Abstract
Technical terms and units used for light and nutrient solution that are sometimes misunderstood or confusing are discussed, and more unified terminology and units are proposed for better communication and understanding among people with different academic and business backgrounds.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The research and development of plant factories with artificial lighting (PFAL) and greenhouses with and without supplemental lighting is a multidisciplinary field, and so similar technical terms are often used with somewhat different definitions and units. To make them easier to understand and remember by people with different academic backgrounds (biology, physics, chemistry, engineering, business, etc.), the technical terms and units as well as their relationship need to be simple but logically and scientifically reasonable.
This chapter discusses the technical terms and units used for light and nutrient solution, which are sometimes misunderstood or confusing, and proposes more unified terminology and units for better communication and understanding among people with different academic and business backgrounds.
The basic rules for terminology and units are (1) each technical term has only one clear meaning or definition, (2) each technical term has only one unit and its derivatives, and (3) mutually related technical terms must be logically understandable. For example, “action spectrum” in plant physiology means “gross photosynthetic rate” over a wavelength of 400–700 nm; however, “action” by itself does not imply “gross photosynthetic rate.” Thus, “gross photosynthetic rate spectrum” may be more clearly understandable. As another example, the unit used for “daily light integral” is either mol m−2 or W m−2, so it is not a suitable term. The word order of the term “photosynthetically active radiation (PAR)” is not a logical match to that of “photosynthetic photon,” so “photosynthetic radiation” may be better than PAR when used with the term “photosynthetic photon.”
2 Light
2.1 Metrics of Light
Basically, the terminology and units used for light need to match with those defined by the International Commission on Illumination (CIE) and the International Electrotechnical Commission (IEC). However, the technical terms for photosynthetic photons and photosynthetic radiation for higher plants are not fully discussed by the CIE and IEC.
2.1.1 Radiometry, Photometry, and Photonmetry
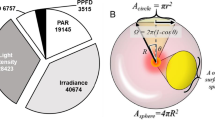
Table 12.1 shows fundamental quantities in radiometry, photometry, and photonmetry and their respective units according to the International System of Units (SI). Photometry is a method of measuring light for human eyes, while photonmetry in this book is a method of measuring photon in relation to plants. Note the difference in units between photometry and photonmetry and between flux and flux density. Photonmetry is used when discussing the properties of lamps for plant lighting and the effect of the light environment on plants. The meaning of each term in Table 12.1 is more fully explained in Fujiwara (2016).
Table 12.2 is a modified version of Table 12.1 with special attention given to radiometry and photonmetry of photosynthetic radiation and photosynthetic photon (wavelength range for both: 400–700 nanometers (nm)). Note the difference in meaning and unit between photosynthetic photon flux (PPF) and photosynthetic photon flux density (PPFD). PPF is a term used for the light source, and PPFD is a term used for the light environment.
The discussion above is a summary of the “Standards for terminology and units of radiometry, photometry, photonmetry and properties of light emitting diodes (LEDs) for plant lighting,” proposed in 2016 by the Committee on LED Lighting for PFAL established by the Japan Plant Factory Association (JPFA) (Goto et al. 2016).
2.1.2 Photosynthetic Radiation Energy Efficacy and Photosynthetic Photon Number Efficiency
The photosynthetic radiation energy efficiency of a lamp is defined as the ratio of the photosynthetic radiation energy flux (unit: W) of the lamp to the effective power consumption of the lamp (unit: W). The effective power consumption measured by a power meter should include the electric power consumed by the lighting unit, power supply, and instruments for controlling the quantity or quality of light, such as a timer clock, dimmer, or computer-programmed control system.
Note the difference between efficiency and efficacy. The term efficiency is used when the numerator and denominator units are the same, so that the unit of efficiency is dimensionless. Efficacy is used when the numerator and denominator units are different. Details about the measurement methods are given in Goto (2016).
2.2 Technical Terms to Be Reconsidered
This section discusses the meanings or definitions of technical terms and units used for light and nutrient solution that are often misunderstood or confused, and some new technical terms are proposed for reconsideration. Confusion or misunderstanding occurs mainly because these technical terms and units have been unsystematically introduced from different scientific fields (meteorology, plant physiology, horticultural science, agricultural engineering, illumination engineering, etc.) into the field of PFAL. Table 12.3 shows a summary of the conventional and proposed technical terms and units to be reconsidered.
2.2.1 PPFD vs PPF
As described above, PPF in units of mol s−1 is used to express the flux of photosynthetic photons emitted from a lamp, while PPFD in units of μmol m−2 s−1 is used to express the flux density of photosynthetic photons received by a virtual or real surface. However, PPF is sometimes used to mean PPFD, which is confusing and should be avoided.
2.2.2 Light Intensity
As shown in Tables 12.1 and 12.2, the terms radiant intensity (W sr−1) and photon intensity (mol sr−1) are, respectively, used to express the amount of radiant energy or photons emitted per solid angle of sr (steradian) of a light source. On the other hand, the term light intensity is still often used to mean either photosynthetic radiation flux density (W m−2), PPFD (μmol m−2 s−1), or both. This misuse of light intensity should be stopped as soon as possible, given the increasing number of people interested in the PPF and radiant/photon intensity of LEDs, in addition to PPFD as a light environment for plants.
2.2.3 PAR and PPF and PRF and PRFD
Although the term PAR has been widely used for many years, it may need to be reconsidered for the following reasons. Firstly, it is necessary to add “flux” as PAR flux when used with the unit W, while “flux” is included in PPF. In other words, “PAR flux” corresponds to PPF. Secondly, if the term “photosynthetically active radiation (PAR)” is used, it may also be necessary to use the term “photosynthetically active photon” so that both terms have the same word structure.
Photosynthetic radiation energy flux and photosynthetic radiation energy flux density are, respectively, often shortened to photosynthetic radiation flux and photosynthetic radiation flux density by omitting the word “energy,” which may not cause any confusion.
The term “photosynthetic radiation flux” or “PRF” can be newly introduced as an alternative to “PAR flux” in the near future. Then, the word order of PRF would be the same as that of PPF, and the relationship between PRF and PPF would be more logical and understandable than that between PAR flux and PPF. The same idea applies to PRFD (photosynthetic radiation flux density) (W m−2) and PPFD (μmol m−2 s−1).
Photosynthetic radiation energy flux is sometimes used in units of μmol s−1, which is confusing with PPF, also used in units of μmol s−1. Thus, photosynthetic radiation energy flux should be used in units of W (= J s−1), not μmol s−1, because the term “radiation” is used in units of energy (joule), not photons (mol).
2.2.4 DLI (Daily Light Integral)
The term DLI (daily light integral) is widely accepted and is used in either units of mol m−2 d−1 or units of W m−2 d−1. Since the word “daily” is part of the term, “d−1” can be or should be removed from the unit.
Another issue is that DLI does not indicate whether it is about photosynthetic photons in units of mol m−2 or photosynthetic radiation in units of W m−2. To make the meaning of DLI clearer, the terms “daily photosynthetic radiation flux density integral” (daily PRFD integral) or DLI-R in units of W m−2 and “daily photosynthetic photon flux density integral” (daily PPFD integral) or DLI-P in units of mol m−2 may be newly introduced in the future.
2.2.5 Lumen (lm) and Color Rendering Index (CRI or Ra)
White LEDs (blue LEDs covered with plastic containing phosphors for wavelength conversion) are increasingly popular as a light source in the PFAL. Strong blue light poses a risk of eye damage due to the strong energy per photon. Meanwhile, the light emitted from red LEDs may affect human psychology when the percentage of red light energy exceeds 70% of the total light energy. This means that the light spectrum of a lamp must be selected by considering not only the growth of plants but also the health of people working in the PFAL and the effect of the color of the plants on humans.
In this sense, in addition to photosynthetic radiant energy efficiency and photosynthetic photon number efficacy, the color rendering index (CRI or Ra) and spectral light distribution in the range of 320–780 nm are important indices for the lamps used in the PFAL. CRI is a quantitative measure of the ability of a light source to faithfully reveal the colors of various objects in comparison with an ideal or natural light source.
2.2.6 Wavelength and Photon Number
Radiation is often characterized by its spectral distribution over a wavelength range, while photon is characterized by the photon number (or wave number), not by the wavelength. However, since the photon number is inversely proportional to the wavelength, there is no confusion even when the wavelength is used to understand the spectral characteristic of photons. It is noted, however, that the shape of the curve for a certain radiant spectrum drawn with the wavelength as the horizontal axis is largely different from that drawn with the photon number as the horizontal axis, because the photon number is not proportional but is inversely proportional to the wavelength.
2.2.7 Wavelength Range of Photosynthetic Radiation and Photon
It is widely accepted that the photosynthetic radiation (or photosynthetic photon) ranges between 400 and 700 nm. It is also well known that the action spectrum curve of a typical green leaf, like the one shown in Fig. 11.2 in Chap. 11, ranges between about 350 and 750 nm. This means that photosynthesis activated by the wavelengths between 350–400 and 700–750 nm has been neglected in the definition of photosynthetic radiation flux (or PPF), probably because the gross photosynthetic rate over these wavelengths was considered to be negligibly small. In reality, however, net photosynthesis in the region between 350–400 and 700–750 nm accounts for over 5% of that in the region between 350 and 750 nm (Fig. 11.2 in Chap. 11).
Recent inexpensive equipment for measuring the net photosynthetic rate has become accurate enough to measure the net photosynthetic rate activated by radiation (or photons) ranging from 350–400 to 700–750 nm. This means that the wavelength range of photosynthetic radiation needs to be reconsidered as 350–750 nm.
2.2.8 Quantum Yield and Photon Yield
Quantum yield is defined as the yield of photochemical products (mol m−2) divided by the total amount of quanta (mol m−2) or photosynthetic radiation energy (J m−2) absorbed during a certain time period. The quantum yield can be renamed as the photosynthetic photon yield or photon yield, since quanta is now also called photons.
2.2.9 Absorptance and Absorption Coefficient
Absorptance of a leaf is defined as the fraction of incident light energy that is absorbed by the leaf, in contrast to the absorption coefficient which is the ratio of the absorbed to incident light energy. Absorption coefficient should not be confused with absorptance. Absorptance spectrum and absorption spectrum mean, respectively, a curve of absorptance or absorption coefficient for a particular wavelength over a certain waveband.
The absorption coefficient, α, is expressed by F(x) = F(x 0) e– α (x − x 0) where F(x) is the photon flux density at point x below the surface of a layer (or a solution) and F(x 0) is the photon flux density at a surface point x 0.
3 Nutrient Solution
In this section, several fundamental technical terms and units of nutrient solution are reconsidered to make them more logically understandable by people with different academic and business backgrounds. Basically, plants absorb inorganic nutrient ions (not molecules) and water from the roots and absorb CO2 and light energy from leaves to grow by photosynthesis. Plants can grow without any organic nutrients, whereas animals and microorganisms need organic nutrients as essential elements for growth.
Note that the roots may absorb a small amount of organic substances such as amino acids, vitamins, and mono-/disaccharides in the nutrient solution, and they may affect the plant growth in some cases (Ge et al., 2009) (this topic is not discussed in this chapter).
3.1 Fertilizer, Nutrient Elements (Nutrients), and (Nutrient) Ions
A fertilizer is any natural or synthetic substance containing one or more plant nutrient elements useful for plant growth. It can be a mixture of inorganic and/or organic substances. The nutrient elements are classified into macro- (major) and micro- (minor) nutrient elements. The macroelements are nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), sulfur (S), and magnesium (Mg), and the microelements are boron (B), chlorine (Cl), manganese (Mn), iron (Fe), zinc (Z), copper (Cu), molybdenum (Mo), and nickel (Ni) (Mohr and Schopfer 1995). The nutrient elements contained in the fertilizer are dissolved in water (rainwater, groundwater, city water, etc.) and ionized before being absorbed by the plant roots.
When organic fertilizer exists in the nutrient solution, a portion of it gradually decomposes to nutrient ions in the presence of specific microorganisms. Since the plant roots do not distinguish the ions originating from inorganic fertilizer from those originating from organic fertilizer, they absorb the ions equally regardless of the origin (Fig. 12.1). On the other hand, in the presence of beneficial bacteria in a hydroponic nutrient solution, the bacteria may live in the plant root system and form a symbiosis, which often affects the root morphology, root function, and nutrient uptake. Then, the root system promotes the growth of aerial parts (Fang 2017). This topic is a challenging area for the next generation of hydroponics.
3.2 Ion, Valence, Equivalent, and Equivalent Concentration
Ions are positively or negatively charged by electrons. Examples of positively charged ions are K+, NH4 +, Ca2 2+, and Mg2+, and those of negatively charged ions are NO3 − and H2PO4 −. KNO3 (potassium nitrate) is ionized to K+ and NO3 − when dissolved in water. Plant roots absorb K+ separately from NO3 −.
The valence of an ion (or an atom) represents the number of electrons to achieve stability by having a full or empty outermost orbital, which is a measure of its capacity to combine with other ions to form chemical compounds or molecules. The valence of K+ and NO3 − is 1, and that of Ca2+ and Mg2+ is 2.
Eq (equivalent or mol equivalent) of ions in solution is calculated by multiplying the number of molecules of each ion (measured in moles) by the charge it carries. If 1 mol of KCl and 1 mol of CaCl2 are dissolved in a solution, there is 1 Eq of K+, 2 Eq of Ca2+, and 3 Eq of Cl− in that solution (the valence of calcium is 2, so that 1 mole of calcium ion has 2 Eq).
Eq concentration is expressed by Eq per kg (or L). mEq (milliequivalent) and mEq kg−1, one thousandth of Eq and Eq kg−1, respectively, are frequently used in hydroponics. Controlling the total sum of mEq kg−1 for all the nutrient ions is a major control variable and is much more important than controlling the total sum of mmol kg−1 or mg kg−1 for all the nutrient ions. Another major control variable is pH (potential of hydrogen: a scale of acidity from 0 to 14). It is to be noted that the sum of each ion concentration is not proportional to the sum of Eq kg−1 for each ion.
3.3 EC Meter
EC (electrical conductivity) quantifies how strongly a given material promotes the flow of electric current. Basically, mEq kg−1 is proportional to the EC of the solution when all the ions are dissociated (separated). The EC meter is robust, relatively inexpensive and can be used for its continuous measurement and control only with occasional calibration.
On the other hand, it is practically impossible to separately control the mEq kg−1 of each ion in nutrient solution, because the ion sensors are generally expensive and also not suited to continuous measurement and control. This is the reason why the EC meter is widely used.
3.4 Why Not Call the EC Meter an Eq kg–1 Meter?
Since the EC meter is widely used in hydroponics to estimate the total Eq kg−1 of the nutrient solution, why not call the EC meter an “Eq meter” simply by changing its scale for the readings? The value we want to know is mEq kg−1, not EC. At least the EC meter should have a reading for mEq kg−1 in addition to the reading for dS m−1. The development of an Eq kg−1 meter for measuring the mEq kg−1 of each ion and/or an Eq kg−1 meter for measuring the mEq kg−1 of nutrient and non-nutrient ions separately is expected. “Section 3.2 Ion balance” describes one such trial.
3.5 Definition of ppm
When substance “A” (e.g., solute) with a mass (or weight) or volume of W1 is contained in “B” (e.g., solvent) with a mass or volume of W2, the concentration of A is expressed by (W1/(W1 + W2) where the units of the numerator and denominator are the same, and thus the unit is dimensionless.
The unit “ppm” (parts per million, 1/106 or 1.0 × 10−6) is widely used for the dilute concentration of a substance such as CO2 in the atmospheric air (about 400 ppm), although ppm is not an SI unit (International System of Units). The SI unit for ppm is μmol mol−1 or μL L−1. Note that the SI unit for CO2 and the air containing CO2 are the same, as mentioned above.
The unit “ppm” is also widely used with the unit mg L−1 for expressing the concentration of a nutrient element in the nutrient solution for hydroponics. In this case, the units of the numerator and denominator are different. However, the numerical value expressed with mol L−1 is almost equal to the value expressed with mol kg−1, since the mass of water per liter is 0.9982 kg at 20 °C and 0.9957 kg at 30 °C. Nowadays, the mass (weight) of a liquid can be measured by an inexpensive electronic balance as accurately as its volume measured with a graduated cylinder. Also, the unit mg kg−1 is more easily understandable and measurable than mg L−1 or mol L−1. Thus, the use of mg kg−1 and mol kg−1 is recommended in an interdisciplinary field such as PFAL.
4 Conclusion
We hope that this chapter contributes the first small step in reconsidering the terminology and units of light and nutrient solution. By using unified terminology and units in the area of PFALs, easier understanding and communication among people with different academic backgrounds can be expected. The same applies for many areas other than light and nutrient solution.
References
Fang W (2017) From hydroponics to bioponics. In: Proceedings of international forum for advanced protected horticulture (2017 IFAPH) Organized by National Taiwan University 1–17
Fujiwara K (2016) Radiometric, photometric and photonmetric quantities and their units (Chapter 26). In: Kozai T, Fujiwara K, Runkle E (eds) LED lighting for urban agriculture). Springer Nature, pp 367–376
Ge T, Song S, Roberts P, Jones DL, Huang D, Iwasaki K (2009) Amino acids as a nitrogen source for tomato seedlings: the use of dual-labeled (13C, 15N) glycine to test for direct uptake by tomato seedlings. Environ Exp Bot 66:357–361
Goto, E. 2016. Guideline for presenting LED grow lights properties. Committee on LED lighting for plant factories with artificial light. Abstract book for annual meeting of the Japanese Society of Agricultural, Biological and Environmental Engineers and Scientists. pp 48–49 (in Japanese)
Goto E, Fujiwara K, Kozai T (2016) Proposed standards developed for LED lighting, vol 16. Urban Ag News online Magazine, pp 73–75
Mohr H and Schopfer H. 1995. Plant nutrition (Chapter 2). In: Plant physiology. (translated into English by G. Lawlor and D.W. Lawlor). Springer, Berlin, pp 9–30
Acknowledgment
We thank all the members of the Committee for LED Lighting for plants (chairperson: Eiji Goto) and those on the Committee for Hydroponic solution controller (chairperson: Yutaka Shinohara). Some important ideas presented in this chapter were obtained during discussions at the committee meetings.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kozai, T., Tsukagoshi, S., Sakaguchi, S. (2018). Reconsidering the Terminology and Units for Light and Nutrient Solution. In: Kozai, T. (eds) Smart Plant Factory. Springer, Singapore. https://doi.org/10.1007/978-981-13-1065-2_12
Download citation
DOI: https://doi.org/10.1007/978-981-13-1065-2_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1064-5
Online ISBN: 978-981-13-1065-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)