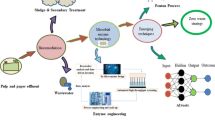
Abstract
Paper and pulp industry is water intensive and has a greater impact on aquatic, surrounding environment and public health. Minimum fresh water usage and emphasis on waste-water recycling/management are key factors for the growth of this industry. Concentration of impurities and toxic substances in processed water mainly limits recycling benefits because it adversely affects processes, equipments and paper quality. Organic wastes are mostly processed through biodegradation and bioremediation using anaerobic digestion (methane production) followed by aerobic digestion (inducing sludge processing). Although, biological processing is economical and eco-friendly but treatment of wastes including non-biodegradable recalcitrant compounds mostly limits its broad application. Therefore, many other innovative approaches have been exploited to tackle this problem. Advanced oxidation process (AOP), novel biodegradable polymeric flocculants, electrocoagulation and photocatalysis etc. are used as alternative ways to facilitate detoxification and recycling. In this chapter, we emphasised and provided an in-depth knowledge about the various wastewater treatment strategies linked to paper and pulp industry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Economic strength of a nation relies on the industrialization however, it also badly affects its environment (Hossain and Rao 2014; Raj et al. 2014). The pulp and paper industry is considered to be one of the most important industrial sectors in the world due to its important contribution in the economic health of a country. Still now, pulp and paper mills are facing challenges with the energy efficiency mechanisms and management of the consequential pollutants, considering the environmental feedbacks and enduring legal requirements (Kamali and Khodaparast 2015). The pulp and paper industry totally relies on the natural resources because it is known to be a major consumer of wood, water and energy (fossil fuels, electricity) along with its major contribution in discharge of toxic effluents and pollutants into the environment. The pulp and paper industry stands at sixth position after oil, cement, leather, textile and steel industries. The pulp and paper industry typically generates a large quantity of wastewater which requires proper treatment and recycling prior to its discharge; otherwise it may lead to serious threat to the environment and economic wealth of a country.
The natural raw materials being used for the manufacturing processes are wood, cellulose, vegetables, bagasses, rice husk, fibers and also waste-paper materials (Fig. 2.1) resulting into large amount of wastewater after processing. The paper making is a water-intensive process because it requires plenty of fresh water for the production processes (about 250–300 m3 per tons of paper) and water consumption depends upon the raw material used in industrial processes. The paper and pulp industry effluent consists of several toxic and recalcitrants including sulphur compounds, organic acids, chlorinated lignin, resin acid, phenolics, unsaturated fatty acids and terpenes (Prasongsuk et al. 2009). The industrial effluents without treatment are hazardous to the environment because of a high Biochemical Oxygen Demand (BOD), Chemical Oxygen Demand (COD), chlorinated compounds measured as adsorbable organic halides (AOX), suspended solids, recalcitrant organics, pH, turbidity, high temperature and intense colour (Chandra et al. 2007). These industries are utilizing a huge amount of lignocellulogic materials and water during the manufacturing process, and release chlorinated lignosulphonic acids, chlorinated resin acids, chlorinated phenols and chlorinated hydrocarbon in the effluent.
The highly toxic and recalcitrant compounds, dibenzo-p-dioxin and dibenzofuran, are formed unintentionally in the effluent of pulp and paper mill. The wastewater resulting from pulp and paper industry contains wood extract, tannin resins, synthetic dyes etc. in form of colouring bodies. The increasing public awareness of the fate of these pollutants and stringent regulations established by the various authorities and agencies are forcing the industry to treat effluents to the required compliance level before discharging in to the environment (DˈSouza et al. 2006). A reduced usage of toxic chemicals and improved wastewater treatment in modern mills, have significantly contributed to reduction in effluent toxicity (Van den Heuvel and Ellis 2002) and thereby are eco-friendly (Sandstrom and Neuman 2003). For decolorization of pulp and paper mill effluents, a number of treatment methods have been applied; these are classified into physical, chemical and biological methods. Physical and chemical methods are less economical and also do not remove BOD and low molecular weight compounds (Singh and Thakur 2004). The biological colour removal process is particularly attractive since in addition to colour and COD it also reduces BOD and low molecular weight chlorolignins (Nagarthnamma et al. 1999). This chapter reviews the pulp and paper mill wastes, characteristics, effects and treatment methods and includes both traditional and advanced processes.
2.2 Pulp and Paper Wastewater
2.2.1 Pollutants Released from Pulp and Paper Industry
Pulp and paper industry heavily consumes raw materials e.g. wood, chemical, energy and water. The waste material resulting from this industry includes 41.8% as bleached pulp, 4.2% as solid waste, 5.25% as dissolved organic matter and 2.3% as suspended solids (Table 2.1) (Nemade et al. 2003). The key pollutants from pulp and paper mill are grouped into following categories:
-
(a)
Organic compounds and chemicals
-
Suspended solids including bark particles, fiber, pigments and dirt.
-
Dissolved colloidal organics like hemicelluloses, sugars, lignin compounds, alcohols, turpentine, sizing agents, adhesives like starch and synthetics.
-
Color bodies, primarily lignin compounds and dyes.
-
Dissolved in organics such as NaOH, Na2SO4 and bleach chemicals.
-
Thermal loads.
-
Toxic chemicals.
-
-
(b)
Gases
-
Malodorous sulphur gases such as mercaptans and H2S released from various stages in kraft pulping and recovery process.
-
Oxide of sulphur from power plants, kraft recovery furnace and lime kiln.
-
Steam.
-
-
(c)
Particulates
-
Fly ash from coal fired power boilers.
-
Chemical particles primarily sodium and calcium based.
-
Char from bark burners.
-
-
(d)
Solid wastes
-
Sludges from primary and secondary treatment and causticizing in kraft mill recovery section.
-
Solids such as grit bark and other mill wastes.
-
Ash from coal fired boilers.
-
2.2.2 Sources of Pulp and Paper Wastewater Pollutants
Paper making process includes five basic steps (Table 2.2) and each step may be carried out through a number of methods. Therefore, the final effluent is resulted from the combination of waste water coming out of the five different unit processes and the methods employed there in. Table 2.3 summarizes the source of pollutants normally produced during several steps manufacturing process of pulp and paper industry (Raj et al. 2014; Karrasch et al. 2006).
2.2.3 Characteristics of Pulp and Paper Industry Wastewater
The effluents from pulp and paper industry are composed of different parameters in terms of COD, BOD and pH etc. contributing to increased water toxicity (Table 2.4).
2.2.4 Impact of Pulp and Paper Wastewater on Surrounding
The paper industry is known to produce plenty of effluents and wastewater. The untreated effluent from the paper and pulp industry impacts the surrounding environment in various ways.
-
(a)
Impact on water
Chemical contamination and reduced level of oxygen, deteriorates the water quality and significantly lowers the survival rate of its aquatic fauna. Oxygen depletion in the aquatic ecosystem occurs due to the high organic load and solid content in the effluent leading to physiological and reproductive alterations in fishes (Springer 2000). These alterations include delayed sexual maturity with lowering in secondary sexual characters in species living in the discharged effluents (Munkittrick et al. 1997). Discharge of greater volume of highly coloured and toxic effluent containing alcohol, chelating agents and inorganic materials that cause hypertrophication of the water bodies. The high molecular weight linin fragments having low biodegradability are responsible for this event leading to an increase in parasitic growth and disease in the species living in the downstream of the discharges (Lehtinen 2004).
The bleaching stage of the process engenders the main effluent bulk containing organic and inorganic compounds primarily comprising of derivatives of lignin or other wood components, such as extractives or carbohydrates. The solid matter usually comprises of fibers and bleaching additives. Resulting wastewater consists of very high biochemical oxygen demand (BOD), total suspended solids, chemical oxygen demands (COD), chlorinated organic compounds and absorbable organic halides (AOX). All these chemicals have an adverse effect on the aquatic life and in the process on the livestock and human population those survive on these water resources.
-
(b)
Impact on atmosphere
Air emissions are usually from recovery broiler. Sulphur dioxide mainly, and particulate emissions of nitrous oxides that comes from nitrogen content in the black liquor – dry solid content and support fuel rate used in recovery broiler. The energy generations process produces majorly particulates sulphur dioxide, nitrous oxide etc. Fly ash, SO2 and NOx are produced from steam and electricity generating units. These gases along with the particulates result into urban smog resulting into serious human health concerns like eyes, nose throat irritation, coughing, breathing difficulties and lungs ailment. The presence of damaging amounts of sulphur and nitrous compounds upon wet precipitation causes acid rain which in turn results in the acidification of soil and water bodies, ensuing in making the water unsuitable for aquatic animals and wildlife. The acid rain is also seen to rapidly deteriorate buildings and heritage sculptures. The high concentration of nitrogen nutrients greatly accelerates algal growth that affects the animal diversity in the aquatic ecosystem. The particulate density in the air causes haze when the sunlight is obstructed by the same. This conceals the clarity, texture and form of the visual perception.
-
(c)
Impact on biodiversity
The spillage of waste water results in the increase of chemicals nutrients like nitrogen and phosphorus. The presence of these nutrients at higher levels promotes excessive algal blooms in water using oxygen and the growth blocks sunlight which hinders the process of photosynthesis of plants under water. The decaying process of these algae also uses oxygen thereby decreasing the oxygen content in water. Blockage of sunlight disrupts the reproductive ability of fish and loss of the natural breeding site. This result in the migration of aquatic organisms to oxygen rich environment and in this process reduction of biodiversity and dead zones are caused.
The waste water pollutants result in the decline of several plant species and increase in the prevalence of hardy species. Also seen are the defoliation, root necrosis, leaves chlorosis, low seedling growth and forest clearance due to premature tree death (Farmer 1990). The studies on mammals and birds show an association between the population decline and loss of relevant food species. The metal contamination correlates to the reduction in reproductive ability in all orders of water dependent species (Tickle et al. 1995). Soil contamination results from the deficiency of microorganisms those play vital roles in soil rejuvenation and fertility thereby affecting micro flora and fauna of the top soil.
-
(d)
Impact on forest
Deforestation is a partial result of this industry. The clearance of growing tress to feed the industry has resulted in the increase in the non-cultivable land. The plains and slopes of the cleared forests also pose erosion threats during the monsoons. The untreated wastewater can also influence the forest reserve area causing damage and mortality of ground plants, killing of trees and soil chemistry changes.
-
(e)
Impact on agriculture
Waste water with high solid content usually leads to problems of waterlogging, desertification, salinization, erosion affecting the irrigated areas. The downstream degradation of water quality by chemicals and toxic leachates has an impact on the agriculture leading to slow growth of crops and final crop output lowers.
-
(f)
Impact on public health
The general health of the human habitation depending on aquatic animals as a source of protein in the diet significantly deteriorates due to low survival rate of aquatic animals resulting from chemical contamination and reduced levels of oxygen in the water.
Surface runoff and consequently non-point source contribute significantly to high level of pathogens in surface water bodies. These usually cause allergies, skin irritation, breathing difficulties, nausea and waterborne infection like diarrhoea. The use of pulp and paper mill wastewater for irrigation contaminates foods during their washing and their consumption as raw vegetables e.g. cabbage, lettuce, strawberries may result in various diseases e.g. cholera, typhoid, amoebiasis and giardiasis etc. due to microbiological contamination.
2.3 Pulp and Paper Production Process
2.3.1 Raw Materials Handling
Paper industry consumes a range of raw materials e.g. cellulosic derived from forest, agricultural residues and waste paper; non-cellulosic coal, chlorine, lime, sodium hydroxide, sodium sulphide, fuel oil, talcum powder etc. Major raw materials used by paper industry are bamboo, wood, bagasse, waste paper and agricultural residue like wheat straw, rice straw, jute sticks, hemp, kenaf, grasses, sea weed etc. Apart from this, paper industry consumes a large amount of chemicals like caustic soda, sodium sulphide, sodium carbonate, chlorine, hypochlorite, mineral acid; coal, talcum powder etc. (Table 2.5).
2.3.2 Pulp Manufacturing
The cellulosic materials are isolated from wood, fibre crops, waste paper and rags using chemical and mechanical methods and are used in formation of pulp which is a key raw material for pulp and paper industry. Pulp formation mostly utilizes heartwood and sapwood. There are a number of methods employed for pulping procedures (given below):
2.3.2.1 Mechanical Pulp
Most modern industries use chips rather than logs and ridged metal discs called refiner plates instead of grindstones. If the chips are just ground up with the plates, the pulp is called refiner mechanical pulp (RMP) and if the chips are steamed while being refined the pulp is called thermomechanical pulp (TMP).
2.3.2.2 Thermomechanical Pulp
Processed wood chips after heat treatment are known as thermomechanical pulp that results from two-step process: stripping of the bark and their conversion into smaller chips.
2.3.2.3 Chemithermomechanical Pulp (CTMP)
The conditions of the chemical treatment are much less vigorous (lower temperature, shorter time, less extreme pH) than in a chemical pulping process since the goal is to make the fibres easier to refine, not to remove lignin as in a fully chemical process. Pulps made using these hybrid processes are known as chemithermomechanical pulps.
2.3.2.4 Chemical Pulp
The high-quality papers are results from these methods because chemical cooking dissolves majority of lignin and hemicelluloses contents found in wood leading to better separation of the cellulose fibres. There are two primary means of chemical pulping.
-
(a)
The sulphite process: This cooks wood chips in sulphurous acid combined with limestone to produce calcium bisulphite. The combination of sulphurous acid and calcium bisulphite dissolves the lignin in the wood and liberates the cellulose fibres. Sulphite pulp is soft and flexible, is moderately strong, and is used to supplement mechanical pulps (most typically in newsprint). In order to overcome the issues raised during the process such as types of trees, rules and regulations of pollution laws etc., latest process have been adopted resulting into new chemicals.
-
(b)
The sulphate process: It is now the most widely used chemical pulping system. It is evolved from the soda processes developed in the nineteenth century, which used strong bases (alkaline solutions) such as lye to digest wood. Pulpers began adding sodium sulphate to the soda process, and a significantly stronger pulp was produced.
-
(c)
The kraft process: It entails treatment of wood chips with a hot mixture of water, sodium hydroxide, and sodium sulphide, known as white liquor that breaks the bonds those link lignin, hemicellulose, and cellulose. This process is more economical, well suited to nearly all known species of trees, increase strength and brightness of pulp. The pulp resulting from this process is much stronger compared to other methods as the name “kraft” suggests and the resulting paper is used in high-speed presses.
To increase pulp whiteness and brightness (unbleached kraft pulp has a dark brown colour), and to remove residual lignin, chemical pulps are bleached. It is at this point that additional non-fibrous materials called fillers are added to the pulp a process called loading and the resulting furnish-the mixture of pulp and fillers-is ready to begin the refining process.
2.3.2.5 Recycled Pulp
Papers with printed ink are recycled using a process termed as deinking, therefore, the recycled pulp is also known as deinked pulp (DIP). A number of industries e.g. newsprint, toilet and tissue paper etc. consumes DIP as a raw material.
2.3.2.6 Organosolv Pulp
Organosolv pulping uses organic solvents e.g. methanol, ethanol, formic acid and acetic acid etc. at temperatures above 140 °C to break down lignin and hemicellulose into soluble fragments. The pulping liquor is easily recovered by distillation.
2.3.2.7 Biological Pulp
In contrast to chemical pulping, biological pulping utilizes a number of microbes e.g. bacteria, algae and fungi those degrade waste lignin (Table 2.6) and cellulose fibres (Ahmad et al. 2011). Lignin peroxidase is a fungal enzyme that selectively degrades lignin (Table 2.7). The treated pulp undergoes bleaching followed by the neutralization step.
2.3.3 Pulp Washing and Screening
Pulping process consumes a high amount of chemicals therefore recovery of chemicals from pulp also known as pulp washing, is required because first they are expensive to replace, second, they interfere with the downstream process and third, they are harmful for the environment.
There are many types of machinery used for pulp washing. Most of them rely on displacing the dissolved solids (inorganic and organic) in a pulp mat by hot water, but some use pressing to squeeze out the chemicals with the liquid. An old, but still common method is to use a rotating drum, covered by a wire mesh, which rotates in a diluted suspension of the fibres. The fibres form a mat on the drum, and showers of hot water are then sprayed onto the fibre mat.
2.3.4 Bleaching
Bleaching process effectively removes total residual lignin content after pulping process because its chromophoric groups contribute in the darkness of the pulp. The modern process employed both bleaching and pulping steps in delignification process. However, traditionally the name ‘bleaching’ is reserved for delignification that is taking place downstream of the pulping process. In practice, there are two separate “bleaching” process steps: oxygen delignification and final bleaching.
2.3.4.1 Oxygen Delignification
This process includes treatment of washed pulp with highly alkaline solution of sodium hydroxide because higher pH favours the oxidation of phenolic groups in the lignin through their ionization and thereafter further depolymerization of resulting partial-degraded lignin into low molecular weight biproducts. These are more soluble in water and can be removed from the fibres. It is important that the pulp has been at least partly washed beforehand because the black liquor solids in unwashed pulp consume oxygen. After the oxygen delignification stage, the pulp has to be washed very well, as otherwise the organics carry over to the final bleaching process, consuming chemicals there and also decreasing the environmental benefits.
The highly alkaline conditions of oxygen delignification also make carbohydrate fractions in the fibres react with oxygen up to a certain extent however radical oxygen species are harmful for carbohydrates. The formation of radicals is promoted by the presence of certain metal ions. However, it has been found that magnesium salts inhibit metal ion activity, and magnesium sulphate is therefore normally added as a protector in oxygen delignification.
Oxygen delignification can significantly decrease the water pollution from the final (normally chlorine or chlorine dioxide based) bleaching. In addition, it is an effluent free process. All dissolved lignin and other organics (as well as the inorganic chemicals) are recovered in the black liquor and returned to the chemical recovery system, rather than being discharged as effluent as they are in chlorine-based bleaching. Finally, oxygen is a fairly cheap bleaching chemical, although the capital costs are high for an efficient system.
2.3.4.2 Final Bleaching
Final bleaching is a multi-stage process that utilizes various commercial bleaching chemicals including chlorine, chlorine dioxide, sodium hypochlorite, oxygen, peroxide, ozone. This process improves strength of the pulp by efficient usage of the chemicals. Elemental chlorine (Cl2) produces a large amount of chlorinated organic compounds in the effluent, and strenuous efforts have, therefore, been made to decrease its usage. Modern bleach plants, therefore, use no elemental chlorine. They are called as ECF plants: elemental chlorine free bleach plants. Despite being much toxic to the environment, chlorine dioxide is more effective in preserving pulp strength but less effective in delignification or bleaching compared to Cl2.
2.3.5 Chemical Recovery
The recovery of the process chemicals and fibres reduces the pollution load to a great extent, where the economy permits; the colour bearing – black liquor is treated for the chemical recovery. However, in this process the lignin is destroyed. The same may also be recovered from the black liquor, by precipitation or acidulation with either CO2 or sulphuric acid. These recovered lignins have got various uses in other industries. The alkaline lignins of kraft process may be used as a dispersing agent in various suspensions. Lignins may be used as raw materials for various other substances like dimethyl sulphoxide, which is used as spinning solvent for polyacrylonitrite fibres. Activated carbons may also be manufactured from the lignins, recovered from the black liquors. The fibres in the white water, from the paper mills are recovered either by sedimentation or by flotation using forced air in the tank.
2.3.6 Paper Making
Paper making process includes mechanical and chemical treatment of pulp fibres resulting into suspension and, successive pressing and drying of cellulosic fibres to get paper after water removal. The mechanical treatment of the fibre normally takes place by passing it between moving steel bars which are attached to revolving metal discs, known as refiners. This treatment has two effects: it shortens the fibre (fibre cutting) and it fibrillates the fibre. The latter action increases the surface area, and as the fibres bond together in the paper sheet by hydrogen bonding, the increased surface area greatly increases the bonding and strength of the paper. Paper strength is dependent on the individual fibre strength and the strength of the bonds between the fibres. It is usually the latter, which is the limiting factor. Refining increases the inter fibre bonding at the expense of the individual fibre strength, but the net result will be an increase in paper strength. Pressing and calendaring (feeding through rollers) increase density and promote smoothness. Various chemicals are added, e.g. to give water resistance, increased strength, produce coloured paper, or to serve as inorganic filters.
2.4 Methods of Pulp and Paper Wastewater Treatment
2.4.1 Physical Methods
Physico-chemical processes are commonly used in the preliminary, primary or tertiary stages of wastewater treatment. The concentration of contaminants present in wastewaters and their desired removal efficiencies are important factors in choosing the type of physico-chemical treatment process. The presence of lignin and its derivatives contribute to strong colour in most pulp-and-paper wastewaters (Dilek and Gokcay 1994). These wastewaters also contain high concentrations of suspended solids and floating matters. Therefore, the use of a primary treatment, commonly sedimentation (Mulligan 2002) is essential for the treatment process. These processes include various techniques:
2.4.1.1 Sedimentation
Sedimentation is the property that helps to remove most of the suspended particles from the wastewater by employing multiple forces e.g. gravitational, centrifugal and electromagnetic etc. (Thompson et al. 2004).
2.4.1.2 Froth Flotation
Froth flotation is a process for selective separation of hydrophobic materials from a mixer containing hydrophilic substances (Hogenkamp 1999). This process is extensively employed in mineral processing, paper recycling and waste-water treatment.
2.4.2 Physiochemical Methods
2.4.2.1 Coagulation-Flocculation
Coagulation-flocculation is a way to treat chemical water in order to remove the particles before it undergoes sedimentation and filtration. Coagulation step results into gelatinous mass after neutralizing charges whereas, flocculation step involves agglomeration of particles into large masses either by stirring or agitation but in both processes these masses are either settled down or trapped in the filter. These processes add an additional step to purify industrial effluents (Wong et al. 2006). Coagulation is an efficient way to remove suspended solids and COD from industrial wastewater prior to further treatment (Dilek and Gokcay 1994).
2.4.2.2 Activated Carbon Filtration
Activated carbon filtration method is an effective way to get rid of organic contaminants from industrial wastewater through their adsorption on carbon filter but it doesn’t involve removal of microbial and other inorganic contaminants. Various parameters such as activated carbon and quality of water etc. determine adsorption efficiency of carbon filters. However, the efficiency and lifetime of carbon filters is drastically increased once they are combined with ozonation process.
2.4.2.3 Chemical Precipitation
Chemical precipitation is the most well known and most commonly used technique for the removal of metals and some anions from wastewater. The aim of precipitation is to precipitate the chemicals from dissolved substances in the wastewater by adding a reagent, which forms an insoluble compound with the to-be-separated matter. Additionally, precipitation also allows to get rid of positive ions e.g. heavy metals and negative ions e.g. phosphates and sulphates.
2.4.3 Biological Methods
Biological treatment is a natural process. Organic matter in water naturally decays as a result of the presence of microorganisms in receiving bodies of water. High organic loads in wastewater will upset the biocenosis (an association of different organisms forming a closely integrated community) of receiving bodies of water and cause other undesirable effects. Biological treatment is engineered to accelerate natural decay processes and neutralize the waste before it is finally discharged to receiving waters. It may be divided in two types:
Aerobic Systems (Activated Sludge (liquid waste), Composting (solid waste))
Anaerobic Systems (UASB (liquid waste), Anaerobic Digester (solid waste))
Aerobic processes are usually operated at low dissolved oxygen (DO) concentrations and microorganisms are subjected to varying periods of time when no DO is present. Therefore, many facultative microorganisms will be found in an aerobic process.
2.4.3.1 Aerobic Process
2.4.3.1.1 Activated Sludge Process
Activated sludge is defined as a suspension of microorganisms, both living and dead, in wastewater. The microorganisms are activated by an input of air and the organics in the waste are metabolised to produce end products and new biomass. Mixing must be adequate to prevent the sedimentation of microorganisms and to mix air, waste and nutrients. The aerobic mode of metabolism is the most efficient in terms of energy recovered by the biomass per unit of substrate processed. This results in a relatively large quantity of sludge production, which is the other primary characteristic of this process.
2.4.3.1.2 Composting
Composting is a biological process that uses naturally occurring microorganisms to convert biodegradable organic matter into a humus-like product and is a suitable method for recycling waste treatment sludge. The composting process destroys pathogens, converts nitrogen from unstable ammonia to stable, organic forms of nitrogen and reduces the volume of waste (Zhu 2006). This process is controlled by environmental parameters (temperature, moisture content, pH, and aeration) and substrate properties (C/N ratio, particle size, and nutrient content) (Kulikowska et al. 2015).
2.4.3.2 Anaerobic Process
Anaerobic processes are more economical in terms of low sludge production and their disposal compared to aerobic processes. This process also yields methane and carbon dioxide, and mostly have been employed during late 1960s e.g. upflow anaerobic sludge blanket (UASB) (Lettinga and Huishoff Pol 1991) expanded granular sludge bed (EGSB) and internal circulation reactor (IC).
2.4.3.2.1 Upflow Anaerobic Sludge Blanket (UASB)
UASB is a methanogenic (methane-producing) digester, evolved from the anaerobic clarigester. It is a single tank anaerobic process for the removal of organic pollutants. As shown in Fig. 2.2, wastewater enters from bottom and flows vertically upwards through sludge blanket filters containing bacteria those help in sludge treatment by anaerobic degradation of organic matter and production of biogas as an energy source. As all aerobic treatments, UASB also require a post-treatment to remove pathogens, but due to a low removal of nutrients, the effluent water as well as the stabilised sludge can be used in agriculture.
2.4.3.2.2 Expanded Granular Sludge Bed (EGSB)
The EGSB reactor is a variant of the UASB for anaerobic treatment of wastewater (Jim and Reyes 2006). A different feature is that a faster rate of upward-flow velocity is designed for the wastewater passing through the sludge bed (Fig. 2.3). EGSB is more suitable for wastewaters with low suspended particles to avoid clogging of sludge bed and less soluble COD values (<1–2 COD/lit).
2.4.3.2.3 Internal Circulation (IC) Reactor
Both USAB and EGSB digestion systems give rise to IC reactor with better digestion rates and biogas production (biogas with around 80% of methane). This reactor typically includes an acidification and hydrolysis tank (Fig. 2.4) and results into effluents requiring aerobic treatment to overcome high BOD and COD contents of the discharge. Water is pumped into the bottom of the reactor where it enters in an efficient water distribution system and then mixed with anaerobic granular sludge in the reactor. In the lower part of the reactor (processing zone), most organics are converted into methane and carbon dioxide. Biogas is collected by the lower part of the three-phase separator, producing gas lift power to push water through up-flow column into gas-liquid separator on the top of the reactor. Biogas is separated in the separator while water flows back to the bottom of the reactor. Hence it is known as internal circulation (IC) reactor. In the second section of the reactor, namely the upper part, wastewater is further treated. In this part, the produced biogas is collected by the three-phase separator and clean water is discharged from the top of the reactor.
2.4.4 Innovative Technologies
2.4.4.1 Ozonation
Ozone readily reacts with the substrate on its own compared to oxygen which requires a catalyst e.g. metal ion to start the reaction. An ozonation was also compared with other methods such as combined ozonation with hydrogen peroxide mediated oxidation and Fenton’s oxidation for the removal of COD and colour from the pulp and paper wastewater (Kishimoto et al. 2010) and found that both, ozonation and ozonation with hydrogen peroxide were successful in decolorization whereas no satisfactory results were found with COD removal. However, the Fenton’s oxidation process was found more economical and more efficient in removing both colour and COD compared to ozonation alone, combined ozonation and hydrogen peroxide oxidation. Chemical precipitation using sulfuric acid followed by ozonation was also tried for the treatment of paper-making wastewater that successfully was able to remove large content of high molecular weight contaminants, 96% and 60–70% of colour and BOD, respectively (Santos Ramos et al. 2009). They showed that the pH level, varied with the quantity of sulfuric acid used in the process, affected the efficiency of ozonation.
2.4.4.2 Ultrafiltration
Ultrafiltration is a type of membrane filtration linked with hydrostatic pressure that forces a liquid through semipermeable membrane and has better removal efficiency but less economical which limits its use in the pulp-and-paper industry (Bhattacharjee et al. 2006).
2.4.4.3 Chemical Oxidation
Chemical oxidation oxidises organic pollutants and inorganic components (cyanide) to less harmful substances and eventually results in CO2 and H2O. This method may also be combined with biological purification leading to partial oxidation (breakdown of complexed compounds into simplified substances) followed by biological degradation. Chemical oxidisation involves addition of oxidants including ozone (O3), hydrogen peroxide (H2O2), bleaching liquor (NaOCl), chlorine dioxide (ClO2), chlorine gas (Cl2), peroxy acetic acid (C2H4O3) and pure oxygen (O2). Among them most active oxidant is hydroxyl radical (OH.) resulting from ozone or hydrogen peroxide after activation with a catalyst (e.g. Fe2+ in a Fenton reaction) or UV light.
2.4.4.4 Electrolysis
Electrolysis involves electricity flow through treatable water or effluent leading to destabilization of dissolved and colloidal particles, suspended matter so that they may undergo electro-coagulation, agglomeration, electro-flotation for their easy removal (Kishimoto et al. 2010).
2.4.5 Alternative Treatment Methods
Biological treatments of paper and pulp wastes are not enough to degrade non-biodegradable wastes. Therefore, alternative treatments are needed to degrade such industrial wastes.
2.4.5.1 Advanced Oxidation Process (AOP)
This treatment method utilizes the properties of highly reactive hydroxyl radicals in order to transform recalcitrant compounds into biodegradable components and inorganic substances such as carbon dioxide and water. These hydroxyl radicals act on aromatic, polyphenols, halogenated compounds, detergents etc. for their proper mineralization. UV radiation along with hydrogen peroxide (H2O2), ozone in combination with UV or H2O2 and Fenton’s reagent (Fe+2 with H2O2) in absence and presence of UV are being utilized to accelerate the production of these hydroxyl radicals for AOP method (Covinich et al. 2014). A combination of AOP with biological treatment enhances its efficiency for the waste-water treatment.
2.4.5.2 Biodegradable Polymeric Flocculants
Highly toxic recalcitrants e.g. dibenjo-p-dioxin and dibenzofuran resulting from pulp and paper industry are threat to water quality and living aquatic fauna. Physio-chemical and biological approaches still not enough to conquer this problem. Therefore, a novel approach bridging between both approaches could be adopted to treat the waste water of this industry using biodegradable anion polymers (Kumar et al. 2015). The basic principal of this approach is based on two steps: bridging and flocculation (Fig. 2.5). Once, small amount of these long chain polymers is administered to the colloidal particle suspension, they get adsorb on those particles and interlink these particles with each other, the phenomena is known as bridging. But over dosage of the polymers results into destabilization of these particles due to insufficient space available on the surface of these particles and steric repulsion among them for further bridging. Therefore, aggregates of these bridged particles are formed, also known as flocculants (Rose and John 1987). This approach is cost-effective, eco-friendly due to use of biodegradable polymers and it serves as an alternate primary treatment to eliminate toxicity, colour and maintain COD and BOD of the water.
2.4.5.3 Electrocoagulation
Low biodegradability index of paper and pulp effluents limits the usage of biochemical method in waste-water treatment of this industry. Therefore, various approaches such as electro-coagulation, electro-floatation and electro-oxidation have been extensively used for the treatment but out of them, electro-coagulation has been emerged as most efficient method due to its salient features e.g. low amount of sludge generation, easy handling and complete degradation of resulting pollutants (Fig. 2.6). Electrocoagulation is an independent and complexed process resulting into coagulants by using aluminium as sacrificial anodes. Various parameters i.e. time, current density, pH, salt concentration, speed of stirrer and electrode distance determine reduction of colour, BOD and COD of resulting wastewater from paper and pulp industry (Sharma 2014).
2.4.5.4 Photocatalysis
Wood is a key raw-material of the paper and pulp industry, resulting into major waste-product lignin in the effluent that has to be degraded effectively for water recycling. Therefore, photo-catalysis has emerged an effective method driving oxidation of lignin contaminant up to 2000 mg/lit using photocatalytic slurry reactor with an optimum temperature of 60 °C (Pawar and Hussain 2016).
2.5 Biological Sources of Pulp and Paper Wastewater Treatment
2.5.1 Bacteria
Bacteria are the primary agents of treatment in any biological treatment process (Table 2.6). Taken as whole, their diverse characteristics and minimal growth requirements allow them to proliferate in a wastewater environment.
2.5.2 Fungi
Yeast and fungi are very commonly used in the treatment processes of paper and pulp wastewater (Table 2.7). Their lower nitrogen requirement and ability to survive at lower pH enable them to be used extensively in wastewater treatment. The white-rot fungi have been extensively employed in various biotechnological areas to explore their degradation abilities. The treatment processes including fungi results into conversion of organics present in wastewater into valuable proteins and by-products along with fungal biomass that could be included in human diet and animal feeds (Guest and Smith 2002; Zheng et al. 2005). Yeast bioconversion of wastewater has been attractive to many researchers for ease of cultivation, ability to grow at pH values lower than 5, and growth rates faster than those of moulds (Gonzalez et al. 1992). Additionally, yeasts yield biomass with greater nutrient values and have lesser susceptibility to be contaminated by other microbes (Satyawali and Balakrishnan 2007). Compared to bacteria, fungi are more favourable to treat pulp and paper wastewater. First, fungi contain a group of extracellular enzymes that facilitate the biodegradation of recalcitrant compounds such as polyphenolic compounds through nonspecific oxidation reaction (D’Annibale et al. 2004; Jaouani et al. 2005). Bacterial cells, by contrast, can produce target-specific enzymes for degrading these recalcitrant contaminants (Chrost and Siuda 2002). Second, Fungi also have a greater resistance to inhibitory compounds than do bacterial species. The hyphal growth of fungi provides a greater protection for their sensitive organelles. Third, the cell walls of fungi, a layer of extra-polysaccharide matrix, protect them from inhibitory compounds through adsorption. Moreover, fungi are eukaryotes, having considerably more genes than bacteria, which make them more versatile in tolerating inhibitory compounds (Guest and Smith 2002). Fourth, the higher number of genes in fungi imparts greater reproductive selectivity, which might result in better adaptations to the environment (Bennett and Lasure 1991). One of the major reasons for using bacterial biomass in biological wastewater treatment is its well-understood growth kinetics.
2.5.3 Algae
Algae are natural water purifier and a number of technologies based on algae are used in treatment of wastewater and production of efficient and economical useful products. Many species of algae have been reported for removal of pollutant from pulp and paper industry wastewater (Table 2.8). Microalgae have been employed for the removal of nutrients from wastewater (Gupta and Rao 1980; Kunikane et al. 1984; Gantar et al. 1991; Queiroz et al. 2007; Rao et al. 2011). The algae-bacterial symbiosis contributes in reduction of electricity demand from aeration which constitutes more than 50% of total energy production form wastewater treatment plants.
2.5.4 Plants
Phytoremediation is an eco-friendly and economical process to convert industrial wastewater into usable water with the help of plants (Lakshmi et al. 2017). The effluents resulting from pulp and paper industry also contaminate surrounding soil with toxic heavy metals those are absorbed by roots of the plants with subsequent transport to aerial plant organs leading to removal of toxins from the affected soil, known as phytoextraction. The sunflower (Helianthus annuus) was found efficient for phytoextraction of lead from industrial effluents (Usha et al. 2011). Similarly, a study demonstrated that channel grass Vallisneria spiralis effectively reduces COD of pulp and paper effluent (Singhal et al. 2003).
Aquatic weeds e.g. Typha latifolia, Eichhornia crassipes, Salvinia molesta and Pistia stratiotes also have been found to be efficient for treatment of effluents under laboratory conditions (Table 2.9) (Sukumaran and Dipu 2013). Eichhornia crassipes and Typha latifolia were found as best options for treatment of the industrial effluents. Eichhornia crassipes prominently removed lead whereas Typha latifolia was found to remove heavy metals like copper and cadmium from industrial effluents (Sukumaran and Dipu 2013). Another comparative study has been done on three tropical native plants named as Scirpus grossus, Azola pinnata and Salvinia molesta on the properties of decolorization and COD removal from pulp and paper mill effluent (Ahmad et al. 2017). The authors found that all plants removed 100% COD but showed variable colour removal property. Among all, Scirpus grossus was found to be a best native aquatic plant for both COD and colour removal (Ahmad et al. 2017). Both water hyacinth (Eichhornia crassipes) and water caltrop (Trapa natans) have been found in biosorption of Pb and Zn from paper mill effluents (Verma et al. 2005; Kumar and Chopra 2016).
2.5.5 Enzymes
Various enzymes resulting from numerous microbes are involved in treatment of paper and pulp mill wastewater (Table 2.10).
2.6 Mechanism of Biodegradation of Major Pollutant of Pulp and Paper Industry
Industrial zones involved in pulp and paper manufacturing and precision machining are contaminated to a large extent by lignin and halogenated hydrocarbons such as dichlororethylene (DCE) and trichloroethylene (TCE), polychlorinated biphenyls (PCBs) and hydroxy-PCBs, polybrominated diphenyl ethers (PBDEs). These chemicals are lipophilic and their release into the environment results in bioaccumulation in adipose tissue and persistence due to their chemical stability and resistance to metabolic breakdown. The more widely used PCBs and benzene hexachloride (lindane) have been identified in almost every component of the global ecosystem including fish, wildlife and humans. Lindane is a persistent organic pollutant. It is relatively long-lived in the environment, transported long distances and can bioaccumulate in food chains. Lindane is used as an insecticide on fruit and vegetable crops. The nervous system is mostly effected form the large amounts of lindane resulting into symptoms like headache and dizziness to seizures, convulsions etc. These polychlorinated hydrocarbons are decomposed in the soil, sediment and water by bacteria, fungi and algae (Tables 2.6, 2.7, and 2.8), also known as biodegradation which results into comparatively less harmful by-products. Biodegradation utilizes microbial metabolic activities for transformation or mineralization of organic contaminants into less toxic and harmful substances those are recycled back into biogeochemical cycles. Bioremediation is an economical and non-destructive method which employs natural biodegradation of containments by optimizing limiting conditions (Alexander 1999).
2.6.1 Biodegradation of Lignin
Lignin is an integral cell wall constituent, which provides plant strength and resistance to microbial degradation. The macromolecular properties and structural characteristics of lignin make biodegradation studies difficult. Lignin is chemically modified by demethylation of its phenolic and nonphenolic units (Eriksson et al. 1990) and limited aromatic hydroxylation and ring cleavage (Kirk and Farrell 1987). The ideal isolation method of lignin would allow the collection of chemically unmodified lignin with quantitative recovery and free of non-lignin contaminants. None of the existing methods fulfil all these requirements. Now a day biodegradation is preferred method of recovery of various natural substrates from lignin complex. A wide range of bacteria and fungi have been isolated from different compost environments for study of lignin degradation (Tables 2.6 and 2.7). Actinomycetes are bacteria which form multicellular filaments, thus they resemble fungi. Actinomycetes are able to degrade some cellulose and solubilize lignin, and they tolerate higher temperatures and pH than fungi. Thus, actinomycetes are important agents of lignocellulose degradation during peak heating, although their ability to degrade cellulose and lignin is not as high as that of fungi (Godden et al. 1992). In fungi the most effective lignin degraders are Basidiomycetes. White-rot fungi belonging to the subdivision Basidiomycetes attack either hardwood or softwood, while Ascomycetes probably degrade only hardwood (Kirk and Farrell 1987). Lignin degradation by white-rot fungi is faster than that of any other organisms and they are responsible for most of the lignin decomposition in nature. However, the growth substrate is not only lignin, but also hemicelluloses and cellulose (Buswell and Odier 1987). The growth of fungi decreases in nitrogen or carbon depleted conditions and ligninolytic activity appears as a form of secondary metabolism (Kirk and Farrell 1987). Fungi degrade plant cell wall lignin using extracellular flavooxidases and generate hydrogen peroxide during redox cycling of non-phenolic aromatic aldehydes. Peroxidase one-electron oxidation of lignin units results in an unstable cation radical that experiences different reactions including breakdown of Cα-Cβ and C4- ether linkage releasing the corresponding aromatic aldehydes that can be intracellularly mineralized (Fig. 2.7).
2.6.2 Biodegradation of Polychlorinated Hydrocarbons
The degradation of aromatic organochlorines occurs via four well characterized mechanisms as follows:
-
(a)
Oxygenolytic dechlorination
This occurs via deoxygenative attack on the aromatic nucleus and it is dependent upon the position of chlorine substituents. The oxygenolytic cleavage of the chlorine atom occurs when both the atoms of molecular oxygen are incorporated into the aromatic nucleus. PCBs are principally hydroxylated on the unsubstituted carbons at positions 2 and 3 and 3 and 4. In either case, the mechanism seems to require the presence of adjacent unchlorinated atoms.
-
(b)
Hydrolytic dechlorination
In this reaction the incorporation of a hydroxyl group leads to the concomitant release of chlorine substituent. Unlike the above the hydroxyl group is derived from water and the reaction is catalysed by a dehalogenase. The requirement for the water rather than molecular oxygen also permits this chlorination to occur in anaerobic denitrifying conditions.
-
(c)
Reductive dechlorination
Reductive dechlorination results in the removal of the chloride substituent with the concurrent addition of electrons to the molecule. Although it has been reported to occur in both aerobic and anaerobic environments but it has been shown to occur primarily in the latter.
-
(d)
Chloride release after cleavage of the aromatic ring
Both aerobic and anaerobic microbial processes of PCBs degradation involved complete ring opening and final cleavage.
The role of anaerobes play in the degradation of PCBs was first shown by Brown et al. (1987). The first finding was that highly chlorinated PCB compounds (with one to ten chlorine atoms) were present in much higher proportion than less chlorinated compounds. Despite this it is clear that the transformations observed specifically involved the removal of ortho, meta and para chlorines. The different position (ortho, meta and para) of chlorine compounds have made advances in the study of anaerobic dechlorination of PCBs. According to Tiedje et al. (1993) the frequency of dechlorination was as follows: meta > para > ortho (Fig. 2.8).
2.7 Pulp and Paper Waste Water Recycling
Increased awareness for environment protection and stringent legislation forced pulp and paper industry to reduce their water consumption and rely on used water recycling. Membrane filtration is one of the methods to clean wastewater to improve its quality. Three types of membrane filters are known to be used here: micro-filtration, ultra-filtration and nano-filtration. Recently, a new type of membrane is also being used, known as ceramic membrane with an advantage on carbon filter which is easy cleaning through backflushing (Laitinen et al. 2002). Wastewater pH is also a limiting factor because acidic pH is more suitable for better permeability compared to neutral pH. The reason is that electrostatic repulsions are present in neutral pH but absent in acidic pH. Another method which can be used for water recycling is membrane bioreactor (MBR). In this technique, wastewater from bleaching process is used for treatment and resulting water may be reused as process water (Tenno and Paulapuro 1999).
Water source diagram (WSD) based on the synthesis of mass exchange network via a heuristical algorithmic procedure is an alternate way to minimize the water consumption and wastewater generation (Gomes et al. 2007). This procedure has an ability to minimize about 46% of water consumption and about 76.8% of reusable water generation with an objective to maximize reuse with low fresh water consumption.
2.8 Future Prospects for Paper and Pulp Wastewater Treatment and Recycling
In the era of digitization, the paper and forest product industry is evolving and changing. The education, industrialization and changing lifestyle still could not reduce the demand of papers and its usage for printing, writing and packaging. Paper is mostly used in the printing and writing sector followed by packaging industry. The demand for tissue paper and pulp in manufacturing hygiene products is also growing throughout the world. The graphic paper has less demand these days but the forest product industry definitely seems to be growing.
Although, the demand for newsprint and coated paper is declined regardless of that demand for specialty papers is rising in the market. The industrial packaging including online shopping, delivery, product safety and fabricating procedures also contribute to increasing demand for paper worldwide. The demand for hardwood and softwood fibres is slowly increasing as they are required as raw materials for stronger and lighter weight packaging stuffs.
The dependency of this industry on the raw materials including wood, agro and reused paper at economical prices may force the industry to accelerate and flourish. However, it also requires innovative strategies for recycling and waste treatment for uninterrupted manufacturing and survival of the industry.
References
Ahmad H, Ahmad FF, Chia YL et al (2011) Lignocellulolytic enzymes produced by tropical white rot fungi during biopulping of Acacia mangium wood chips. J Biochem Technol 3:245–250
Ahmad J, Abdullah SRS, Hassan HB, Rahman RAA, Idris M (2017) Screening of tropical native aquatic plants for polishing pulp and paper mill final effluent. Malays J Anal Sci 21:105–112
Alexander M (1999) Biodegradation and bioremediation, 2nd edn. Academic, San Diego ISBN: 978012049861, p 453
Anuranjana Jaya JG, Vijayan N (2016) Microbial degradation and nutrient optimization of pulp and paper industry waste water. Int Res J Eng Technol 3:1919–1923
Bajpai P (1999) Application of enzymes in the pulp and paper industry. Biotechnol Prog 15:147–157
Barapatre A, Jha H (2016) Decolourization and biological treatment of pulp and paper mill effluent by lignin-degrading fungus Aspergillus flavus strain F10. Int J Curr Microbiol App Sci 5:19–32
Bennett JW, Lasure LL (1991) More gene manipulations in fungi. Academic, San Diego
Bhardwaj NK, Bajpai P, Bajpai PK (1996) Use of enzymes in modification of fibres for improved beat ability. J Biotechnol 51:21–26
Bhattacharjee S, Bhattacharjee C, Datta S (2006) Studies on the fractionation of β-lactoglobulin from casein whey using ultrafiltration and ion-exchange membrane chromatography. J Membr Science 275:141–150
Brown JF, Wanger RE, Feng H et al (1987) Environmental dechlorination of PCBs. Environ Toxicol Chem 6:579–593
Buswell JA, Odier E (1987) Lignin biodegradation. CRC Crit Rev Biotechnol 6:1–60
Chandra R, Bharagava RN (2013) Bacterial degradation of synthetic and kraft lignin by axenic and mixed culture and their metabolic products. J Environ Biol 34:991–999
Chandra R, Raj A, Purohit HJ et al (2007) Characterisation and optimisation of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere 67:839–846
Chrost RJ, Siuda W (2002) Ecology of microbial enzymes in lake ecosystems. In: Burns RG, Dick RF (eds) Enzymes in the environment: activity, ecology and applications. CRC Press, New York, pp 35–72
Covinich LG, Bengoechea DI, Fenoglio RJ et al (2014) Advanced oxidation processes for wastewater treatment in the pulp and paper industry: a review. Am J Environ Eng 4:56–70
D’Annibale A, Ricci M, Quaratino D et al (2004) Panus tigrinus efficiently removes phenols, color and organic load from olive-mill wastewater. Res Microbiol 155:596–603
Dilek FB, Gokcay CF (1994) Treatment of effluents from hemp-based pulp and paper industry: I e waste characterization and physico-chemical treatability, Proceedings of the IAWQ International Specialized Conference on Pretreatment of Industrial Wastewaters. Pergamon Press, Athens, pp 161–163
D'Souza DT, Tiwari R, Sah AK et al (2006) Enhanced production of laccase by a marine fungus during treatment of coloured effluents and synthetic dyes. Enzyme Microb Technol 38:504–511
Ekendahl S (2015) Algae culturing at pulp- and paper industries for sustainable production of bio-oil. Project Rep 66:1–55
Eriksson KEL, Blanchette RA, Ander P (1990) Microbial and enzymatic degradation of wood components. Springer, Berlin
Fabienne M (2001) Pectin methyl esterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6:414–419
Farmer AM (1990) The effect of lake acidification on aquatic macrophytes-a review. Environ Pollut 65:219–240
Ferdowshi Z (2013) Screening of fresh water microalgae and Swedish pulp and paper mill waste waters with the focus on high algal biomass production. Master of Science Thesis
Fukunaga N, Kita Y (1990) Elimination of ink from reclaimed paper. Jpn Pat 683:2–80
Gantar M, Obreht Z, Dalmacija B (1991) Nutrient removal and algae succession during the growth of Spirulina platensis and Scenedesmus quadricauda on swine wastewater. Bioresour Technol 36:167–171
Gauthier F, Neufeld JD, Driscoll BT (2000) Coliform bacteria and nitrogen fixation in pulp and paper mill effluent treatment systems. Appl Environ Microbiol 66:5155–5160
Godden B, Ball AS, Helvenstein P (1992) Towards elucidation of lignin degradation pathway in actinomycetes. J Gen Microbiol 138:2441–2448
Gomes JFS, Queiroz EM, Pessoa FLP (2007) Design procedure for water/wastewater minimization: single contaminant. J Clean Prod 15:474–485
Gonzalez MP, Siso MIG, Murado MA et al (1992) Depuration and valuation of mussel-processing wastes: characterization of amylolytic postincubates from different species grown on an effluent. Bioresour Technol 42:133–140
Guest RK, Smith DW (2002) A potential new role for fungi in a wastewater MBR biological nitrogen reduction system. J Environ Eng Sci 1:433–437
Gupta SK, Rao PVSS (1980) Treatment of urea by algae, activated sludge and flocculation algal bacterial system – a comparative study. Indian J Environ Health 22:103–112
Gurumoorthy P, Saravanan A (2016) Biofuel production from marine microalgae using paper and pulp industry waste water. Int J Chem Sci 14:3249–3255
Guy Yare JA, Lucrelk M, Sakaguchi H (1990) Removal of ink from recycled paper. Jap Pat 150:984–990
Hogenkamp H (1999) Flotation: the solution in handling effluent discharge. Pap Asia 15:16–18
Hong Y, Dashtban M, Chen S et al (2015) Lignin in paper mill sludge is degraded by white-rot fungi in submerged fermentation. J Microb Biochem Technol 7:177–181
Hooda R, Bhardwaj NK, Singh P (2015) Screening and identification of ligninolytic bacteria for the treatment of pulp and paper mill effluent. Water Air Soil Pollut 226:1–11
Hossain K, Rao AR (2014) Environmental change and it’s affect. Eur J Sustain Dev 3:89–96
Jaouani A, Guillen F, Penninckx MJ et al (2005) Role of Pycnoporus coccineus laccase in the degradation of aromatic compounds in olive oil mill wastewater. Enzym Microb Technol 36:478–486
Jerusik RJ (2010) Fungi and paper manufacture. Fungal Biol Rev 24:68–72
Jim F, Reyes S (2006) Anaerobic granular sludge bed reactor technology. University of Arizona, Tucson, Archived from the original on 2006
Kamali M, Khodaparast Z (2015) Review on recent developments on pulp and paper mill wastewater treatment. Ecotoxicol Environ Saf 114:326–342
Karrasch B, Parra O, Cid H et al (2006) Effects of pulp and paper mill effluents on the microplankton and microbial self-purification capabilities of the Bibyo River. Chile Sci Total Environ 359:194–208
Kibblewhite RP, Wong KKY (1999) Modification of a commercial radiata pine kraft pulp using carbohydrate degrading enzymes. Appita J 52:300–311
Kirk TK, Farrell RL (1987) Enzymatic combustion: the microbial degradation of lignin. Annu Rev Microbiol 41:465–505
Kishimoto N, Nakagawa T, Okada H et al (2010) Treatment of paper and pulp mill wastewater by ozonation combined with electrolysis. J Water Environ Technol 8:99–109
Kreetachat T, Chaisan O, Vaithanomsat P (2016) Decolorization of pulp and paper mill effluents using wood rotting Fungus Fibrodontia sp. RCK783S. Int J Environ Sci Dev 7:321–324
Kshirsagar AD (2013) Bioremediation of wastewater by using microalgae: an experimental study. Int J Life Sci Pharma Res 2:340–346
Kulikowska D, Gusiatin ZM, Bułkowska K et al (2015) Feasibility of using humic substances from compost to remove heavy metals (Cd, Cu, Ni, Pb, Zn) from contaminated soil aged for different periods of time. J Hazard Mater 300:882–891
Kumar V, Chopra AK (2016) Reduction of pollution load of paper mill effluent by phytoremediation technique using water caltrop (Trapa natans L.). Cogen Environ Sci 2:1–12
Kumar V, Dhall P, Naithani S et al (2014) Biological approach for the treatment of pulp and paper industry effluent in sequence batch reactor. J Bioremed Biodegr 5:1–10
Kumar S, Saha T, Sharma S (2015) Treatment of pulp and paper mill effluents using novel biodegradable polymeric flocculants based on anionic polysaccharides: a new way to treat the waste water. Int Res J Eng Technol 2:1415–1428
Kunikane S, Kaneko M, Maehara R (1984) Growth and nutrient uptake of green alga, Scenedesmus dimorphus, under a wide range of nitrogen-phosphorus ratio (I): experimental study. Water Res 18:1299–1311
Laitinen N, Kulovaara M, Levänen E et al (2002) Ultrafiltration of stone cutting mine waste water with ceramic membranes-a case study. Desalination 149:121–125
Lakshmi KS, Sailaja VH, Reddy MA (2017) Phytoremediation - a promising technique in waste water treatment. Int J Sci Res Manage 5:5480–5489
Lehtinen K (2004) Relationship of the technical development of pulping and bleaching to effluent quality and aquatic toxicity. Destech Publications, Lancaster
Lettinga G, Huishoff Pol LW (1991) UASB process design for various types of waste water. Water Sci Technol 24:87–107
Luisa M, Goncalves FC, Steiner W (1996) Purification and characterisation of laccase from a newly isolated wood-decaying fungus: enzymes for pulp and paper processing. Am Chem Soc 20:258–263
Machmud MN, Fadi F, Fuadi Z et al (2014) Alternative fiber sources from Gracilaria Sp and Eucheuma Cottonii for papermaking. Int J Sci Eng 6:1–10
Marquina D (2005) Biomass production of cellulolytic fungi for degradation of waste lignocellulosics. Univ Complutense Madrid 1:41–47
Mehta J, Sharma P, Yadav A (2014) Screening and identification of bacterial strains for removal of COD from pulp and paper mill effluent. Adv Life Sci Health 1:34–42
Micheli F (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 9:414–419
Mulligan CN (2002) Environmental biotreatment: technologies for air, water, soil, and waste. ABS Consulting/Government Institutes, Rockville, p 395 ISBN-13: 978-0865878907
Munkittrick KR, Servos MR, Carey JH et al (1997) Environmental impacts of pulp and paper wastewater: evidence for a reduction in environmental effects at North American pulp mills since 1992. Water Sci Technol 35:329–338
Nagarathnamma R, Bajpai P, Bajpai PK (1999) Studies on decolourization, degradation and detoxification of chlorinated lignin compounds in kraft beaching effluents by Ceriporiopsis subyermispora. Process Biochem 34:939–948
Nemade PD, Kumar S, Louis D et al (2003) Application of anaerobic technology for biomethanation of paper and pulp mill effluent – an insight. Environ Pollut Control 6:6–15
Nilsson T Asserson A (1969) Treating wood chips with fungi to enhance enzymatic hydrolysis. US Patent 3:486
Nomura Y, Shoji S (1988) Digestion of pulp (Honshu Paper Mfg. Co. Ltd.) Jpn Pat 59:494–498
Ordaz-Diaz LA, Rojas-Contreras JA, Rutiaga-Quinones OM et al (2014) Microorganism degradation efficiency in BOD analysis formulating a specific microbial consortium in a pulp and paper mill effluent. Biol Resour 9:7189–7197
Pawar SN, Hussain M (2016) Photo induced catalytic treatment of pulp and paper industry wastewater. Int J Innov Eng Technol 7:494–498
Prasongsuk S, Lotrakul P, Imai T, Punnapayak H (2009) Decolourization of pulp millwastewater using thermotolerant white rot fungi. Sci Asia 35:37–41
Queiroz MI, Lopes EJ, Zepka LQ et al (2007) The kinetics of the removal of nitrogen and organic matter from parboiled rice effluent by cyanobacteria in a stirred batch reactor. Bioresour Technol 98:2163–2169
Ragunathan R, Swaminathan K (2004) Biological treatment of a pulp and paper industry effluent by Pleurotus spp. World J Microbiol Biotechnol 20:289–293
Raj A, Kumar S, Haq I et al (2014) Bioremediation and toxicity reduction in pulp and paper mill effluent by newly isolated ligninolytic Paenibacillus sp. Eco Eng 71:355–362
Rao HP, Kumar RR, Raghavan BG et al (2011) Application of phycoremediation technology in the treatment of wastewater from a leather-processing chemical manufacturing facility. Water Soil Air 37:7–14
Reid ID, Paice MG (1998) Effects of manganese peroxidase on residual lignin of softwood kraft pulp. Appl Environ Microbiol 64:2273–2274
Rose GR, St. John MR (1987) Flocculation in encyclopaedia of polymer science and engineering, vol 7. Wiley, New York, p 211
Sandstrom O, Neuman E (2003) Long-term development in a Baltic fish community exposed to bleached pulp mill effluent. Aqua Ecol 37:267–276
Santos Ramos WDL, Tatyana P, Chairez I et al (2009) Remediation of lignin and its derivatives from pulp and paper industry wastewater by the combination of chemical precipitation and ozonation. J Haz Mater 169(1–3):428–234. https://doi.org/10.1016/j.jhazmat.2009.03.152
Saraswathi R, Saseetharan MK (2010) Investigation on microorganisms and their degradation efficiency in paper and pulp mill effluent. J Water Resour Prot 2:660–664
Saritha V, Maruthi YA, Mukkanti K (2010) Decolourization of higher concentrations of industry effluent by Fomes lividus. World J Microbiol Biotechnol 19:591–593
Satyawali Y, Balakrishnan M (2007) Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: a review. J Environ Manag 86:481–497
Selvam K, Shanmuga Priya M (2013) Biological treatment of pulp and paper industry effluent by white rot fungi Schizophyllum commune and Lenzites eximia. Int J Pharm Biol Arch 3:121–126
Selvam K, Swaminathan K, Hoon Song M et al (2002) Biological treatment of a pulp and paper industry effluent by Fomes lividus and Trametes versicolor. World J Microbiol Biotechnol 18:523–526
Senthilkumar S, Perumalsamy M, Prabhu HJ (2014) Decolourization potential of white-rot fungus Phanerochaete chrysosporium on synthetic dye bath effluent containing Amido black 10B. J Saudi Chem Soc 18:845–853
Seo JK, Park TS, Kwon IH et al (2013) Characterization of cellulolytic and xylanolytic enzymes of Bacillus licheniformis JK7 isolated from the rumen of a native korean goat. Asian-Australas J Ani Sci 26:50–58
Shanthi J, Krubakaran CTB, Balagurunathan R (2012) Characterization and isolation of paper mill effluent degrading microorganisms. J Chem Pharm Res 4:4436–4439
Sharma D (2014) Treatment of pulp and paper effluent by electrocoagulation. Int J Chem Technol Res 6:860–870
Sharma N, Gupta VC (2012) Batch biodegradation of phenol of paper and pulp effluent by Aspergillus Niger. Int Chem Eng Appl 3:182–186
Sharma R, Chandra S, Singh A et al (2014) Degradation of pulp and paper mill effluents. IIOAB J 5:6–12
Sharyo M, Sakaguchi H (1990) Deinking used paper with incorporation of lipase. Jap Pat 2:160–168
Sigoillot C, Record E, Belle V et al (2004) Natural and recombinant fungal laccases for paper pulp bleaching. Appl Microbiol Biotechnol 64:346–352
Singh P, Thakur S (2004) Removal of color and detoxification of pulp and paper mill effluent by microorganism in two step bioreactor. J Sci Ind Res 63:944–948
Singhal V, Kumar A, Rai JPN (2003) Phytoremediation of pulp and paper mill and distillery effluents by channel grass (Vallisneria spiralis). J Sci Ind Res 62:319–328
Springer AM (2000) Industrial environmental control: pulp and paper industry, 3rd edn. TAPPI Press, Atlanta
Suutarinen J, Honkapää K, Heiniö R et al (2002) Modeling of calcium chloride and pectin methylesterase prefreezing treatments of strawberries and jams. J Food Sci 67:1240–1248
Sukumaran, Dipu (2013) Phytoremediation of heavy metals from industrial effluent using constructed wetland technology. Appl Eco Environ Sci 5:92–97
Tenno R, Paulapuro H (1999) Removal of dissolved organic compounds from paper machine whitewater by membrane bioreactors: a comparative analysis. Espoo, Finland
Thompson RC, Olsen Y, Mitchell RP et al (2004) Lost at sea: where is all the plastic? Science 304:838–838
Tickle A, Malcolm F, Graham D (1995) Acid rain and natural conservation in Europe: a preliminary study of areas at risk from acidification. WWF International, Morges
Tiedje JM, Quensen JF, Chee-Sanford et al (1993) Microbial reductive dechlorination of PCBs. Biodegradation 4:231–240
Tyagi S, Kumar V, Singh J et al (2014) Bioremediation of pulp and paper mill effluent by dominant aboriginal microbes and their consortium. Int J Environ Res 8:561–568
Usha R, Vasavi A, Thishya K, Rani SJ, Supraja P (2011) Phytoextraction of lead from industrial effluents by sunflower (Helianthus Annuus. L). Rasayan J Chem 4:8–12
Van den Heuvel MR, Ellis RJ (2002) Timing of exposure to a pulp and paper effluent influences the manifestation of reproductive effects in rainbow trout. Environ Toxicol Chem 21:2338–2347
Verma VK, Gupta RK, Rai JPN (2005) Biosorption of Pb and Zn from pulp and paper industry effluents by water hyacinth (Eichhornia crassipes). J Sci Ind Res 64:778–781
Wong SS, Teng TT, Ahmad AL et al (2006) Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J Hazard Mater 135:378–388
Wu J, Xiao Y, Yu H (2005) Degradation of lignin in pulp mill wastewaters by white-rot fungi on biofilm. Bioresour Technol 96:1357–1363
Xiang Z, Gao W, Chen L et al (2016) A comparison of cellulose nanofibrils produced from Cladophora glomerata algae and bleached eucalyptus pulp. Cellulose 23:493–503
Yamuna M, Selvam K, Meenakshi R (2016) Treatment of a pulp and paper industry effluent by Daldenia concentrica, Lepiota sp. and Trametes serialis -a biological approach. Int J Sci Eng Res 7:1112–1119
Yerkes WD (1968) Process for the digestion of cellulosic materials by enzymatic action of Trametes suaveolens. United States Patent 3:406–489
Zabel RA, Morrell JJ (1992) Wood microbiology: decay and its prevention. Academic, San Diego
Zheng S, Yang M, Yang Z (2005) Biomass production of yeast isolated from salad oil manufacturing wastewater. Bioresour Technol 96:1183–1187
Zhu N (2006) Composting of high moisture content swine manure with corncob in a pilot-scale aerated static bin system. Bioresour Technol 97:1870–1875
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gupta, A., Gupta, R. (2019). Treatment and Recycling of Wastewater from Pulp and Paper Mill. In: Singh, R., Singh, R. (eds) Advances in Biological Treatment of Industrial Waste Water and their Recycling for a Sustainable Future. Applied Environmental Science and Engineering for a Sustainable Future. Springer, Singapore. https://doi.org/10.1007/978-981-13-1468-1_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-1468-1_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1467-4
Online ISBN: 978-981-13-1468-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)