Abstract
Refrigerant significantly influences the performance of air-conditioning and refrigeration system as well as it has some environmental issues that need to be considered before selection. These systems can be made eco-friendly, if it is powered by solar energy or low-grade thermal energy and they use environment-friendly refrigerants. In this chapter, low GWP (global warming potential) refrigerants have been explored for the domestic air-conditioning applications. Refrigerants with high GWP are mostly used in environment control applications such as heating, ventilation, air-conditioning (HVAC), and refrigeration systems. Some refrigerants contribute to significant environmental issues and the Montreal Protocol and Kyoto Protocol have been signed to address the threats of ozone layer depletion and global warming potential. To fulfil the commitments of Kyoto Protocol, meanwhile, governments in many countries instituted phase-out plan for the use of environmentally harmful gasses in heat pump systems. For instance, EU MAC Directives, F-gas regulation, and Japan METI directives, which clearly declared their target year to use new refrigerant of GWP below 150 for mobile air conditioner and GWP below 750 for the residential air conditioner. Research interest has been stimulated to find alternative refrigerants with low or ultra-low GWP for energy conservation and environmental sustainability. Hydrofluoroolefins (HFOs) have a very low environmental impact, and thus HFOs are considering as potential candidates to replace the hydrofluorocarbon (HFC) refrigerants such as R410A, a near-azeotropic mixture of difluoromethane (R-32) and pentafluoroethane (R-125) and is commonly used refrigerant in air-conditioning applications. The limited number of pure fluids sometimes cannot meet the excellent heat transfer criteria due to their low volumetric capacity and moderate flammability or toxicity. Mixing of HFOs and HFCs refrigerants, in this case, allows the adjustment of the most desirable properties of the refrigerant by varying the molar fraction of the components. Different combination of mixture presented here cannot be claimed as the best mixture, but it might be a good choice for further study. This chapter focuses on the research trend in finding low GWP refrigerants and its application in different heat pump system considering the system performance, safety, and the overall environmental impact. The conventional vapor compression system, thermally driven adsorption system, and sorption-compression hybrid system have been taken into consideration.
Shigeru Koyama: Deceased on August 4, 2018.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Global warming potential
- Hydrofluoro-olefins (HFOs)
- Refrigerant blend
- Temperature glide
- Volumetric capacity
1 Introduction
The air-conditioning and refrigeration system are widely used in a domestic and industrial application for providing thermal comfort and storage facilities (Calm and Didion 1998). According to the Japan Refrigeration and Air Conditioning Industry Association (JRAIA), the demand for room air conditioners (RAC) is estimated to reach 88.81 million units in 2016 which is 2.9% higher than the previous year. Figure 15.1 presents the huge demand of RAC and in a country or region which reflect the significance of environmental concern in this sector in terms of greenhouse gas emission and electrical energy consumption.
World room air-conditioning demand (RAC) in 2016 (JRAIA 2017)
Refrigerants as heat carrier substance significantly influence the performance of the RAC and refrigeration systems. Thermophysical properties of such refrigerants are crucial in designing such a system. Additionally, flammability, toxicity, and environmental friendliness are equally important. From the beginning of commercial production of the system, most of the popular refrigerants such as CFCs and HCFCs possess show excellent system performance. These refrigerants are not flammable and toxic but possess higher ODP and GWP characteristics. Following the Montreal Protocol (1987), a gradual phase-out of refrigerants that deplete the ozone layer has been implemented and are substituted with more environment-friendly refrigerants which have almost zero ODP (UNEP 2016; Benhadid-Dib and Benzaoui 2012). The air-conditioning and refrigeration industry have taken many initiatives after signing the Montreal Protocol to overcome challenges in developing suitable refrigerants to replace the popular CFCs and HCFCs refrigerants (Calm and Didion 1998; McMullan 2002). Remarkably, the CFCs phase-out has been accomplished in 1996 in the developed countries whilst the targeted deadline for the developing countries is the year 2010 whereas HCFCs phase-out will be accomplished in 2030 or earlier (Powell 2002). Today, most acceptable choice of refrigerants as an alternative to the ozone-depleting refrigerants (CFCs and HCFCs), includes R410A, R134a, R407C, etc., particularly, for residential and automobile air-conditioning systems.
However, the global warming potential (GWP) of these refrigerants are considered very high. For example, hydrofluorocarbon refrigerant R134a and R410A have zero ODP but their GWP values are 1300 and 1900, respectively (Cabello et al. 2015; Xu et al. 2013). Refrigerant R410A has good thermal and transport properties with high volumetric capacity for air-conditioning applications, but it has an unfavorable effect on the environment in case of leakage that might be resulted during maintenance and unavoidable wear and tear. The Kyoto Protocol in 1997 (COP3-1997) (Parties to the Protocol 1998) commits the state parties will reduce the greenhouse gas emission to the atmosphere at least 5% below 1990 levels in the first commitment period, i.e., from 2008 to 2012. In the second commitment period, from 2013 to 2020 (agreed in 2012, known as Doha Amendment to the protocol), 37 countries including the European Union agreed to reduce 18% greenhouse gas emission. According to the European Environment Agency report (2016), the emission of F-gas, which includes HFCs, PFCs, and SF6, will be reduced by two-thirds of its 2014 value by 2030. In the USA, the Montreal Protocol is amended and now is implementing as the Clean Air Act via the US Environment Protection Agency (EPA). The EPA (1990) decided to limit the use of R134a in a newly manufactured light-duty vehicle from the model year 2021 and HVAC units using HCFC-22 can continue to be serviced until 2020. After the model year 2025, R134a will not be accepted in MVAC system although its production will continue for servicing older vehicles contain R134a. The Australian government has imposed 50$/kg carbon tax for R410A (Pham and Rajendran 2012).
Finding low GWP refrigerants with excellent energy efficiency has become an urgent task of the present generation whilst the total climate impact associated with refrigerants consists of direct and indirect contributions. Realizing the long-term environmental commitments and to comply with global regulations, the auto industry began to look for new, low GWP air conditioner refrigerants in approximately 2011. There are a number of refrigerants available which have a low environmental impact but not suitable to replace the existing halogenated refrigerants. The phase-out plan of high GWP refrigerants used in domestic and automobile application is listed in Table 15.1.
This chapter will focus both the pure and blend refrigerants which have ultra-low and low GWP but system performance equivalent or better to the existing refrigerant in domestic and automobile air-conditioning application. According to the taxonomy of RTOC 2014 Assessment Report (UNEP 2016), the 100 year GWP levels are classified in five levels which is shown in Table 15.2.
2 Refrigerant Selection
Currently, the widely used refrigerant in the domestic air-conditioning system is R410A which has no ODP but higher GWP. Alternative refrigerant with low GWP is desirable to fulfil the directive of Kyoto Protocol and some countries local guideline. Low GWP refrigerant mixture is being studied by many authors around the world considering the system performance, flammability, and toxicity. The refrigerants R32, R1234yf, R1234ze(E), R744, R152a, and hydrocarbon (HC) possesses very low GWP and within safety limit. Sometime refrigerant blend is found suitable to replace the widely used R410A. Blend is usually a mixture of two or more refrigerants. Some characteristics features of the pure refrigerants are furnished in Table 15.3. Along with the GWP and safety limit, the volumetric capacity and cycle performance are equally important. For blend, the temperature glide needs to be under consideration.
2.1 Global Warming Effect
GWP100 is measured by the amount of heat traps in a refrigerant compared to the heat trap by the same mass of CO2 over 100-year horizon. A clear definition of GWP calculation is to be found in the IPCC’s 2001 Third Assessment Report. The widely used refrigerants in domestic and automobile applications are R410A and R134a, have the GWP of 1900 and 1300, respectively. After Kyoto Protocol, research interest on low GWP refrigerant intensified. Initially, HFCs and HFOs are put into the domain. But in most of the cases, single component low GWP cannot meet the requirements to drop-in replacement of R410A. Now refrigerant blend is considering as an alternative refrigerant. Blend are formed by mixing two or more single component refrigerants, the GWP of a refrigerant mixture is calculated as the mass weighted average of GWPs of individual components in the mixture. To respond the international guideline, refrigeration industry is looking for ultra-low GWP refrigerant either pure or blend. The GWP value of the mixture can be adjusted varying the composition of constituents in a mixture. Table 15.4 lists the most widely used refrigerants with respective ODP and GWP in the residential and automobile applications.
Following the number of components in the blend the GWP can be calculated as:
2.2 Volumetric Capacity
Volumetric capacity is defined by the cooling capacity per unit of vapor volume at the exit of evaporator. It is calculated by the product of evaporation enthalpy (∆heva) and vapor density at evaporator outlet (ρv). A refrigerant with high volumetric capacity gives high cooling capacity for a given swept volume in compressor (Granryd 2001). Generally, low GWP HFOs have found low volumetric capacity also compared to HFCs. By mixing HFOs with higher volumetric HFCs can solve the problem of low volumetric capacity. So the mixture of high GWP HFCs with low GWP HFOs increases the volumetric capacity but it reduces the GWP. Table 15.5 shows the volumetric capacity of some pure refrigerant at a temperature −3 °C.
Volumetric capacity,
2.3 Flammability and Toxicity
Most potential refrigerant having lower GWP and toxicity possess higher flammability, which is a problem to replace HCFC refrigerants. A special security arrangement is required to make the system when flammable refrigerant is used. The LFL and UFL (lower and upper flammable limit) range the basic combustion characteristics of flammable refrigerants. ASHRAE (American Society of Heating, Refrigeration and air conditioning) updated two standards, NSI/ASHRAE standard 15 Safety Standard for Refrigeration Systems and standard 34 Designation and Safety Classification of Refrigerants. In the latest version, classification “2L” was added to highlight the lower flammability refrigerants with a maximum burning velocity <10 cm/s which benefit the promotion of HFOs and R32 (Yang and Wu 2013). The burning velocity and safety group of some refrigerants are presented in Table 15.6.
2.4 Refrigerant Mixture
To use pure refrigerant in a system is always easy because of its azeotropic nature and the available thermophysical properties. Nowadays, HFO refrigerants get attention due to its suitable properties like extremely low GWP, lower toxicity, and moderate flammability (A2L). But some researcher reported that pure HFOs are not good choice as an alternative of R410A in residential scale air-conditioning due to their low vapor pressure (Kojima et al. 2015; Barve and Cremaschi 2012; Koyama et al. 2011). The improvement of overall system performance is achieved by many researchers when added with HFC or natural refrigerant (Han et al. 2007; Mota-Babiloni et al. 2015; Maczek et al. 1997; Wu et al. 2009). Refrigerant mixture can solve the problem of very limited number of fluids which have suitable properties. But there is a chance to alter the other properties of mixture. There are three categories of mixtures which can be used as working fluids: azeotropes, near-azeotropes, and non-azeotropes. This chapter highlighted some important parameters of binary and ternary mixture which need to be considered during mixture formation such as temperature glide, volumetric capacity, GWP, and cycle performance. In this chapter, few new mixture properties are presented keeping in mind the performance of widely used R410A (Table 15.7).
2.5 Temperature Glide
During the evaporation process, refrigerant begins to boil at a saturated liquid temperature, called bubble point, and ended with saturated vapor pressure, called dew point. At constant pressure, the difference between dew point and bubble point is called temperature glide. Pure refrigerant boils and condenses at a constant temperature so it has no gliding temperature. A mixture of two or more different fluids is classified as azeotropes and has long been used in the refrigeration industry; this refrigerant behaves like a pure refrigerant so it has no gliding temperature. The boiling point and condensation point curves unite at a point at which vapor and liquid have the same concentration. Near-azeotropes have a much greater potential for drop-in alternatives to HFCs (Morrison and McLinden 1993). The temperature change during evaporation and condensation is negligible (1–2 K) for near-azeotropic mixture. Non-azeotropic (zeotropic) mixtures have a separate curve for bubble point and dew point over the full concentration range. During the evaporation, a more volatile component of the mixture starts to boil first where less volatile component boils at last, so there is a concentration change with temperature, that creates temperature glide (3–20 K). Sometime leakage may alter their composition and properties so as to Tglide. At composition X0 in Fig. 15.2, the refrigerant begins to boil at T1 and ended at T2 (Tglide = T2 − T1), the temperature varies because the evaporating liquid continuously changing its composition and thus the boiling point.
Temperature glide
2.6 Cycle Performance
The cycle performance of the refrigerant is very important and can be calculated theoretically and experimentally. The coefficient of performance (COP) can be evaluated theoretically employing thermophysical properties of refrigerant from REFPROP database which contain so many refrigerants. Considering the application area, cycle simulation requires operating condition for the system. As the mixture shows temperature glide during evaporation and condensation, so pressure selection for simple calculation is difficult. Sometimes average values for temperature are considered for evaporation and condensation in the simulation process. In this chapter, mixture performance are shown using constant operating parameters which are shown in Table 15.8. The following equations can be used where suffix 1, 2, 2a, 3, and 4 are picked from Fig. 15.3.
Coefficient of Performance (COP) for heating of an ideal cycle is presented in Eq. 15.3.
where
For refrigeration or cooling COP,
Volumetric capacity during evaporation can be calculated as
Temperature glide at evaporator can be calculated as shown in Eq. 15.7, where the temperatures are shown in Fig. 15.4. Basic thermodynamic cycle.
3 Low GWP Refrigerants
There is an urgent need to find a low GWP refrigerant to develop sustainable technologies. The current single component low GWP refrigerant may increase energy consumption, introduce safety risk and sometimes require significant system modification. Refrigerant blend can be an effective alternative to achieve sustainable building technology reducing energy consumption and greenhouse gas emissions by 50% compared to the current best refrigerants. This section discusses the ongoing research activities about some pure and blend refrigerants which are considered as a promising alternative.
3.1 Pure Refrigerants
3.1.1 R1234yf
The R1234yf (CF3CF = CH2) is a refrigerant of GWP < 1. It is low in toxicity and mildly flammable (A2L) (Minor et al. 2010; Honeywell Technical Bulletin 2012). It has no ODP but has excellent life cycle climate performance (LCCP) compared to R134a and R744 (Spatz and Minor 2008). The critical temperature and critical pressure of the refrigerant are 94.7 °C and 3.38 MPa, respectively (Tanaka and Higashi 2010; Akasaka et al. 2010; Lai et al. 2011). In 2007, the SAE International launched CRP 1234 program to investigate the safety and performance of HFO 1234yf for the use in mobile air-conditioning. It has got attention as a prospective alternative candidate of R134a, the mostly used refrigerant in automobiles (Spatz and Minor 2008; SAE-CRP 1234 2009; Lee and Jung 2012) though the performance of R11234yf is slightly lower than R134a (Navarro-Esbrí et al. 2013; Zilio et al. 2011; Qi 2015). General Motors started using R1234yf for vehicles in 2012 (Sciance 2013). However, when this refrigerant is used as an alternative to R410A, it shows lower coefficient of performance (COP) and also it requires larger unit bodies related to R410A (Barve and Cremaschi 2012; Fukuda et al. 2016). Figure 15.5 shows that thermodynamic cycle of R1234yf positioned much lower than R410A. The volumetric capacity of R1234yf is lower compared to R410A. Insufficient production capacity and higher price are another constraints to use this refrigerant in the larger scale.
3.1.2 R1234ze(E)
The (R1234ze(E) (CF3CH = CHF) got attention due to its ultra-low GWP (<1) and it is investigated as a candidate refrigerant for industrial centrifugal chillers (Ueda et al. 2011; Yang et al. 2015). The critical temperature and critical pressure of the refrigerant are 109.3 °C and 3.63 MPa, respectively (Higashi et al. 2010; Brown et al. 2010). It is a potential refrigerant for high-temperature heat pumps which works as hot dryers and steam generators for industrial purposes, such as the concentration of beverages, sterilization of foods, drying lumber, solvent recovery and distillation of petrochemical products. The possibility of using the refrigerant R1234ze(E) and R1234ze(Z) into high-temperature heat pump system has been investigated by Fukuda et al. (2014). The flammability of the refrigerant was studied by the same authors and found all the properties are suitable for the future application. Brown et al. (2009) predicted the performance potential of R1234ze(Z) in high-temperature heat pumping applications and suggested for further research as a possible alternative of R114. Experimental studies revealed that due to its low volumetric capacity, pure R1234ze(E) is not suitable as an alternative for R410A (Koyama et al. 2011) but it is suitable for turbo refrigeration system if the impeller size of the compressor is enlarged as compared to that of R134a (Koyama et al. 2010a). The energy saving potential of the refrigerant is found to 9–15% compared to R134a (Kabeel et al. 2016; Lai 2014). Figure 15.6 shows the schematic diagram of water-cooled experimental set up which is used to study the performance of new refrigerants and their blends (Fukuda et al. 2016).
Experimental set up to test new refrigerant (Koyama et al. 2010a)
Figure 15.7 shows the coefficient of performance (COP) of R1234ze(E) compared to R410A and R32, where it is found that the COP of R1234ze(E) is lower than other two. The COP is the ratio of heat transfer in the condenser or evaporator to the compressor/inverter input and can be estimated based on the cycle level and system level. The COPcycle and the COPsystem are calculated using the following equations.
Thermodynamic cycle COP for heating,
Thermodynamic cycle COP for refrigeration,
System COP for heating,
System COP for refrigeration or cooling,
3.1.3 R152a
HFC-152a (1,1 difluoroethane, C2H4F2) has been used for many years as a component in refrigerant blends but not as a single compound. The critical temperature and pressure of the refrigerant are 113 °C and 4.52 MPa, respectively (Higashi et al. 1987; Tamatsu et al. 1993; Van Poolen et al. 1997). It has zero ODP. The special advantage of this refrigerant is the low global warming potential (=138, Stocker et al. 2013) and reduced price compared to other HFC and HFO refrigerants. Compared to R134a, R152a possesses very similar volumetric cooling capacity and pressure levels, while the energy efficiency, the mass flow, and the vapor density are even more favorable (SAE-CRP 1234 2009). Bolaji (2010) and Cabello et al. (2015) reported superior system performance of R152a in a vapor compression system while comparing to the experimental result with R134a. Their test results revealed an improvement in COP ranges from 4.7 to 13% with a decrease in cooling capacity of about 0–10%. This refrigerant has been used for a long time as an aerosol spray propellant and foam blowing agent, as well as a component in some refrigerant blends (R401A, R415A, R430A, R500, etc.). However, its flammability is ranked as A2 by ASHRAE (Wilson et al. 2010), and hence flammable hazards could be the major reason in hindering its usage as a pure refrigerant until now.
3.1.4 R744
R744 (Carbon dioxide, CO2) is a natural refrigerant used in vapor compressor systems of many types for over 130 years (Pearson 2005; Austin and Sumathy 2011; Bolaji and Huan 2013). The cost of this refrigerant is low and it is not necessary for recovery or disposal but it requires extremely high pressure as it operates at transcritical refrigeration cycle. There is a renewed interest in R744 as it is free of toxicity and flammability (Austin and Sumathy 2011; Maina and Huan 2015). Gustav Lorentzen was the pioneer of the revival of R744 in the early 1990s. Lorentzen and Pettersen (1994) developed a laboratory prototype of car air-conditioning system to evaluate the cycle performance of R744 and R12. Authors suggested that the higher energy density of high-pressure refrigerants may give considerable advantages in terms of cost and practicality due to the reduced dimension and weight. Xue et al. (2010) developed a steady state model of the R744 transcritical cycle for air-conditioning to estimate the heating and cooling performance. The performance of R744 both theoretically and experimentally as well as the comparison with other refrigerants has been conducted by many authors as a viable alternative of synthetic fluids (Brown et al. 2002; Jing-yang et al. 2003; Girotto et al. 2004; He et al. 2016; Pitarch et al. 2016; Chen et al. 2017). Hwang et al. (2004) measured the performance of different two-stage compressor R744 cycles and found 18–35% improved COP over the basic cycles at 7.2 °C evaporating temperature. Girotto et al. (2004) found a possibility to improve the efficiency of the CO2 system approaching the efficiency of the R404A system though the installation cost is 20% higher. Maina and Huan (2015), and Nekså (2002) reviewed numerous area of applications for R744 including hot water production, commercial refrigeration, and heat pump dryers.
3.1.5 HC
HC (hydrocarbon) is a natural refrigerant which offers in general, high efficiency, good miscibility with mineral oils, lower compressor discharge pressure and good heat transfer criteria, but its highly flammable behavior limits the usage of this refrigerant in a larger scale. The use of HCs as refrigerant is confined to Europe because many other countries elsewhere banned the use of flammable gas in the presence of public. Lampugnani and Zgliczynski (1996) studied the performances of R290 in comparison with R22 theoretically and experimentally. The experimental result showed that R290 is an excellent candidate to replace R22 from the thermodynamic point of view. Granryd (2001) reviewed the possibilities and problems of using hydrocarbons as the refrigerant for refrigeration and heat pump applications. It is found a number of HC have favorable characteristics as refrigerants from the thermodynamic and heat transfer point of view. Halimic et al. (2003) concluded in their study that R290 is an attractive alternative to R12 in small domestic refrigerators after correcting technical operation and safety factors. Bjerre and Larsen (2006) evaluated the potential of R600 for household applications and found 10% better performance than R134a. Palm (2008) reviewed the excellent performance of HC comparing R134a, R22, and ammonia. The author suggested that the safety risk can be reduced by designing the systems as hermetic type with the minimum number of connections and a minimum charge of refrigerant or it can be used as an indirect system. Corberán et al. (2008) reviewed the standard of using HC in vapor compression system. It is stated that the IEC355.2.20 standard allows up to approximately 150 g of HC sealed in the typical refrigerator and small freezers are permitted to be located anywhere regardless of the room size incorporating a few special safety measures. This standard has opened the way for some European refrigerator manufacturers to produce household refrigerator with flammable HCs.
3.1.6 R32
R32 is a difluoromethane having GWP of 677. The critical temperature and pressure of this refrigerant is 78.2 °C and 5.8 MPa, respectively. Though the safety class of this refrigerant is 2L, it is higher hazardous than R1234yf due to its faster flame propagation. The volumetric capacity (VC) of R32 is higher than R410A (see Table 15.4) which shows the potentiality of higher COP than R410A. The larger VC reduces the charging amount even to achieve similar COP of that of R410A. The direct equivalent greenhouse gas emission of R32 is also lower due to low charging. But the higher compressor discharge temperature of R32 adversely affects its vast application (Mota-Babiloni et al. 2017; Bolaji 2010; Leck 2010).
3.2 Refrigerant Blend
Pure refrigerants for vapor compression systems can be considered convenient due to azeotropic nature and mostly well-developed thermophysical properties. For pure refrigerants, HFOs are good choices in terms of toxicity, flammability, and ultra-low GWP. Bella and Kaemmer (2011) reported that pure HFOs are not efficient alternatives for R410A from the viewpoint of system performance because redesigning the system is required with larger compressor, piping, and heat exchangers. Mixing HFOs with some other refrigerants is one approach to solve the aforementioned problems. Generally, the addition of two or more single component refrigerants is termed as blend, which can be of two types: azeotropic and zeotropic. Azeotropic blends behave like a single component refrigerant, in that they boil and condense at respective constant temperature at any given pressure. Whereas zeotropic blends boil and condense through a range of temperatures at a given pressure. Such temperature range is called “temperature glide” which it is basically the difference between the bubble point and the dew point of the refrigerant compound.
3.2.1 Binary Mixtures
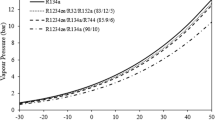
The addition of two single component refrigerants is termed as binary mixtures. Figure 15.8 shows the temperate glides of R32/R1234ze(E) binary mixtures at a constant pressure. It can be seen that the temperature glide depends on the mixture component. About 20–30% of R32 in the mixture provides maximum temperature glide.
3.2.1.1 R32/R1234ze(E)
Figure 15.7 shows that the COP of R1234ze(E) is lower than that of commonly used refrigerant, i.e., R410A and R32, mainly due to the low volumetric capacity and higher pressure drop (Mota-Babiloni et al. 2016). To increase vapor density as well as the performance of R1234ze(E), R32 is normally mixed using various ratios (Koyama et al. 2010a; Wang and Amrane 2014). The various refrigerants mixture and their respective GWP values, typically less than 300. In the cycle performance analysis, Koyama et al. (2010b) showed that the addition of R32 (50% mass) with R1234ze(E) improved the volumetric capacity keeping COP higher than that of R410A which suggested this mixture is a strong candidate to replace R410A.
3.2.1.2 R32/R1234yf
The refrigerant R1234yf can be considered as a promising next-generation refrigerant with its ultra-low GWP and comparable performances with R134a. Hitherto, some shortcomings of R1234yf, especially the volumetric capacity, which is considerably lower than that of R134a. On the other hand, pure R32 is a good choice for residential air-condition but it shows very high temperature at the compressor outlet and considerably higher GWP. So mixing of these two refrigerants offers solutions to overcome their individual shortcomings. In a drop-in experiment, Kojima et al. (2016) studied the performance of this mixture considering the GWP values 300 and 200. The experimental results show the performance of R32/R1234yf binary mixture of 42/58 (by mass) is considerably higher than that of R410A. An irreversible loss by parts and total irreversible loss for different mixtures are also compared where results show that the irreversible loss for R32/R1234yf (42/58) mixture is lower than R32/R1234ze(E) (28/72). Figure 15.9 shows that after mixing with R32, the cycle operation area for binary mixture match with R410A. It seems these mixtures can replace R410A.
3.2.1.3 R32/R1123
In 2014, AGC (Asahi Glass Co., Ltd.) developed a new refrigerant for air-conditioning systems that can reduce GWP further, which adopts hydrofluoroolefin R1123 as a main component. R1123 (trifluoroethylene) is strongly attractive because of its performance, which is equivalent to that of conventional refrigerants, along with extremely low GWP (=0.3) (Tanaka et al. 2014). Considering the safety and stability, the mixture of R1123 and R32 is considered as an alternative to R410A. According to Asahi Glass Co. Ltd., the new refrigerant mixture is azeotropic and can achieve good performance when replacing R410A for domestic and commercial air-conditioning system (Fukushima and Hashimoto 2015).
3.2.1.4 HC Mixtures
HC mixtures are environment-friendly natural refrigerants and can be used in the existing systems. Due to high flammable (A3) properties, HC mixtures are preferred to use in a small system where the charged amount is very small compared to halogenated refrigerants. Many researchers studied R290/R600a mixture as a substitute for R12 and found higher COP and refrigeration capacity of the hydrocarbon mixture than R12 (Richardson and Butterworth 1995; Dalkilic and Wongwises 2010; Mani and Selladurai 2008; Jung et al. 2000). Kim et al. (2007, 2008) experimentally studied the mixture of R744/R290 with three different compositions and found the ratio of 3:1 (by mass) enhanced the COP or 12.8% than that of R744 when the temperature glide matches with the change in fluid temperature. Tian et al. (2015) studied the performance of R32/R290 mixtures (68/32) as a drop-in replacement for R410A and found the COP of the mixture is 6–7% lower than that of R410A, whereas the charge amount is reduced by 30–35%. Mohanraj et al. (2011) reviewed the development of HC mixtures and identified that these mixtures can be suitable alternatives to phase-out halogenated based refrigerants in vapor compression systems.
3.2.2 Ternary Mixtures
3.2.2.1 R744/R32/R1234ze(E)
The binary mixture of R32/R1234yf and R32/R1234ze(E) show better performance than R410A when the mass fraction of R32 is above 50% in the mixtures. The mass fraction of R32 is below 50% in the binary mixture gives the GWP value less than 300; however, the COP drastically decreases as a consequence of the insufficient volumetric capacity. Fukuda et al. (2014, 2016), added R744 into the binary mixture to increase the volumetric capacity. In a drop-in experiment, authors studied the performance of R744/R32/R1234ze(E) mixtures having GWP 300 and GWP 200 and found the COP of GWP 300 mixture is comparable to that of R410A in both heating and cooling modes.
3.2.2.2 R744/R32/R1234yf
Fukuda et al. (2016) experimentally studied two ternary mixtures by adding R744 into R32/R1234yf mixture to increase the volumetric capacity and then compared the cycle performance with R410A. It is also found that the addition of R744 decreases the GWP but increases the temperature glide. But when the temperature glide of mixtures matches with the temperature changes in the heat sink and heat source during condensation and evaporation, they found that the irreversible loss is minimum. The ternary mixture of GWP nearly 300 show very good performance in both heating and cooling modes which means the ternary mixture of 4/44/52 (by mass) can be used as a drop-in alternative of R410A (Fukuda et al. 2016). Figure 15.10 shows the COPs of the ternary mixtures at optimum charge condition which are comparable with R410A.
3.2.2.3 Other Ternary Mixture
Maczek et al. (1997) studied R744/R32/R134a (7/31/62) mixture theoretically and experimentally to compare the performance with R22. Authors found the mixture is promising as a drop-in replacement of R22 with 10% better COP suggested using in low-temperature heat pump because of its excessive condensing pressure. Hakkaki-fard et al. (2014) theoretically compared the performance of R744/R32/Propane (10/80/10, GWP ~ 540) mixture with R410A and found the heating capacity of the mixture is higher than that of R410A.
4 Environmental Sustainability
The conventional vapor compression air-conditioning and refrigeration technology are responsible for both direct and indirect greenhouse gas emission. The warming potential of heat transfer fluid and the input electrical energy to run the system are accountable for environmental sustainability. Here, the emission is calculated considering system lifetime 10 years. Other related data are taken from the “Kyushu Electric Power Environmental Action Report 2013”.
4.1 Direct Emission
The direct emission is a function of GWP, the charge amount, and the emission due to leakages from the air-conditioning system and those associated with the servicing and equipment disposal. Leakage can be reduced by using a small and tight system with sealed compressor but the servicing and equipment disposal cannot be stopped. Figure 15.11 shows the comparison of GWP for newly proposed binary and ternary mixtures with currently used blend refrigerants. Figure 15.12 shows the direct equivalent CO2 emission for pure refrigerant considering different application area. Figure 15.13 shows the comparison of direction emission among the widely used R410A with newly proposed binary and ternary mixtures when applied in domestic air condition system. It can be seen from these two figures that direct emission significantly reduced when blend refrigerants are considered as an alternative.
Direct CO2 equivalent emission for some popular pure refrigerant (Pal et al. 2018)
4.2 Indirect Emission
The conventional air-conditioning and refrigeration devices are run by the electricity. The indirect emission is related to the operational activities of the system such as the emission during the production of electricity in a power plant, i.e., kg CO2-equivalent emission generated during production of electrical energy which is consumed by the air-conditioning system. Usually, the higher performance of the system means lower electricity consumption. The proposed refrigerant blend is attractive not only due to its very low GWP but also its excellent system performance. Figure 15.14 shows the indirect emission considering the annual use for heating is 1183 h and for cooling 1008 h.
The environmental impacts of the air-conditioning and refrigeration system for its lifetime can be calculated by LCCP (Life Cycle Climate performance) or TEWI (Total Equivalent Warming Impact) (Mohanraj et al. 2011; Islam et al. 2017). LCCP accounts the energy embodied in product materials, greenhouse gas emissions during chemical manufacturing, and end of life disposal of the unit which is ignored for the case of TEWI. The small sources of emissions generated over the course of the lifetime of the unit are explicitly accounted for in LCCP. This study compared the TEWI value of blend with widely used R410A to avoid the emission related to the manufacturing of the component of the system.
where
- a :
-
Annual refrigerant leak rate [%/year]
- b :
-
Refrigerant recovery rate (based on residual refrigerant at disposal) [%]
- c :
-
Carbon dioxide emission coefficient [kg-CO2/kWh]
- CC:
-
Rated cooling capacity [kW]
- HC:
-
Rated heating capacity [kW]
- LE:
-
Refrigerant leakage during disposal [kg-CO2]
- LL:
-
Lifetime refrigerant leakage [kg-CO2]
- M :
-
Refrigerant charge amount [kg]
- t c :
-
System use in cooling mode [h/year]
- t h :
-
System use in heating mode [h/year]
- Y :
-
System lifetime [year]
4.3 Energy Efficiency
Energy efficiency for a refrigeration system is related to the selection of refrigerant, system configuration, and component efficiencies. For a specific refrigerant, there is need of suitable configuration of evaporator, condenser, and compressor to achieve maximum performance. There is different approach to assess the energy efficiency for a specific refrigerant, which are theoretical or semi-theoretical cycle simulations, detailed equipment simulation models and laboratory test of the system. The operating condition, system capacity, and system hardware also influence the energy efficiency. In practice, the cost of the system is another important parameter as the success in the market depends on a cost-performance trade-off.
4.4 Nonconventional System
There is another approach to building a sustainable environment by changing the design of the vapor compression system. The adsorption cooling/heating system is one of them. This system is able to use natural refrigerants and is driven by renewable or waste energy. Water, ethanol, ammonia, and methanol known as natural refrigerants, are getting attention in domestic and automobile heat pump applications (Saha et al. 1995; El-Sharkawy et al. 2014; Tamainot-Telto et al. 2009; Wang et al. n.d.). These refrigerants have no GWP and toxicity. As the system is driven by waste energy so it is free from direct and indirect greenhouse gas emission. Water (R-718) and ethanol are not familiar refrigerant in vapor compression systems due to poor volumetric efficiency, but they can be considered as popular refrigerants in sorption-based systems (Wang et al. 2014; Uddin et al. 2014). Adsorption cooling systems can be operational utilizing low-temperature heat sources such as solar energy and low-grade process waste heat, e.g., engine exhaust, industrial waste heat (Khan et al. 2006; Kai and Edward 2011). When the system is applied in automobiles, the exhausted heat can be utilized without compromising any mechanical energy output from the engine. Hybrid vapor compression–adsorption system is also feasible when low-grade heat is available (Banker et al. 2008; Uddin et al. 2013). Figure 15.15 shows the conventional mechanical system and nonconventional adsorption heat pump system. The thermal compressor in nonconventional system works based on adsorption and desorption phenomenon.
5 Summary
To summarize, the most important factors determining the environmental sustainability are the low GWP refrigerant with higher volumetric capacities. Figure 15.16 shows the progression of refrigerants from the beginning of commercial production to current condition. The fourth generation refrigerant should be zero ODP, ultra-low GWP, should have a shorter lifetime in atmosphere, and high efficiency. The HFOs and their mixture can be a good solution to reduce the direct and indirect greenhouse gas emission. The study presented here will help the reader to find a suitable composition of the mixture that is desirable for a specific application.
The adsorption cooling system using natural refrigerant is also a promising alternative to traditional vapor compression system, in terms of primary energy source diversification and reducing the overall environmental effect. Vapor compression and adsorption hybrid system can also be a promising alternative of current mechanical vapor compressor system using conventional refrigerants.
References
Akasaka R, Tanaka K, Higashi Y (2010) Thermodynamic property modeling for 2,3,3,3-tetrafluoropropene (HFO-1234yf). Int J Refrig 33:52–60. https://doi.org/10.1016/j.ijrefrig.2009.09.004
Austin BT, Sumathy K (2011) Transcritical carbon dioxide heat pump systems: a review. Renew Sustain Energy Rev 15:4013–4029. https://doi.org/10.1016/j.rser.2011.07.021
Banker ND, Dutta P, Prasad M, Srinivasan K (2008) Performance studies on mechanical + adsorption hybrid compression refrigeration cycles with HFC 134a. Int J Refrig 31:1398–1406. https://doi.org/10.1016/j.ijrefrig.2008.03.009
Barve A, Cremaschi L (2012) Drop-in performance of low GWP refrigerants in a heat pump system for residential applications. Int Refrig Air Cond Conf 1–9. Purdue
Bella B, Kaemmer N (2011) An assessment of low GWP refrigerants in different applications. 23rd IIR Congr Refrig 8. Prague, Czech Republic
Benhadid-Dib S, Benzaoui A (2012) Refrigerants and their environmental impact Substitution of hydro chlorofluorocarbon HCFC and HFC hydro fluorocarbon. Search for an adequate refrigerant. Energy Procedia 18:1611–1623. https://doi.org/10.1016/j.egypro.2012.05.096
Bjerre P, Larsen P (2006) Evaluation of N-butane as a potential refrigerant for household compressors. Int Compress Eng Conf 1–6. Purdue
Bolaji BO (2010) Experimental study of R152a and R32 to replace R134a in a domestic refrigerator. Energy 35:3793–3798. https://doi.org/10.1016/j.energy.2010.05.031
Bolaji BO, Huan Z (2013) Ozone depletion and global warming: case for the use of natural refrigerant—a review. Renew Sustain Energy Rev 18:49–54. https://doi.org/10.1016/j.rser.2012.10.008
Brown JS, Yana-motta SF, Domanski PA (2002) Comparative analysis of an automotive air conditioning systems operating with CO2 and R134a. Int J Refrig 25:19–32. https://doi.org/10.1016/s0140-7007(01)00011-1
Brown JS, Zilio C, Cavallini A (2009) The fluorinated olefin R-1234ze(Z) as a high-temperature heat pumping refrigerant. Int J Refrig 32:1412–1422. https://doi.org/10.1016/j.ijrefrig.2009.03.002
Brown JS, Zilio C, Cavallini A (2010) Thermodynamic properties of eight fluorinated olefins. Int J Refrig 33:235–241. https://doi.org/10.1016/j.ijrefrig.2009.04.005
Cabello R, Sánchez D, Llopis R, Arauzo I, Torrella E (2015) Experimental comparison between R152a and R134a working in a refrigeration facility equipped with a hermetic compressor. Int J Refrig 60:92–105. https://doi.org/10.1016/j.ijrefrig.2015.06.021
Calm JM, Didion DA (1998) Trade-offs in refrigerant selections: past, present, and future. Int J Refrig 21:308–321. https://doi.org/10.1016/S0140-7007(97)00089-3
Chen J, Yu J (2008) Performance of a new refrigeration cycle using refrigerant mixture R32/R134a for residential air-conditioner applications. Energy Build 40:2022–2027. https://doi.org/10.1016/j.enbuild.2008.05.003
Chen G, Volovyk O, Zhu D, Ierin V, Shestopalov K (2017) Theoretical analysis and optimization of a hybrid CO2 transcritical mechanical compression—ejector cooling cycle. Int J Refrig 74:86–94. https://doi.org/10.1016/j.ijrefrig.2016.10.002
Corberán JM, Segurado J, Colbourne D, Gonzálvez J (2008) Review of standards for the use of hydrocarbon refrigerants in A/C, heat pump and refrigeration equipment. Int J Refrig 31:748–756. https://doi.org/10.1016/j.ijrefrig.2007.12.007
Dalkilic AS, Wongwises S (2010) A performance comparison of vapour-compression refrigeration system using various alternative refrigerants. Int Commun Heat Mass Transf 37:1340–1349. https://doi.org/10.1016/j.icheatmasstransfer.2010.07.006
Devotta S, Waghmare AV, Sawant NN, Domkundwar BM (2001) Alternatives to HCFC-22 for air conditioners. Appl Therm Eng 21:703–715
El-Sharkawy III, Uddin K, Miyazaki T, Saha BB, Koyama S, Miyawaki J et al (2014) Adsorption of ethanol onto parent and surface treated activated carbon powders. Int J Heat Mass Transf 73:445–455. https://doi.org/10.1016/j.ijheatmasstransfer.2014.02.046
EPA (1990) 40 CFR Part 82—protection of stratospheric ozone: change of listing status for certain substitutes under the significant new alternative policy program. Final Rule. Fed Regist 80:1–91
European Environment Agency (2016) Company reporting for Regulation (EU) No 517/ 2014 on fluorinated greenhouse gases
Fukuda S, Kondou C, Takata N, Koyama S (2014a) Low GWP refrigerants R1234ze(E) and R1234ze(Z) for high temperature heat pumps. Int J Refrig 40:161–173. https://doi.org/10.1016/j.ijrefrig.2013.10.014
Fukuda S, Kondou C, Takata N, Koyama S (2014b) Cycle performance of low GWP refrigerant mixtures R-32/1234ze(E) and R-744/1234ze(E). Asian Conf Refrig Air Cond. Jeju, Korea
Fukuda S, Kojima H, Kondou C, Takata N, Koyama S (2016) Comparative assessment on irreversible losses in heat pumps using R744/R32/R1234yf and R744/R32/R1234ze(E). Sci Technol Built Environ 22:1118–1127. https://doi.org/10.1080/23744731.2016.1206452
Fukushima M, Hashimoto M (2015) Next generation low-GWP refrigerants “AMOLEA-TM”. Res Reports. Asahi Glas Co. Ltd
Girotto S, Minetto S, Neksa P (2004) Commercial refrigeration system using CO2 as the refrigerant. Int J Refrig 27:717–723. https://doi.org/10.1016/j.ijrefrig.2004.07.004
Granryd E (2001) Hydrocarbons as refrigerants—an overview. Int J Refrig 24:15–24. https://doi.org/10.1016/S0140-7007(00)00065-7
Hakkaki-fard ALI, Aidoun Z, Ouzzane M (2014) Air-source heat pumps with refrigerant mixtures for cold climates. 11th IEA Heat Pump Conf 1–11. Monteal, Canada
Halimic E, Ross D, Agnew B, Anderson A, Potts I (2003) A comparison of the operating performance of alternative refrigerants. Appl Therm Eng 23:1441–1451. https://doi.org/10.1016/S1359-4311(03)00081-4
Han XH, Wang Q, Zhu ZW, Chen GM (2007) Cycle performance study on R32/R125/R161 as an alternative refrigerant to R407C. Appl Therm Eng 27:2559–2565. https://doi.org/10.1016/j.applthermaleng.2007.01.034
He Y, Deng J, Zheng L, Zhang Z (2016) Performance optimization of a transcritical CO2 refrigeration system using a controlled ejector. Int J Refrig 75:250–261. https://doi.org/10.1016/j.ijrefrig.2016.12.015
Higashi Y, Akasaka R (2016) Measurements of thermodynamic properties for R1123 and R1123 + R32 Mixture. Int Refrig Air Cond Conf Purdue 1–10. Purdue, Indiana
Higashi Y, Ashizawa M, Kabata Y, Majima T, Uematsu M, Watanabe K (1987) Measurements of vapor pressure, vapor-liquid coexistence curve and critical parameters of refrigerant 152a. Trans Japan Soc Mech Eng Ser B 53:1379–1385. https://doi.org/10.1299/kikaib.53.1379
Higashi Y, Tanaka K, Ichikawa T (2010) Critical parameters and saturated densities in the critical region for trans-1,3,3,3-Tetrafluoropropene (HFO-1234ze(E)). J Chem Eng Data 55:1594–1597
Honeywell Technical Bulletin (2012) Honeywell SolsticeTM yf refrigerants
Hwang YH, Celik A, Radermacher R (2004) Performance of CO2 cycles with a two-stage compressor. Int Refrig Air Cond Conf 1–8. Purdue
Islam MA, Srinivasan K, Thu K, Saha BB (2017) Assessment of total equivalent warming impact (TEWI) of supermarket refrigeration systems. Int J Hydrogen Energy 1–11. https://doi.org/10.1016/j.ijhydene.2017.07.035
Jing-yang M, Jiang-ping C, Zhi-jiu C (2003) System design and analysis of trans-critical carbon-dioxide automotive air-conditioning system. J Zhejiang Univ Sci 4:305–308
Joudi KA, Al-Amir QR (2014) Experimental Assessment of residential split type air-conditioning systems using alternative refrigerants to R-22 at high ambient temperatures High ambient performance parameters refrigerants R290 R407C and R410A in residential split A/C systems. Energy Convers Manag 86:496–506. https://doi.org/10.1016/j.enconman.2014.05.036
JRAIA 2017 (2017) World air conditioner demand by region
Jung D, Kim CB, Song K, Park B (2000) Testing of propane/isobutane mixture in domestic refrigerators. Int J Refrig 23:517–527. https://doi.org/10.1016/S0140-7007(99)00084-5
Kabeel AE, Khalil A, Bassuoni MM, Raslan MS (2016) Comparative experimental study of low GWP alternative for R134a in a walk-in cold room. Int J Refrig 69:303–312. https://doi.org/10.1016/j.ijrefrig.2016.06.017
Kai W, Edward AV (2011) New opportunities for solar: adsorption refrigeration. ASHRAE J 14–24
Khan MZI, Alam KCA, Saha BB, Hamamoto Y, Akisawa A, Kashiwagi T (2006) Parametric study of a two-stage adsorption chiller using re-heat—the effect of overall thermal conductance and adsorbent mass on system performance. Int J Therm Sci 45:511–519. https://doi.org/10.1016/j.ijthermalsci.2005.08.003
Kim JH, Cho JM, Lee IH, Lee JS, Kim MS (2007) Circulation concentration of CO2/propane mixtures and the effect of their charge on the cooling performance in an air-conditioning system. Int J Refrig 30:43–49. https://doi.org/10.1016/j.ijrefrig.2006.06.008
Kim JH, Cho JM, Kim MS (2008) Cooling performance of several CO2/propane mixtures and glide matching with secondary heat transfer fluid. Int J Refrig 31:800–806. https://doi.org/10.1016/j.ijrefrig.2007.11.009
Kojima H, Fukuda S, Kondou C, Takata N, Koyama S (2015) Comparative assessment of heat pump cycle operated with R32/R1234ze(E) and R32/R1234yf mixtures. Int Congr Refrig 1–8. Yokohama
Kojima H, Arakaki S, Fukuda S, Takata N, Kondou C, Koyama S (2016) comparative study on heat pump cycle using R32/R1234yf and R744/R32/R1234yf. 8th Asian Conf Refrig Air Cond 8–11. Taiwan
Koyama S, Takata N, Fukuda S (2010a) Drop-in experiments on heat pump cycle using HFO-1234ze(E) and its mixtures with HFC-32. Int Refrig Air Cond Conf 1–7. Purdue
Koyama S, Takata N, Matsuo Y, Yoshitake D, Fukuda S (2010b) Possibility to introduce HFO-1234ze(E) and its mixture with HFC-32 as low-GWP alternatives for heat pump/refrigeration systems. Int Symp Next-generation Air Cond Refrig Technol 1–10. Tokyo
Koyama S, Takata N, Fukuda S (2011) An experimental study on heat pump cycle using zeotropic binary refrigerant of HFO-1234ze(E) and HFC-32. 10th IEA Heat Pump Conf 1–10
Lai NA (2014) Equations of state for HFO-1234ze(E) and their application in the study on refrigeration cycle. Int J Refrig 43:194–202. https://doi.org/10.1016/j.ijrefrig.2013.11.011
Lai NA, Vrabec J, Raabe G, Fischer J, Wendland M (2011) Description of HFO-1234yf with BACKONE equation of state. Fluid Phase Equilib 305:204–211. https://doi.org/10.1016/j.fluid.2011.04.005
Lampugnani G, Zgliczynski M (1996) R290 as a substitute of R502 and R22 in commercial refrigeration and air conditioning. Int Compress Eng Conf 83–88. Purdue
Leck TJ (2010) New high performance, low GWP refrigerants for stationary AC and refrigeration. Int Refrig Air Cond Conf 8
Lee Y, Jung D (2012) A brief performance comparison of R1234yf and R134a in a bench tester for automobile applications. Appl Therm Eng 35:240–242. https://doi.org/10.1016/j.applthermaleng.2011.09.004
Lemmon EW, Huber ML, Mclinden MO (2013) NIST Reference fluid thermodynamic and transport properties—REFPROP (Version 9.1)
Lorentzen G (1994) Revival of carbon dioxide as a refrigerant. Int J Refrig 17:292–301. https://doi.org/10.1016/0140-7007(94)90059-0
Maczek K, Muller J, Wojtas K, Domanski PA (1997) Ternary zeotropic mixture with CO2 component for R-22 heat pump application. CLIMA 2000, Brussels
Maina P, Huan Z (2015) A review of carbon dioxide as a refrigerant in refrigeration technology. S Afr J Sci 111:1–10. https://doi.org/10.17159/sajs.2015/20140258
Mani K, Selladurai V (2008) Experimental analysis of a new refrigerant mixture as drop-in replacement for CFC12 and HFC134a. Int J Therm Sci 47:1490–1495. https://doi.org/10.1016/j.ijthermalsci.2007.11.008
McMullan JT (2002) Refrigeration and the environment—issues and strategies for the future. Int J Refrig 25:89–99. https://doi.org/10.1016/S0140-7007(01)00007-X
Meno A (2015) Laws and regulation for fluorocarbons in Japan. Ministry of Economy, Trade and Industry, Japan
Minjares R (2011) Refrigerants for light-duty passenger vehicle air conditioning systems Technical assessment of alternatives to HFC-134a. Int Counc Clean Transp
Minor BH, Herrmann D, Gravell R (2010) Flammability characteristics of HFO-1234yf. Process Saf Prog 29:150–154. https://doi.org/10.1002/prs.10347
Mohanraj M, Muraleedharan C, Jayaraj S (2011) A review on recent developments in new refrigerant mixtures for vapour compression-based refrigeration, air-conditioning and heat pump units. Int J Energy Res 35:647–669. https://doi.org/10.1002/er.1736
Morrison G, McLinden MO (1993) Azeotropy in refrigerant mixtures. Int J Refrig 16:129–138. https://doi.org/10.1016/0140-7007(93)90069-K
Mota-Babiloni A, Navarro-Esbrí J, Barragán-Cervera Á, Molés F, Peris B (2015) Experimental study of an R1234ze(E)/R134a mixture (R450A) as R134a replacement. Int J Refrig 51:52–58. https://doi.org/10.1016/j.ijrefrig.2014.12.010
Mota-Babiloni A, Navarro-Esbrí J, Molés F, Cervera ÁB, Peris B, Verdú G (2016) A review of refrigerant R1234ze(E) recent investigations. Appl Therm Eng 95:211–222. https://doi.org/10.1016/j.applthermaleng.2015.09.055
Mota-Babiloni A, Navarro-Esbrí J, Makhnatch P, Molés F (2017) Refrigerant R32 as lower GWP working fluid in residential air conditioning systems in Europe and the USA. Renew Sustain Energy Rev 80:1031–1042. https://doi.org/10.1016/j.rser.2017.05.216
Navarro-Esbrí J, Mendoza-Miranda JM, Mota-Babiloni A, Barragán-Cervera A, Belman-Flores JM (2013) Experimental analysis of R1234yf as a drop-in replacement for R134a in a vapor compression system. Int J Refrig 36:870–880. https://doi.org/10.1016/j.ijrefrig.2012.12.014
Nekså P (2002) CO2 heat pump systems. Int J Refrig 25:421–427. https://doi.org/10.1016/S0140-7007(01)00033-0
Pal A, Uddin K, Thu K, Saha BB (2018) Environmental assessment and characteristics of next generation refrigerants. Evergr Jt J Nov Carbon Resour Sci Green Asia Strateg 5:58–66
Palm B (2008) Hydrocarbons as refrigerants in small heat pump and refrigeration systems—a review. Int J Refrig 31:552–563. https://doi.org/10.1016/j.ijrefrig.2007.11.016
Park K-J, Seo T, Jung D (2007) Performance of alternative refrigerants for residential air-conditioning applications. Appl Energy 84:985–991. https://doi.org/10.1016/j.apenergy.2007.05.002
Park KJ, Shim YB, Jung D (2008) Performance of R433A for replacing HCFC22 used in residential air-conditioners and heat pumps. Appl Energy 85:896–900. https://doi.org/10.1016/j.apenergy.2007.11.003
Park KJ, Shim YB, Jung D (2009a) A “drop-in” refrigerant R431A for replacing HCFC22 in residential air-conditioners and heat pumps. Energy Convers Manag 50:1671–1675. https://doi.org/10.1016/j.enconman.2009.03.027
Park KJ, Shim YB, Jung D (2009b) Experimental performance of R432A to replace R22 in residential air-conditioners and heat pumps. Appl Therm Eng 29:597–600. https://doi.org/10.1016/j.applthermaleng.2008.02.019
Parties to the Protocol (1998) Kyoto protocol to the united nations framework convention on climate change
Pearson A (2005) Carbon dioxide—new uses for an old refrigerant. Int J Refrig 28:1140–1148. https://doi.org/10.1016/j.ijrefrig.2005.09.005
Pham H, Rajendran R (2012) R32 and HFOs as low-GWP refrigerants for air conditioning. Int Refrig Air Cond Conf. Purdue, Indiana, 1–10
Pitarch M, Navarro-Peris E, Gonzalvez J, Corberan JM (2016) Analysis and optimisation of different two-stage transcritical carbon dioxide cycles for heating applications. Int J Refrig 70:235–242. https://doi.org/10.1016/j.ijrefrig.2015.08.013
Powell RL (2002) CFC phase-out: have we met the challenge? J Fluor Chem 114:237–250. https://doi.org/10.1016/S0022-1139(02)00030-1
Qi Z (2015) Performance improvement potentials of R1234yf mobile air conditioning system. Int J Refrig 58:35–40. https://doi.org/10.1016/j.ijrefrig.2015.03.019
R-32 (n.d.) Next-generation refrigerant/benefits of daikin technology. Daikin Global. http://www.daikin.com/about/why_daikin/benefits/r-32/. Accessed 4 July 2017
Richardson RN, Butterworth JS (1995) The performance of propane/isobutane mixtures in a vapour-compression refrigeration system. Int J Refrig 18:58–62. https://doi.org/10.1016/0140-7007(94)P3712-A
SAE-CRP1234 (2009) Industry evaluation of low global warning potential refrigerant HFO-1234yf
Saha BB, Boelman EC, Kashiwagi T (1995) Computer simulation of a silica gel water adsorption refrigeration cycle-the influence of operating conditions on cooling output and COP. Am Soc Heating, Refrig Air-Conditioning Eng ASHRAE Trans 101:348–357
Sciance F (2013) The transition from HFC-134a to a low-GWP refrigerant in mobile air conditioners HFO-1234yf. Gen Mot Public Policy Cent 1–15
Spatz M, Minor B (2008) HFO-1234yf low GWP refrigerant : a global sustainable solution for mobile air conditioning 1–26
Spatz MW, Motta SFY, Yana Motta SF (2004) An evaluation of options for replacing HCFC-22 in medium temperature refrigeration systems. Int J Refrig 27:475–483. https://doi.org/10.1016/j.ijrefrig.2004.02.009
Stocker TF, Qin D, Plattner G-K, Tignor MMB, Allen SK, Boschung J et al (2013) Climate change 2013 the physical science basis working group I contribution to the fifth assessment Report of the intergovernmental panel on climate change. Cambridge University Press
Takizawa K, Tokuhashi K, Kondo S (2009) Flammability assessment of CH2 = CFCF3: comparison with fluoroalkenes and fluoroalkanes. J Hazard Mater 172:1329–1338. https://doi.org/10.1016/j.jhazmat.2009.08.001
Tamainot-Telto Z, Metcalf SJ, Critoph RE, Zhong Y, Thorpe R (2009) Carbon-ammonia pairs for adsorption refrigeration applications: ice making, air conditioning and heat pumping. Int J Refrig 32:1212–1229. https://doi.org/10.1016/j.ijrefrig.2009.01.008
Tamatsu T, Sato H, Watanabe K (1993) An equation of state for 1,1-difluoroethane (HFC 152a). Int J Refrig 16:347–352
Tanaka K, Higashi Y (2010) Thermodynamic properties of HFO-1234yf (2,3,3,3-tetrafluoropropene). Int J Refrig 33:474–479. https://doi.org/10.1016/j.ijrefrig.2009.10.003
Tanaka T, Hidekazu O, Katsuya U, Jun I, Otsuka T, Nogami T et al (2014) Development of a new low-GWP refrigerant composed of HFO-1123 (trifluoroethylene). AIChE Annu Meet, Atlanta, GA
Tian Q, Cai D, Ren L, Tang W, Xie Y, He G et al (2015) An experimental investigation of refrigerant mixture R32/R290 as drop-in replacement for HFC410A in household air conditioners. Int J Refrig 57:216–228. https://doi.org/10.1016/j.ijrefrig.2015.05.005
Tiwari A, Gupta RC (2011) Recent development of domestic refrigerator—a review. Int J Eng Sci Technol 3:4233–4239
Uddin K, Miyazaki T, Koyama S, Saha BB (2013) Performance investigation of adsorption-compression hybrid refrigeration system. Int J Air-Conditioning Refrig 21:1350024. https://doi.org/10.1142/S2010132513500247
Uddin K, El-Sharkawy III, Miyazaki T, Saha BB, Koyama S, Kil H-S et al (2014) Adsorption characteristics of ethanol onto functional activated carbons with controlled oxygen content. Appl Therm Eng 72:211–218. https://doi.org/10.1016/j.applthermaleng.2014.03.062
Ueda K, Wajima K, Yokoyama A, Akimasa SA, Kenji U, Kazuki W, Akimasa AS, Yokoyama A (2011) Low GWP refrigerant application for the centrifugal chiller- study for application of HFO-1234ze(E). Trans Japan Soc Refrig Air Cond Eng 28:503–508. https://doi.org/10.11322/tjsrae.28.503
UNEP (2016) Montreal protocol on substances that deplete the ozone layer (report of the technology and economic assessment panel)
Vaghela JK (2017) Comparative evaluation of an automobile air-conditioning system using R134a and its alternative refrigerants. Energy Procedia 109:153–160. https://doi.org/10.1016/j.egypro.2017.03.083
Van Poolen LJ, Holcomb CD, Niesen VG (1997) Critical temperature and density from liquid-vapor coexistence data: application to refrigerants R32, R124, and R152a. Fluid Phase Equilib 129:105–111. https://doi.org/10.1016/S0378-3812(96)03171-8
Wang X, Amrane K (2014) AHRI Low global warming potential alternative refrigerants evaluation program (low-GWP AREP)—summary of phase I testing results. 15th Int Refrig Air Cond Conf 1–10. Purdue
Wang LW, Wu JY, Wang RZ, Xu YX, Wang SG, Li XR (n.d.) Study of the performance of activated carbon–methanol adsorption systems concerning heat and mass transfer. https://doi.org/10.1016/s1359-4311(03)00104-2
Wang D, Zhang J, Yang Q, Li N, Sumathy K (2014) Study of adsorption characteristics in silica gel-water adsorption refrigeration. Appl Energy 113:734–741. https://doi.org/10.1016/j.apenergy.2013.08.011
Wilson DP, Kujak S, Leary JMO, Kennoy DH, Kusmierz A, Patnaik V et al (2010) Designation and Safety Classification of Refrigerants. ANSI/ASHRAE Stand 34-2010 8400:9
Wu J, Chu Y, Hu J, Liu Z (2009) Performance of mixture refrigerant R152a/R125/R32 in domestic air-conditioner. Int J Refrig 32:1049–1057. https://doi.org/10.1016/j.ijrefrig.2008.10.009
Xu X, Hwang Y, Radermacher R (2013) Performance comparison of R410A and R32 in vapor injection cycles. Int J Refrig 36:892–903. https://doi.org/10.1016/j.ijrefrig.2012.12.010
Xue J, Koyama S, Kuwahara K (2010) Performance prediction of a R744 transcritical cycle for air conditioning. Int Symp Next-generation Air Cond Refrig Technol 17–19
Yang Z, Wu X (2013) Retrofits and options for the alternatives to HCFC-22. Energy 59:1–21. https://doi.org/10.1016/j.energy.2013.05.065
Yang Z, Wu X, Tian T (2015) Flammability of Trans-1, 3, 3, 3-tetrafluoroprop-1-ene and its binary blends. Energy 91:386–392. https://doi.org/10.1016/j.energy.2015.08.037
Yun R, Heo JH, Kim Y (2006) Evaporative heat transfer and pressure drop of R410A in microchannels R410A. Int J Refrig 29:92–100. https://doi.org/10.1016/j.ijrefrig.2005.08.005
Zilio C, Brown JS, Schiochet G, Cavallini A (2011) The refrigerant R1234yf in air conditioning systems. Energy 36:6110–6120. https://doi.org/10.1016/j.energy.2011.08.002
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Uddin, K., Saha, B.B., Thu, K., Koyama, S. (2019). Low GWP Refrigerants for Energy Conservation and Environmental Sustainability. In: Tyagi, H., Agarwal, A., Chakraborty, P., Powar, S. (eds) Advances in Solar Energy Research. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-13-3302-6_15
Download citation
DOI: https://doi.org/10.1007/978-981-13-3302-6_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3301-9
Online ISBN: 978-981-13-3302-6
eBook Packages: EnergyEnergy (R0)