Abstract
Stem cells proliferate by undergoing self-renewal and differentiate into multiple cell lineages in response to biochemical and biophysical stimuli. Various biochemical cues such as growth factors, nucleic acids, chemical reagents, and small molecules have been used to induce stem cell differentiation or reprogramming or to maintain their pluripotency. Moreover, biophysical cues such as matrix stiffness, substrate topography, and external stress and strain play a major role in modulating stem cell behavior. In this chapter, we have summarized microenvironmental regulation of stem cell behavior through biochemical and biophysical stimulation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
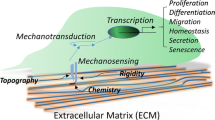
1 Stem Cells
Stem cells are classified into totipotent, pluripotent, multipotent, and unipotent stem cells based on their differentiation potential (Revel 2009). Stem cells can self-renew and differentiate into other cell types, suggesting their use in various applications such as cell therapy, tissue engineering, and regenerative medicine. Therefore, it is important to develop methods to expand stem cells and induce their differentiation by using biochemical and/or biophysical stimulation to realize this potential.
1.1 Pluripotent Stem Cells
Pluripotent stem cells (PSCs) can proliferate perpetually and can differentiate into cells that form the three germ layers, namely, the endoderm, mesoderm, and ectoderm. PSCs are a valuable tool for stem cell therapy, in vitro drug screening, and disease modeling. PSCs include embryonic stem cells (ESCs), ESCs produced by somatic cell nuclear transfer (SCNT-ESCs), and induced PSCs (iPSCs). ESCs are derived from embryos at the developmental stage, SCNT-ESCs are produced by transferring nuclei of somatic cells into enucleated eggs, and iPSCs are artificially generated by reprogramming adult cells. In 2006, Takahashi and Yamanaka achieved a seminal breakthrough in stem cell production (Takahashi and Yamanaka 2006). They found that mouse embryonic fibroblasts (MEFs) can be reprogrammed into iPSCs by exogenous transcription of four factors, Oct4, Sox2, c-Myc, and Klf4. iPSCs are very similar to ESCs but are associated with less ethical concerns and show enhanced patient specificity. For iPSCs, increasing the reprogramming efficiency without the risk from genetic manipulation should be overcome.
1.2 Multipotent Stem Cells
Multipotent stem cells such as mesenchymal stem cells (MSCs) derived from the bone marrow, adipose tissue, umbilical cord blood, nerve tissue, dental pulp, hair follicle, or brain can also self-renew and differentiate into different cell types after biochemical and/or biophysical stimulation. MSCs derived from mesodermal tissues differentiate into mesodermal cells such as osteoblasts, chondrocytes, or adipocytes. However, some studies indicate that MSCs can also trans-differentiate into ectodermal or endodermal lineage cells in vitro when cultured in an induction medium containing some soluble factors (Brzoska et al. 2005; Damien and Allan 2015; Gao et al. 2014; Li et al. 2013). Some MSCs express growth factors and chemokines that induce cell proliferation and angiogenesis (Chen et al. 2008; Doorn et al. 2011; Haynesworth et al. 1996) and exert anti-inflammatory and immunomodulatory effects (Aggarwal and Pittenger 2005; Iyer and Rojas 2008). MSCs have been used for treating various disorders such as spinal cord injury, bone fracture, autoimmune disorder, rheumatoid arthritis, and hematopoietic defects.
2 Biochemical Stimulation
Biochemical components such as growth factors, cytokines, enzymes, peptides, chemical reagents, and small molecules are commonly added to cell culture medium to regulate stem cell differentiation. Moreover, biochemical components can be immobilized or precoated on cell culture substrates or scaffolds to induce the differentiation of stem cells into different cell lineages. Biochemical factors bind to receptors present on stem cells or enter stem cells to activate different cellular signaling pathways, thus modulating their behavior. Here, we will explore some existing methods for inducing stem cell differentiation with biochemical factors, as listed in Table 9.1.
2.1 Biochemical Differentiation of Multipotent Stem Cells
Osteogenic differentiation can be induced using soluble factors such as ascorbic acid, β-glycerophosphate, bone morphogenetic proteins (BMPs), dexamethasone, NEL-like molecule-1 (NELL-1), phenamil, or tauroursodeoxycholic acid (TUDCA). BMP-2 stimulates the expression of major osteogenic genes such as those encoding osteopontin, osteocalcin, and Runt-related transcription factor 2 (Sun et al. 2015). Although BMPs are suggested to be the most potent osteoinductive proteins, they also induce pro-adipogenesis (Hata et al. 2003; Jin et al. 2006). NELL-1 induces highly specific osteogenic differentiation of MSCs both in vitro and in vivo (Zhang et al. 2010). TUDCA, an endogenous hydrophilic bile acid, suppresses adipogenesis and promotes angiogenesis and osteogenesis by reducing ER stress, preventing unfolded protein response dysfunction, and stabilizing mitochondria (Cha et al. 2014; Cho et al. 2015; Kim et al. 2017; Vang et al. 2014; Yoon et al. 2016). Wnt protein, specifically Wnt3a and Wnt4, is another factor that induces osteogenic differentiation by activating YAP/TAZ accumulation in MSCs (Byun et al. 2014; Park et al. 2015).
Transforming growth factor-β1 (TGF-β1), TGF-β3, kartogenin (KGN), and matrilin-3 are used to enhance chondrogenic differentiation. TGF-β1-tethered photocrosslinkable hydrogel system enhances sulfated glycosaminoglycan accumulation in vitro and cartilage regeneration in vivo (Choi et al. 2015). TGF-β3 is more effective for inducing the chondrogenesis of MSCs than TGF-β1 and TGF-β2 (Barry et al. 2001; Estes et al. 2006). KGN, a new low-molecular-mass heterocyclic molecule, induced selective differentiation of MSCs into chondrocytes and promoted cartilage repair after its intra-articular injection into an animal model of osteoarthritis (Johnson et al. 2012). KGN-conjugated chitosan nanoparticles and microparticles also show potential as efficient intra-articular drug delivery systems for treating osteoarthritis (Kang et al. 2014). Matrilin-3, a non-collagenous extracellular matrix (ECM) protein, enhances the chondrogenic differentiation of adipose tissue-derived MSCs both in vitro and in vivo (Muttigi et al. 2017).
Poly-l-lysine (PLL) is coated on cell culture dishes to enhance cell adhesion through interaction between positive charges on PLL and negative charges on cell membrane (De Kruijff and Cullis 1980; Pachmann and Leibold 1976). Immobilization of PLL on cell culture plates increases the expansion and erythroid differentiation of human hematopoietic stem cells (HSCs) (Fig. 9.1) (Park et al. 2014). Moreover, PLL induces neural differentiation of MSCs (Cai et al. 2012).
2.2 Biochemical Differentiation of Pluripotent Stem Cells
Biochemical differentiation of PSCs in vitro is traditionally achieved by inducing uncontrolled spontaneous differentiation or directed differentiation of these cells into specific cell lineages (Ding et al. 2017). Spontaneous differentiation produces a mixed population of cell lineages from all three germ layers, and the differentiation is uncontrollable. Directed differentiation of PSCs by using soluble factors can be successfully used to generate various cell types such as cardiomyocytes, neural cells, pancreatic beta cells, and hepatocytes. However, the efficiency of and purity of cell types obtained through directed differentiation are low.
Functional cardiomyocytes can be produced by culturing EBs in a differentiation medium containing non-essential amino acids such as l-glutamine, β-mercaptoethanol, and 20% fetal bovine serum (FBS), followed by microdissection of beating areas (Zhang et al. 2009). Addition of small-molecule Wnt signaling inhibitors or activators, BMP inhibitors, or shRNA also induces the differentiation of PSCs into cardiomyocytes (Aguilar et al. 2015; Fonoudi et al. 2015; Zhang et al. 2013).
Various protocols have been developed for the neurogenic differentiation of PSCs. Differentiation of PSCs to neuroectoderms can be mediated with CHCHD2, a mitochondrial protein, that suppresses the TGF-β signaling pathway (Zhu et al. 2016). Highly pure astrocyte-like cells have been generated by adding retinoic acid, sonic hedgehog, epidermal growth factor, basic fibroblast growth factor (bFGF), ciliary neurotrophic factor, and 10% FBS to cell culture medium (Krencik et al. 2011). Neural cells can also be generated from PSCs by adding small-molecules to inhibit dual SMAD signaling and activate Wnt signaling (Chambers et al. 2009, 2016). Numerous clinical trials have assessed the potential of human iPSCs and ESCs to undergo neurogenesis for treating spinal cord injury and retinal diseases. However, generation of mature neural cells from PSCs remains a challenge.
Pancreatic hormone-expressing endocrine cells can be successfully produced from human ESCs by adding and/or removing growth factors such as activin, Wnt, FGF-10, KAAD-cyclopamine (CYC), all-trans retinoic acid, γ-secretase inhibitor DAPT, exendin-4, insulin-like growth factor 1, and hepatocyte growth factor to and/or from cell culture medium over five-stages protocol (D’Amour et al. 2006). ViaCyte Inc. (San Diego, CA) is performing clinical trials to assess the efficacy of hESC-derived pancreatic endodermal cells for treating type I diabetes (Agulnick et al. 2015; Kimbrel and Lanza 2015). Addition of activin A, FGF, retinoic acid, BMP inhibitor (LDN), and some gene inhibitors also induces the pancreatic differentiation of PSCs (Pagliuca et al. 2014; Rezania et al. 2014). However, the complexity of these multistep protocols, cost of production, and scaling up should be overcome before using these strategies in clinical practice.
2.3 Biochemical Reprogramming Into iPSCs
Takahashi and Yamanaka showed that MEFs could be reprogrammed into iPSCs by inducing forced expression of four transcription factors, namely, OCT4, SOX2, c-MYC, and KLF4 (Yamanaka 4 factors), that are important for ESC function (Takahashi and Yamanaka 2006). This seminal development gave Yamanaka the 2012 Nobel Prize in Physiology or Medicine. Since this pioneering discovery, many researchers have developed various methods to enhance reprogramming efficiency by using biochemical factors. Overexpressed epithelial-cadherin can replace OCT4 during cellular reprogramming, thus enhancing reprogramming efficiency (Chen et al. 2010; Redmer et al. 2011). Addition of high concentration of FBS (>20%), ascorbic acid (vitamin C), histone deacetylase inhibitors, DNA methyltransferase inhibitor (5-azacytidine), or SB431542 (a TGF-β signaling inhibitor) to cell culture medium also enhances reprogramming efficiency (Esteban et al. 2010; Kwon et al. 2016). ALK5 inhibitor, LY364947 or E-616452, can be used to replace Sox2 to reprogram MEFs into iPSCs (Huangfu et al. 2008a, b; Ichida et al. 2009; Lee et al. 2012; Lin et al. 2009; Mikkelsen et al. 2008; Staerk et al. 2011), and CCAAT/enhancer-binding protein alpha (C/EBPα) can boost up the iPSC reprogramming efficiency by upregulating Klf4 and increase several chromatin-modifying complex proteins that activates pluripotency program (Di Stefano et al. 2016).
3 Biophysical Stimulation
Many researchers have extensively investigated the effects of various biophysical factors, including matrix stiffness, nanotopography, three-dimensionality, external stress and strain, electrical stimulation, hydrostatic pressure, electromagnetic field, ultrasound, and photostimulation, on cell behavior, as listed in Table 9.2.
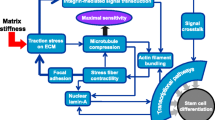
3.1 Stiffness
In 2006, Engler showed that substrate stiffness regulated stem cell fates and was correlated with in vivo ECM elasticity (Fig. 9.2) (Engler et al. 2006). Human MSCs preferred neurogenesis, myogenesis, and osteogenesis on a soft gel (0.1−1 kPa) mimicking the mechanical stiffness of brain, on an intermediate gel (8−17 kPa) mimicking the mechanical stiffness of muscle, and on very stiff gel (25−40 kPa) mimicking the mechanical stiffness of bone, respectively. Human adipose tissue-derived MSCs undergo adipogenesis on a soft substrate (2 kPa) in the absence of inductive soluble biochemical factors (Young et al. 2013). Neural stem cells (NSCs) expressed high levels of neurogenic biomarker β-tubulin III on substrates having stiffness similar to the brain tissue (Saha et al. 2008). Increase in substrate stiffness increases the expression of type A lamin, a mechanosensitive cellular molecule (Swift et al. 2013). Skeletal muscle stem cells rapidly lose their regenerative potential when grown on stiff culture dishes but retain their self-renewal and regenerative capacities when grown on soft hydrogels (Gilbert et al. 2010). Substrates with different stiffness induce the differentiation of different stem cells in a similar manner. Optimal substrate stiffness for the differentiation of stem cells into specific lineages differs based on stem cell source, substrate used, and differentiation protocol used. PSCs also sense and respond with the stiffness of microenvironments. Soft microenvironments (0.1−0.7 kPa) promote early neurogenic differentiation of human PSCs without affecting their proliferation (Keung et al. 2012).
Generation of iPSCs is also affected by substrate stiffness. Soft substrates enhance reprogramming efficiency by increasing the expression of MET and pluripotent markers (Fig. 9.3) (Choi et al. 2016).
3.2 Topography
Stem cell adhesion, phenotype, and differentiation are highly sensitive to substrate topography (Dalby et al. 2014; Ding et al. 2017; Griffin et al. 2015; Park and Im 2015). The effect of surface topography on stem cell phenotype depends on the shape (pillars, pits, and gratings), dimension (feature size, spacing, and height), arrangement, and composition of a substrate (Dalby et al. 2007; Lapointe et al. 2013; Murphy et al. 2014; Wang et al. 2015). Presence of highly ordered nanoscale pitted patterns in a substrate inhibits the adhesion of cells to the substrate (Dalby et al. 2007). Expression of human bone marrow-derived MSC (BMSC) and adipose-derived MSC (ASC) markers depends on the size of pits (McMurray et al. 2011). High-density arrangement of smaller topographical features promotes the proliferation and pluripotency of human iPSCs (Reimer et al. 2016).
Substrates with 120-nm diameter pits and an average 300-nm spacing (randomly offset by 50 nm) induce the osteogenesis of human BMSCs (Dalby et al. 2007). Human ASCs cultured on a polystyrene surface containing nanopores (NPo; 200-nm diameter/400-nm depth) undergo enhanced adipogenic differentiation, while those cultured on a polystyrene surface containing nanopillars (NPi; 200-nm diameter/650-nm height) undergo osteogenic differentiation (Fig. 9.4) (Park et al. 2012). Disordered nanotopographies enhance the osteogenesis of human ESCs (Kingham et al. 2013). Fibronectin-coated microgrooves (4-μm width/10-μm depth/10-μm spacing) improve the maturation and function of human iPSC-derived cardiomyocytes (Rao et al. 2013). Human MSCs cultured on 350-nm PDMS nanogratings show significantly upregulated expression of neuronal markers β-tubulin III and microtubule-associated protein 2 compared with human MSCs cultured on microgratings and flat surface (Yim et al. 2007).
Behavior of ASCs cultured on NPo- and NPi-containing substrates. Fabrication of each nano-featured polystyrene substrates were established using fabricated nickel stamp, and ASC differentiation trends show each flat, NPo, and NPi surface enhances chondrogenic, adipogenic, and osteogenic differentiation, respectively
Substrate topography also affects the reprogramming of MEFs into iPSCs (Downing et al. 2013). Elongation of MEFs on parallel microgrooved surfaces modulates epigenetic states and improves reprogramming efficiency.
3.3 External Stress and Strain
In addition to the intrinsic physical properties of the stem cell microenvironment, such as substrate stiffness, extrinsic mechanical stimuli such as stress or strain are important for regulating the differentiation of stem cells (Keung et al. 2010).
Cyclic strain inhibits the differentiation of hESCs by upregulating the phosphorylation of TGF-β1, activin A, Nodal, and SMAD2/3 and promotes the myogenesis of BMSCs (Gong and Niklason 2008). Cyclic uniaxial tension induces the osteogenesis and dynamic compression induces the chondrogenesis of human BMSCs (Haudenschild et al. 2009). Dynamic culturing with orbital shaking at 100 rpm significantly improves the reprogramming efficiency of iPSCs (Sia et al. 2016). In the presence of uniaxial tensile strain, vascular smooth muscle cells derived from human iPSCs and human ESCs align perpendicular to the strain axis and show increased ECM gene expression (Wanjare et al. 2015). Compressive and tensile forces induced by fluid flow, cell–cell interaction, and cell–matrix interaction regulate MSC behavior in vivo (Hao et al. 2015; Liu and Lee 2014). Human BMSCs with high intracellular tension differentiate into osteoblasts, whereas those with low intracellular tension or low actin–myosin interaction differentiate into adipocytes (McMurray et al. 2011). Shear stress stimulates the differentiation of human MSCs obtained from different tissues into endothelial-like cells (Dan et al. 2015).
3.4 Non-contact-Dependent Factors: Electric Field, Ultrasound, and Photostimulation
In addition to cell–matrix interaction-dependent factors such as substrate stiffness and topography, non-contact-dependent factors such as electromagnetic field, low-intensity pulsed ultrasound (LIPUS), and light of varying wavelengths affect stem cell behavior.
Electrical stimulation is of interest for both cardiac and neural differentiation because of its importance in embryonic development. Pulsed biphasic electrical field of 2 V/cm every 4 ms promotes human iPSC-derived cardiomyocytes to develop a phenotype similar to native cardiomyocytes (Hirt et al. 2014). Human ESC-derived NSCs migrate toward positive charged regions in the presence of a small direct-current electrical field (Feng et al. 2012). Application of an electrical field to NSCs or BMSCs grown on an electroconductive matrix enhances their neurogenesis (Pires et al. 2015; Thrivikraman et al. 2014). Extremely low-frequency electromagnetic fields replace SOX2, KLF4, and c-MYC during somatic cell reprogramming of iPSCs (Baek et al. 2014).
Ultrasound frequencies also regulate stem cell behavior. LIPUS enhances the osteogenic differentiation of human ASCs and is used for bone fracture healing and callus distraction (Claes and Willie 2007; Kang et al. 2013). LIPUS stimulation enhances the proliferation and neural differentiation of human iPSC-derived neural crest stem cells (Lv et al. 2013).
Photostimulation also modulates stem cell behavior. Irradiation with helium–neon lasers (632.8 nm), which are used clinically to promote wound healing, induces the proliferation and migration of human epidermal stem cells (Liao et al. 2014). Irradiation with visible blue light (405 nm) enhances the osteogenesis of and bone formation by mouse MSCs (Kushibiki and Awazu 2009).
4 Conclusion
Stem cells are a very promising cell source for the cell therapy of various diseases because of their self-renewal and differentiation capacities. For successful application of stem cells and biomaterials in tissue engineering and regenerative medicine, stem cell behavior such as adhesion, proliferation, survival, and differentiation in response to biochemical and biophysical cues must be precisely regulated. Furthermore, results of biochemical and biophysical stimulation studies involving three-dimensional microenvironments will play an important role in more accurately predicting the in vivo behavior of stem cells.
References
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822. https://doi.org/10.1182/blood-2004-04-1559
Aguilar JS, Begum AN, Alvarez J, Zhang XB, Hong Y, Hao J (2015) Directed cardiomyogenesis of human pluripotent stem cells by modulating Wnt/beta-catenin and BMP signalling with small molecules. Biochem J 469(2):235–241. https://doi.org/10.1042/BJ20150186
Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, Kelly JR, Haakmeester C, Srijemac R, Wilson AZ, Kerr J, Frazier MA, Kroon EJ, D’Amour KA (2015) Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Transl Med 4(10):1214–1222. https://doi.org/10.5966/sctm.2015-0079
Baek S, Quan X, Kim S, Lengner C, Park J-K, Kim J (2014) Electromagnetic fields mediate efficient cell reprogramming into a pluripotent state. ACS Nano 8(10):10125–10138 %@ 1936-0851
Barry F, Boynton RE, Liu B, Murphy JM (2001) Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res 268(2):189–200. https://doi.org/10.1006/excr.2001.5278
Brzoska M, Geiger H, Gauer S, Baer P (2005) Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun 330(1):142–150. https://doi.org/10.1016/j.bbrc.2005.02.141
Byun MR, Hwang JH, Kim AR, Kim KM, Hwang ES, Yaffe MB, Hong JH (2014) Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death Differ 21(6):854–863. https://doi.org/10.1038/cdd.2014.8
Cai L, Lu J, Sheen V, Wang S (2012) Optimal poly (L-lysine) grafting density in hydrogels for promoting neural progenitor cell functions. Biomacromolecules 13(5):1663–1674 %@ 1525-7797
Cha BH, Kim JS, Ahn JC, Kim HC, Kim BS, Han DK, Park SG, Lee SH (2014) The role of tauroursodeoxycholic acid on adipogenesis of human adipose-derived stem cells by modulation of ER stress. Biomaterials 35(9):2851–2858. https://doi.org/10.1016/j.biomaterials.2013.12.067
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27(3):275–280. https://doi.org/10.1038/nbt.1529
Chambers SM, Mica Y, Lee G, Studer L, Tomishima MJ (2016) Dual-SMAD inhibition/WNT activation-based methods to induce neural crest and derivatives from human pluripotent stem cells. Methods Mol Biol 1307:329–343. https://doi.org/10.1007/7651_2013_59
Chen L, Tredget EE, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3(4):e1886. https://doi.org/10.1371/journal.pone.0001886
Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, Gu Y, Li J, Xiao L, Pei G (2010) E-cadherin-mediated cell–cell contact is critical for induced pluripotent stem cell generation. Stem Cells 28(8):1315–1325 %@ 1549-4918
Cho JG, Lee JH, Hong SH, Lee HN, Kim CM, Kim SY, Yoon KJ, Oh BJ, Kim JH, Jung SY, Asahara T, Kwon SM, Park SG (2015) Tauroursodeoxycholic acid, a bile acid, promotes blood vessel repair by recruiting vasculogenic progenitor cells. Stem Cells 33(3):792–805. https://doi.org/10.1002/stem.1901
Choi B, Kim S, Fan J, Kowalski T, Petrigliano F, Evseenko D, Lee M (2015) Covalently conjugated transforming growth factor-beta1 in modular chitosan hydrogels for the effective treatment of articular cartilage defects. Biomater Sci 3(5):742–752. https://doi.org/10.1039/c4bm00431k
Choi B, Park KS, Kim JH, Ko KW, Kim JS, Han DK, Lee SH (2016) Stiffness of hydrogels regulates cellular reprogramming efficiency through mesenchymal-to-epithelial transition and Stemness markers. Macromol Biosci 16(2):199–206
Claes L, Willie B (2007) The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol 93(1):384–398 %@ 0079-6107
D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24(11):1392–1401. https://doi.org/10.1038/nbt1259
Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO (2007) The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 6(12):997–1003. https://doi.org/10.1038/nmat2013
Dalby MJ, Gadegaard N, Oreffo RO (2014) Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater 13(6):558–569. https://doi.org/10.1038/nmat3980
Damien P, Allan DS (2015) Regenerative therapy and immune modulation using umbilical cord blood–derived cells. Biol Blood Marrow Transplant 21(9):1545–1554 %@ 1083-8791
Dan P, Velot E, Decot V, Menu P (2015) The role of mechanical stimuli in the vascular differentiation of mesenchymal stem cells. J Cell Sci 128(14):2415–2422. https://doi.org/10.1242/jcs.167783
De Kruijff B, Cullis PR (1980) The influence of poly (l-lysine) on phospholipid polymorphism evidence that electrostatic polypeptide-phospholipid interactions can modulate bilayer/non-bilayer transitions. Biochim Biophys Acta Biomembr 601:235–240 %@ 0005-2736
Di Stefano B, Collombet S, Jakobsen JS, Wierer M, Sardina JL, Lackner A, Stadhouders R, Segura-Morales C, Francesconi M, Limone F, Mann M, Porse B, Thieffry D, Graf T (2016) C/EBPalpha creates elite cells for iPSC reprogramming by upregulating Klf4 and increasing the levels of Lsd1 and Brd4. Nat Cell Biol 18(4):371–381. https://doi.org/10.1038/ncb3326
Ding S, Kingshott P, Thissen H, Pera M, Wang PY (2017) Modulation of human mesenchymal and pluripotent stem cell behavior using biophysical and biochemical cues: a review. Biotechnol Bioeng 114(2):260–280. https://doi.org/10.1002/bit.26075
Doorn J, van de Peppel J, van Leeuwen JP, Groen N, van Blitterswijk CA, de Boer J (2011) Pro-osteogenic trophic effects by PKA activation in human mesenchymal stromal cells. Biomaterials 32(26):6089–6098. https://doi.org/10.1016/j.biomaterials.2011.05.010
Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, Chu J, Patel S, Schaffer DV, Li S (2013) Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater 12(12):1154 %@ 1476-1122
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689. https://doi.org/10.1016/j.cell.2006.06.044
Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, Chen K, Li Y, Liu X, Xu J, Zhang S, Li F, He W, Labuda K, Song Y, Peterbauer A, Wolbank S, Redl H, Zhong M, Cai D, Zeng L, Pei D (2010) Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6(1):71–79. https://doi.org/10.1016/j.stem.2009.12.001
Estes BT, Wu AW, Guilak F (2006) Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum 54(4):1222–1232. https://doi.org/10.1002/art.21779
Feng JF, Liu J, Zhang XZ, Zhang L, Jiang JY, Nolta J, Zhao M (2012) Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells 30(2):349–355. https://doi.org/10.1002/stem.779
Fonoudi H, Ansari H, Abbasalizadeh S, Larijani MR, Kiani S, Hashemizadeh S, Zarchi AS, Bosman A, Blue GM, Pahlavan S, Perry M, Orr Y, Mayorchak Y, Vandenberg J, Talkhabi M, Winlaw DS, Harvey RP, Aghdami N, Baharvand H (2015) A universal and robust integrated platform for the scalable production of human cardiomyocytes from pluripotent stem cells. Stem Cells Transl Med 4(12):1482–1494. https://doi.org/10.5966/sctm.2014-0275
Gao Y, Bai C, Wang K, Sun B, Guan W, Zheng D (2014) All-trans retinoic acid promotes nerve cell differentiation of yolk sac-derived mesenchymal stem cells. Appl Biochem Biotechnol 174(2):682–692. https://doi.org/10.1007/s12010-014-1100-2
Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM (2010) Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329(5995):1078–1081. https://doi.org/10.1126/science.1191035
Gong Z, Niklason LE (2008) Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J 22(6):1635–1648. https://doi.org/10.1096/fj.07-087924
Griffin MF, Butler PE, Seifalian AM, Kalaskar DM (2015) Control of stem cell fate by engineering their micro and nanoenvironment. World J Stem Cells 7(1):37
Hao J, Zhang Y, Jing D, Shen Y, Tang G, Huang S, Zhao Z (2015) Mechanobiology of mesenchymal stem cells: perspective into mechanical induction of MSC fate. Acta Biomater 20:1–9. https://doi.org/10.1016/j.actbio.2015.04.008
Hata K, Nishimura R, Ikeda F, Yamashita K, Matsubara T, Nokubi T, Yoneda T (2003) Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell 14(2):545–555. https://doi.org/10.1091/mbc.E02-06-0356
Haudenschild AK, Hsieh AH, Kapila S, Lotz JC (2009) Pressure and distortion regulate human mesenchymal stem cell gene expression. Ann Biomed Eng 37(3):492–502. https://doi.org/10.1007/s10439-008-9629-2
Haynesworth SE, Baber MA, Caplan AI (1996) Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol 166(3):585–592. https://doi.org/10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6
Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Börnchen C, Müller C, Schulz H, Hubner N, Stenzig J, Stoehr A (2014) Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol 74:151–161 %@ 0022-2828
Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA (2008a) Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol 26(7):795–797. https://doi.org/10.1038/nbt1418
Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA (2008b) Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 26(11):1269–1275. https://doi.org/10.1038/nbt.1502
Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K (2009) A small-molecule inhibitor of Tgf-β signaling replaces Sox2 in reprogramming by inducing Nanog. Cell Stem Cell 5(5):491–503 %@ 1934-5909
Iyer SS, Rojas M (2008) Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther 8(5):569–581. https://doi.org/10.1517/14712598.8.5.569
Jin W, Takagi T, Kanesashi SN, Kurahashi T, Nomura T, Harada J, Ishii S (2006) Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell 10(4):461–471. https://doi.org/10.1016/j.devcel.2006.02.016
Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC, Meeusen S, Althage A, Cho CY, Wu X, Schultz PG (2012) A stem cell-based approach to cartilage repair. Science 336(6082):717–721. https://doi.org/10.1126/science.1215157
Kang KS, Hong JM, Kang JA, Rhie JW, Cho DW (2013) Osteogenic differentiation of human adipose-derived stem cells can be accelerated by controlling the frequency of continuous ultrasound. J Ultrasound Med 32(8):1461–1470. https://doi.org/10.7863/ultra.32.8.1461
Kang ML, Ko JY, Kim JE, Im GI (2014) Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 35(37):9984–9994. https://doi.org/10.1016/j.biomaterials.2014.08.042
Keung AJ, Kumar S, Schaffer DV (2010) Presentation counts: microenvironmental regulation of stem cells by biophysical and material cues. Annu Rev Cell Dev Biol 26:533–556. https://doi.org/10.1146/annurev-cellbio-100109-104042
Keung AJ, Asuri P, Kumar S, Schaffer DV (2012) Soft microenvironments promote the early neurogenic differentiation but not self-renewal of human pluripotent stem cells. Integr Biol 4(9):1049–1058
Kim BJ, Arai Y, Park EM, Park S, Bello AB, Han IB, Lee SH (2017) Osteogenic potential of tauroursodeoxycholic acid (TUDCA) as an alternative to rhBMP-2 in a mouse spinal fusion model. Tissue Eng Part A 24:407–417. https://doi.org/10.1089/ten.TEA.2016.0349
Kimbrel EA, Lanza R (2015) Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov 14(10):681–692. https://doi.org/10.1038/nrd4738
Kingham E, White K, Gadegaard N, Dalby MJ, Oreffo RO (2013) Nanotopographical cues augment mesenchymal differentiation of human embryonic stem cells. Small 9(12):2140–2151. https://doi.org/10.1002/smll.201202340
Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC (2011) Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol 29(6):528–534. https://doi.org/10.1038/nbt.1877
Kushibiki T, Awazu K (2009) Blue laser irradiation enhances extracellular calcification of primary mesenchymal stem cells. Photomed Laser Surg 27(3):493–498. https://doi.org/10.1089/pho.2008.2343
Kwon D, Kim J-S, Cha B-H, Park K-S, Han I, Park K-S, Bae H, Han M-K, Kim K-S, Lee S-H (2016) The effect of fetal bovine serum (FBS) on efficacy of cellular reprogramming for induced pluripotent stem cell (iPSC) generation. Cell Transplant 25(6):1025–1042 %@ 0963-6897
Lapointe VL, Fernandes AT, Bell NC, Stellacci F, Stevens MM (2013) Nanoscale topography and chemistry affect embryonic stem cell self-renewal and early differentiation. Adv Healthc Mater 2(12):1644–1650. https://doi.org/10.1002/adhm.201200382
Lee J, Xia Y, Son MY, Jin G, Seol B, Kim MJ, Son MJ, Do M, Lee M, Kim D (2012) A novel small molecule facilitates the reprogramming of human somatic cells into a pluripotent state and supports the maintenance of an undifferentiated state of human pluripotent stem cells. Angew Chem Int Ed 51(50):12509–12513 %@ 1521-3773
Li J, Zhu L, Qu X, Li J, Lin R, Liao L, Wang J, Wang S, Xu Q, Zhao RC (2013) Stepwise differentiation of human adipose-derived mesenchymal stem cells toward definitive endoderm and pancreatic progenitor cells by mimicking pancreatic development in vivo. Stem Cells Dev 22(10):1576–1587. https://doi.org/10.1089/scd.2012.0148
Liao X, Xie GH, Liu HW, Cheng B, Li SH, Xie S, Xiao LL, Fu XB (2014) Helium-neon laser irradiation promotes the proliferation and migration of human epidermal stem cells in vitro: proposed mechanism for enhanced wound re-epithelialization. Photomed Laser Surg 32(4):219–225. https://doi.org/10.1089/pho.2013.3667
Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, Ding S (2009) A chemical platform for improved induction of human iPSCs. Nat Methods 6(11):805–808. https://doi.org/10.1038/nmeth.1393
Liu YS, Lee OK (2014) In search of the pivot point of mechanotransduction: mechanosensing of stem cells. Cell Transplant 23(1):1–11. https://doi.org/10.3727/096368912X659925
Lv Y, Zhao P, Chen G, Sha Y, Yang L (2013) Effects of low-intensity pulsed ultrasound on cell viability, proliferation and neural differentiation of induced pluripotent stem cells-derived neural crest stem cells. Biotechnol Lett 35(12):2201–2212. https://doi.org/10.1007/s10529-013-1313-4
McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, Murawski K, Kingham E, Oreffo RO, Dalby MJ (2011) Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater 10(8):637–644. https://doi.org/10.1038/nmat3058
Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, Bernstein BE, Jaenisch R, Lander ES, Meissner A (2008) Dissecting direct reprogramming through integrative genomic analysis. Nature 454(7200):49–55. https://doi.org/10.1038/nature07056
Murphy WL, McDevitt TC, Engler AJ (2014) Materials as stem cell regulators. Nat Mater 13(6):547–557 %@ 1476–1122
Muttigi MS, Kim B, Choi B, Yoshie A, Kumar H, Han I, Park H, Lee SH (2017) Matrilin-3 co-delivery with adipose-derived mesenchymal stem cells promotes articular cartilage regeneration in a rat osteochondral defect model. J Tissue Eng Regen Med 12:667. https://doi.org/10.1002/term.2485
Pachmann K, Leibold W (1976) Insolubilization of protein antigens on polyacrylic plastic beads using poly-L-lysine. J Immunol Methods 12(1–2):81–89 %@ 0022-1759
Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA (2014) Generation of functional human pancreatic beta cells in vitro. Cell 159(2):428–439. https://doi.org/10.1016/j.cell.2014.09.040
Park S, Im GI (2015) Stem cell responses to nanotopography. J Biomed Mater Res A 103(3):1238–1245. https://doi.org/10.1002/jbm.a.35236
Park KS, Cha KJ, Han IB, Shin DA, Cho DW, Lee SH, Kim DS (2012) Mass-producible nano-featured polystyrene surfaces for regulating the differentiation of human adipose-derived stem cells. Macromol Biosci 12(11):1480–1489. https://doi.org/10.1002/mabi.201200225
Park K-S, Ahn J, Kim JY, Park H, Kim HO, Lee S-H (2014) Poly-l-lysine increases the ex vivo expansion and erythroid differentiation of human hematopoietic stem cells, as well as erythroid enucleation efficacy. Tissue Eng A 20(5–6):1072–1080 %@ 1937-3341
Park HW, Kim YC, Yu B, Moroishi T, Mo J-S, Plouffe SW, Meng Z, Lin KC, Yu F-X, Alexander CM, Wang C-Y, Guan K-L (2015) Alternative Wnt signaling activates YAP/TAZ. Cell 162(4):780–794. https://doi.org/10.1016/j.cell.2015.07.013
Pires F, Ferreira Q, Rodrigues CA, Morgado J, Ferreira FC (2015) Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim Biophys Acta 1850(6):1158–1168. https://doi.org/10.1016/j.bbagen.2015.01.020
Rao C, Prodromakis T, Kolker L, Chaudhry UA, Trantidou T, Sridhar A, Weekes C, Camelliti P, Harding SE, Darzi A, Yacoub MH, Athanasiou T, Terracciano CM (2013) The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials 34(10):2399–2411. https://doi.org/10.1016/j.biomaterials.2012.11.055
Redmer T, Diecke S, Grigoryan T, Quiroga-Negreira A, Birchmeier W, Besser D (2011) E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep 12(7):720–726 %@ 1469-221X
Reimer A, Vasilevich A, Hulshof F, Viswanathan P, van Blitterswijk CA, de Boer J, Watt FM (2016) Scalable topographies to support proliferation and Oct4 expression by human induced pluripotent stem cells. Sci Rep 6:18948. https://doi.org/10.1038/srep18948
Revel A (2009) Multitasking human endometrium: a review of endometrial biopsy as a diagnostic tool, therapeutic applications, and a source of adult stem cells. Obstet Gynecol Surv 64(4):249–257. https://doi.org/10.1097/OGX.0b013e318195136f
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ (2014) Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 32(11):1121–1133. https://doi.org/10.1038/nbt.3033
Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE (2008) Substrate modulus directs neural stem cell behavior. Biophys J 95(9):4426–4438. https://doi.org/10.1529/biophysj.108.132217
Sia J, Sun R, Chu J, Li S (2016) Dynamic culture improves cell reprogramming efficiency. Biomaterials 92:36–45. https://doi.org/10.1016/j.biomaterials.2016.03.033
Staerk J, Lyssiotis CA, Medeiro LA, Bollong M, Foreman RK, Zhu S, Garcia M, Gao Q, Bouchez LC, Lairson LL (2011) Pan-Src family kinase inhibitors replace Sox2 during the direct reprogramming of somatic cells. Angew Chem Int Ed 50(25):5734–5736 %@ 1521-3773
Sun J, Li J, Li C, Yu Y (2015) Role of bone morphogenetic protein-2 in osteogenic differentiation of mesenchymal stem cells. Mol Med Rep 12(3):4230–4237. https://doi.org/10.3892/mmr.2015.3954
Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin J-W, Tewari M (2013) Nuclear Lamin-a scales with tissue stiffness and enhances matrix-directed differentiation. Science 341(6149):1240104 %@ 0036-8075
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. https://doi.org/10.1016/j.cell.2006.07.024
Thrivikraman G, Madras G, Basu B (2014) Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. Biomaterials 35(24):6219–6235. https://doi.org/10.1016/j.biomaterials.2014.04.018
Vang S, Longley K, Steer CJ, Low WC (2014) The unexpected uses of Urso- and Tauroursodeoxycholic acid in the treatment of non-liver diseases. Glob Adv Health Med 3(3):58–69. https://doi.org/10.7453/gahmj.2014.017
Wang P-Y, Clements LR, Thissen H, Tsai W-B, Voelcker NH (2015) Screening rat mesenchymal stem cell attachment and differentiation on surface chemistries using plasma polymer gradients. Acta Biomater 11:58–67 %@ 1742-7061
Wanjare M, Agarwal N, Gerecht S (2015) Biomechanical strain induces elastin and collagen production in human pluripotent stem cell-derived vascular smooth muscle cells. Am J Physiol Cell Physiol 309(4):C271–C281. https://doi.org/10.1152/ajpcell.00366.2014
Yim EK, Pang SW, Leong KW (2007) Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res 313(9):1820–1829. https://doi.org/10.1016/j.yexcr.2007.02.031
Yoon YM, Lee JH, Yun SP, Han YS, Yun CW, Lee HJ, Noh H, Lee SJ, Han HJ, Lee SH (2016) Tauroursodeoxycholic acid reduces ER stress by regulating of Akt-dependent cellular prion protein. Sci Rep 6:39838. https://doi.org/10.1038/srep39838
Young DA, Choi YS, Engler AJ, Christman KL (2013) Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 34(34):8581–8588 %@ 0142-9612
Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ (2009) Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res 104(4):e30–e41. https://doi.org/10.1161/CIRCRESAHA.108.192237
Zhang X, Zara J, Siu RK, Ting K, Soo C (2010) The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res 89(9):865–878. https://doi.org/10.1177/0022034510376401
Zhang Y, Liang X, Lian Q, Tse HF (2013) Perspective and challenges of mesenchymal stem cells for cardiovascular regeneration. Expert Rev Cardiovasc Ther 11(4):505–517. https://doi.org/10.1586/erc.13.5
Zhu L, Gomez-Duran A, Saretzki G, Jin S, Tilgner K, Melguizo-Sanchis D, Anyfantis G, Al-Aama J, Vallier L, Chinnery P, Lako M, Armstrong L (2016) The mitochondrial protein CHCHD2 primes the differentiation potential of human induced pluripotent stem cells to neuroectodermal lineages. J Cell Biol 215(2):187
Acknowledgements
This work was supported by a grant from the National Research Foundation (NRF) of Korea funded by the Ministry of Science, ICT & Future Planning (MSIP) (No. NRF-2016R1A2A1A05004987) and the Ministry of Education, Science and Technology (MEST) (No. NRF-2014R1A6A3A04055123).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Choi, B., Kim, D., Han, I., Lee, SH. (2018). Microenvironmental Regulation of Stem Cell Behavior Through Biochemical and Biophysical Stimulation. In: Noh, I. (eds) Biomimetic Medical Materials. Advances in Experimental Medicine and Biology, vol 1064. Springer, Singapore. https://doi.org/10.1007/978-981-13-0445-3_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-0445-3_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0444-6
Online ISBN: 978-981-13-0445-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)