Abstract
Microalgae are being increasingly considered as a potential biomass feedstock for various biofuels, biodiesel in particular. Microalgal biomass for biofuel production purposes can be derived by cultivation using several waste resources, such as wastewater or flue gases, due mainly to the absence of the stringent regulations usually applied for food grade health supplements from microalgae. Anaerobic digestion and dark fermentation, the two highly used biomass digestion processes, generate biogas (a mixture of CH4, CO2 and other gases) and a COD (chemical oxygen demand)-rich effluent with leftover organic acids from the fermentation process. Microalgae can utilize the CO2 present in the biogas stream, thus increasing the methane content and improving the fuel properties of biogas. Several reports indicate that certain microalgae are highly tolerant to the high concentrations of methane present in the biogas stream and can effectively utilize the CO2 in photoautotrophic/mixotrophic mode of cultivation to obtain microalgal biomass. The organic acids of the effluent can also be used as a carbon source for mixotrophic/heterotrophic mode of microalgal cultivation, thus providing a cleanup of both the liquid and gaseous effluents of the fermentation process. This chapter describes in detail the capability of microalgae for carbon capture from biogas and their efficiency in the utilization of organic acids from various effluent streams. A biorefinery concept, integrating anaerobic digestion and microalgal cultivation is proposed, and the future perspectives are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Renewable energy in the form of biofuels is steadily gaining research momentum and finding its way into the energy mix for consumption. This invigorating change is driven by the necessity to replace fast depleting fossil fuel resources, improve energy security, and combat the environmental effects caused by the imprudent use of fossil fuels. It has been predicted that renewable energy might become prominent in the energy mix, mainly due to the advent of new technologies and State support (Annual Energy Outlook 2018, EIA). Substantial research has focused on liquid biofuels, of which biodiesel and bioethanol dominate the renewable energy market. Biohydrogen and biomethane are the most promising gaseous biofuel candidates. Biomethane (defined as >97% of methane of biological origin) is currently being viewed as an important alternative energy source and has potential applications in the transport sector (Åhman 2010), or it can be converted to electricity or heat via combined heat and power stations (Weiland 2010). Biogas is a prominent source of biomethane, which is derived from the Anaerobic Digestion (AD) of organic matter. AD occurs in nature under anaerobic conditions in ocean sediments, ruminant intestines, and anthropogenic methane emissions in sites like landfills and livestock agriculture, contributing to an annual release of 0.55–1.3 billion tons of CH4 to the atmosphere (Braun 2007). Despite the fact that the GHG reduction potential of other biofuels is questionable based on the feedstock, energy consumption, and emissions profile (Haberl et al. 2012), biogas production by AD can markedly contribute to reduction in GHG emissions, with negative GHG emissions when used as a fuel in particular (Tilche and Galatola 2008; Uusitalo et al. 2014). Atmospheric methane concentrations due to anthropogenic emissions are projected to increase to a staggering 405 Tg (terragram) CH4 per year by 2030 (Abbasi et al. 2012), and biogas production by AD is an effective way of capturing the released CH4, since CH4 is almost 25 times more potent than CO2 as a GHG.
The major components in the biogas include CH4 and CO2, along with numerous other compounds like H2S, NH3, water vapor, and certain trace elements. The effective composition of biogas is influenced by the nature of feedstock used and the reaction conditions applied for efficient digestion of the feedstock. Commercial biogas production plants generally operate at wastewater treatment plants for the AD of sewage sludge, at landfill sites for degradation of garbage, and at animal husbandry sites for the AD of manure, and also separate digesters can be set up for AD of agricultural biomass. Of the 18,000 AD plants in Europe, around 12,000 installations operate on agricultural feedstock (European Biogas Association Statistical report, 2017), while about 1200 of the 2200 AD plants in the USA are located in wastewater treatment plants (American Biogas Council). The composition of biogas varies depending on the feedstock used, along with the presence of other impurities, as summarized in Table 15.1. The major energy carrier of biogas is methane, and the other substances are regarded as impurities. Based on the end use application, additional components in biogas needs to be removed and the methane content in biogas enhanced. The biogas generated in a fermenter in an AD plant can be used in a CHP station with a desulfurizing step, and the CHP station that generated heat and electricity could be used directly within the plant or supplied elsewhere (Patterson et al. 2011). However, when it comes to the use of biogas as a transportation fuel, stringent regulations are applied as the various extraneous impurities present in raw biogas can impede the performance of combustion engines. The European standard for transportation grade biogas is presented in Table 15.1, and usually the biogas needs upgrading of its methane content to increase the fuel performance.
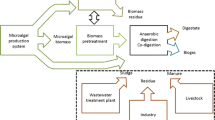
The schematic of an anaerobic digestion plant for the production of biogas is illustrated in Fig. 15.1. The feedstock, like organic matter, animal manure, sewage sludge, microalgae, macroalgae, or even food waste, is treated in an appropriate manner to enhance the methane generation potential and fed into the digester. Raw biogas in generally cleaned up of the toxic compounds like H2S and siloxanes, which could then be used in a CHP station for the generation of heat and electricity for onsite use. Further, the biogas can be upgraded for its methane content and purified of all impurities to be used as a fuel. It can then be integrated with the natural gas grid or be used as a transportation fuel (American Biogas Council). The leftover digestate from the fermenter is then separated as solid and liquid fractions, which can then be further reused as fertilizers. The prospect of utilization of liquid digestate from AD as a nutrient source is discussed in detail in Sect. 3. Biogas upgrading can be performed by various physical, chemical, and biological methods, and detailed information regarding these have been reviewed earlier in detail (Muñoz et al. 2015; Kadam and Panwar 2017; Angelidaki et al. 2018). Successful physical/chemical processes applied in commercial biogas plants include water scrubbing by physical adsorption, chemical absorption with amine solutions, pressure swing adsorption, membrane separation, cryogenic processes, and scrubbing with organic physical scrubbers (Angelidaki et al. 2018). The methane recovery with the physical/chemical processes is over 96%, and the upgraded biogas usually has a methane content of 95–97% meeting the fuel standard specifications. Biological processes for biogas upgrading include (a) chemoautotrophic conversion of CO2 to CH4 using H2 as electron donor, (b) photosynthetic CO2 capture by microalgae or cyanobacteria, (c) microbial conversion of CO2 into valuable liquid products like ethanol, and (d) microbial electrochemical conversion of CO2 to CH4. Of these, this chapter deals with the upgrading of biogas by microalgal carbon capture. Biogas upgrading by microalgae is an eco-friendly, zero waste, and green technology that could simultaneously remove CO2 from biogas and the organic nutrients present in the liquid AD digestate (Chen et al. 2018). This chapter presents the basic principles of biogas production by AD and the carbon capture potential of microalgae. The utilization of organic acids by microalgae via mixotrophic metabolism is discussed in detail, and an integrated biorefinery for AD and microalgal cultivation is proposed.
2 Anaerobic Digestion and Biogas Production
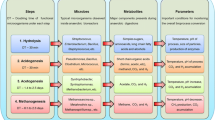
Anaerobic digestion is the fermentation of complex organic matter in the absence of oxygen, resulting in the decomposition of organic matter to CH4, CO2, H2, and some volatile fatty acids. AD is a multi-step process, and it occurs in a sequential order, as defined by the dominant microbial population in the digester. The four major stages of AD are hydrolysis, acidogenesis, acetogenesis, and methanogenesis, and the major activities in these stages are illustrated in Fig. 15.2. Hydrolysis is the first step of AD and results in the dissolution or disintegration of the complex organic matter to simple monomers, increasing their bioavailability to the fermentative bacteria. The predominant bacterial species in this phase are generally found to be strict or facultative anaerobes of the genera Clostridium, Bacteroides, Butyrivibrio, Bifidobacterium, Bacillus, Streptococcus, and members of the Enterobacteriaceae family (Amani et al. 2010; Merlin Christy et al. 2014). These organisms are endowed with an array of hydrolytic enzymes like amylase, cellulase, cellobioase, protease, and lipase which act on carbohydrates, proteins, and lipids, eventually degrading them into monosaccharides, long-chain fatty acids, and amino acids. The feedstock for AD is highly versatile (animal manure, food waste, sewage sludge, lignocellulosic biomass, microalgae, macroalgae), and hydrolysis is essential for the liquefaction and subsequent solubilization of the solid organic matter. Hydrolysis of all the compounds present is crucial, since certain materials are highly recalcitrant, or they cannot be hydrolyzed by bacterial depolymerases (lignocellulose in particular), and hence it is often dubbed as the “rate-limiting” step (Park et al. 2005). A pretreatment step can greatly enhance hydrolysis efficiency and improve the methane generation potential of the applied feedstock. The pretreatment step is chosen based on the feedstock used, energy requirements, and the feasibility for use in large-scale applications (Carrere et al. 2016).
Acidogenesis is the principal phase of the conversion of monomers to higher organic acids, alcohols, aldehydes, and gaseous products. Fermentative bacteria (both obligate and facultative) use the monosaccharides derived from sugars and convert them to organic acids like lactate, propionate, butyrate, propionate, and acetate, along with alcohols like ethanol or methanol, accompanied by the evolution of CO2 and H2. Fatty acids and amino acids arising from lipids and proteins can be utilized as carbon sources by anaerobic bacteria, further converting them into simpler compounds. The major bacterial species present in this stage are from the genera Bacillus, Clostridium, Micrococcus, Pseudomonas, Lactobacillus, Salmonella, Corynebacterium, Eubacterium, Escherichia coli, Desulfobacter, Desulfomonas, and Desulfovibrio (Merlin Christy et al. 2014). Acidogenic bacteria are generally the fast growing in the reactor, with an operational pH value of about 4.5–5.5 as defined by the production of acids in the medium. Of the organic acids produced in the acidogenesis phase, acetate and butyrate are preferred for methane generation. Acidogenic and hydrolytic microbes are linked closely to each other based on their growth rate and pH requirements, and together they are the fastest-growing organisms in the reactor, completing the hydrolysis and acidogenesis within 10–15 days (Cirne et al. 2007).
The next phase, acetogenesis, is characterized by the conversion of the higher organic acids to acetate and hydrogen by acetogenic bacteria. Acetogenic bacteria are slow-growing obligate anaerobes, and an optimal pH of around 6 is preferred (Merlin Christy et al. 2014). The growth rate is lower for these bacteria, with prolonged lag periods required for the adjustment to their immediate environments. Hydrogen evolution in acidogenesis phase is accompanied by the accumulation of electron sinks in the form of higher acids and alcohols, and acetogenic bacteria catalyze the conversion of these electron sinks to acetate, CO2 and H2 (Merlin Christy et al. 2014). The major acetogenic bacteria are the following: Syntrophomonas wolfeii, Syntrophobacter wolinii, S. fumaroxidans, Pelotomaculum sp., Smithella sp., and Clostridium aceticum (Amani et al. 2010). The hydrogen evolved during acetogenesis is toxic for acetogenic bacteria, and a low partial pressure of hydrogen is preferred. A syntrophic association exists between hydrogen-evolving acetogenic bacteria and hydrogen-consuming methanogenic bacteria, and this relationship in combination with the efficient conversion of the organics to acetate determines the efficiency of biogas production (Weiland 2010). Higher hydrogen concentration favors methane formation, while lower hydrogen concentrations favor acetate formation from CO2 and H2 by homoacetogenic bacteria. Notable homoacetogenic bacteria include Acetobacterium, Butyribacterium, Clostridium, Eubacterium, Peptostreptococcus, and Sporomusa (Saady 2013). However, homoacetogens can outgrow methanogens in an AD process at low temperature and other thermodynamically unfavorable conditions (Ye et al. 2014).
The final phase is the methane-generating phase, defined as methanogenesis. Archaea dominate the methanogenesis phase due to their unusual metabolic capability of utilizing acetate, CO2/H2, formate, or other methylated carbons as a source of energy and carbon, evolving methane in the process (Enzmann et al. 2018). Methanogenic organisms in AD can be acetoclastic methanogens or hydrogenotrophic methanogens. Acetoclastic methanogens generate methane by acetate decarboxylation and produce methane and CO2. Very few species are capable of acetoclastic methanogenesis including Methanosarcina barkeri, Methanococcus mazei, Methanotrix soehngenii (Weiland 2010), Methanosaeta concilii, and Methanosarcina acetivorans (Amani et al. 2010). Hydrogenotrophic methanogens generate methane via the reduction of CO2/H2, and most methanogens are capable of this function including species of the genera Methanospirillum, Methanococcus, Methanobrevibacter, Methanococcus, Methanoculleus, and so on (Amani et al. 2010). The efficiency of the AD process is determined by the methanogens and their ability to outcompete homoacetogens and methanotrophs in a bacterial consortia,; hence, it is essential to control the process parameters in AD favoring methanogens. At the end of AD, the resultant products are biogas (a mixture of CO2 and CH4) and the residual digestate. The digestate can be further divided into solid and liquid fractions. The solid digestate is easy to handle with higher bioavailable nitrogen for plants and is usually applied as a bio fertilizer (Möller and Müller 2012). The liquid part is particularly rich in the leftover organic acids from the fermentation and other macronutrients like NH3 and phosphorus. The amount of total nitrogen (N), total phosphorus (P), and chemical oxygen demand (COD) levels in liquid anaerobic digestate can range from 139 to 3496 mg/L (65–98% of ammonia nitrogen), 7–381 mg/L (82–95% phosphate), and 210–6900 mg/L, respectively (Xia and Murphy 2016a). This liquid digestate can be used as a carbon source for the cultivation of microalgae, since microalgae can assimilate organic carbon in the presence/absence of light under mixotrophic/heterotrophic conditions, respectively. The nitrogen and phosphorus are used for growth and biomass accumulation as well. Hence, after microalgal treatment, the liquid digestate has relatively low concentrations of N, P, and COD aiding in subsequent environmental release without the fear of eutrophication of surrounding water bodies.
Dark fermentation (DF) for biohydrogen production is another most commonly used anaerobic fermentation process, with hydrogen as the principal product and COD-rich leftover fermentation liquor as a by-product. The basic biochemical pathway for dark fermentation is similar to the first three stages of AD, accomplished by both obligate and facultative anaerobic bacteria. Methanogenesis is usually inhibited in such processes by careful control of the reaction parameters like temperature and pH (Ghimire et al. 2015). The organic acids present in the fermentation liquor, particularly acetate and butyrate can be assimilated by microalgae in mixotrophic mode of cultivation (Liu et al. 2013a). AD digestate and DF liquor are both needed to be processed further to enhance the energy recovery in each process.
3 Microalgae and Carbon Capture
Microalgae is an umbrella term for the countless unicellular/simple multicellular, prokaryotic and eukaryotic organisms that can fix the atmospheric CO2 via photosynthesis into organic biomass. The estimated number of classified algal species were around 75,000 in 2012 (Guiry 2012) and is currently at 150,000 species as described by Algaebase (http://www.algaebase.org/). All these include properly named and characterized species, and still numerous algal species could be isolated and characterized. This huge number explains the diversity that can be seen in algae related to their habitats, morphology, physiology, phylogeny, and carbon metabolism. Microalgae are now considered as the third-generation feedstock for the production of biofuels, because of their higher photosynthetic efficiency. The theoretical maximum for photosynthetic efficiency (PE) of a green plant in bright sunlight is estimated to be 13% and a practical PE around 8–9% is attainable under optimal conditions (Bolton and Hall 2008), while reported global average PE for terrestrial plants is around 1–2%. Microalgae can have higher PE, anywhere between 1% and 21% based on various reports (Brennan and Owende 2010). Higher PE results in higher oil productivity close to 136,900 L oil/ha year in high oil microalgae. A biodiesel productivity of 121,104 kg biodiesel/ha year can be achieved with high oil microalgae, whereas it is very low in traditional oil crops like jatropha and soybean (Mata et al. 2010). These two traits set microalgae apart from other potential biofuel resources, and additionally microalgal cultivation requires minimal nutrients, atmospheric CO2 as carbon source, minimal requirements for land and water, noninterference with local agriculture, and no land use changes. Microalgae fixes atmospheric carbon via a series of reactions in the presence of sunlight in the light and dark reactions of photosynthesis. An estimated 180 tons of CO2 is required for the production of about 100 tons of microalgal biomass (Chisti 2008) and other than atmospheric carbon dioxide (which is currently at 407 ppm), various relatively inexpensive gases rich in CO2 can be used for microalgal cultivation.
Carbon capture by microalgae is an economically viable option for biological carbon mitigation, and microalgae can be cultivated in CO2-rich gases like industrial flue gases (cement industries, coal fired power plants) and CO2 emissions from ethanol industries. Certain microalgae can tolerate very high concentrations of CO2, as high as 50–70%, previously reported for Chlorella species (Maeda et al. 1995; Sung et al. 1999; Yue and Chen 2005). The CO2-rich off-gas from ethanol fermentation has been used for the cultivation of Arthrospira platensis (Bezerra et al. 2013) and Chlorella vulgaris (Zhang et al. 2017a). The fermentation CO2 from acetone-butanol-ethanol fermentation for biobutanol production has been used successfully for the cultivation of capnophilic E. coli-based succinic acid production, with a maximum succinic acid concentration and productivity of 65.7 g/L and 0.76 g/l/h, respectively. The CO2 capture from this fermentation off-gas has enriched the hydrogen content of the gas to up to 92.7% (He et al. 2016). The CO2 released during an integrated dark-photo fermentation for hydrogen production has been used for the cultivation of C. vulgaris, and microalgal biomass rich in proteins (48.6% by weigh of biomass) was obtained (Lo et al. 2010). The VFA-rich fermentation effluents from a dark fermentation reaction and the CO2 rich off-gas were both used as a carbon source for the mixotrophic cultivation of Chlorella vulgaris ESP6, and CO2 content of the off-gas was reduced from 34% to 5% with complete consumption of acetate and butyrate in the liquid effluent. The resultant carbohydrate-rich microalgal biomass was used for biohydrogen production, thus enhancing the energy recovery from the initial energy input (Liu et al. 2013b).
While the CO2 released during fermentation reactions is relatively pure and can be directly used for the cultivation of microalgae (Xu et al. 2010), the composition of CO2-rich industrial flue gases vary depending upon the source, and an additional 142 compounds are known to be present with around 3–25% by volume of CO2 (Van Den Hende et al. 2012). The most important compounds present include SOx, NOx, unburned carbohydrates, CO, water vapor, O2, chlorine, fluorine, heavy metals, and other related compounds. The SOx and NOx can dissolve in culture medium leading to a drop in medium pH, and other impurities might be lethal to microalgae. Selection of a microalgal strain resistant to high CO2, fluctuations in medium pH, robust growth characteristics, and simple pretreatment of flue gases can help attain high biomass productivities when using flue gas as a carbon source for growth (Cheah et al. 2016). Life cycle analysis and design parameters for microalgae-based carbon capture indicates that microalgal biodiesel production with flue gas capture can be profitable based on the microalgal strain chosen and fuel production pathway (Gebreslassie Berhane et al. 2013; Gong and You 2014; Hernández-Calderón et al. 2016; Gutiérrez-Arriaga et al. 2014). Flue gas has been successfully used for the cultivation of Chlorella sp. (Kao et al. 2014), Desmodesmus sp. (Aslam et al. 2017), and Desmodesmus abundans (Lara-Gil et al. 2016). Biogas contains even higher concentrations of gaseous CO2, in the range of 20–50% depending on the AD feedstock used. Biogas does not contain many toxic compounds like flue gas, and it is the product of anaerobic fermentation; hence, it is available at ambient temperature alleviating the need for thermotolerant strains. The utilization of biogas CO2 by microalgae for biogas upgrading is discussed in detail in Sect. 5.
4 Utilization of Volatile Fatty Acids from Fermentation Effluents by Microalgae
The acid fermentation pathways of anaerobic bacteria lead to the breakdown of the input carbon source into organic acids in the acidogenic and acetogenic phase, which is then converted to methane by the archaeal methanogens. The major volatile fatty acids from the typical mixed acid fermentations of anaerobic bacteria include formate, acetate, lactate, butyrate, propionate, valerate, and isovalerate. Alcohols like ethanol, methanol, propanol, and isopropanol can also be found in smaller quantities based on the fermentative organism and fermentation conditions (Zhou et al. 2018). Microalgae are capable of assimilating these volatile fatty acids via the central carbon metabolic pathway, similar to bacteria and higher eukaryotes. Microalgae are endowed with certain transporters for the effective transport of VFAs at the expense of energy, and inside the cell, these VFAs enter carbon catabolic pathways.
The principal VFA in majority of effluents is acetate, and it is also the most commonly used carbon source for the mixotrophic/heterotrophic cultivation of microalgae. Acetate enters the cell via a monocarboxylic carbon/proton transport protein under aerobic conditions. The transporter is not specific for acetate but a general transporter for monocarboxylic acids (Perez-Garcia et al. 2011). In the cytoplasm, acetate is converted to acetyl coenzyme A (acetyl CoA) by acetyl CoA synthetase at the expense of an ATP molecule. Acetyl CoA can be further metabolized via the glyoxylate cycle for the formation of C4 metabolites, or it can enter the tricarboxylic acid (TCA) cycle for the generation of ATP and carbon skeletons for anabolism and reducing equivalents (Perez-Garcia et al. 2011). Acetyl CoA is also the major precursor for fatty acid synthesis in microalgae; hence, acetate availability is the rate-limiting step for lipid accumulation in microalgae (Ramanan et al. 2013). Under nitrogen deprivation, cells reduce protein synthesis due to the unavailability of nitrogen-arresting cell division. Nitrogen limitation also activates certain deaminases that act on AMP; hence AMP concentration declines leading to the reduced activity of isocitrate dehydrogenase of TCA cycle, resulting in the accumulation of citrate which is then converted to acetyl CoA. Thus, funneling of available carbon as acetyl CoA under nitrogen deprivation helps in increased lipid accumulation in eukaryotic oleaginous microalgae (Ratledge 2004). In Chlamydomonas reinhardtii, starchless mutants exhibit higher oil accumulation, but the wild-type strains depend totally on external acetate availability for enhanced lipid accumulation. Acetate addition, seven times higher than the standard conditions, resulted in a steady increase in lipid accumulation (Fan et al. 2012). Chlorella sorokiniana could outcompete aerobic and anaerobic bacteria for acetate consumption in heterotrophic growth with unsterilized dark fermentation effluents, with a 55% carbon yield on acetate (Turon et al. 2015a). Acetate addition is also known to stimulate carotenogenesis in Haematococcus pluvialis, triggering the conversion to heterologous cysts compared to that of non-acetate-based growth (Kobayashi et al. 1991). Acetate concentrations as high as 10 g/L has been used for the cultivation of Chlorella vulgaris, but low acetate concentration aids in the use of acetate as a sole carbon source by microalgae. This may be due to the fact that higher acetate concentrations (particularly the sodium or potassium salts) can cause an increase in culture pH upon acetate consumption and a pH- stat culture is required (Perez-Garcia et al. 2011).
Butyrate can be consumed by microalgae in the same manner as acetate, via the monocarboxylate/proton transporter. Butyrate is believed to be converted to acetyl CoA via crotonyl CoA, but the mechanism of conversion is not clearly understood (Baroukh et al. 2017). It could be possible that butyrate is metabolized by the beta-oxidation pathway of fatty acids in the peroxisomes as previously reported for the yeast Candida tropicalis (Kurihara et al. 1992). Once converted to acetyl CoA, it can be further processed by the glyoxylate cycle or tricarboxylic acid cycle. Butyrate is not a preferred carbon source for microalgae, and clear diauxic pattern of growth has been observed in the presence of acetate in Chlorella sorokiniana (Baroukh et al. 2017). Butyrate above a concentration of 0.1 g/L is inhibitory for microalgal growth (Liu et al. 2013a), while a concentration of 0.3 g/L can be tolerated in mixotrophic growth (Baroukh et al. 2017). However, butyrate inhibition is believed to be relieved in the presence of acetate, owing to initial biomass accumulation by acetate consumption and secondary consumption of butyrate for energy generation. A higher acetate, butyrate ration, could enable butyrate consumption in microalgae, as high as 8:1 as reported for Chlorella protothecoides (Fei et al. 2015). An increase in substrate to microorganism ratio is also believed to overcome butyrate inhibition. A food to microorganism ratio of 4.5 was optimal for total VFA assimilation (Liu et al. 2013b), while a ratio of 1.1 was observed to be optimal for the use of butyrate as a sole carbon source for Chlorella vulgaris ESP6 (Liu et al. 2013a).
Propionate is the third major VFA to be produced during the acidogenesis phase. Mechanism of propionate utilization in microalgae is unknown, but in certain photosynthetic bacteria, it is assimilated by conversion to propionyl-CoA which is then carboxylated to methylmalonyl-CoA. A molecular rearrangement of methylmalonyl-CoA leads to succinyl-CoA, which then enters TCA cycle (Neilson and Lewin 1974). Propionate as a sole carbon source (at a concentration of 10 g/L carbon equivalent to glucose) did not support biomass production and lipid accumulation in Scenedesmus sp. R-16, but the high concentration of this acid used could also inhibit microalgal growth. The effect of propionate concentration on microalgal growth was not shown (Ren et al. 2013). Propionate at a concentration of 3000 mg/L did not support biomass and lipid accumulation of a microalgal consortium (Venkata Mohan and Prathima Devi 2012). Propionate could be consumed by microalgae at very low concentrations in a diauxic pattern in the presence of acetate. An acetate-butyrate-propionate ratio of 8:1:1 works well for both Chlamydomonas reinhardtii (Moon et al. 2013) and Chlorella protothecoides (Fei et al. 2015). Lactate was inhibitory for the growth of Chlorella vulgaris ESP6 at a concentration of above 0.5 g/L (Liu et al. 2013a), and this could happen due to the acidification of the intracellular environment upon import of this acidic metabolite. However it has been shown that lactate was never transported inside the cell or utilized for growth in Chlorella vulgaris (Liu et al. 2013a), Chlorella sorokiniana, and Auxenochlorella protothecoides (Turon et al. 2015b). Valeric and isovaleric acids have been utilized by Chlorella vulgaris and Scenedesmus sp. R16 (Cho et al. 2015; Ren et al. 2014), while isovalerate was reported to be the second best carbon source next to acetate for C. protothecoides FACHB-3 (Wen et al. 2013).
5 Microalgae-Based Biogas Upgrading and Biomass Production
Microalgae have been used for effective nutrient removal from wastewaters of domestic and industrial origin in high rate algal ponds for over a century. The nature of the AD slurry and the role of microalgae in bioremediation of the AD slurry before further processing are described in detail in Sect. 2. Microalgae-based biogas upgrading of real biogas (raw/desulfurized) and synthetic or simulated biogas are summarized in Tables 15.2 and 15.3, respectively. The nature of the organic acids present in most wastewaters are not presented in detail, and still the high COD is contributed primarily by the presence of VFAs as discussed previously. The removal and utilization of COD by microalgae represents the organic acid fraction that is utilized. Real biogas from AD plants have all the impurities and other toxic components of unknown nature that could influence microalgal growth; hence they were summarized separately (Table 15.2). Studies on biogas upgrading of simulated biogas (which mainly consists of CH4, CO2 and sometimes H2S) for evaluating their effect and tolerance levels in microalgae provide valuable insight into the metabolism of these compounds by microalgae (Table 15.3).
The chief component of biogas is methane (CH4) present in about 40–70% v/v, and generally most microalgae chosen for biogas upgrading are tolerant to the levels of CH4 seen in biogas. Biological consumption of CH4 by microalgae has been shown in some reports, but the mechanism of such consumption is unknown (Prandini et al. 2016; Lebrero et al. 2016). Since the axenic status of these cultures is questionable and the carryover of microorganisms from the AD slurry or even in biogas is possible, such biological consumption of methane could be attributed to microorganisms other than microalgae. A marine microalga Nannochloropsis gaditana CCMP 567 (wild type) was grown in methane concentrations of 0%, 50%, and 100%, and it was found that the biomass concentrations and specific growth rate (1 g/L and 0.1 day−1, respectively) were not affected by the increasing concentrations of methane (Meier et al. 2015). Three microalgal strains, C. protothecoides TISTR 8243, Chlorella sp. TISTR 8263, and marine Chlorella sp., capable of high growth potential in 50% CO2, were evaluated for their ability to grow in the presence of 50% CH4 and 50% CO2 simulating the biogas composition (Tongprawhan et al. 2014a). Of these the marine Chlorella sp. fared well, with no significant differences in biomass and lipid production in the presence of 50% CH4. The CO2 removal efficiency from 50% CO2 in air and 50% CO2 in methane were 70.4% and 68.9%, respectively (Tongprawhan et al. 2014a). Another related study for screening microalgae for tolerance to high levels of CH4 in biogas led to the isolation of a Scenedesmus sp. with high biomass and lipid productivity. Scenedesmus sp. showed 99.3% CO2 removal efficiency in simulated biogas (CH4:CO2 = 60:40), with a CO2 fixation rate of 2.59 g-CO2 day/L (Srinuanpan et al. 2017). The biomass concentration and lipid productivity were estimated to be 2.83 g/L and 96.18 mg/L/day, respectively, with lipids that could produce biodiesel with high stability and ignition quality (Srinuanpan et al. 2017). Other than the wild types, mutant strains were developed by random mutagenesis for tolerance to CH4. A mutant Chlorella sp. MM-2 was developed by random mutagenesis which was resistant to up to 80% CH4 retaining 70% of the growth potential compared to growth in the absence of CH4, with a biomass productivity of 0.116 g/L/day (Kao et al. 2012a). Another mutant, Chlorella sp. MB-9, also could grow in the presence of 80% CH4 and 20% CO2 retaining 82% of growth potential and biomass productivity of 0.243 g/L/day (Kao et al. 2012b). It has been shown that even in biogas-tolerant strains, presence of moderate levels of CH4 in the range of 45–55% can enhance biogas upgrading (Yan et al. 2014).
The second important component in the biogas that could severely influence the outcome of biogas upgrading is H2S. The concentrations of H2S in biogas vary from 0 to 10,000 ppm (Table 15.1), and dissolution of H2S in the culture medium could reduce the pH of the medium drastically inhibiting microalgal growth. The tolerance of microalgae to H2S could be attributed to the presence of certain sulfur oxidizing bacteria carried over from the AD slurry. A Scenedesmus sp. was reported to be tolerant to H2S up to 3000 ppm with complete removal of CO2 and H2S. However, it must be noted that the microalga was grown in raw unsterilized AD digestate with the fermentation microbes from the AD process (Prandini et al. 2016). A high rate algal pond (HRAP) at pH 10 with Spirulina platensis and an alkaliphilic H2S oxidizing bacterial consortium could remove up to 5000 ppm H2S effectively, and it was unaffected by the presence of other components in the AD slurry used as nutrient source (Bahr et al. 2014). Another HRAP harboring Chlorella vulgaris and nitrifying-denitrifying activated sludge showed 100% removal efficiency for 0.5% v/v H2S (Serejo et al. 2015a). Also, mutant microalgal strains resistant to biogas level H2S has not been isolated yet. Since desulfurization of biogas is a routine procedure of biogas purification for feeding into the CHP stations, most studies use desulfurized biogas where the H2S concentrations are reduced to 50–100 ppm to which most microalgae are generally tolerant (Table 15.2).
The CO2 removal efficiencies of microalgae from biogas are in the range of 50–99% based on the culture conditions and the microalgae used (Tables 15.1 and 15.2). All experiments based on simulated biogas used CO2 at 30%, and it was efficiently removed by microalgae. Algal bacterial bioreactors or microalgae with fungi or bacterial co-culture performed better than mono-algal culture, due to the synergistic effect of bacteria on algal growth and their pollutant removal efficiency to an extent. A wild-type strain Nannochloropsis gaditana which can tolerate up to 100% methane was inhibited by CO2 concentrations of 9% (Meier et al. 2015). Chlorella vulgaris, Chlorella protothecoides, Chlorococcum sp., Chlorella sp., and Scenedesmus armatus were evaluated for their biogas upgrading potential by growing in 50% CO2 in air under phototrophic conditions. Of these, the marine Chlorella sp. TISTR 8263 showed better tolerance to 50% CO2 with a specific growth rate, biomass content, lipid content, and lipid productivity of 0.457 day−1, 601 mg/L, 28.2% DW, and 21.3 mg/L/day, respectively (Tongprawhan et al. 2014b). A similar screening was performed with another set of strains comprising freshwater Chlorella sp., marine Chlorella sp., Nannochloropsis sp., Scenedesmus sp. and Botyrococcus sp. (Srinuanpan et al. 2017). The culture was phototrophic with 40% CO2, and among the strains Scenedesmus sp. showed better performance based on biomass, lipid content, and lipid productivity. Even though the lipid content of Botyrococcus sp. (42%) was higher than Scenedesmus sp. (27%), the specific growth of Botyrococcus sp. was the lowest at 0.21 day−1 thereby reducing the lipid productivity (Srinuanpan et al. 2017). In the presence of 60% CH4, Scenedesmus sp. showed 98% CO2 removal efficiency, escalating the methane content in the simulated biogas to 99.39% (Srinuanpan et al. 2017). Three microalgae, Chlorella vulgaris FACHB 31, Scenedesmus obliquus FACHB 416, and Neochloris oleoabundans UTEX 1185, were co-cultured with activated sludge on biogas slurry and evaluated for CO2 and H2S removal from biogas. The CO2 removal efficiency was over 95% for all the strains 45–55% CO2 and 55–75% CH4. The H2S present in the simulated biogas were also removed from the biogas at an efficiency ranging from 70% to 80% (Sun et al. 2016). Hence CO2 tolerance and carbon fixation efficiency are highly strain dependent. As it can be seen form Tables 15.1 and 15.2, Chlorella sp. dominate the scene for biogas upgrading, closely followed by Scenedesmus sp. Chlorella sp. are known to be robust, easily adaptable to any environment with higher growth rates. Chlorella vulgaris is known to be rich in proteins, lipids, carbohydrates, pigments, antioxidants, and other vitamins and minerals (Safi et al. 2014). They are a perfect feedstock for any valuable product generation and the methodology has been perfected over the years. Scenedesmus sp. are also appreciated as potential bio-mitigation candidates and can be applied for biogas upgrading (Ho et al. 2010).
6 Factors Affecting Nutrient Removal from the Effluents of AD and DF
Microalgal biomass can be obtained by cultivation in open systems or closed photobioreactors (PBR). Open pond systems are the most preferred method for microalgal cultivation, because of its inexpensive nature and easier methods. However, the stringent requirements for pharmaceutical compounds insist the use of closed PBRs for axenic cultivation of specific microalgae, which will yield the desired product of interest (Chang et al. 2017). PBRs also offer the advantage of proper control of the process parameters like temperature, pH, light intensity, and mixing. It must be noted that the optimal process parameters for the cultivation is chosen based on the microalgal strain used. Biogas upgrading by microalgae works on the same principle, and optimization of the process occurs based on the microalgal strains used. Here we discuss some important external factors affecting microalgae-based biogas upgrading.
6.1 Light Intensity
Light intensity is essential for microalgal cultivation, and the supply of optimal light intensity is one of the major challenges in microalgal cultivation. Light is the source of energy for photosynthesis, the primary metabolism in microalgae. Increase in light intensity may result in light limitation and subsequent inhibition of growth, while a decrease in light intensity cannot sustain biomass growth. It has also been shown that light intensity is a key factor regulating lipid accumulation in microalgae. Under high light intensities, lipid accumulation serves as an electron sink for the over-reduced photosynthetic apparatus (Liu et al. 2012). Scenedesmus sp. 11-1 accumulated 40% by weight as lipids under a light intensity of 400 μmol m−2 s−1, while only 26% was obtained at 40 μmol m−2 s−1 light intensity (Liu et al. 2012). Lipid synthesis requires uninterrupted supply of ATP and NADP(H), which is provided by photosynthesis under high light intensities, simultaneously protecting the cells from photo oxidative damage (He et al. 2015). The neutral lipid content of both Chlorella sp. L1 and Monoraphidium dybowskii Y2 were higher at high light intensities, which was 71% and 60% of the total lipids at 100 μmol m−2 s−1 (He et al. 2015). Also, high light intensities (600 μmol m−2 s−1) resulting in moderate photoinhibition could promote neural lipid accumulation in Pseudochlorococcum sp. LARB-1 (Li et al. 2011a). However, high light intensities could inhibit the uptake of organic carbon in microalgae (Perez-Garcia et al. 2011). Chlorella sorokiniana UTEX 1230 could rapidly import and metabolize glucose in the absence of light under heterotrophic growth conditions with a 9 h doubling time, accumulation of 39% total lipids and TAG productivity of 28.9 mg L/day. In the presence of light under mixotrophic conditions, TAG productivity was reduced to 18.2 mg L/day (Rosenberg et al. 2014). Light was also known to inhibit glucose uptake in Chlorella vulgaris, even in a non-photosynthetic mutant (Kamiya and Kowallik 1987). Low light intensities under mixotrophic conditions might help overcome light inhibition of both photosynthesis and organic carbon uptake, making it an effective strategy for microalgal cultivation in the presence of VFAs (Chen et al. 2018). Also, choosing the strains without light inhibition is of importance in mixotrophic cultivation (Perez-Garcia et al. 2011). Moderate light intensities were preferred for efficient biogas upgrading in microalgae. Scenedesmus sp. obtained high nutrient removal rates from biogas slurry at moderate light intensities of 150–170 μmol m−2 s−1 (Ouyang et al. 2015). Chlorella sp. showed higher biogas CO2 removal and better biogas upgrading at 350 μmol m−2 s−1 compared to 400 μmol m−2 s−1 (Yan and Zheng 2013). Illumination of the microalgal culture with lights at different wavelengths revealed that red light was found optimal for Chlorella sp.. Some studies indicate an optimal light intensity of 400–1000 μmol m−2 s−1 (Yan et al. 2016a), while another related study reported an optimal light intensity if 1200–1600 μmol m−2 s−1 (Zhao et al. 2013). A mixture of red and blue lights at a ratio of 5:5 was shown to be optimal for many studies (Yan et al. 2014, 2016b; Zhang et al. 2017b; Yan and Zheng 2014). Also, moderate photoperiod of 14 h light/10 h dark was preferred for biogas upgrading by Scenedesmus obliquus FACHB-31 (Wang et al. 2016) and Chlorella sp. (Yan and Zheng 2013). The introduction of photoperiod for the culture of Chlorella sorokiniana enhanced biogas CO2 removal even in the dark conditions, and the authors speculated that a decrease in the culture temperature in the dark can increase CO2 solubility with biogas CO2 removal even in dark periods (Meier et al. 2017). The photoperiod or difference in light/dark periods did not influence biogas upgrading by an alkali-tolerant microalgal culture of Picochlorum sp. and Halospirulina sp. in a high rate algal pond (Franco-Morgado et al. 2017b).
6.2 Culture pH
The pH of the culture medium is another important factor governing microalgal growth, metabolism and other cellular functions. The optimal pH for each microalgal strain might vary depending on the natural habitat and subsequent laboratory conditions for which they were primarily adapted. Variations in the medium pH might interfere with nutrient uptake, as pH of the medium determines the available form of inorganic carbon as CO2 or bicarbonates (Juneja et al. 2013). Alkaline conditions are best suited for biogas upgrading by microalgae, as alkaline conditions can enhance the solubility of CO2 from biogas. Under alkaline AD conditions, the CO2 generated in the fermentation process remains as dissolved carbonate in the fermentation medium generating highly pure biogas (Nolla-Ardevol et al. 2015). Also alkaline conditions could promote the absorption of other impurities present in biogas via chemical reactions (Franco-Morgado et al. 2017a). Maintenance of the medium pH at slightly alkaline conditions of pH 7.8 enhanced CO2 removal from biogas by Chlorella sp. TISTR 8263 (Tongprawhan et al. 2014b). The pH of AD effluents ranges from acidic to alkaline depending on the process conditions and microbial inoculum, so the microalgal cultivation medium pH should be maintained at the optimal pH of the microalga cultivated. Also, VFAs uptake by microalgae together with CO2 solubilization from biogas might reduce the medium pH drastically which could be highly inhibitory for microalgal growth (Chen et al. 2018). Microalgal photosynthesis makes the medium alkaline, and hence maintenance of the optimal pH via acidification of the medium could be required. Maintaining the optimal pH of the cultivation medium at 7 greatly enhanced the biomass productivity and nutrient removal efficiency of C. vulgaris when cultivated in undiluted AD effluent of activated sludge. In pH controlled cultures (maintained at pH 7), a biomass productivity of 433 mg/L/day was achieved, whereas in pH uncontrolled cultures biomass productivity was reduced to around 296 mg/L/day (Cho et al. 2015). High ammonia concentrations and the high pH (pH = 9) in piggery wastewaters could also affect microalgal growth and nutrient removal efficiency (Tan et al. 2016). It was also observed that the high pH levels could protect the microalgal culture from extraneous contaminants, and alkaline pH could be considered as a stress factor for triggering lipid accumulation in microalgae (Bartley et al. 2014). It has been shown that the CO2 removal efficiency of an algal-bacterial co-culture comprising of Chlorella sp. and activated sludge increased from 23% at pH 7 to 62% at pH 8.1 (Lebrero et al. 2016). However, for microalgae that have an optimal around 6–7, pH over 9 is severely inhibitory. The culture pH of a microalgal consortium grown in undiluted piggery wastewater was maintained under 8 with CO2 acidification for effective nutrient removal and biogas upgrading (Ayre et al. 2017).
6.3 Temperature
Temperature is another important factor governing microalgal growth and beneficial product accumulation in microalgae. In biogas upgrading by microalgae, temperature of the process is mainly chosen based on the optimal growth temperature of the microalgal strain. Biogas is fermentation off-gas of AD process, and it is at ambient temperature. Hence, cooling of the biogas to reduce the temperature or selection of thermotolerant microalgal strains is not needed. As it can be seen form the table on biogas upgrading, most of the processes occur at ambient temperature or the temperature being controlled at the optimum level of the microalgal strain. An attempt to determine the optimal temperature for biogas upgrading by Leptolyngbya sp. indicated that temperature influences the biomass growth, but not biogas upgrading by carbon capture (Choix et al. 2017). A central composite design for the determination of optimal temperature and light intensity revealed that light intensity significantly influences carbon capture and carbon assimilation, while the effect of temperature is statistically insignificant for the same. Leptolyngbya sp. is known to grow in a temperature range of 20–45 °C, and an optimal temperature of 27.1 °C was best suited for biogas upgrading and biomass accumulation (Choix et al. 2017). However, temperature is also known to influence the solubility of biogas CO2 in the culture medium. The growth rate of Chlorella sorokiniana increased during the light period of light/dark cycle (12 h:12 h), when grown on M8a medium using biogas (65% CH4, 32% CO2) from a laboratory scale brewery wastewater AD process (Meier et al. 2017). The authors also stated that CO2 solubility is inversely related to the culture temperature; as temperature decreases, solubility increases and vice versa. Hence, CO2 solubility, desorption, and accumulation performs well under dark conditions and that biogas feeding can be continued in the dark period to enhance biogas upgrading (Meier et al. 2017). The effect of temperature on organic acid accumulation by microalgae has not been studied in detail; however it has been stated that suboptimal temperatures are preferred for growth on inhibitory VFAs like butyrate, since optimal or close to optimal temperatures can exacerbate the inhibitory effect (Turon et al. 2016).
7 Bottlenecks in Microalgae-Based Biogas Upgrading and Future Perspectives
Microalgae are currently being touted as the ultimate solution for most pressing problems like global warming, climate change, and the search for alternative energy. Some researchers feel that microalgae could not fit the bill as a potential carbon mitigation candidate or as an effective carbon sink for emission reduction due to the difficulties in longtime carbon storage (Acien Fernandez et al. 2012). Still, they are the best known sustainable alternative for biofuels, pigments, and fatty acids. Also, microalgae can be effectively used for the treatment of various wastewaters before release into the environment. Competent design of microalgal cultivation in wastewaters with minimal requirement of valuable resources like water, nutrients, or CO2 can greatly enhance the energy balances of wastewater treatment and turn them into potential power houses (Menger-Krug et al. 2012). In this book chapter, we discussed extensively about the integration of microalgal cultivation with anaerobic digestion as a solution for treating nutrient-rich AD slurry with concomitant improvement in biogas quality. The microalgal biomass composition can be manipulated (high carbohydrate/lipid) by carefully controlling the process parameters and the microalgal strain chosen for cultivation (Srinuanpan et al. 2018a; Serejo et al. 2015b). Also, they make an excellent feed for aquaculture or animal husbandry. The major problem associated with any microalgae-based bioremediation/energy generation system is the constraints in commercialization of the proof-of-concept level studies carried out under controlled laboratory conditions with skilled personnel. Technology carryover to commercialization at this stage should also consider the economics of the process, cost competitiveness with available alternatives in the market, and the availability of skilled individuals for operation. Biogas upgrading by microalgae also face considerable challenges at the cultivation level, and some of the major bottlenecks in biogas upgrading by microalgae and the potential solutions are listed in Table 15.4.
Integration of microalgal cultivation with AD is mainly challenging due to the difficulties in choosing an appropriate algal strain that is capable of mixotrophic growth in the presence of organic and inorganic carbon. In our previous review, we have pointed out the essential qualities required in a microalgal strain to be used for biogas upgrading and nutrient removal from slurry: (1) the strain should be capable of mixotrophic growth, utilizing both inorganic and organic carbon under low light intensities; (2) the strain must possess robust growth properties, with high tolerance to extreme conditions like high CO2, high CH4, variations in pH, and certain toxic compounds present in biogas; and (3) the strain should be capable of accumulating higher levels of either carbohydrates or lipids for subsequent energy generation in the form of biofuels. Apart from these characteristics of the microalgal strain, the cultivation process itself needs to be optimized based on the chosen strain minimizing energy input and lowering the carbon footprint of the total system (Chen et al. 2018). Microalgae are very diverse with very flexible metabolic potentials, and hence it has always been the way to prospect for microalgal strains in natural habitats that could be used in a particular process. A Scenedesmus sp. was isolated from an open pond in a wastewater treatment plant for effective nutrient removal from swine water digestate. The microalga could grow well in raw unsterilized digestate, with a maximum CO2 assimilation from biogas at 219 mg/L/day (Prandini et al. 2016). A Chlorella vulgaris strain was isolated form an open pond used for the storage of vinasse in a sugar industry. The strain was slowly acclimated for growth in vinasse AD digestate for 21 days under optimal light intensity of 61 μmol m−2 s−1 before being introduced in an HRAP for biogas upgrading (Serejo et al. 2015b). On the other hand, acclimation of the microalgal cultures for growth in biogas slurry has been carried out. A co-culture of C. vulgaris FAHCB31 with fungi Ganoderma lucidum and Pleurotus ostreatus were acclimated or slowly adapted to diluted biogas slurry until the cells were tolerant to slurry conditions along with higher growth rates, which were then used for slurry nutrient removal (Cao et al. 2017). Specific genetic engineering of microalgae for tolerance to biogas components or flue gas components have not been performed yet due to the unclear nature of the mechanisms involved. However, random mutagenesis has been performed to improve tolerance to high CO2 or high methane concentrations (Kao et al. 2012a, b). Thus, choice of the microalgal strain is crucial for the success of the integrated AD-microalgal system. Another efficient strategy would be to control the inflow rate of biogas for low tolerance strains, which could improve the biomass production. Biogas might contain up to 70% methane based on the feedstock used, and hence control of biogas loading in the culture is a practical way to overcome methane inhibition. Nutrient removal from slurry and CO2 removal from biogas by Chlorella were shown to be higher when the influent methane concentration ranged from 45% to 55% (Yan et al. 2014). The other important component present in biogas that could inhibit microalgal growth is hydrogen sulfide (H2S). Desulfurization of biogas is a common biogas cleaning step, and hence most of the studies applied desulfurized biogas for microalgal cultivation. The presence of 5 mg S/L reduced the photosynthetic oxygen production rate of a microalgal consortium by 43%, and inhibitory effects were observed above a sulfide concentration of 20 mg S/L (Gonzalez-Camejo et al. 2017). The sulfides present in the biogas were known to be converted to sulfates under alkaline conditions and illumination, and sulfates were shown to be assimilated by Chlorella sp. resulting in enhanced growth rates (González-Sánchez and Posten 2017).
Liquid digestates are very high in COD, in the range of 210–6900 mg/L (Xia and Murphy 2016b). Such high COD levels results in increased turbidity of the liquid, which will severely affect light penetration in microalgal cultures. Hence, cultivation of microalgae under low light intensities in mixotrophic mode is a viable option for overcoming this hindrance. Also, mixotrophic mode enables microalgae to utilize both organic and inorganic carbon sources in the presence of light. The initial growth utilized the organic carbon via respiration, and when the organic carbon level decreases, photosynthesis is initiated, resulting in higher biomass productivities compared to autotrophic and heterotrophic modes of cultivation (Zhan et al. 2017). C. vulgaris was shown to grow with glucose as carbon source under respiratory mode, and in the presence of light, it is funneled to lipid synthesis, increasing lipid productivities under mixotrophic mode. The lipid productivity of C. vulgaris UTEX 259 under photoautotrophic mode and mixotrophic mode with glucose were shown to be 4 mg/L/day and 54 mg/L/day, respectively, under similar light intensities (Liang et al. 2009). Liquid digestates are also rich in total nitrogen (TN – 139–3456 mg/L) and total phosphorus (TP – 7–381 mg/L), with over 90% of both available as ammonia and phosphates (Xia and Murphy 2016b). Such high concentration of N and P combined with the high COD could be inhibitory to microalgal growth, and hence dilution of the digestate to obtain optimal level of these essential nutrients in the culture medium can improve light penetration as well. S. obliquus (FACHB-31) was grown in piggery wastewater AD digestate with a COD of 3200 mg/L, and the COD, N, and P removal efficiencies were 65%, 63% and 71%, respectively, with a biomass productivity of 241 mg/L/day. When the liquid digestate was diluted to COD 1600 mg/L, the COD, N, and P removal efficiencies increased to 75%, 74% and 81%, respectively, with a simultaneous increase in biomass productivity of 311 mg/L/day (Xu et al. 2015). Similarly, C. vulgaris (FACHB-31) performed well in biogas upgrading and nutrient removal from sewage treatment plants when the COD and total nitrogen levels were maintained at relatively lower concentrations (Xu et al. 2017). The total COD, N, and P removal efficiencies were 72%, 71%, and 69% with a medium influent COD of 200 mg/L, and the biogas methane was increased to 92% from 67%. When the COD level raised to 400 mg/L, the removal efficiencies were considerably lower. And when the total nitrogen was maintained at a medium level of 40 mg/L, the total COD, N, and P removal efficiencies were 77%, 77%, and 73% respectively, with an increase in biogas methane to 93%, and it was considerably higher compared to the high TN levels of 80 mg/L (Xu et al. 2017). Thus, maintaining the COD and TN concentrations in optimal levels enhances both light penetration and nutrient removal efficiencies. Another interesting option is to isolate microalgal strains from local environments that could be resistant to the extreme conditions of the digestate to be treated. A Chlorella strain was isolated from centrate (highly concentrated municipal wastewater), and the strain was able to grow in raw centrate with COD, TN, TP, and ammonia removal efficiencies of 90.8%, 89.1%, 80.9%, and 93.9%, respectively. The lipid-rich algal biomass obtained could be used for biodiesel production with a yield of 0.12 g-biodiesel/ for every liter of algae culture (Li et al. 2011b). The microalgal culture was scaled up to 25 L in a coil reactor, and a net biomass productivity of 0.92 g algae/L/day was achieved (Li et al. 2011b). Isolation and screening of microalgae specifically for remediation of wastewater might increase the chances of success for economic biofuel production (Li et al. 2011c; Zhou et al. 2011). Enhanced COD levels of liquid digestate are mainly due to the presence of increased concentration of VFAs as well. Acetate is the primary VFA present in most digestates, in addition to butyrate, isobutyrate, propionate, and valerate, along with certain alcohols. As discussed in detail in Sect. 4, butyrate and lactate are known to be inhibitory to microalgal growth at concentrations above 0.1 g/L and 0.5 g/L, respectively. An increase in the food-to-microorganism ratio or an increase in the acetate/butyrate ration can aid in overcoming the inhibition, and the details are discussed in Sect. 4 as well. Light intensity is also known to affect the uptake and utilization of organic acids by microalgae, and hence optimization of light intensity should be carried out while determining the process parameters for microalgal cultivation in AD slurry.
Another major issue in the use of AD digestate for microalgal cultivation is the carryover of pathogenic or harmful anaerobic bacteria from the AD process. The microbial community in AD process is very diverse, ranging from facultative anaerobes to obligate sporulating anaerobes that could survive extreme environmental conditions, and it could be present in the slurry after processing. Hence, autoclaving is essential to destroy the pathogenic bacteria (Zhu et al. 2016), but it is both expensive and energy intensive. If the anaerobic fermentation is carried out with a single nonpathogenic bacterium like dark fermentation for hydrogen production, pretreatment of effluent for pathogen removal could be deemed unnecessary. C. sorokiniana was cultivated in undiluted raw dark fermentation effluent containing acetate and butyrate under heterotrophic mode. C. sorokiniana grew efficiently in the raw effluent consuming acetate, and the presence of any contaminating bacteria present in the raw effluent did not affect the biomass productivity when compared to the use of sterile effluent (Turon et al. 2015a). Raw unsterilized centrate has also been used for biogas upgrading and slurry nutrient removal by Scenedesmus sp. (Prandini et al. 2016) and Chlorella sp. (Posadas et al. 2017b).
The inability to produce microalgae-based bioproducts and biofuels in a cost-competitive manner compared to the available products in the market is a major barrier in commercialization of the same, mainly owing to the high cost associated with microalgal cultivation, harvesting, and processing. This applies to integration of microalgal cultivation to biogas upgrading, and effective measures needs to be taken for economic cultivation of microalgae. Outdoor cultivation of microalgae is known to be economic and has been adapted by various commercial organizations for mass production. Culture contamination could be prevented by growing the microalgae in alkaline conditions, which is inhibitory to many of the common contaminants. Biogas upgrading has been performed in pilot scale in HRAPs under alkaline conditions with the use of a microalgal consortium comprising of Leptolyngbya lagerheimii, Chlorella vulgaris, Parachlorella kessleri, Tetradesmus obliquus, and Chlorella minutissima (Marín et al. 2018). The alkaline conditions helped increase the solubility of CO2, and summer months proved to be best for both nutrient removal and biogas upgrading. Maximum biogas removal occurred in May with the resultant biogas with 0.1% CO2, while the biogas with highest methane concentrations (99.6%) was achieved in August (Marín et al. 2018). Chlorella pyrenoidosa FACHB-9 was cultivated in outdoor rectangular photobioreactors on anaerobically digested activated sludge, and effective nutrient removal was achieved in summer months. The authors also proposed an innovative method to control contamination: by cutting off CO2 supply intermittently the medium pH tends to rise to 8.5–9.8 before resuming CO2 supply, which would inhibit contaminants (Tan et al. 2015). Other simulated and outdoor pilot scale studies have been reported indicating the feasibility of outdoor cultures for AD waste treatment (Tan et al. 2016; Posadas et al. 2017b; Sheets et al. 2014). Water is an essential resource required for microalgal cultivation and the water footprint of microalgae-based biodiesel production ranges from 1600 to 3360 L water/L biodiesel without recycling (Farooq et al. 2015). Recycling is an effective way to reduce the water footprint of microalgal cultivation, and care should be taken about the carryover of growth-inhibiting substances present in the recycled water resulting in the crash of the cultivation. Microalgal allelopathy is a well-reported phenomenon, and the secondary metabolites released by certain harmful algae can totally inhibit other related algae (Bacellar Mendes and Vermelho 2013). Even in the absence of harmful bacteria and allelopathy, harvest water can be recycled only once or twice based on the buildup of growth inhibitory substances in harvest water (Zhu et al. 2016). Extracellular polysaccharides and certain nitrogen-rich small organic molecules were observed to accumulate in the recycled culture media of C. vulgaris, and the water was recycled for over 60 days without any significant inhibition of growth (Hadj-Romdhane et al. 2013). The liquid digestate could provide other essential nutrients like N, P, and carbon in the place of expensive fertilizers, making the cultivation more economic.
Proper utilization of the biomass obtained can greatly improve the economics of microalgal cultivation, and proficient harvesting and downstream processing techniques are pivotal for cost-cutting measures. It has been reported that algal cultivation, harvest, and dewatering might contribute to up to 70% of the production costs of any algae-based product. So effective harvesting and dewatering strategies with lower energy input might help decrease the associated production costs (Chen et al. 2011). The composition of the biomass obtained (lipid/carbohydrate/protein rich) might vary depending on the microalgal strain chosen, and proper control of process parameters can result in high accumulation of beneficial component. Carbohydrate-rich biomass can be used for production of biofuels like bioethanol, biobutanol, and biohydrogen by fermentation of the sugars released after simple pretreatment (Serejo et al. 2015b; Nwoba et al. 2016). Lipid-rich algae can be used for the production of biodiesel (Srinuanpan et al. 2018b). Microalgal biomass rich in protein can be used as animal feed components (Singh et al. 2011). The residual biomass after product extraction can be processed by a number of thermochemical ways like pyrolysis, hydrothermal liquefaction, gasification, or torrefaction for complete energy recovery from the biomass (Chen et al. 2015). Pyrolysis resulting in the production of algal biochar has been proposed as the most effective option for the treatment of residual biomass in integrated AD-microalgal cultivation systems, since soil amendment of biochar can result in closing of the carbon cycle (Chen et al. 2018). Also, biochar can be used as a sustainable adsorbent for the removal of various harmful pollutants (Ho et al. 2017). Another similar study integrates microalgal cultivation with biogas production, and the microalgal biomass obtained was returned as AD feedstock in a loop. Life cycle analysis indicated that the use of obtained algal biomass as AD feedstock has a net energy ratio of 1.54, with reduced land use changes compared to other terrestrial crops. This strategy could help increase the annual biomethane production of the proposed Sweden biogas plant by 9.4% (Wang et al. 2013). An integrated AD of distillery stillage with microalgal cultivation providing biogas upgrade and nutrient removal was proposed. The microalga Chlorella sp. consumed CO2 from simulated flue gas and raw biogas in the range of 2–50%, simultaneously removing ammonia from biogas slurry with higher growth rates (Doušková et al. 2010). The obtained biogas was designed to be used at the plant for heat and electricity, while the microalgal biomass obtained was to be processed as food or feed supplement making this a closed technology (Doušková et al. 2010). Integration of microalgal cultivation with AD of cattle manure resulted in an annual production of 160–190 ton of microalgal biomass, with valuable components like lipids, proteins, and carbohydrates (Ledda et al. 2016). Hence, integration of microalgal cultivation with biogas production is technically feasible and economically viable. A possible integration scenario of microalgal cultivation with AD is illustrated in Fig. 15.3. All the specifics had been discussed previously, and the integration will be sustainable and beneficial giving high precedence to the following: choice of microalgal strain (preferably indigenous and robust), effective upstream and downstream process design, and complete energy recovery from the resultant biomass.
8 Conclusions
Microalgae-based carbon capture is a superior method for biogas upgrading in terms of environmental impacts and process operating conditions, when compared to the available chemical-based methods. The major constraint in realizing the potential of the technology is the development of cost-competitive methods for commercialization. With a boom in biogas production plants particularly in Europe and the USA, simultaneous development of microalgal technology to suit the needs of the biogas industry is essential. Isolation of an indigenous strain capable of tolerating the extreme condition of biogas slurry and biogas is pivotal for biogas upgrading. The existing information gap between microalgal genetic engineering and biogas upgrading needs much research attention, developing genetically engineered strains for excellent carbon capture from biogas- and slurry-based nutrient removal. With the ideal strain, the desired metabolic potential and optimal process parameters, outstanding performances in biogas upgrading, and bioremediation of biogas slurry can be accomplished. The biomass composition of the obtained microalgal biomass can help reduce the economic burden of the process, and the production of value-based chemicals in a biorefinery-based concept could be pragmatic. The return of the obtained biomass to soil in the form of biochar or fertilizer can help decrease carbon footprint of the system with long-term carbon sequestration. It has been shown that many closed loop sustainable technologies for microalgal cultivation and biogas upgrading have been carried out at the pilot scale. Commercial-scale carbon capture from flue gas has already been established at the St. Mary’s cement factory in Canada, and similar attempts are needed in biogas plants for the realization of microalgae-based biogas upgrading.
References
Abbasi T, Tauseef SM, Abbasi SA. Anaerobic digestion for global warming control and energy generation—an overview. Renew Sust Energ Rev. 2012;16(5):3228–42. https://doi.org/10.1016/j.rser.2012.02.046.
Acien Fernandez FG, Gonzalez-Lopez CV, Fernandez Sevilla JM, Molina Grima E. Conversion of CO2 into biomass by microalgae: how realistic a contribution may it be to significant CO2 removal? Appl Microbiol Biotechnol. 2012;96(3):577–86. https://doi.org/10.1007/s00253-012-4362-z.
Åhman M. Biomethane in the transport sector—an appraisal of the forgotten option. Energy Policy. 2010;38(1):208–17. https://doi.org/10.1016/j.enpol.2009.09.007.
Amani T, Nosrati M, Sreekrishnan TR. Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects — a review. Environ Rev. 2010;18. (NA:255–78. https://doi.org/10.1139/A10-011.
Angelidaki I, Treu L, Tsapekos P, Luo G, Campanaro S, Wenzel H, Kougias PG. Biogas upgrading and utilization: current status and perspectives. Biotechnol Adv. 2018;36(2):452–66. https://doi.org/10.1016/j.biotechadv.2018.01.011.
Aslam A, Thomas-Hall SR, Mughal TA, Schenk PM. Selection and adaptation of microalgae to growth in 100% unfiltered coal-fired flue gas. Bioresour Technol. 2017;233:271–83. https://doi.org/10.1016/j.biortech.2017.02.111.
Ayre JM, Moheimani NR, Borowitzka MA. Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Res. 2017;24:218–26. https://doi.org/10.1016/j.algal.2017.03.023.
Bacellar Mendes LB, Vermelho AB. Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol Biofuels. 2013;6(1):152. https://doi.org/10.1186/1754-6834-6-152.
Bahr M, Diaz I, Dominguez A, Gonzalez Sanchez A, Munoz R. Microalgal-biotechnology as a platform for an integral biogas upgrading and nutrient removal from anaerobic effluents. Environ Sci Technol. 2014;48(1):573–81. https://doi.org/10.1021/es403596m.
Baroukh C, Turon V, Bernard O. Dynamic metabolic modeling of heterotrophic and mixotrophic microalgal growth on fermentative wastes. PLoS Comput Biol. 2017;13(6):e1005590. https://doi.org/10.1371/journal.pcbi.1005590.
Bartley ML, Boeing WJ, Dungan BN, Holguin FO, Schaub T. pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. J Appl Phycol. 2014;26(3):1431–7. https://doi.org/10.1007/s10811-013-0177-2.
Bezerra RP, Matsudo MC, Sato S, Converti A, de Carvalho JCM. Fed-batch cultivation of arthrospira platensis using carbon dioxide from alcoholic fermentation and urea as carbon and nitrogen sources. Bioenerg Res. 2013;6(3):1118–25. https://doi.org/10.1007/s12155-013-9344-1.
Bolton JR, Hall DO. The maximum efficiency of photosynthesis. Photochem Photobiol. 2008;53(4):545–8. https://doi.org/10.1111/j.1751-1097.1991.tb03668.x.
Braun R. Anaerobic digestion: a multi-faceted process for energy, environmental management and rural development. In: Ranalli P, editor. Improvement of crop plants for industrial end uses. Dordrecht: Springer; 2007. p. 335–416. https://doi.org/10.1007/978-1-4020-5486-0_13.
Brennan L, Owende P. Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev. 2010;14(2):557–77. https://doi.org/10.1016/j.rser.2009.10.009.
Cao W, Wang X, Sun S, Hu C, Zhao Y. Simultaneously upgrading biogas and purifying biogas slurry using cocultivation of Chlorella vulgaris and three different fungi under various mixed light wavelength and photoperiods. Bioresour Technol. 2017;241:701–9. https://doi.org/10.1016/j.biortech.2017.05.194.
Carrere H, Antonopoulou G, Affes R, Passos F, Battimelli A, Lyberatos G, Ferrer I. Review of feedstock pretreatment strategies for improved anaerobic digestion: from lab-scale research to full-scale application. Bioresour Technol. 2016;199:386–97. https://doi.org/10.1016/j.biortech.2015.09.007.
Chang JS, Show PL, Ling TC, Chen CY, Ho SH, Tan CH, Nagarajan D, Phong WN. 11 – photobioreactors A2 – Larroche, Christian. In: Sanromán MÁ, Du G, Pandey A, editors. Current developments in biotechnology and bioengineering. Amsterdam/Boston: Elsevier; 2017. p. 313–52. https://doi.org/10.1016/B978-0-444-63663-8.00011-2.
Cheah WY, Ling TC, Juan JC, Lee D-J, Chang J-S, Show PL. Biorefineries of carbon dioxide: from carbon capture and storage (CCS) to bioenergies production. Bioresour Technol. 2016;215(Supplement C):346–56. https://doi.org/10.1016/j.biortech.2016.04.019.
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol. 2011;102(1):71–81. https://doi.org/10.1016/j.biortech.2010.06.159.
Chen W-H, Lin B-J, Huang M-Y, Chang J-S. Thermochemical conversion of microalgal biomass into biofuels: a review. Bioresour Technol. 2015;184:314–27. https://doi.org/10.1016/j.biortech.2014.11.050.
Chen Y-d, Ho S-H, Nagarajan D, Ren N-q, Chang J-S. Waste biorefineries — integrating anaerobic digestion and microalgae cultivation for bioenergy production. Curr Opin Biotechnol. 2018;50:101–10. https://doi.org/10.1016/j.copbio.2017.11.017.
Chisti Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008;26(3):126–31. https://doi.org/10.1016/j.tibtech.2007.12.002.
Cho HU, Kim YM, Choi Y-N, Xu X, Shin DY, Park JM. Effects of pH control and concentration on microbial oil production from Chlorella vulgaris cultivated in the effluent of a low-cost organic waste fermentation system producing volatile fatty acids. Bioresour Technol. 2015;184:245–50. https://doi.org/10.1016/j.biortech.2014.09.069.
Choix FJ, Snell-Castro R, Arreola-Vargas J, Carbajal-Lopez A, Mendez-Acosta HO. CO2 removal from biogas by Cyanobacterium leptolyngbya sp. CChF1 isolated from the Lake Chapala, Mexico: optimization of the temperature and light intensity. Appl Biochem Biotechnol. 2017;183(4):1304–22. https://doi.org/10.1007/s12010-017-2499-z.
Cirne DG, Lehtomäki A, Björnsson L, Blackall LL. Hydrolysis and microbial community analyses in two-stage anaerobic digestion of energy crops. J Appl Microbiol. 2007;103(3):516–27. https://doi.org/10.1111/j.1365-2672.2006.03270.x.
Converti A, Oliveira RPS, Torres BR, Lodi A, Zilli M. Biogas production and valorization by means of a two-step biological process. Bioresour Technol. 2009;100(23):5771–6. https://doi.org/10.1016/j.biortech.2009.05.072.
Doušková I, Kaštánek F, Maléterová Y, Kaštánek P, Doucha J, Zachleder V. Utilization of distillery stillage for energy generation and concurrent production of valuable microalgal biomass in the sequence: biogas-cogeneration-microalgae-products. Energy Convers Manag. 2010;51(3):606–11. https://doi.org/10.1016/j.enconman.2009.11.008.
Enzmann F, Mayer F, Rother M, Holtmann D. Methanogens: biochemical background and biotechnological applications. AMB Express. 2018;8(1):1. https://doi.org/10.1186/s13568-017-0531-x.
Fan J, Yan C, Andre C, Shanklin J, Schwender J, Xu C. Oil accumulation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii. Plant Cell Physiol. 2012;53(8):1380–90. https://doi.org/10.1093/pcp/pcs082.
Farooq W, Suh WI, Park MS, Yang J-W. Water use and its recycling in microalgae cultivation for biofuel application. Bioresour Technol. 2015;184:73–81. https://doi.org/10.1016/j.biortech.2014.10.140.
Fei Q, Fu R, Shang L, Brigham CJ, Chang HN. Lipid production by microalgae Chlorella protothecoides with volatile fatty acids (VFAs) as carbon sources in heterotrophic cultivation and its economic assessment. Bioprocess Biosyst Eng. 2015;38(4):691–700. https://doi.org/10.1007/s00449-014-1308-0.
Franco-Morgado M, Alcantara C, Noyola A, Munoz R, Gonzalez-Sanchez A. A study of photosynthetic biogas upgrading based on a high rate algal pond under alkaline conditions: influence of the illumination regime. Sci Total Environ. 2017a;592:419–25. https://doi.org/10.1016/j.scitotenv.2017.03.077.
Franco-Morgado M, Alcantara C, Noyola A, Muñoz R, Gonzalez-sanchez A. A study of photosynthetic biogas upgrading based on a high rate algal pond under alkaline conditions: influence of the illumination regime. Sci Total Environ. 2017b;592:419–25. https://doi.org/10.1016/j.scitotenv.2017.03.077.
Gebreslassie Berhane H, Waymire R, You F. Sustainable design and synthesis of algae-based biorefinery for simultaneous hydrocarbon biofuel production and carbon sequestration. AICHE J. 2013;59(5):1599–621. https://doi.org/10.1002/aic.14075.
Ghimire A, Frunzo L, Pirozzi F, Trably E, Escudie R, Lens PNL, Esposito G. A review on dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products. Appl Energy. 2015;144:73–95. https://doi.org/10.1016/j.apenergy.2015.01.045.
Gong J, You F. Optimal design and synthesis of algal biorefinery processes for biological carbon sequestration and utilization with zero direct greenhouse gas emissions: MINLP model and global optimization algorithm. Ind Eng Chem Res. 2014;53(4):1563–79. https://doi.org/10.1021/ie403459m.
Gonzalez-Camejo J, Serna-Garcia R, Viruela A, Paches M, Duran F, Robles A, Ruano MV, Barat R, Seco A. Short and long-term experiments on the effect of sulphide on microalgae cultivation in tertiary sewage treatment. Bioresour Technol. 2017;244(Pt 1):15–22. https://doi.org/10.1016/j.biortech.2017.07.126.
González-Sánchez A, Posten C. Fate of H2S during the cultivation of Chlorella sp. deployed for biogas upgrading. J Environ Manag. 2017;191:252–7. https://doi.org/10.1016/j.jenvman.2017.01.023.
Guiry MD. How many species of algae are there? J Phycol. 2012;48(5):1057–63. https://doi.org/10.1111/j.1529-8817.2012.01222.x.
Gutiérrez-Arriaga CG, Serna-González M, Ponce-Ortega JM, El-Halwagi MM. Sustainable integration of algal biodiesel production with steam electric power plants for greenhouse gas mitigation. ACS Sustain Chem Eng. 2014;2(6):1388–403. https://doi.org/10.1021/sc400436a.
Haberl H, Sprinz D, Bonazountas M, Cocco P, Desaubies Y, Henze M, Hertel O, Johnson RK, Kastrup U, Laconte P, Lange E, Novak P, Paavola J, Reenberg A, van den Hove S, Vermeire T, Wadhams P, Searchinger T. Correcting a fundamental error in greenhouse gas accounting related to bioenergy. Energy Policy. 2012;45:18–23. https://doi.org/10.1016/j.enpol.2012.02.051.
Hadj-Romdhane F, Zheng X, Jaouen P, Pruvost J, Grizeau D, Croué JP, Bourseau P. The culture of Chlorella vulgaris in a recycled supernatant: effects on biomass production and medium quality. Bioresour Technol. 2013;132:285–92. https://doi.org/10.1016/j.biortech.2013.01.025.
He Q, Yang H, Wu L, Hu C. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour Technol. 2015;191:219–28. https://doi.org/10.1016/j.biortech.2015.05.021.
He A, Kong X, Wang C, Wu H, Jiang M, Ma J, Ouyang P. Efficient carbon dioxide utilization and simultaneous hydrogen enrichment from off-gas of acetone-butanol-ethanol fermentation by succinic acid producing Escherichia coli. Bioresour Technol. 2016;214:861–5. https://doi.org/10.1016/j.biortech.2016.04.073.
Hernández-Calderón OM, Ponce-Ortega JM, Ortiz-del-Castillo JR, Cervantes-Gaxiola ME, Milán-Carrillo J, Serna-González M, Rubio-Castro E. Optimal design of distributed algae-based biorefineries using CO2 emissions from multiple industrial plants. Ind Eng Chem Res. 2016;55(8):2345–58. https://doi.org/10.1021/acs.iecr.5b01684.
Ho SH, Chen WM, Chang JS. Scenedesmus obliquus CNW-N as a potential candidate for CO2 mitigation and biodiesel production. Bioresour Technol. 2010;101(22):8725–30. https://doi.org/10.1016/j.biortech.2010.06.112.
Ho S-H, Y-D C, Z-K Y, Nagarajan D, Chang J-S, N-Q R. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour Technol. 2017;246:142–9. https://doi.org/10.1016/j.biortech.2017.08.025.
Juneja A, Ceballos MR, Murthy SG. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies. 2013;6(9):4607–38. https://doi.org/10.3390/en6094607.
Kadam R, Panwar NL. Recent advancement in biogas enrichment and its applications. Renew Sust Energ Rev. 2017;73:892–903. https://doi.org/10.1016/j.rser.2017.01.167.
Kamiya A, Kowallik W. Photoinhibition of glucose uptake in Chlorella. Plant Cell Physiol. 1987;28:611–9. https://doi.org/10.1093/oxfordjournals.pcp.a077336.
Kao C-Y, Chiu S-Y, Huang T-T, Dai L, Wang G-H, Tseng C-P, Chen C-H, Lin C-S. A mutant strain of microalga Chlorella sp. for the carbon dioxide capture from biogas. Biomass Bioenerg. 2012a;36:132–40. https://doi.org/10.1016/j.biombioe.2011.10.046.
Kao C-Y, Chiu S-Y, Huang T-T, Dai L, Hsu L-K, Lin C-S. Ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Appl Energy. 2012b;93:176–83. https://doi.org/10.1016/j.apenergy.2011.12.082.
Kao CY, Chen TY, Chang YB, Chiu TW, Lin HY, Chen CD, Chang JS, Lin CS. Utilization of carbon dioxide in industrial flue gases for the cultivation of microalga Chlorella sp. Bioresour Technol. 2014;166:485–93. https://doi.org/10.1016/j.biortech.2014.05.094.
Kobayashi M, Kakizono T, Nagai S. Astaxanthin production by a green alga Haematococcus pluvialis accompanied with morphological changes in acetate media. J Ferment Bioeng. 1991;71(5):335–9. https://doi.org/10.1016/0922-338X(91)90346-I.
Kurihara T, Ueda M, Okada H, Kamasawa N, Naito N, Osumi M, Tanaka A. Beta-oxidation of butyrate, the short-chain-length fatty acid, occurs in peroxisomes in the yeast Candida tropicalis. J Biochem. 1992;111(6):783–7.
Lara-Gil JA, Senés-Guerrero C, Pacheco A. Cement flue gas as a potential source of nutrients during CO2 mitigation by microalgae. Algal Res. 2016;17:285–92. https://doi.org/10.1016/j.algal.2016.05.017.
Lebrero R, Toledo-Cervantes A, Muñoz R, del Nery V, Foresti E. Biogas upgrading from vinasse digesters: a comparison between an anoxic biotrickling filter and an algal-bacterial photobioreactor. J Chem Technol Biotechnol. 2016;91(9):2488–95. https://doi.org/10.1002/jctb.4843.
Ledda C, Schievano A, Scaglia B, Rossoni M, Acién Fernández FG, Adani F. Integration of microalgae production with anaerobic digestion of dairy cattle manure: an overall mass and energy balance of the process. J Clean Prod. 2016;112:103–12. https://doi.org/10.1016/j.jclepro.2015.07.151.
Li Y, Han D, Sommerfeld M, Hu Q. Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol. 2011a;102(1):123–9. https://doi.org/10.1016/j.biortech.2010.06.036.
Li Y, Chen Y-F, Chen P, Min M, Zhou W, Martinez B, Zhu J, Ruan R. Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol. 2011b;102(8):5138–44. https://doi.org/10.1016/j.biortech.2011.01.091.
Li Y, Zhou W, Hu B, Min M, Chen P, Ruan RR. Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: strains screening and significance evaluation of environmental factors. Bioresour Technol. 2011c;102(23):10861–7. https://doi.org/10.1016/j.biortech.2011.09.064.
Liang Y, Sarkany N, Cui Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett. 2009;31(7):1043–9. https://doi.org/10.1007/s10529-009-9975-7.
Liu J, Yuan C, Hu G, Li F. Effects of light intensity on the growth and lipid accumulation of microalga Scenedesmus sp. 11-1 under nitrogen limitation. Appl Biochem Biotechnol. 2012;166(8):2127–37. https://doi.org/10.1007/s12010-012-9639-2.
Liu C-H, Chang C-Y, Liao Q, Zhu X, Chang J-S. Photoheterotrophic growth of Chlorella vulgaris ESP6 on organic acids from dark hydrogen fermentation effluents. Bioresour Technol. 2013a;145:331–6. https://doi.org/10.1016/j.biortech.2012.12.111.
Liu C-H, Chang C-Y, Liao Q, Zhu X, Liao C-F, Chang J-S. Biohydrogen production by a novel integration of dark fermentation and mixotrophic microalgae cultivation. Int J Hydrog Energy. 2013b;38(35):15807–14. https://doi.org/10.1016/j.ijhydene.2013.05.104.
Lo Y-C, Chen C-Y, Lee C-M, Chang J-S. Sequential dark–photo fermentation and autotrophic microalgal growth for high-yield and CO2-free biohydrogen production. Int J Hydrog Energy. 2010;35(20):10944–53. https://doi.org/10.1016/j.ijhydene.2010.07.090.
Maeda K, Owada M, Kimura N, Omata K, Karube I. CO2 fixation from the flue gas on coal-fired thermal power plant by microalgae. Energy Convers Manag. 1995;36(6):717–20. https://doi.org/10.1016/0196-8904(95)00105-M.
Mann G, Schlegel M, Schumann R, Sakalauskas A. Biogas-conditioning with microalgae. Agron Res. 2009;7:33–8.
Marín D, Posadas E, Cano P, Pérez V, Lebrero R, Muñoz R. Influence of the seasonal variation of environmental conditions on biogas upgrading in an outdoors pilot scale high rate algal pond. Bioresour Technol. 2018;255:354–8. https://doi.org/10.1016/j.biortech.2018.01.136.
Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev. 2010;14(1):217–32. https://doi.org/10.1016/j.rser.2009.07.020.
Meier L, Pérez R, Azócar L, Rivas M, Jeison D. Photosynthetic CO2 uptake by microalgae: an attractive tool for biogas upgrading. Biomass Bioenerg. 2015;73:102–9. https://doi.org/10.1016/j.biombioe.2014.10.032.
Meier L, Barros P, Torres A, Vilchez C, Jeison D. Photosynthetic biogas upgrading using microalgae: effect of light/dark photoperiod. Renew Energy. 2017;106:17–23. https://doi.org/10.1016/j.renene.2017.01.009.
Menger-Krug E, Niederste-Hollenberg J, Hillenbrand T, Hiessl H. Integration of microalgae systems at municipal wastewater treatment plants: implications for energy and emission balances. Environ Sci Technol. 2012;46(21):11505–14. https://doi.org/10.1021/es301967y.
Merlin Christy P, Gopinath LR, Divya D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew Sust Energ Rev. 2014;34:167–73. https://doi.org/10.1016/j.rser.2014.03.010.
Möller K, Müller T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng Life Sci. 2012;12(3):242–57. https://doi.org/10.1002/elsc.201100085.
Moon M, Kim CW, Park W-K, Yoo G, Choi Y-E, Yang J-W. Mixotrophic growth with acetate or volatile fatty acids maximizes growth and lipid production in Chlamydomonas reinhardtii. Algal Res. 2013;2(4):352–7. https://doi.org/10.1016/j.algal.2013.09.003.
Muñoz R, Meier L, Diaz I, Jeison D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev Environ Sci Biotechnol. 2015;14(4):727–59. https://doi.org/10.1007/s11157-015-9379-1.
Neilson AH, Lewin RA. The uptake and utilization of organic carbon by algae: an essay in comparative biochemistry. Phycologia. 1974;13(3):227–64. https://doi.org/10.2216/i0031-8884-13-3-227.1.
Nolla-Ardevol V, Strous M, Tegetmeyer H. Anaerobic digestion of the microalga Spirulina at extreme alkaline conditions: biogas production, metagenome, and metatranscriptome. Front Microbiol. 2015;6:597. https://doi.org/10.3389/fmicb.2015.00597.
Nwoba EG, Ayre JM, Moheimani NR, Ubi BE, Ogbonna JC. Growth comparison of microalgae in tubular photobioreactor and open pond for treating anaerobic digestion piggery effluent. Algal Res. 2016;17:268–76. https://doi.org/10.1016/j.algal.2016.05.022.
Ouyang Y, Zhao Y, Sun S, Hu C, Ping L. Effect of light intensity on the capability of different microalgae species for simultaneous biogas upgrading and biogas slurry nutrient reduction. Int Biodeterior Biodegrad. 2015;104:157–63. https://doi.org/10.1016/j.ibiod.2015.05.027.
Park C, Lee C, Kim S, Chen Y, Chase HA. Upgrading of anaerobic digestion by incorporating two different hydrolysis processes. J Biosci Bioeng. 2005;100(2):164–7. https://doi.org/10.1263/jbb.100.164.
Patterson T, Esteves S, Dinsdale R, Guwy A. Life cycle assessment of biogas infrastructure options on a regional scale. Bioresour Technol. 2011;102(15):7313–23. https://doi.org/10.1016/j.biortech.2011.04.063.
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y. Heterotrophic cultures of microalgae: metabolism and potential products. Water Res. 2011;45(1):11–36. https://doi.org/10.1016/j.watres.2010.08.037.
Posadas E, Szpak D, Lombó F, Domínguez A, Díaz I, Blanco S, García-Encina PA, Muñoz R. Feasibility study of biogas upgrading coupled with nutrient removal from anaerobic effluents using microalgae-based processes. J Appl Phycol. 2016;28(4):2147–57. https://doi.org/10.1007/s10811-015-0758-3.
Posadas E, Marin D, Blanco S, Lebrero R, Munoz R. Simultaneous biogas upgrading and centrate treatment in an outdoors pilot scale high rate algal pond. Bioresour Technol. 2017a;232:133–41. https://doi.org/10.1016/j.biortech.2017.01.071.
Posadas E, Marín D, Blanco S, Lebrero R, Muñoz R. Simultaneous biogas upgrading and centrate treatment in an outdoors pilot scale high rate algal pond. Bioresour Technol. 2017b;232:133–41. https://doi.org/10.1016/j.biortech.2017.01.071.
Prandini JM, da Silva MLB, Mezzari MP, Pirolli M, Michelon W, Soares HM. Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour Technol. 2016;202:67–75. https://doi.org/10.1016/j.biortech.2015.11.082.
Ramanan R, Kim B-H, Cho D-H, Ko S-R, Oh H-M, Kim H-S. Lipid droplet synthesis is limited by acetate availability in starchless mutant of Chlamydomonas reinhardtii. FEBS Lett. 2013;587(4):370–7. https://doi.org/10.1016/j.febslet.2012.12.020.
Ratledge C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie. 2004;86(11):807–15. https://doi.org/10.1016/j.biochi.2004.09.017.
Ren HY, Liu BF, Ma C, Zhao L, Ren NQ. A new lipid-rich microalga Scenedesmus sp. strain R-16 isolated using Nile red staining: effects of carbon and nitrogen sources and initial pH on the biomass and lipid production. Biotechnol Biofuels. 2013;6(1):143. https://doi.org/10.1186/1754-6834-6-143.
Ren H-Y, Liu B-F, Kong F, Zhao L, Xing D, Ren N-Q. Enhanced energy conversion efficiency from high strength synthetic organic wastewater by sequential dark fermentative hydrogen production and algal lipid accumulation. Bioresour Technol. 2014;157:355–9. https://doi.org/10.1016/j.biortech.2014.02.009.
Rosenberg JN, Kobayashi N, Barnes A, Noel EA, Betenbaugh MJ, Oyler GA. Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the Microalga C. sorokiniana. PLoS One. 2014;9(4):e92460. https://doi.org/10.1371/journal.pone.0092460.
Ryckebosch E, Drouillon M, Vervaeren H. Techniques for transformation of biogas to biomethane. Biomass Bioenerg. 2011;35(5):1633–45. https://doi.org/10.1016/j.biombioe.2011.02.033.
Saady NMC. Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: unresolved challenge. Int J Hydrog Energy. 2013;38(30):13172–91. https://doi.org/10.1016/j.ijhydene.2013.07.122.
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C. Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sust Energ Rev. 2014;35:265–78. https://doi.org/10.1016/j.rser.2014.04.007.
Serejo ML, Posadas E, Boncz MA, Blanco S, Garcia-Encina P, Munoz R. Influence of biogas flow rate on biomass composition during the optimization of biogas upgrading in microalgal-bacterial processes. Environ Sci Technol. 2015a;49(5):3228–36. https://doi.org/10.1021/es5056116.
Serejo ML, Posadas Olmos E, Boncz M, Blanco S, García-Encina P, Muñoz R. Tailoring biomass composition during the optimization of the integral upgrading of biogas in microalgal-bacterial processes. Environ Sci Technol. 2015b;49(5):3328–36.
Sheets JP, Ge X, Park SY, Li Y. Effect of outdoor conditions on Nannochloropsis salina cultivation in artificial seawater using nutrients from anaerobic digestion effluent. Bioresour Technol. 2014;152:154–61. https://doi.org/10.1016/j.biortech.2013.10.115.
Singh M, Reynolds DL, Das KC. Microalgal system for treatment of effluent from poultry litter anaerobic digestion. Bioresour Technol. 2011;102(23):10841–8. https://doi.org/10.1016/j.biortech.2011.09.037.
Srinuanpan S, Cheirsilp B, Kitcha W, Prasertsan P. Strategies to improve methane content in biogas by cultivation of oleaginous microalgae and the evaluation of fuel properties of the microalgal lipids. Renew Energy. 2017;113:1229–41. https://doi.org/10.1016/j.renene.2017.06.108.
Srinuanpan S, Cheirsilp B, Prasertsan P. Effective biogas upgrading and production of biodiesel feedstocks by strategic cultivation of oleaginous microalgae. Energy. 2018a;148:766–74. https://doi.org/10.1016/j.energy.2018.02.010.
Srinuanpan S, Cheirsilp B, Prasertsan P. Effective biogas upgrading and production of biodiesel feedstocks by strategic cultivation of oleaginous microalgae. Energy. 2018b;148:766–74. https://doi.org/10.1016/j.energy.2018.02.010.
Sun S, Ge Z, Zhao Y, Hu C, Zhang H, Ping L. Performance of CO2 concentrations on nutrient removal and biogas upgrading by integrating microalgal strains cultivation with activated sludge. Energy. 2016;97:229–37. https://doi.org/10.1016/j.energy.2015.12.126.
Sung KD, Lee JS, Shin CS, Park SC. Isolation of a new highly CO2 tolerant fresh water microalga Chlorella sp. KR-1. Renew Energy. 1999;16(1):1019–22. https://doi.org/10.1016/S0960-1481(98)00362-0.
Surendra KC, Takara D, Hashimoto AG, Khanal SK. Biogas as a sustainable energy source for developing countries: opportunities and challenges. Renew Sust Energ Rev. 2014;31:846–59. https://doi.org/10.1016/j.rser.2013.12.015.
Takabe Y, Himeno S, Okayasu Y, Minamiyama M, Komatsu T, Nanjo K, Yamasaki Y, Uematsu R. Feasibility of microalgae cultivation system using membrane-separated CO2 derived from biogas in wastewater treatment plants. Biomass Bioenerg. 2017;106:191–8. https://doi.org/10.1016/j.biombioe.2017.09.004.
Tan XB, Yang LB, Zhang YL, Zhao FC, Chu HQ, Guo J. Chlorella pyrenoidosa cultivation in outdoors using the diluted anaerobically digested activated sludge. Bioresour Technol. 2015;198:340–50. https://doi.org/10.1016/j.biortech.2015.09.025.
Tan XB, Zhang YL, Yang LB, Chu HQ, Guo J. Outdoor cultures of Chlorella pyrenoidosa in the effluent of anaerobically digested activated sludge: the effects of pH and free ammonia. Bioresour Technol. 2016;200:606–15. https://doi.org/10.1016/j.biortech.2015.10.095.
Tilche A, Galatola M. The potential of bio-methane as bio-fuel/bio-energy for reducing greenhouse gas emissions: a qualitative assessment for Europe in a life cycle perspective. Water Sci Technol. 2008;57(11):1683.
Toledo-Cervantes A, Estrada JM, Lebrero R, Muñoz R. A comparative analysis of biogas upgrading technologies: photosynthetic vs physical/chemical processes. Algal Res. 2017a;25:237–43. https://doi.org/10.1016/j.algal.2017.05.006.
Toledo-Cervantes A, Madrid-Chirinos C, Cantera S, Lebrero R, Munoz R. Influence of the gas-liquid flow configuration in the absorption column on photosynthetic biogas upgrading in algal-bacterial photobioreactors. Bioresour Technol. 2017b;225:336–42. https://doi.org/10.1016/j.biortech.2016.11.087.
Tongprawhan W, Srinuanpan S, Cheirsilp B. Biocapture of CO2 from biogas by oleaginous microalgae for improving methane content and simultaneously producing lipid. Bioresour Technol. 2014a;170:90–9. https://doi.org/10.1016/j.biortech.2014.07.094.
Tongprawhan W, Srinuanpan S, Cheirsilp B. Biocapture of CO2 from biogas by oleaginous microalgae for improving methane content and simultaneously producing lipid. Bioresour Technol. 2014b;170:90–9. https://doi.org/10.1016/j.biortech.2014.07.094.
Turon V, Trably E, Fayet A, Fouilland E, Steyer JP. Raw dark fermentation effluent to support heterotrophic microalgae growth: microalgae successfully outcompete bacteria for acetate. Algal Res. 2015a;12:119–25. https://doi.org/10.1016/j.algal.2015.08.011.
Turon V, Baroukh C, Trably E, Latrille E, Fouilland E, Steyer JP. Use of fermentative metabolites for heterotrophic microalgae growth: yields and kinetics. Bioresour Technol. 2015b;175:342–9. https://doi.org/10.1016/j.biortech.2014.10.114.
Turon V, Trably E, Fouilland E, Steyer JP. Potentialities of dark fermentation effluents as substrates for microalgae growth: a review. Process Biochem. 2016;51(11):1843–54. https://doi.org/10.1016/j.procbio.2016.03.018.
Uusitalo V, Havukainen J, Manninen K, Höhn J, Lehtonen E, Rasi S, Soukka R, Horttanainen M. Carbon footprint of selected biomass to biogas production chains and GHG reduction potential in transportation use. Renew Energy. 2014;66:90–8. https://doi.org/10.1016/j.renene.2013.12.004.
Van Den Hende S, Vervaeren H, Boon N. Flue gas compounds and microalgae: (bio-)chemical interactions leading to biotechnological opportunities. Biotechnol Adv. 2012;30(6):1405–24. https://doi.org/10.1016/j.biotechadv.2012.02.015.
Venkata Mohan S, Prathima Devi M. Fatty acid rich effluent from acidogenic biohydrogen reactor as substrate for lipid accumulation in heterotrophic microalgae with simultaneous treatment. Bioresour Technol. 2012;123:627–35. https://doi.org/10.1016/j.biortech.2012.07.004.
Wang X, Nordlander E, Thorin E, Yan J. Microalgal biomethane production integrated with an existing biogas plant: a case study in Sweden. Appl Energy. 2013;112:478–84. https://doi.org/10.1016/j.apenergy.2013.04.087.
Wang Z, Zhao Y, Ge Z, Zhang H, Sun S. Selection of microalgae for simultaneous biogas upgrading and biogas slurry nutrient reduction under various photoperiods. J Chem Technol Biotechnol. 2016;91(7):1982–9. https://doi.org/10.1002/jctb.4788.
Wang X, Bao K, Cao W, Zhao Y, Hu CW. Screening of microalgae for integral biogas slurry nutrient removal and biogas upgrading by different microalgae cultivation technology. Sci Rep. 2017;7(1):5426. https://doi.org/10.1038/s41598-017-05841-9.
Weiland P. Biogas production: current state and perspectives. Appl Microbiol Biotechnol. 2010;85(4):849–60. https://doi.org/10.1007/s00253-009-2246-7.
Wen Q, Chen Z, Li P, Duan R, Ren N. Lipid production for biofuels from hydrolyzate of waste activated sludge by heterotrophic Chlorella protothecoides. Bioresour Technol. 2013;143:695–8. https://doi.org/10.1016/j.biortech.2013.06.085.
Xia A, Murphy JD. Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol. 2016a;34(4):264–75. https://doi.org/10.1016/j.tibtech.2015.12.010.
Xia A, Murphy JD. Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol. 2016b;34(4):264–75. https://doi.org/10.1016/j.tibtech.2015.12.010.
Xu Y, Isom L, Hanna MA. Adding value to carbon dioxide from ethanol fermentations. Bioresour Technol. 2010;101(10):3311–9. https://doi.org/10.1016/j.biortech.2010.01.006.
Xu J, Zhao Y, Zhao G, Zhang H. Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl Microbiol Biotechnol. 2015;99(15):6493–501. https://doi.org/10.1007/s00253-015-6537-x.
Xu J, Wang X, Sun S, Zhao Y, Hu C. Effects of influent C/N ratios and treatment technologies on integral biogas upgrading and pollutants removal from synthetic domestic sewage. Sci Rep. 2017;7(1):10897. https://doi.org/10.1038/s41598-017-11207-y.
Yan C, Zheng Z. Performance of photoperiod and light intensity on biogas upgrade and biogas effluent nutrient reduction by the microalgae Chlorella sp. Bioresour Technol. 2013;139:292–9. https://doi.org/10.1016/j.biortech.2013.04.054.
Yan C, Zheng Z. Performance of mixed LED light wavelengths on biogas upgrade and biogas fluid removal by microalga Chlorella sp. Appl Energy. 2014;113:1008–14. https://doi.org/10.1016/j.apenergy.2013.07.012.
Yan C, Zhang L, Luo X, Zheng Z. Influence of influent methane concentration on biogas upgrading and biogas slurry purification under various LED (light-emitting diode) light wavelengths using Chlorella sp. Energy. 2014;69:419–26. https://doi.org/10.1016/j.energy.2014.03.034.
Yan C, Muñoz R, Zhu L, Wang Y. The effects of various LED (light emitting diode) lighting strategies on simultaneous biogas upgrading and biogas slurry nutrient reduction by using of microalgae Chlorella sp. Energy. 2016a;106:554–61. https://doi.org/10.1016/j.energy.2016.03.033.
Yan C, Zhu L, Wang Y. Photosynthetic CO2 uptake by microalgae for biogas upgrading and simultaneously biogas slurry decontamination by using of microalgae photobioreactor under various light wavelengths, light intensities, and photoperiods. Appl Energy. 2016b;178:9–18. https://doi.org/10.1016/j.apenergy.2016.06.012.
Ye R, Jin Q, Bohannan B, Keller JK, Bridgham SD. Homoacetogenesis: a potentially underappreciated carbon pathway in peatlands. Soil Biol Biochem. 2014;68:385–91. https://doi.org/10.1016/j.soilbio.2013.10.020.
Yue L, Chen W. Isolation and determination of cultural characteristics of a new highly CO2 tolerant fresh water microalgae. Energy Convers Manag. 2005;46(11):1868–76. https://doi.org/10.1016/j.enconman.2004.10.010.
Zhan J, Rong J, Wang Q. Mixotrophic cultivation, a preferable microalgae cultivation mode for biomass/bioenergy production, and bioremediation, advances and prospect. Int J Hydrog Energy. 2017;42(12):8505–17. https://doi.org/10.1016/j.ijhydene.2016.12.021.
Zhang Q, Nurhayati CC-L, Nagarajan D, Chang J-S, Hu J, Lee D-J. Carbon capture and utilization of fermentation CO2: integrated ethanol fermentation and succinic acid production as an efficient platform. Appl Energy. 2017a;206:364–71. https://doi.org/10.1016/j.apenergy.2017.08.193.
Zhang Y, Bao K, Wang J, Zhao Y, Hu C. Performance of mixed LED light wavelengths on nutrient removal and biogas upgrading by different microalgal-based treatment technologies. Energy. 2017b;130:392–401. https://doi.org/10.1016/j.energy.2017.04.157.
Zhao Y, Wang J, Zhang H, Yan C, Zhang Y. Effects of various LED light wavelengths and intensities on microalgae-based simultaneous biogas upgrading and digestate nutrient reduction process. Bioresour Technol. 2013;136:461–8. https://doi.org/10.1016/j.biortech.2013.03.051.
Zhou W, Li Y, Min M, Hu B, Chen P, Ruan R. Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresour Technol. 2011;102(13):6909–19. https://doi.org/10.1016/j.biortech.2011.04.038.
Zhou M, Yan B, Wong JWC, Zhang Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: a mini-review focusing on acidogenic metabolic pathways. Bioresour Technol. 2018;248(Pt A):68–78. https://doi.org/10.1016/j.biortech.2017.06.121.
Zhu L, Yan C, Li Z. Microalgal cultivation with biogas slurry for biofuel production. Bioresour Technol. 2016;220:629–36. https://doi.org/10.1016/j.biortech.2016.08.111.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Nagarajan, D., Lee, DJ., Chang, JS. (2019). Biogas Upgrading by Microalgae: Strategies and Future Perspectives. In: Alam, M., Wang, Z. (eds) Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment. Springer, Singapore. https://doi.org/10.1007/978-981-13-2264-8_15
Download citation
DOI: https://doi.org/10.1007/978-981-13-2264-8_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2263-1
Online ISBN: 978-981-13-2264-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)