein by enzyme digestion. The aggregation of LL-37 might have blocked the enzyme cleavage site. Fortunately, the fusion protein was successfully cleaved by formic acid (Li et al. 2006b). By making use of the oligomerization property of LL-37 (Li et al. 2007), we improved the peptide yield and obtained additional 15N-labeled or 15N, 13C-labeled peptides. These labeled peptides in complex with deuterated SDS micelles provided the needed sample stability for recording a suite of triple-resonance NMR spectra, such as HNCACB, CBCA(CO)NH, HNCO, and HNCA (Kay et al. 2011). The 3D structure of LL-37 determined by 3D NMR reveals a long amphipathic helix (residues 2–31) and a C-terminal tail (residues 32–37) (Fig. 12.2). To validate the structure, we measured the ps-ns peptide backbone dynamics using a 15N-labeled LL-37 sample. The dynamics data indicate the helical region is rigid, while the C-terminal tail is mobile, fully consistent with the structure (Wang 2008). There are also other structures for LL-37 and its fragments. Additional information can be found in other articles (Wang 2008; Wang et al. 2014b).
3D structure of human innate immune peptide LL-37 bound to bacteria membrane-mimetic micelles determined by 3D NMR (PDB ID: 2K6O). (a) A backbone view of the long helix corresponding to residues 2-31 of LL-37 with the C-terminal tail disordered. (b) A stick view of the LL-37 structure with hydrophobic and hydrophilic residues selectively labeled. In both views, hydrophobic amino acids are in green. A discontinuation of the hydrophobic surface at S9, rather than the helix, is thus evident (Wang 2008)
3.3 Structural Basis of Bacterial Membrane Binding of LL-37
We also asked whether the high-resolution structure determined in SDS micelles could be applied to bacterial membranes. First, we used dioctanoyl phosphatidylglycerol (D8PG) for NMR studies because it has the same head group as the major anionic lipid in bacteria (Wang et al. 2004; Keifer et al. 2004). Based on all the NMR data, the structure of LL-37 in complex with D8PG is the same as that bound to SDS micelles, implying that the detergent/lipid head groups play little role (Wang 2008). Because D8PG is not deuterated, it enabled us to observe intermolecular NOE cross peaks between peptide and lipid as well. In particular, aromatic F5, F6, F17, and F27 as well as basic R23 of LL-37 show direct interactions with the phosphatidylglycerol, confirming their important role in bacterial membrane binding (Wang 2008). The significance of R23 in interaction with bacteria was initially observed using the central fragment of LL-37 (Wang 2007) and later confirmed by alanine scan (Wang et al. 2012b) as well as the lysine/arginine swap (Wang et al. 2018). Second, we also studied the interaction of LL-37 with E. coli LPS. While the signals for the helical region are absent, the signals for the C-terminal tail are evident. Thus, the disordered tail of LL-37 observed in micelles recurs when bound to LPS. We conclude that human LL-37 utilizes the hydrophobic surface of a long amphipathic helix (residues 2–31) to interact with bacterial membranes for antimicrobial killing without the need of the C-terminal tail.
Because the SDS-bound structure reflects the bacterial membrane-bound form, the 3D NMR-derived structure can be used to explain peptide LPS-binding and activity data. The synergistic binding to LPS by an ovine cathelicidin SMAP-29 can be explained by a helix break caused by a proline (Tack et al. 2002). This is not the case for LL-37 (Turner et al. 1998) because of a lack of proline in the middle and the continuity of the helix. However, S9 separates the hydrophobic surface of the long helix of LL-37 into two domains (Fig. 12.2), providing a novel mechanism for synergistic binding of human LL-37 to LPS (Wang 2008). In addition, S9 is also important for antibacterial activity since an effort to render the hydrophobic surface continuous reduced the peptide activity (Wang et al. 2012a). The 3D structure determined by 3D NMR also laid a foundation for discovering active fragments within LL-37.
4 Discovery of Antibacterial Regions Within LL-37
This section highlights LL-37 fragments generated under natural conditions and in laboratories. The natural conditions include protease processing from both the host and pathogens. Peptides are also chemically synthesized in laboratories to understand sequence-activity relationship.
4.1 Skin Processing of Human LL-37
The Gallo lab studied the protease cleavage of LL-37 in human skin by kallikreins. They found multiple fragments of LL-37, including both active and inactive forms. Some examples are KR-20, LL-23, KS-30, and RK-31 (Table 12.1). Interestingly, KS-30 and RK-31 are more active than their parent LL-37 in bacterial killing, indicating the N-terminal region is less important (Murakami et al. 2004). This processing may be a natural regulatory mechanism that enhances peptide antimicrobial potency but reduces unwanted immune responses via the intact molecule. This mechanism further enriches the molecule reservoir of human cathelicidin-based defense line. Future studies may elucidate the details of this skin regulatory mechanism of human cathelicidin.
4.2 Pathogen Degradation of LL-37
As a resistance mechanism, there are multiple pathogen proteases that can degrade human defense peptide LL-37. However, only in a few cases were the resultant fragments documented. Sieprawska-Lupa et al. (2004) studied the digested products of S. aureus proteases such as aureolysin and V8 proteases. Aureolysin cleaved LL-37 between R19-I20, R23-I24, and L31-V32 peptide bonds, leading to the instant inactivation of LL-37. In contrast, the V8 protease cleaves the E16-F17 peptide bond of LL-37 (Fig. 12.3), leading to the accumulation and isolation of FK-21 (Table 12.1), a fragment corresponding to residues 17-37 of LL-37. Interestingly, FK-21 is more potent than LL-37 in killing E. coli, B. subtilis, P. aeruginosa, and E. faecalis. This is understandable since FK-21 contains the major antimicrobial region FK-16 discovered by NMR (Li et al. 2006a). In another study, Rapala-Kozik et al. (2015) isolated an intermediate peptide LL-25 (Table 12.1) from the C. albicans cleavage of LL-37. LL-25 appears to have a different immune modulating role compared to LL-37 (see below).
Host-pathogen interaction at the peptide level. Listed are select LL-37 peptides discovered from structure and library approaches (Li et al. 2006a, b; Nell et al. 2006; Wang 2008; Wang et al. 2008). It is amazing that the core antimicrobial region of human LL-37 is also recognized by bacteria for degradation or to release virulence factors (see the text)
4.3 Identification of Active Regions Within Human LL–37 in Laboratories
One of the major barriers toward peptide therapeutics is production cost. LL-37 is relatively long with 37 amino acids. To reduce the synthesis cost, it is necessary to trim unwanted regions. Two major methods are used to locate the active regions of LL-37. The first method is library screening. There are numerous studies with a goal of locating the active region of LL-37. The sequence relationship of these peptides with LL-37 is summarized in Table 12.1. For example, Braff et al. (2005) made a peptide library to identify the antibacterial, antifungal, and antiviral regions. Two long peptides, KS-30 and RK-31 (Table 12.1), are more potent than LL-37. Nell et al. (2006) identified a 24mer peptide P60 (i.e., residues 13-36 of LL-37 in Fig. 12.3; IG-24 in Table 12.1). A peptide P60.4 with the C-terminal four-residues changed from PRTE to RPLR has antimicrobial activity, LPS and LTA neutralization ability comparable to LL-37. Kanthawong et al. (2010) found IG-19, RK-25, and LL-31 (Table 12.1) against Gram-negative Burkholderia pseudomallei both in planktonic and biofilm forms. Among these peptides, LL-31 is most potent.
The second method is structure-based. Based on the helix-forming propensity of each amino acid along the sequence, Sigurdardottir et al. (2006) found GKE-21 with reduced cytotoxicity than LL-37. Li et al. (2006a) identified multiple LL-37 peptides based on NMR structural studies. In that study, LL-37 was initially split into LL-12 and IG-25 for structural studies (Table 12.1). While LL-12 is inactive, IG-25 is antibacterial. The study of a micelle-bound form of IG-25 led to the discovery of the major antimicrobial peptide FK-16 of LL-37 by using the NMR-trim technology that removes nonessential membrane-binding regions, such as the disordered C-terminal tail (Li et al. 2006a). Compared to the library approach that requires the synthesis of at least dozens of peptides, this NMR technology is efficient since it arrived at the major antimicrobial region using only a couple of synthetic peptides. In particular, IG-25 is only one residue longer than IG-24 found above via library screening (Nell et al. 2006). An N-terminally glycine appended version of FK-16 is called GF-17. FK-16 and GF-17 have very similar antibacterial activity (Table 12.2). A comparison of the antibacterial activity of multiple peptides described above confirmed GF-17 is most potent against methicillin-resistant Staphylococcus aureus (MRSA) USA300 (Wang et al. 2014a). This result may be due to a sufficient hydrophobicity of the major antimicrobial peptide of LL-37 (Table 12.1). In addition, a balance in charged and hydrophobic amino acids in the sequence led to a near zero GRAVY value in Table 12.1. Our NMR studies also led to the identification of FK-13, the core antibacterial region of LL-37 corresponding to residues 17–29 (Li et al. 2006a). Using a series of shorter peptides, it was found that the antibacterial region of FK-13 could be further shortened to 12-residue KR-12, the smallest antibacterial peptide of LL-37 (Wang, 2008). KR-12 is active against Gram-negative E. coli K12 (MIC 40 μM) but not Gram-positive S. aureus USA300. In 2018, Jessen lab made several 12-residue peptides along the LL-37 sequence. Their study showed no activity (>100 μg/mL) for N-terminal fragments LL-12, SK-12, C-terminal fragments VQ-12, IK-12, and DF-12 (net charge = 0, Table 12.1). This is not surprising since most of these fragments do not have the minimal hydrophobicity set by KR-12, the minimal antibacterial peptide we found (Wang 2008). In contrast, weak antibacterial activity (MIC 50 μg/mL) was observed for central fragments KR-12 and FK-12 against S. epidermidis. Note that both KR-12 and FK-12 (Saporito et al. 2018) were derived from FK-13 (Table 12.1), further validating the antimicrobial core region (Fig. 12.3) of LL-37 (Li et al. 2006a).
5 Peptide Design Based on Select LL-37 Peptides
The ESKAPE pathogens, including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species, are responsible for the majority of hospital-acquired infections (Boucher et al. 2009). For MRSA alone, the estimated total deaths are already comparable to those caused by HIV-1/AIDS (Klevens et al. 2007). Therefore, new antimicrobials are needed. There is also a high interest in developing AMPs into therapeutic molecules (Zasloff 2002). Being linear, human cathelicidin LL-37 is an important template. The therapeutic implication of LL-37 is evident. First, a lack of LL-37 in neutrophils may be responsible for periodontal disease in patients with morbus Kostmann (Pütsep et al. 2002). Second, knockout of the homologous cathelicidin gene CRAMP makes the mice more susceptible to infection (Nizet et al. 2001). As a complement strategy, expression of additional cathelicidin protects the skin from infection (Lee et al. 2005) and restores bacterial killing in a cystic fibrosis xenograft model (Bals et al. 1999). Third, topical treatment of chronic wounds such as venous leg ulcers with LL-37 (0.5 or 1.6 mg/mL) markedly reduces the mean ulcer area without safety concerns (Grönberg et al. 2014). Furthermore, LL-37 can inhibit biofilm formation probably by opsonization that enhances bacterial clearance (Overhage et al. 2008; Sol et al. 2013). In addition, LL-37 boosts immune response to clear pathogens (Overhage et al., 2014). Fourth, LL-37 may also be induced to help eliminate pathogens (van der Does et al. 2012; Jiang et al. 2013; Schögler et al. 2016). These observations laid a solid basis for peptide design based on LL-37 and its derived fragments.
5.1 IG-24 Derived P60.4
Serum/plasma binding is believed to be a key factor that might have limited the systemic use of cationic AMPs. Based on the P60.4 template originally identified from a library screen (Nell et al. 2006), OP-145 or P60.4Ac (Table 12.1) was initially obtained via further amino acid modification, including N-terminal acetylation. OP-145 is effective against S. aureus clinical strains, but its activity can be reduced in the presence of plasma (de Breij et al. 2016). Recently, de Breij et al. (2018) made additional peptide mutants to search for candidates with reduced binding to plasma. SAAP-148, with such a property, is found to have antimicrobial and antibiofilm ability against the ESKAPE pathogens, including persisters, which cannot be killed by traditional antibiotics.
5.2 The FK-16-Derived GF-17 Template
Using GF-17 as a template, we studied the role of basic amino acids by alanine scan (Wang et al. 2012b). One important finding is that the five basic amino acids in this peptide are not equal, but all involved in lipid clustering (Epand et al. 2009). In the case of membrane permeation, the R23A variant of GF-17 failed to cross either the outer or inner membranes of E. coli, indicative of an essential role of R23 for membrane damage (Wang et al. 2012b), consistent with the observation of intermolecular NOE cross peaks between R23 and D8PG by NMR spectroscopy (Wang 2007). As a conservative change, we also swapped the positions between a pair of lysine and arginine in GF-17. Interestingly, the charged swapped peptides showed reduced killing efficiency and increased cytotoxicity to human cells, revealing the evolutional significance of the native sequence of the peptide (Wang et al. 2017).
Cytotoxicity also limits the applications of cationic AMPs. It is commonly observed that peptide cytotoxicity results from high hydrophobicity (Zasloff 2002; Boman 2003). In the case of GF-17, the major antimicrobial region of LL-37 (Fig. 12.3), two approaches were utilized to reduce the peptide hydrophobicity. The first method is peptide truncation that led to the discovery of KR-12 (Wang 2008). The second method is to incorporate D-amino acids. The structural basis for this has been elucidated for GF-17d3, which contains D-amino acids at positions 20, 24, and 28 of GF-17 (Table 12.1). The D-amino acids distorted the backbone of GF-17 and caused non-coherent packing of side chains, leading to hydrophobic defects (Li et al. 2006a). A hydrophobic gap exists on the hydrophobic surface of LL-23, explaining its poor antibacterial activity (Wang et al. 2012a).
Another hurdle in peptide development is protease stability. Any peptide that is cleaved prior to bacterial killing is not useful. We used a library screen method to identify a peptide template with stability to chymotrypsin. This was initially accomplished during antibacterial assays in duplicated wells containing proteases; a stable peptide remains bacterial inhibition even in the presence of proteases. In this experiment, GF-17d3, with 3 D-amino acids incorporated, is active against E. coli K12, but not GF-17d1 (one D-amino acid at position 20) or GF-17d2 (two D-amino acids at positions 20 and 24) containing 1-2 D-amino acids. This chymotrypsin-resistant template, GF-17d3, was used to design peptide analogs to combat resistant pathogens. One of the peptides, 17BIPHE2, with both F17 and F27 replaced with biphenylalanines, is potent, selective, and stable (Wang et al. 2014a). This is the first LL-37-derived peptide illustrated to kill the ESKAPE pathogens (MIC 3.1–6.2 μM) and can inhibit biofilm formation in vivo. 17BIPHE2 showed a 50% hemolytic concentration (HL50) between 150 and 220 μM depending on the types of blood cells used. Our further investigation of 17BIPHE2 confirmed its stability to chymotrypsin digestion. In addition, it is not degraded after digestion with pathogen protease S. aureus V8 or fungal protease K, but can be cleaved by trypsin. In contrast, our recently designed Trp-rich peptide TetraF2W-RK with eight amino acids is inherently stable to trypsin and S. aureus V8 but can be cleaved by chymotrypsin (Mishra et al. 2017a). However, TetraF2W-RK, synthesized in D-amino acids, is stable to both trypsin and chymotrypsin. Peptide stability to such proteases from the digestive system can be important for future engineering peptides as oral drugs.
With the idea of personalized medicine, it may be necessary to design peptides with a desired activity spectrum. Recently, we found it possible to convert the broad-spectrum GF-17 into narrow-spectrum AMPs. First, by partially incorporating D-amino acids, we obtained GF-17d3 (Li et al. 2006a), which is only active against Gram-negative E. coli ATCC 25922 and A. baumannii B28-16, but not Gram-positive pathogens we tested (Wang et al. 2017). Alternatively, hydrophobic truncation of GF-17 led to KR-12 that is only active against E. coli K12. Second, 17BIPHE2-3RA, where three arginines are changed to alanines, is only active against Gram-positive staphylococcal strains such as S. aureus USA300 and S. epidermidis 1457. 17BIPHE2-3RA, with a net charge of +2 and hydrophobic content of 64%, resembles the database designed anti-MRSA peptide DFTamP1 that kills only Gram-positive pathogens (Mishra and Wang 2012). These results underscore the importance of amino acid composition in determining the peptide activity spectrum (Wang et al. 2018).
5.3 FK-13 and KR-12 Templates
NMR studies established a helical structure for KR-12 in complex with D8PG (Wang 2008). Understanding the role of each residue in KR-12 is important for peptide design. We investigated the effect of a single alanine substitution of basic amino acids on KR-12 activity (Mishra et al. 2013). Consistent with our finding for GF-17 that R23 and K25 are important for antimicrobial action, KR-12R23A and KR-12K25A are less effective in binding to anionic D8PG (Mishra et al. 2013). We did not replace hydrophobic amino acids because KR-12 is already the smallest, and such substitutions would lead to inactive peptides. This is indeed the case when we substituted I20, I24, or L28 of KR-12 into an alanine (Wang 2010). Recently, Gunasekera et al. (2018) made a systematic amino acid substitution within KR-12. It seems the culture media and bacterial strains used for antimicrobial assays can substantially influence the MIC values and make the results incomparable with the previously published results (Mishra et al. 2013).
Because of its low cytotoxicity and short length, KR-12 (Fig. 12.3) becomes an attractive template for peptide engineering. Jacob et al. (2013) generated several variants of KR-12 by increasing basic and hydrophobic residues at positions 27, 26, 22, 23, 25, and 18. KR-12-a1 to KR-12-a6 contains 1–6 changes of the listed residues of KR-12. KR-12a1 (a F27W variant) showed MIC values in the range of 1–8 μM using six Gram-positive and negative bacteria. Of note, additional substitutions did not increase peptide activity substantially against S. aureus, S. typhimurium, B. subtilis, and S. epidermidis, and in the case of KR-12-a6 with all six changes (K18L, Q22K, R23L, K25L, D26K, and F27W), antibacterial activity of the peptide against E. coli and P. aeruginosa actually reduced. Meanwhile, KR-12-a5 and KR-12-a6 became more hemolytic, indicating the K25L change is not favorable. Of note, a topical use of KR-12-a2 to treat MRSA otorrhea found no hearing loss or cochlear damage in guinea pigs (Sung et al. 2017), while KR-12-a5 is potent against oral pathogens (Caiaffa et al. 2017). A similar mutational study was also conducted using FK-13 (Rajasekaran et al. 2017). Da Silva et al. (2017) also utilized KR-12 as a template to design antibiofilm peptides against oral pathogens such as Streptococcus mutans. Substitution of I20 with W and appending KAEK at the C-terminus of KR-12 led to a more potent peptide against biofilms. These studies indicate a great potential of KR-12 as a template or a sequence unit for peptide design.
6 Potential Applications of LL-37 Peptides
6.1 Antibiofilm Peptides
Many pathogens are able to form biofilms, where a surface-anchored bacterial community shares a tower-like structure decorated with polymers (e.g., polysaccharides and DNA). Such biofilms, once formed on medical devices, are very challenging to remove. Therefore, much research effort of AMPs was oriented toward the development of potent antibiofilm agents and infection-free implants to combat these pathogens. Biofilm inhibition by human LL-37 at a low concentration was initially observed by the Hancock Group (Overhage et al. 2008). As one advantage of peptide design, however, both GF-17 and 17BIPHE2 possess antibiofilm capability superior to LL-37 (Mishra et al. 2016). In addition, we have demonstrated the antibiofilm ability of the engineered peptide 17BIPHE2 in a mouse catheter model (Wang et al. 2014a). As an independent path, a European group demonstrated antibiofilm ability of OP-145 and SAAP-148 (de Breij et al. 2016, 2018). However, combination treatment may be necessary and a natural choice for preformed biofilms that are difficult to remove. Our recent antipseudomonal biofilm results are summarized here: (1) Antibiotics are not effective, while AMPs are active, underscoring the importance of such peptides as a new antibiofilm agent. (2) Combined use of 17BIPHE2 with antibiotics is more effective to remove the P. aeruginosa biofilms. (3) Early treatment prior to biofilm maturation (<10 h) is advantageous since monotherapy of either antibiotic or peptide is effective (Mishra and Wang 2017). Based on these observations, there is now a growing interest in developing biofilm prevention approaches.
6.2 Antibiofilm Coating of LL-37 Peptides
An attractive antimicrobial strategy is to coat human cathelicidin LL-37 to the surfaces of medical devices to prevent infection, especially when biofilms form. In particular, peptide surface immobilization confers some advantages such as a local high peptide concentration, reduced cytotoxicity, and increased host cell adhesion. This section describes covalent coupling of LL-37 and its peptides (Table 12.3). There are multiple factors that affect the antimicrobial activity of immobilized peptides. These include the types of materials (metals or polymers), the linker or spacer, and the site for peptide coupling (terminus vs. side chain). There is no consensus as to the nature of linkers (short vs. long).
The full-length LL-37 molecule, with a cysteine added at the N-terminus, was first immobilized on a titanium surface via the maleimide chemistry. Gabriel et al. (2006) found that site-specific coupling of LL-37 with a flexible hydrophilic poly(ethylene glycol) (PEG) spacer is better than randomized coating. The LL-37-coated surface shows antimicrobial activity against E. coli. Recently, LL-37 has also been immobilized on other types of surfaces, including nanoparticles (Gustafsson et al. 2010; Cassin et al. 2016; Niemirowicz et al. 2017; Comune et al. 2017). The chemistries are summarized in Table 12.3. These studies illustrated (1) antifungal activity of LL-37 against C. albicans, (2) anti-adhesion properties, (3) LPS binding, (4) no toxicity to mammalian cells, (5) immune modulatory activity such cell migration, and (6) treatment of wounds in a mouse model.
Several fragments (IG-25, FK-16, and KR-12) initially reported by us (Li et al. 2006a; Wang 2008) have also been surface immobilized. Santos et al. (2013) immobilized IG-25 (Table 12.1) on fluorous thin films and fluorosilicone contact lens with activity against an ocular pathogen P. aeruginosa (Table 12.3). KR-12 was also covalently immobilized via a site-specific maleimide chemistry on electrospun silk fibroin nanofiber. The surface could inhibit S. aureus, S. epidermidis, E. coli, and P. aeruginosa. Compared to the free peptide described above, this surface-coated KR-12 appeared to have gained antibacterial activity. In addition, the immobilized peptide suppressed the LPS-induced TNF-α expression of monocytes (RAW264.7), helped the proliferation of fibroblasts and keratinocytes, and promoted the differentiation of keratinocytes with enhanced cell-cell attachment (Song et al. 2016). Likewise, KR-12 was also immobilized on the titanium surface (Nie et al. 2016), which shows antimicrobial and antibiofilm activities against S. epidermidis. Importantly, the peptide-coated surface is able to increase the adhesion and proliferation of human bone marrow mesenchymal stem cells (Nie et al. 2016). To obtain a surface with broad-spectrum activity, we recently immobilized FK-16, the major antimicrobial peptide of LL-37, on the titanium surface using a similar maleimide chemistry (Mishra and Wang 2017). FK-16, with a higher coating density than LL-37 on the titanium surface, shows potent activity against the ESKAPE pathogens but is nontoxic to human erythrocytes and epidermal keratinocytes HaCaT cells. Significantly, the FK-16-coated surface is able to inhibit biofilm formation of S. aureus USA300 (initial inoculation at 103 CFU) for up to 72 h. A lower CFU (103) used here should be medically more relevant considering the sterile condition of the surgery room. This study provides a proof-of-concept example for generating a broad-spectrum antimicrobial surface to prevent bacterial adhesion and biofilm formation on medical implants.
6.3 Antiviral Peptides
Viral infection is life threatening and has raised concerns from the public. This ranges from the well-known human immunodeficiency virus type 1 (HIV-1) to the recent Zika and Ebola viruses in the news. Often, we do not have therapeutic molecules to treat such outbreaks. Infected patients can only be sent to a containment facility and wait for miracle medicine. The development of new vaccines is slow. Therefore, alternative antiviral strategies are clearly needed. Current rise in HIV patients with coinfections has required researchers to revisit AMPs with a hope to overcome ever-increasing cases of antibiotic resistance and to increase the immune response status. It is interesting to explore how nature has devised AMPs for protection against various viruses. As of June 2018, the APD registered 182 antiviral peptides (Wang et al. 2016). While a systematic review on HIV inhibitory AMPs (Wang 2012) can be found online, this section focuses on antiviral effects of human cathelicidin LL-37. Up to date, LL-37 has been demonstrated to have inhibitory effects against at least ten types of viruses, including herpes simplex virus (HSV; Yasin et al. 2000), smallpox vaccinia virus (Howell et al. 2004), HIV-1 (Bergman et al. 2007), respiratory syncytial virus (RSV; Tian et al. 2011), varicella zoster virus (VZV; Crack et al. 2012), human adenovirus (Uchio et al. 2013), influenza A virus (IAV; Tripathi et al. 2013), dengue virus type 2 (DENV-2; Alagarasu et al. 2017), human rhinovirus (HRV; Sousa et al. 2017), and Zika virus (He et al. 2018). To facilitate our discussion, these LL-37 inhibited viruses are classified in Table 12.4 based on the type of nucleic acids (DNA/RNA) and whether they are enveloped. Thus, LL-37 has an inhibitory effect on both enveloped and non-enveloped viruses.
It is natural to ask whether the antibacterial segments derived from LL-37 also effectively inhibit viruses. While fragments from either the N-terminus or C-terminus are inactive, a central fragment of LL-37 is active against HIV-1 (Wang et al. 2008). Different from the anti-MRSA case, GI-20 (Fig. 12.3) has the highest therapeutic index, indicating that the central region of LL-37 is also important to inhibit viruses. In collaboration with ImQuest Biosciences, we also identified the minimal anti-HIV peptide of LL-37. Different from the bacteria case, the LL-37 core peptide FK-13 retains anti-HIV activity but not KR-12. This fact implies a significant role of F17 in inhibiting HIV-1. There are also other differences from the antibacterial case. In particular, after reversal of the FK-13 sequence or incorporation of D-amino acids into GF-17, the peptides remain active against bacteria but not the virus, implying a different molecular target. It appears that LL-37 and its peptides IG-25 and FK-16 are able to inhibit HIV-1 reverse transcriptase in vitro at IC50 of 15, 7, and 70 μM, respectively (Wong et al. 2011). The weak enzyme inhibition activity of these peptides observed here (20–30% inhibition at 100 μM) does not sufficiently explain anti-HIV activity observed below 1 μM. Therefore, additional mechanistic studies are needed to better understand peptide activity. It is clear that the sequence requirement for inhibiting HIV-1 differs from that for bacterial inhibition. Other cathelicidins can also be useful. While BMAP-27 is toxic, a C-terminal truncated peptide is not (Skerlavaj et al. 1996). BMAP-18 is also demonstrated to be inhibitory to HIV-1 (Wang et al. 2008). Future studies may further define the mechanism of action and evaluate the therapeutic potential of these anti-HIV peptides in animal models.
Considering other benefits of LL-37 such as spermicidal effects and selective killing of invading pathogens without damaging commensal bacteria (Tanphaichitr et al. 2016), these LL-37 peptides may be promising candidates as topical spermicides/microbicides. However, further studies are required to resolve the complication from coinfected viruses such as HSV-2 that appears to alter the defense role of LL-37 into an offense role. The mechanistic studies revealed that LL-37 produced by HSV-2-infected epithelial cells upregulates HIV receptors (CD4 and CCR5) on the surface of monocyte-derived Langerhans cells (the mucosal epithelium resident dendritic cells), thereby enhancing HIV infection (Ogawa et al. 2013).
LL-37 peptides also have inhibitory effects on other RNA viruses (Table 12.4). LL-37 can directly disrupt enveloped influenza A virus (Tripathi et al. 2013). In the case of the pandemic H1N1 strain of 2009 (A/California/04/09/H1N1 or “Cal09”), LL-37 is inactive. However, GI-20, a central LL-37 fragment, retains anti-IAV activity against this strain (Tripathi et al. 2015b). This observation indicates the advantage of LL-37 reengineering.
Mosquito-borne Zika is another enveloped RNA virus first isolated in Uganda in 1947 near the Zika forest. After its outbreak in 2007, it became a rapidly emerging public health threat. Although clinical infection is frequently mild, significant neurological manifestations have been demonstrated in infants born to Zika virus (ZIKV)-infected mothers (McArthur 2017). Currently, there is no drug to treat ZIKV infection. Although vaccines are under active development, other effective countermeasures may also be considered. Recently, the efficacy of LL-37 and its derived peptides (e.g., GI-20 and GF-17) against ZIKA has been demonstrated in vitro, whereas RI-10 (Fig. 12.3) is ineffective (He et al. 2018). Further characterization reveals that GF-17 (Fig. 12.3) can directly inactivate this virus and work via the interferon pathway. In addition, 17BIPHE2, an engineered version of GF-17, also inhibits ZIKA effectively. However, its antiviral effect is reduced when R23 is altered to ornithine, indicating the important role of this arginine in inhibiting ZIKA.
Other labs also searched active antiviral regions of LL-37 against respiratory syncytial virus (RSV), which is responsible for lower respiratory tract infections of children. A low level of human cathelicidin is directly correlated with human RSV infection. Harcourt et al. (2016) found a better anti-RSV effect when LL-37 is used prophylactically (treat before infection) than therapeutically (treat after infection). Currie et al. (2016) also compared the effect of treatment time. They found that co-administration of LL-37 with virus clearly protects animals better against infection either when the peptide is treated before or after viral colonization. It is established that LL-37 can directly damage the viral envelope, disrupt virus particles, and inhibit infection of epithelial cells in vitro (Currie et al. 2016). The direct anti-RSV effect of LL-37 stimulates the interest in identifying the active region of LL-37. As we observed in the antibacterial study (Li et al. 2006a), Tian et al. (2011) found that the N-terminal fragment LL-12 is inactive while the C-terminal fragment IG-25 is. Moreover, among the four 22mer LL-37 peptides corresponding to residues 13–34 (IG-22), 14–35 (GK-22), 15–36 (KE-22), and 16–37 (EF-22), EF-22 shows an anti-RSV effect similar to LL-37. Currie et al. (2013) also showed that, while neither the N-terminal fragment LL-22 nor the C-terminal fragment EF-22 is inhibitory, a central peptide KI-22 (Table 12.1) is effective against viral particles. Despite the conflicting results between Tian et al. (2011) and Currie et al. (2013) regarding EF-22, these studies also point at the important antiviral role of the central fragment of LL-37 previously found based on bacteria (Li et al. 2006a; Nell et al. 2006) and later demonstrated against HIV-1 (Wang et al. 2008). It seems that the central region of LL-37 plays a general protective role against both bacterial and viral infection (Fig. 12.3).
Because peptides have a relatively short half-life, potential cytotoxicity, and non-specific interactions with cells, one possible way to improve the treatment outcome is to combine LL-37 with nanoparticles, which can be internalized, increasing the uptake of the peptide. LL-37 containing liposomes (size 106.8 ± 10.1 nm, shelf-life stability >1 year) are found to be superior to its free form in protecting keratinocytes from RSV infection without displaying cytotoxicity even at 400 μM (Ron-Doitch et al. 2016). In future studies, the central fragment of LL-37 may be formulated in the same manner. Other preventative measures may include the administration of vitamin D and increased exposure to sunlight to boost the expression of LL-37. However, the interaction of LL-37 with combustion-derived carbon nanoparticles (especially in winter) can compromise peptide antiviral and antibacterial activity, making immunocompromised people more susceptible to infection in highly polluted environments (Findlay et al. 2017).
6.4 Antifungal Peptides
High mortality and morbidity rates due to invasive mycosis have been increasing over the last 20 years. Medically significant pathogenic fungi (~300 species) are almost always molds. Opportunistic fungal infections create therapeutic challenges, particularly in high-risk immunocompromised patients with AIDS, cancer, and those undergoing transplantation. In light of growing resistance to antifungal drugs, novel medicine and treatment approaches are required.
The current APD registered 1067 antifungal peptides (Wang et al. 2016). Cathelicidin α-helical peptides have shown activity (BMAP-27 and BMAP-28 from cows) against Candida spp. and C. neoformans, but they are less active against filamentous fungi. Both LL-37 and a close mouse analog mCRAMP have a similar MIC range (15–20 μM) against C. albicans. In one study, mCRAMP was induced by C. albicans at the skin surface in a mouse model, demonstrating that these peptides provide a natural barrier to fungal infection. In addition, LL-37 can also regulate the immune response to better clear fungal infection. Gallo and colleagues tested antifungal activity of LL-37 and its fragments against C. albicans and found that KS-30 and RK-31 are more active (Braff et al. 2005). Using fluorescein-labeled peptides, den Hertog et al. (2006) investigated the cell location of LL-37-derived peptides. While LL-37 and its C-terminally truncated peptide LL-31 (Table 12.1) are found at the perimeter of C. albicans, the N-terminally truncated peptide RK-31 (Table 12.1) enters the cytoplasm within 30 min. LL-25 can enter cells faster than RK-31. It seems that both the N-terminal helix and the central helix play a role in determining the peptide location on the cell perimeter since all the phenylalanines are important bacterial membrane anchors (Wang 2008). In addition, these peptides can all induce lipid phase separation in fungal membranes.
6.5 Immune Modulating Peptides
Human LL-37 also plays an important role in regulating the immune response (Scott et al. 2002; Choi et al. 2012; Kahlenberg and Kapplan 2013). This section highlights some differences between LL-37 and its derived peptides. The LPS neutralization property of LL-37 forms the basis for its use to treat sepsis. Arginines are important in this neutralization as citrullination of LL-37 makes it inactive in a sepsis mouse model (Koziel et al. 2014). Using a designed peptide 17BIPHE2, it is shown that an alteration of R23 to ornithine slightly reduces LPS neutralization (Wang et al. 2018). In addition, synergistic LPS binding of LL-37 requires two domains (See Fig. 12.2). This explains the reduced LPS-binding ability of LL-37 peptides without the N-terminal domain, including the 24mer peptide P60 found by Nell et al. (2006) and IG-19 (Nan et al. 2012) (sequences in Table 12.1). Increasing basic/hydrophobic amino acids and changing F17 and F27 to W, however, enhance the LPS-binding ability of IG-19. By binding to LPS, LL-37 suppresses the LPS binding to receptors such as CD14 and TLR-4, thereby reducing the apoptosis of liver endothelial cells (Suzuki et al., 2011). In addition, LL-37 also plays a role in LPS clearance. In this process, LL-37 enhances LPS uptake by liver cells via endocytosis (Suzuki et al. 2016).
Chemotaxis is a recognized role of LL-37. There is no correlation between immune modulation (e.g., IL-8 release from keratinocytes) and antimicrobial activity (Braff et al. 2005). Nell et al. (2006) also found that, although P60.4 has antimicrobial activity comparable to LL-37, it loses its chemotactic ability, while P60 and LL-37 are nearly equivalent in inducing neutrophil migration. Thus, an alteration of the follow-up sequence behind the central helix (Fig. 12.3) regulates chemotaxis. Chemotaxis of LL-37 is important for host defense against pathogen invasion. As a counteracting strategy, fungi can cleave LL-37 using aspartic proteases. LL-25 is identified as an intermediate peptide that shows a lower chemotactic activity to neutrophils than LL-37, reducing the recruitment of neutrophils to the infection sites (Rapala-Kozik et al. 2015).
Interestingly, LL-37 can also be cleaved into LL-23 in human skin (Murakami et al. 2004). There is a clear difference between LL-37 and its N-terminal fragment LL-23 in immune regulation (Wang et al. 2012a). Immune modulation by LL-37 also plays a role in viral control. For example, LL-37 can inhibit the release of IL-8 from neutrophils induced by IAV (Tripathi et al. 2014). Of note, the central peptide GI-20 is equally effective in reducing IL-8 (Tripathi et al. 2015a). LL-37 can also stimulate immune response by binding to nucleic acids. In this process, LL-37 forms oligomers and serves as a carrier. A comparison of LL-37 with its fragments reveals that both the N- and C-terminal regions are required in this process (Singh et al. 2014). This sequence requirement agrees with the NMR study of free LL-37 at pH 7 that the entire region of LL-37 is involved in the oligomerization into tetramers (Wang 2017).
It is interesting to note that there is also a sequence difference required for antimicrobial action and pathogen response. While FK-13 (Table 12.1) is the minimal peptide to inhibit HIV-1, KR-12 (obtained by deleting the N-terminal F17 of FK-13) retains antibacterial activity against E. coli (Wang 2008). Interestingly, RI-10 (Table 12.1), obtained by deleting one residue from the N- and C-termini of KR-12, lost antimicrobial activity (Wang 2008; He et al. 2018). However, RI-10 retains the minimal sequence information of LL-37 that triggers the bacterial two-component system. A direct interaction between LL-37 and the CSrRS receptor increases the virulence factor expression of group A streptococcus (GAS) (Velarde et al. 2014). With the expansion in our investigation, other functional sequence motifs of human LL-37 may emerge, further enriching our understanding of this innate immune peptide.
Taken together, there are different sequence requirements for antimicrobial action and cell response from both the host and pathogen sides. Such sequence differences are determined by different molecular targets: usually bacterial membranes for antimicrobial activity but cell receptors for host immune stimulation and pathogen response.
6.6 Anticancer Peptides
Anticancer activity of AMPs was demonstrated rather early (Ohsaki et al. 1992). AMPs with anticancer activity are discussed elsewhere in this book. An updated list of anticancer peptides can be found in the APD (Wang et al. 2016). Human cathelicidin LL-37 is linked to cancers in different manners. Its level is increased in ovarian, breast, and lung cancers, but LL-37 suppresses colon and gastric cancers (Wu et al. 2010b). Whether and how LL-37 promotes cancer and metastasis deserves further studies. This section discusses anticancer activity of LL-37 as a basis for developing alternative cancer treatment approaches. Such methods can be important for cancers that are resistant to existing anticancer drugs. However, the poor cell selectivity between normal and malignant cells may make it challenging to put them into practical use directly (Ohsaki et al. 1992; Li et al. 2006a; Mishra et al. 2018). Advanced engineering strategies discussed elsewhere may be helpful (Mishra et al. 2017b).
6.6.1 Colon Cancers
Human cathelicidin LL-37 is expressed strongly in normal colon mucosa but downregulated in colon cancer tissues. Kuroda et al. (2012) showed that LL-37 and a peptide analog FF/CAP18 suppresses colon cancer cell (HCT116) proliferation. FF/CAP18 corresponds to residues 5–32 of LL-37 with residues E16 and K25 changed to F. The peptide works by depolarization of the mitochondrial membrane independent of the p53 pathway. Ren et al. (2012) showed that treatment of colon cancer cells with LL-37 induces anionic phosphatidylserine (PS) exposure and DNA fragmentation, indicative of apoptosis. Previously, FK-16 is found to be the major antimicrobial and anticancer peptide of LL-37 (Li et al. 2006a). It is interesting that FK-16 shows a similar anti-colon cancer effect independent of caspase activation (Ren et al. 2013). Mechanistically, FK-16 causes the upregulation of Bax and downregulation of Bcl-2 by activating p53. As an alternative anticancer strategy, Cheng et al. (2014) observed tumor size shrinking when cathelicidin-expressing adeno-associated virus was administered intravenously into HT-29-derived subcutaneous tumors in nude mice.
6.6.2 Gastric Cancer
Helicobacter pylori is linked with gastric cancers (Li and Perez Perez 2018). Hase et al. (2003) noticed that the level of LL-37 in various types of gastric cancers is significantly reduced. During Helicobacter pylori infection, the level of LL-37/hCAP-18 secreted into gastric juice is increased. This is understandable since the H. pylori-induced expression of LL-37 exerts bactericidal effects. Wu et al. (2010a) showed that LL-37 suppresses gastric cancer by increasing the tumor-suppressing bone morphogenetic protein signaling via inhibiting proteasome. Schauber et al. (2004) detected upregulation of LL-37 in both colonic and gastric cells when histone-deacetylase (HDAC) inhibitors (e.g., butyrate and trichostatin A) were administered. Induction of LL-37 appears to be promising also for treatment of colon cancer.
7 Concluding Remarks
Human cathelicidin LL-37 is an interesting moonlighting peptide with multiple functional roles and involvement in numerous diseases. The wide functions of this peptide provide a scientific basis for developing its potential applications. The high interest in the therapeutic potential of antimicrobial peptides originates from their potency against drug-resistant bacteria, RNA viruses, and cancer. The design of AMPs based on LL-37 starts from the identification of its active regions (Table 12.1 and Fig. 12.3). Both library screen and structure-based approaches are utilized. It is also possible to combine the library screen with structure-based design (e.g., Wang et al. 2004). What we have learned to date from LL-37 peptide design can be summarized below:
-
1.
Through multiple studies, the benefits of LL-37 engineering emerge. While the activity of full-length LL-37 is media dependent, the designer peptides such as GF-17 and 17BIPHE2 are not (Li et al. 2006a; Wang et al. 2014, 2018; Mishra et al. 2016). While LL-37 is poor in preformed biofilm disruption, both GF-17 and 17BIPHE2 work well (Mishra et al. 2016). While LL-37 is inactive against a seasonal flu virus, GI-20 remains active (Tripathi et al. 2015a, b). While the effect of LL-37 on cancer is controversial, FK-16 is anticancer and works superior to LL-37 (Li et al. 2006a; Ren et al. 2013). These examples underscore the medical significance of LL-37 fragments as well as peptide engineering.
-
2.
There is a consensus that the antimicrobial action of LL-37 is achieved primarily via its central region. This central antimicrobial region (residues 13–32) becomes remarkably evident in the 3D structure of LL-37 (Fig. 12.2) because it is sandwiched between two punctuation signals of the LL-37 sequence: hydrophilic S9 (which splits the hydrophobic surface into two domains) and P33 (which ends the helical region) (Wang 2008). Peptides KR-12, FK-13, GF-17, and GI-20 are all derived from this region (Fig. 12.3). The sequence of IG-24 (Nell et al. 2006), the template for SAAP-148, however, extends beyond this central region by including four residues from the non-membrane-targeting C-terminal tail of LL-37.
-
3.
An alteration of the amino acid composition affects the peptide activity spectrum. The wide-spectrum GF-17 (i.e., killing both Gram-positive and Gram-negative bacteria) has been converted to narrow-spectrum peptides that kill only either Gram-positive or Gram-negative bacteria. These results underscore the importance of basic amino acids for antimicrobial activity against Gram-negative bacteria and hydrophobic residues for killing Gram-positive pathogens, consistent with the database findings (Wang et al. 2018).
-
4.
Consistent with the classic view, hydrophobic amino acids of LL-37 peptides are important for membrane anchoring. The protruding aromatic rings of F17 and F27 imply their interdigitating membranes. While all basic amino acids participate in lipid clustering (Epand et al. 2009), they are not equal. R23 of LL-37 is essential for pathogen recognition, LPS neutralization, membrane permeation, pathogen killing, and antibiofilm effects (Wang 2007; Wang et al. 2012b, 2017, 2018; He et al. 2018). Even a lysine-arginine positional swap can affect such properties of LL-37 peptides, revealing the evolutional significance of the native sequence (Wang et al. 2017). As an unwanted effect, such an interfacial arginine also contributes to hemolysis and an change to ornithine improves peptide selectivity (Wang et al. 2018).
-
5.
To reduce peptide production cost, there has been a desire to identify the minimally active regions of LL-37. Interestingly, the shortest active peptide varies with biological activity (i.e., RI-10 for receptor binding, KR-12 against bacteria, FK-13 against HIV-1, and GF-17 against MRSA).
-
6.
While NMR studies have accurately mapped the core antimicrobial region of LL-37 (Li et al. 2006a), bacteria also counteract on this region. S. aureus can secrete aureolysin to cut this region of LL-37 (Sieprawska-Lupa et al. 2004). In addition, another Gram-positive pathogen GAS can recognize RI-10 via the CsrRS receptor (Velarde et al. 2014) to increase the expression of virulence factors (Fig. 12.3). Such bacterial recognition regions shed new light on the significance of the core antimicrobial region of LL-37 as well as host-pathogen interactions at the molecular level.
-
7.
While an increase of excessive basic amino acids can make the peptide more toxic (e.g., Jacob et al. 2013), a general and classic wisdom to improve cell selectivity of peptides is to decrease hydrophobicity. Sequence mutation, deletion, and truncation are routinely utilized for this purpose. Partial incorporation of D-amino acids can alter peptide conformation and generate incoherent side chain packing, reducing hydrophobicity (Li et al. 2006a). The incoherent packing of side chains leads to hydrophobic defects in the structure, the basis for cell selectivity. The importance of peptide hydrophobicity for targeting bacterial membranes sets a limit on the degree of hydrophobic reduction for cell selectivity.
-
8.
Usually, peptides made of D-amino acids are more resistant to proteases (Boman 2003). Protease-stable peptides can also be screened from a peptide library followed by structure-guided peptide design to enhance activity against the ESKAPE pathogens. 17BIPHE2 can kill bacteria in the presence of host chymotrypsin, S. aureus V8 protease, or fungal proteinase K (Wang et al. 2014).
-
9.
Likewise, plasma binding to SAAP-148 has been minimized (de Breij et al. 2018). This is regarded as a main reason for the failure of AMPs in vivo. Whether this is a general requirement remains to be validated.
-
10.
To date, the therapeutic use of LL-37 is limited to topical treatment due to poor bioavailability, production cost, and potential cytotoxicity. The template for engineering 17BIPHE2 is obtained based on structure, whereas the template for SAAP-148 originates from peptide library screen (Wang et al. 2014a; de Breij et al. 2018). Both peptides show effects in vitro and in vivo on resistant pathogens and biofilms.
-
11.
It is preferred to prevent biofilm formation because preformed biofilms (e.g., P. aeruginosa) are notoriously difficult to get rid of. Combined treatment using 17BIPHE2 and antibiotics give better results. This practical approach not only potentiates the effect of traditional antibiotics but also reduces the peptide needed, reducing cost and potential cytotoxicity (Wang 2017).
-
12.
Antimicrobial activity and immune modulation are the two faces of the same coin. These innate immune peptides use different molecular targets (e.g., bacterial membranes for antimicrobial effects and host receptors for signal transduction and immune modulation). In a catheter-associated mouse biofilm model, 17BIPHE2 is not only antibacterial but also chemotactic to monocytes (Wang et al. 2014). GF-17 can directly inactivate Zika virus. It also acts via the interferon pathway (He et al. 2018). Through peptide design, it is possible to retain antibacterial activity and tune host immune response (Nell et al. 2006).
We anticipate that research interest in human cathelicidin will continue to expand, and new functions of LL-37 may be discovered. Both prevention and treatment strategies are under active development. To avoid infection, we anticipate continued research interest in covalent immobilization of AMPs on medical implants. The basis for this is that peptide injection or non-covalent coating of LL-37 peptides can reduce S. aureus infection (Wang et al. 2014a; de Breij et al. 2016). Efforts will also continue to find the optimal approach for LL-37 induction at a right time and location. In winter, sunlight and vitamin D supplement can be beneficial. Our results indicate the importance of early treatment. In addition, the anti-infective potential of LL-37-derived peptides may be expanded by combining them with traditional antibiotics or other approved simple compounds. As a milestone, topical treatment of infections using LL-37-derived peptides has been demonstrated in animal models (e.g., Wang et al. 2014a; de Breij et al. 2018), and OP-145 was used to treat chronic otitis media in a clinical phase 2 trial in 2009 (de Breij et al. 2018). These achievements will reignite the hope to develop the peptide into a new systemic antibiotic, the holy grail of future LL-37 engineering.
References
Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH (1995) FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A 92(1):195–199
Alagarasu K, Patil PS, Shil P, Seervi M, Kakade MB, Tillu H, Salunke A (2017) In-vitro effect of human cathelicidin antimicrobial peptide LL-37 on dengue virus type 2. Peptides 92:23–30
Bals R, Weiner DJ, Meegalla RL, Wilson JM (1999) Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Invest 103:1113–1117
Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Söderlund J (2007) The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res 5(4):410–415
Boman HG (2003) Antibacterial peptides: basic facts and emerging concepts. J Intern Med 254(3):197–215
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12
Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, Lin K, Streib JE, Dorschner R, Leung DY, Gallo RL (2005) Structure-function relationships among human cathelicidin peptides: disso ciation of antimicrobial properties from host immunostimulatory activities. J Immunol 174(7):4271–4278
Caiaffa KS, Massunari L, Danelon M, Abuna GF, Bedran TBL, Santos-Filho NA, Spolidorio DMP, Vizoto NL, Cilli EM, Duque C (2017) KR-12-a5 is a non-cytotoxic agent with potent antimicrobial effects against oral pathogens. Biofouling 33(10):807–818
Cassin ME, Ford AJ, Orbach SM, Saverot SE, Rajagopalan P (2016) The design of antimicrobial LL37-modified collagen-hyaluronic acid detachable multilayers. Acta Biomater 40:119–129
Chen G, Zhou M, Chen S, Lv G, Yao J (2009) Nanolayer biofilm coated on magnetic nanoparticles by using a dielectric barrier discharge glow plasma fluidized bed for immobilizing an antimicrobial peptide. Nanotechnology 20(46):465706
Cheng M, Ho S, Yoo JH, Tran DH, Bakirtzi K, Su B, Tran DH, Kubota Y, Ichikawa R, Koon HW (2014) Cathelicidin suppresses colon cancer development by inhibition of cancer associated fibroblasts. Clin Exp Gastroenterol 8:13–29
Choi KY, Chow LN, Mookherjee N (2012) Cationic host defence peptides: multifaceted role in immune modulation and inflammation. J Innate Immun 4(4):361–370
Comune M, Rai A, Chereddy KK, Pinto S, Aday S, Ferreira AF, Zonari A, Blersch J, Cunha R, Rodrigues R, Lerma J, Simões PN, Préat V, Ferreira L (2017) Antimicrobial peptide-gold nanoscale therapeutic formulation with high skin regenerative potential. J Control Release 262:58–71
Cowland JB, Johnsen AH, Borregaard N (1995) hCAP-18, a cathelin/probactenecin-like protein of human neutrophil specific granules. FEBS Lett 368(1):173–176
Crack LR, Jones L, Malavige GN, Patel V, Ogg GS (2012) Human antimicrobial peptides LL-37 and human β-defensin-2 reduce viral replication in keratinocytes infected with varicella zoster virus. Clin Exp Dermatol 37(5):534–543
Currie SM, Findlay EG, McHugh BJ, Mackellar A, Man T, Macmillan D, Wang H, Fitch PM, Schwarze J, Davidson DJ (2013) The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS One 8(8):e73659
Currie SM, Gwyer Findlay E, McFarlane AJ, Fitch PM, Böttcher B, Colegrave N, Paras A, Jozwik A, Chiu C, Schwarze J, Davidson DJ (2016) Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J Immunol 196(6):2699–2710
da Silva BR, Conrado AJS, Pereira AL, Evaristo FFV, Arruda FVS, Vasconcelos MA, Lorenzón EN, Cilli EM, Teixeira EH (2017) Antibacterial activity of a novel antimicrobial peptide [W7]KR12-KAEK derived from KR-12 against Streptococcus mutans planktonic cells and biofilms. Biofouling 33(10):835–846
de Breij A, Riool M, Kwakman PH, de Boer L, Cordfunke RA, Drijfhout JW, Cohen O, Emanuel N, Zaat SA, Nibbering PH, Moriarty TF (2016) Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J Control Release 222:1–8
de Breij A, Riool M, Cordfunke RA, Malanovic N, de Boer L, Koning RI, Ravensbergen E, Franken M, van der Heijde T, Boekema BK, Kwakman PHS, Kamp N, El Ghalbzouri A, Lohner K, Zaat SAJ, Drijfhout JW, Nibbering PH. (2018) Sci Transl Med. 10(423). pii: eaan4044
den Hertog AL, van Marle J, Veerman EC, Valentijn-Benz M, Nazmi K, Kalay H, Grün CH, Van’t Hof W, Bolscher JG, Nieuw Amerongen AV (2006) The human cathelicidin peptide LL-37 and truncated variants induce segregation of lipids and proteins in the plasma membrane of Candida albicans. Biol Chem 387(10–11):1495–1502
Dürr UH, Sudheendra US, Ramamoorthy A (2006) Membrane fragmentation by an amyloidogenic fragment of human islet amyloid polypeptide detected by solid-state NMR spectroscopy of membrane nanotubes. Biochim Biophys Acta 1768(9):2026–2029
Dutta D, Kumar N, D P Willcox M (2016) Antimicrobial activity of four cationic peptides immobilised to poly-hydroxyethylmethacrylate. Biofouling 32(4):429–438
Epand RF, Wang G, Berno B, Epand RM (2009) Lipid segregation explains selective toxicity of a series of fragments derived from the human cathelicidin LL-37. Antimicrob Agents Chemother 53(9):3705–3714
Findlay F, Pohl J, Svoboda P, Shakamuri P, McLean K, Inglis NF, Proudfoot L, Barlow PG (2017) Carbon nanoparticles inhibit the antimicrobial activities of the human cathelicidin LL-37 through structural alteration. J Immunol 199(7):2483–2490
Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H, Gudmundsson GH (1997) The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 272(24):15258–15263
Gabriel M, Nazmi K, Veerman EC, Nieuw Amerongen AV, Zentner A (2006) Preparation of LL-37-grafted titanium surfaces with bactericidal activity. Bioconjug Chem 17(2):548–550
Grönberg A, Mahlapuu M, Ståhle M, Whately-Smith C, Rollman O (2014) Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: a randomized, placebo-controlled clinical trial. Wound Repair Regen 22:613–621
Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R (1996) The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem 238:325–332
Gunasekera S, Muhammad T, Strömstedt AA, Rosengren KJ, Göransson U (2018) Alanine and lysine scans of the LL-37-derived peptide fragment KR-12 reveal key residues for antimicrobial activity. Chembiochem 19(9):931–939
Gustafsson A, Olin AI, Ljunggren L (2010) LPS interactions with immobilized and soluble antimicrobial peptides. Scand J Clin Lab Invest 70(3):194–200
Harcourt JL, McDonald M, Svoboda P, Pohl J, Tatti K, Haynes LM (2016) Human cathelicidin, LL-37, inhibits respiratory syncytial virus infection in polarized airway epithelial cells. BMC Res Notes 9:11
Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L, Kagnoff MF (2003) Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 125(6):1613–1625
He M, Zhang H, Li Y, Wang G, Tang B, Zhao J, Huang Y, Zheng J (2018) Cathelicidin-derived antimicrobial peptides inhibit Zika virus through direct inactivation and interferon pathway. Front Immunol 9:722
Henzler Wildman KA, Lee DK, Ramamoorthy A (2003) Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry 42(21):6545–6558
Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY (2004) Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol 172(3):1763–1767
Jacob B, Park IS, Bang JK, Shin SY (2013) Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity. J Pept Sci 19(11):700–707
Jayaram LN, Chen JY (2015) Antimicrobial peptides: possible anti-infective agents. Peptides 72:88–94
Jiang W, Sunkara LT, Zeng X, Deng Z, Myers SM, Zhang G (2013) Differential regulation of human cathelicidin LL-37 by free fatty acids and their analogs. Peptides 50:129–138
Kahlenberg JM, Kaplan MJ (2013) Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol 191(10):4895–4901
Kanthawong S, Bolscher JG, Veerman EC, van Marle J, Nazmi K, Wongratanacheewin S, Taweechaisupapong S (2010) Antimicrobial activities of LL-37 and its truncated variants against Burkholderia thailandensis. Int J Antimicrob Agents 36(5):447–452
Kay LE, Ikura M, Tschudin R, Bax A (2011) Three-dimensional triple-resonance NMR Spectroscopy of isotopically enriched proteins. 1990. J Magn Reson 213(2):423–441
Keifer PA, Peterkofsky A, Wang G (2004) Effects of detergent alkyl chain length and chemical structure on the properties of a micelle-bound bacterial membrane targeting peptide. Anal Biochem 331:33–39
Klevens RM, Morrison MA, Nadle J et al (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771
Koziel J, Bryzek D, Sroka A, Maresz K, Glowczyk I, Bielecka E, Kantyka T, Pyrć K, Svoboda P, Pohl J, Potempa J (2014) Citrullination alters immunomodulatory function of LL-37 essential for prevention of endotoxin-induced sepsis. J Immunol 192(11):5363–5372
Kuroda K, Fukuda T, Yoneyama H, Katayama M, Isogai H, Okumura K, Isogai E (2012) Anti-proliferative effect of an analogue of the LL-37 peptide in the colon cancer derived cell line HCT116 p53+/+ and p53−/−. Oncol Rep 28(3):829–834
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC (1995) Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun 63(4):1291–1297
Lee PH, Ohtake T, Zaiou M, Murakami M, Rudisill JA, Lin KH, Gallo RL (2005) Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc Natl Acad Sci U S A 102:3750–3755
Lee CC, Sun Y, Qian S, Huang HW (2011) Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys J 100(7):1688–1696
Lehrer RI, Lu W (2012) α-Defensins in human innate immunity. Immunol Rev 245(1):84–112
Li J, Perez Perez GI. (2018) Is there a role for the non-helicobacter pylori bacteria in the risk of developing gastric cancer? Int J Mol Sci 19(5) pii: E1353
Li X, Li Y, Han H, Miller DW, Wang G (2006a) Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J Am Chem Soc 128:5776–5785
Li Y, Li X, Wang G (2006b) Cloning, expression, isotope labeling, and purification of human antimicrobial peptide LL-37 in Escherichia coli for NMR studies. Protein Expr Purif 47:498–505
Li Y, Li X, Li H, Lockridge O, Wang G (2007) A novel method for purifying recombinant human host defense cathelicidin LL-37 by utilizing its inherent property of aggregation. Protein Expr Purif 54:157–165
McArthur MA (2017) Zika virus: Recent advances toward the development of vaccines and therapeutics. Viruses 9(6) pii: E143
Meister M, Lemaitre B, Hoffmann JA (1997) Antimicrobial peptide defense in Drosophila. BioEssays 19(11):1019–1026
Mishra B, Wang G (2012) Ab initio design of potent anti-MRSA peptides based on database filtering technology. J Am Chem Soc 134:12426–12429
Mishra B, Wang G (2017) Individual and combined effects of engineered peptides and antibiotics on the Pseudomonas aeruginosa biofilms. Pharmaceuticals 10(3):58
Mishra B, Epand RF, Epand RM, Wang G (2013) Structural location determines functional roles of the basic amino acids of KR-12, the smallest antimicrobial peptide from human cathelicidin LL-37. RSC Adv 3:19560–19571
Mishra B, Golla R, Lau K, Lushnikova T, Wang G (2016) Anti-Staphylococcal biofilm effects of human cathelicidin peptides. ACS Med Chem Lett 7:117–121
Mishra B, Lushnikova T, Golla RM, Wang X, Wang G (2017a) Design and surface immobilization of short anti-biofilm peptides. Acta Biomater 49:316–328
Mishra B, Reiling S, Zarena D, Wang G (2017b) Host defense antimicrobial peptide as antibiotics: design and application strategies. Curr Opin Chem Biol 38:87–96
Mishra B, Wang X, Lushnikova T, Zhang Y, Golla RM, Narayana JL, Wang C, McGuire TR, Wang G (2018) Antibacterial, antifungal, anticancer activities and structural bioinformatics analysis of six naturally occurring temporins. Peptides 106:9–20
Murakami M, Lopez-Garcia B, Braff M, Dorschner RA, Gallo RL (2004) Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J Immunol 172:3070–3077
Murakami M, Kameda K, Tsumoto H, Tsuda T, Masuda K, Utsunomiya R, Mori H, Miura Y, Sayama K (2017) TLN-58, an additional hCAP18 processing form, found in the lesion vesicle of palmoplantar pustulosis in the skin. J Invest Dermatol 137(2):322–331
Nan YH, Bang JK, Jacob B, Park IS, Shin SY (2012) Prokaryotic selectivity and LPS-neutralizing activity of short antimicrobial peptides designed from the human antimicrobial peptide LL-37. Peptides 35(2):239–247
Nell MJ, Tjabringa GS, Wafelman AR, Verrijk R, Hiemstra PS, Drijfhout JW, Grote JJ (2006) Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides 27(4):649–660
Nie B, Ao H, Chen C, Xie K, Zhou J, Long T, Tang T, Yue B (2016) Covalent immobilization of KR-12 peptide onto a titanium surface for decreasing infection and promoting osteogenic differentiation. RSC Adv 6(52):46733–46743
Niemirowicz K, Durnaś B, Tokajuk G, Piktel E, Michalak G, Gu X, Kułakowska A, Savage PB, Bucki R (2017) Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13. Sci Rep 7(1):4610
Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL (2001) Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414(6862):454–457
Ogawa Y, Kawamura T, Matsuzawa T, Aoki R, Gee P, Yamashita A, Moriishi K, Yamasaki K, Koyanagi Y, Blauvelt A, Shimada S (2013) Antimicrobial peptide LL-37 produced by HSV-2-infected keratinocytes enhances HIV infection of Langerhans cells. Cell Host Microbe 13(1):77–86
Ohsaki Y, Gazdar AF, Chen HC, Johnson BE (1992) Antitumor activity of magainin analogues against human lung cancer cell lines. Cancer Res 52(13):3534–3538
Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y (1999) Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J 341(Pt 3):501–513
Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE (2008) Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun 76(9):4176–4182
Overhage PE, McHugh B, Gwyer Findlay E, Mackellar A, Mackenzie KJ, Gallo RL, Govan JR, Simpson AJ, Davidson DJ (2014) Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PLoS One 9:e99029
Peschel A, Sahl HG (2006) The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4:529–536
Pütsep K, Carlsson G, Boman HG, Andersson M (2002) Deficiency of antibacterial peptides in patients with morbus kostmann: an observation study. Lancet 360:1144–1149
Rajasekaran G, Kim EY, Shin SY (2017) LL-37-derived membrane-active FK-13 analogs possessing cell selectivity, anti-biofilm activity and synergy with chloramphenicol and anti-inflammatory activity. Biochim Biophys Acta 1859(5):722–733
Rapala-Kozik M, Bochenska O, Zawrotniak M, Wolak N, Trebacz G, Gogol M, Ostrowska D, Aoki W, Ueda M, Kozik A (2015) Inactivation of the antifungal and immunomodulatory properties of human cathelicidin LL-37 by aspartic proteases produced by the pathogenic yeast Candida albicans. Infect Immun 83(6):2518–2530
Ren SX, Cheng AS, To KF, Tong JH, Li MS, Shen J, Wong CC, Zhang L, Chan RL, Wang XJ, Ng SS, Chiu LC, Marquez VE, Gallo RL, Chan FK, Yu J, Sung JJ, Wu WK, Cho CH (2012) Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res 72(24):6512–6523
Ren SX, Shen J, Cheng AS, Lu L, Chan RL, Li ZJ, Wang XJ, Wong CC, Zhang L, Ng SS, Chan FL, Chan FK, Yu J, Sung JJ, Wu WK, Cho CH (2013) FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS One 8(5):e63641
Ron-Doitch S, Sawodny B, Kühbacher A, David MMN, Samanta A, Phopase J, Burger-Kentischer A, Griffith M, Golomb G, Rupp S (2016) Reduced cytotoxicity and enhanced bioactivity of cationic antimicrobial peptides liposomes in cell cultures and 3D epidermis model against HSV. J Control Release 229:163–171
Saar-Dover R, Bitler A, Nezer R, Shmuel-Galia L, Firon A, Shimoni E, Trieu-Cuot P, Shai Y (2012) D-alanylation of lipoteichoic acids confers resistance to cationic peptides in group B streptococcus by increasing the cell wall density. PLoS Pathog 8:e1002891
Santos CM, Kumar A, Kolar SS, Contreras-Caceres R, McDermott A, Cai C (2013) Immobilization of antimicrobial peptide IG-25 onto fluoropolymers via fluorous interactions and click chemistry. ACS Appl Mater Interfaces 5(24):12789–12793
Saporito P, Vang Mouritzen M, Løbner-Olesen A, Jenssen H (2018) LL-37 fragments have antimicrobial activity against Staphylococcus epidermidis biofilms and wound healing potential in HaCaT cell line. J Pept Sci 8:e3080
Schauber J, Iffland K, Frisch S, Kudlich T, Schmausser B, Eck M, Menzel T, Gostner A, Lührs H, Scheppach W (2004) Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol 41(9):847–854
Schögler A, Muster RJ, Kieninger E, Casaulta C, Tapparel C, Jung A, Moeller A, Geiser T, Regamey N, Alves MP (2016) Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur Respir J 47:520–530
Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE (2002) The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol 169(7):3883–3891
Sevcsik E, Pabst G, Richter W, Danner S, Amenitsch H, Lohner K (2008) Interaction of LL-37 with model membrane systems of different complexity: influence of the lipid matrix. Biophys J 94:4688–4699
Shah NR, Hancock RE, Fernandez RC (2014) Bordetella pertussis lipid A glucosamine modification confers resistance to cationic antimicrobial peptides and increases resistance to outer membrane perturbation. Antimicrob Agents Chemother 58(8):4931–4934
Sieprawska-Lupa M, Mydel P, Krawczyk K, Wójcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, Shafer W, McAleese F, Foster T, Travis J, Potempa J (2004) Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother 48(12):4673–4679
Sigurdardottir T, Andersson P, Davoudi M, Malmsten M, Schmidtchen A, Bodelsson M (2006) In silico identification and biological evaluation of antimicrobial peptides based on human cathelicidin LL-37. Antimicrob Agents Chemother 50(9):2983–2989
Singh D, Vaughan R, Kao CC (2014) LL-37 peptide enhancement of signal transduction by Toll-like receptor 3 is regulated by pH: identification of a peptide antagonist of LL-37. J Biol Chem 289(40):27614–27624
Skerlavaj B, Gennaro R, Bagella L, Merluzzi L, Risso A, Zanetti M (1996) Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J Biol Chem 271(45):28375–28381
Sol A, Ginesin O, Chaushu S, Karra L, Coppenhagen-Glazer S, Ginsburg I, Bachrach G (2013) LL-37 opsonizes and inhibits biofilm formation of Aggregatibacter actinomycetemcomitans at subbactericidal concentrations. Infect Immun 81:3577–3585
Song DW, Kim SH, Kim HH, Lee KH, Ki CS, Park YH (2016) Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: implications for wound healing. Acta Biomater 39:146–155
Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS et al (2001) Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97(12):3951–3959
Sørensen OE, Gram L, Johnsen AH, Andersson E, Bangsbøll S, Tjabringa GS, Hiemstra PS, Malm J, Egesten A, Borregaard N (2003) Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: a novel mechanism of generating antimicrobial peptides in vagina. J Biol Chem 278(31):28540–28546
Sousa FH, Casanova V, Findlay F, Stevens C, Svoboda P, Pohl J, Proudfoot L, Barlow PG (2017) Cathelicidins display conserved direct antiviral activity towards rhinovirus. Peptides 95:76–83
Spencer JD, Schwaderer AL, Becknell B, Watson J, Hains DS (2014) The innate immune response during urinary tract infection and pyelonephritis. Pediatr Nephrol 29(7):1139–1149
Sung CM, Kim HC, Cho YB, Shin SY, Jang CH (2017) Evaluating the ototoxicity of an anti-MRSA peptide KR-12-a2. Braz J Otorhinolaryngol pii: S1808-8694(17)30078–2
Suzuki K, Murakami T, Kuwahara-Arai K, Tamura H, Hiramatsu K, Nagaoka I (2011) Human anti-microbial cathelicidin peptide LL-37 suppresses the LPS-induced apoptosis of endothelial cells. Int Immunol 23(3):185–193
Suzuki K, Murakami T, Hu Z, Tamura H, Kuwahara-Arai K, Iba T, Nagaoka I (2016) Human host defense cathelicidin peptide LL-37 enhances the lipopolysaccharide uptake by liver sinusoidal endothelial cells without cell activation. J Immunol 196(3):1338–1347
Tack BF, Sawai MV, Kearney WR, Robertson AD, Sherman MA, Wang W, Hong T, Boo LM, Wu H, Waring AJ, Lehrer RI (2002) SMAP-29 has two LPS-binding sites and a central hinge. Eur J Biochem 269(4):1181–1189
Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S (1999) Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11(4):443–451
Tanphaichitr N, Srakaew N, Alonzi R, Kiattiburut W, Kongmanas K, Zhi R, Li W, Baker M, Wang G, Hickling D (2016) Potential use of antimicrobial peptides as vaginal spermicides/microbicides. Pharmaceuticals 9:13
Tian M, Zhao DY, Wang HW (2011) Studies on the core functional region of antimicrobial peptide LL-37 for inhibition of RSV replication. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 25(5):355–357. In Chinese
Tripathi S, Tecle T, Verma A, Crouch E, White M, Hartshorn KL (2013) The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J Gen Virol 94(Pt 1):40–49
Tripathi S, Verma A, Kim EJ, White MR, Hartshorn KL (2014) LL-37 modulates human neutrophil responses to influenza A virus. J Leukoc Biol 96(5):931–938
Tripathi S, Wang G, White M, Rynkiewicz M, Seaton B, Hartshorn K (2015a) Identifying the critical domain of LL-37 involved in mediating neutrophil activation in the presence of influenza virus: functional and structural analysis. PLoS One 10(8):e0133454
Tripathi S, Wang G, White M, Qi L, Taubenberger J, Hartshorn KL (2015b) Antiviral activity of the human cathelicidin, LL-37, and derived peptides on seasonal and pandemic influenza A viruses. PLoS One 10:e0124706
Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI (1998) Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother 42(9):2206–2214
Uchio E, Inoue H, Kadonosono K (2013) Anti-adenoviral effects of human cationic antimicrobial protein-18/LL-37, an antimicrobial peptide, by quantitative polymerase chain reaction. Korean J Ophthalmol 27(3):199–203
van der Does AM, Bergman P, Agerberth B, Lindbom L (2012) Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J Leukoc Biol 92(4):735–742
Vandamme D, Landuyt B, Luyten W, Schoofs L (2012) A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol 280(1):22–35
Velarde JJ, Ashbaugh M, Wessels MR (2014) The human antimicrobial peptide LL-37 binds directly to CsrS, a sensor histidine kinase of group A Streptococcus, to activate expression of virulence factors. J Biol Chem 289(52):36315–36324
Wang G (2006) Structural biology of antimicrobial peptides by NMR spectroscopy. Curr Org Chem 10:569–581
Wang G (2007) Determination of solution structure and lipid micelle location of an engineered membrane peptide by using one NMR experiment and one sample. Biochim Biophys Acta 1768:3271–3281
Wang G (2008) Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem 283:32637–32643
Wang G (2010) Structure, dynamics and mapping of membrane-binding residues of micelle-bound antimicrobial peptides by natural abundance 13C NMR spectroscopy. Biochim Biophys Acta 1798:114–121
Wang G (2012) Natural antimicrobial peptides as promising anti-HIV candidates. Curr Top Peptide Protein Res 13:93–110
Wang G (2014) Human antimicrobial peptides and proteins. Pharmaceuticals 7:545–594
Wang G (2017) In: Wang G (ed) Antimicrobial peptides. CABI, UK, pp 1–19. and 169–187
Wang Z, Wang G (2004) APD: the antimicrobial peptide database. Nucleic Acids Res 32:D590–D592
Wang G, Keifer PA, Peterkofsky A (2004) Short-chain diacyl phosphatidylglycerols: which one to choose for NMR structural determination of a membrane-associated peptide from Escherichia coli? Spectroscopy 18:257–264
Wang G, Waston K, Buckheit R Jr (2008) Anti-human immunodeficiency virus type 1 (HIV-1) activities of antimicrobial peptides derived from human and bovine cathelicidins. Antimicrob Agents Chemother 52:3438–3440
Wang G, Li X, Wang Z (2009) APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res 37:D933–D937
Wang G, Elliott M, Cogen AL, Ezell EL, Gallo RL, Hancock REW (2012a) Structure, dynamics, antimicrobial and immune modulatory activities of human LL-23 and its single residue variants mutated based on homologous primate cathelicidins. Biochemistry 51:653–664
Wang G, Epand RF, Mishra B, Lushnikova T, Thomas VC, Bayles KW, Epand R (2012b) Decoding the functional roles of cationic side chains of the major antimicrobial region of human cathelicidin LL-37. Antimicrob Agents Chemother 56:845–856
Wang G, Hanke ML, Mishra B, Lushnikova T, Heim CE, Chittezham Thomas V, Bayles KW, Kielian T (2014a) Transformation of human cathelicidin LL-37 into selective, stable, and potent antimicrobial compounds. ACS Chem Biol 9:1997–2002
Wang G, Mishra B, Epand RF, Epand RM (2014b) High-quality 3D Structure shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim Biophys Acta 1838:2160–2172
Wang G, Mishra B, Lau K, Lushnikova T, Golla R, Wang X (2015) Antimicrobial peptides in 2014. Pharmaceuticals 8:123–150
Wang G, Li X, Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44:D1087–D1093
Wang X, Junior JCB, Mishra B, Lushnikova T, Epand RM, Wang G (2017) Arginine-lysine positional swap of the LL-37 peptides reveals evolutional advantages of the native sequence and leads to bacterial probes. Biochim Biophys Acta 1859:1350–1361
Wang X, Mishra B, Lushinikova T, Narayana JL, Wang G (2018) Amino acid composition determines peptide activity spectrum and hot spot-based design of merecidin. Adv Biosyst 2:1700259
Wong JH, Legowska A, Rolka K, Ng TB, Hui M, Cho CH, Lam WW, Au SW, Gu OW, Wan DC (2011) Effects of cathelicidin and its fragments on three key enzymes of HIV-1. Peptides 32(6):1117–1122
Wu WK, Sung JJ, To KF, Yu L, Li HT, Li ZJ, Chu KM, Yu J, Cho CH (2010a) The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J Cell Physiol 223(1):178–186
Wu WK, Wang G, Coffelt SB, Betancourt AM, Lee CW, Fan D, Wu K, Yu J, Sung JJ, Cho CH (2010b) Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int J Cancer 127(8):1741–1747
Yasin B, Pang M, Turner JS, Cho Y, Dinh NN, Waring AJ, Lehrer RI, Wagar EA (2000) Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis 19(3):187–194
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A 84(15):5449–5453
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389–395
Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, Gallo RL (2015) Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 347:67–71
Acknowledgments
This study is supported by the NIAID/NIH grant R01 AI105147 and AI128230 to GW. This chapter reflects the point of view of the authors and may not represent the funding agency.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Wang, G. et al. (2019). Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. In: Matsuzaki, K. (eds) Antimicrobial Peptides. Advances in Experimental Medicine and Biology, vol 1117. Springer, Singapore. https://doi.org/10.1007/978-981-13-3588-4_12
Download citation
DOI: https://doi.org/10.1007/978-981-13-3588-4_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3587-7
Online ISBN: 978-981-13-3588-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)