Abstract
The cellular microenvironment has been known to direct the cell behaviour through biochemical and mechanical signalling. Different biomaterials have been fabricated to study the impact of biophysical cues on proliferation and stem cell differentiation in vitro. Stem cells have immense promise in regenerative medicine. Therefore, there is a pressing need to understand the interdependency of biophysical signals and biochemical signals in regulating stem cell potency and differentiation. In this chapter, we explore the different types of biomaterials commonly used for studying mechanobiology in stem cells and highlight the primary mechanism and pathways behind extracellular matrix (ECM)-mediated cellular response. Furthermore, we discuss how the understanding of stem cell mechanobiology influences the fields of tissue engineering and regenerative medicine. We also touch upon the importance of mechanobiology in cancer. In short, we have tried to convey to our readers that although current expansion and differentiation methods use biochemical molecules alone, it is crucial to understand that biophysical cues from the stem cell microenvironment can also regulate the proliferation and differentiation of stem cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Human pluripotent stem cells (hPSCs), which include both human-induced pluripotent stem cells (hiPSCs) and human embryonic stem cells (hESCs), have a unique ability to differentiate into cells of three germ layers and have unlimited expansion potential; hence, they can be used for tissue engineering. Multipotent stem cells, for example, mesenchymal stem cells and hematopoietic stem cells, are often used for various clinical researches, and there are several clinical trials conducted with these cells. However, most applications remain at the clinical trial stage due to the non-functionality of transplanted cells, cell death after transplantation, deposition of cells into the lungs, or teratoma formation (Lodi et al. 2011; Naji et al. 2019). This can be due to sudden changes in the microenvironment from in vitro to in vivo. Many researchers have been trying to study interactions between stem cells and their surrounding microenvironment to overcome this.
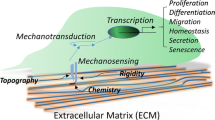
In vivo, stem cells reside in a specific microenvironment, also known as “niche.” This niche maintains an equilibrium between stem cell self-renewal and differentiation and is unique to every stem cell type. The critical regulatory components within the niche include dynamic and complex interactions between cells, macromolecules of extracellular matrix (ECM), biochemical components such as signaling molecules and hormones, and biophysical components such as ECM stiffness, pressure, shear fluid flow, stress, and strain (Pelham and Wang 1997; Vining and Mooney 2017). While the role of biochemical factors is well established, recent scientific literature points to evidence which indicates that the mechanical and biophysical signals generated from the extracellular milieu affect stem cell proliferation and differentiation (Gerardo et al. 2019; Gungordu et al. 2019). All cells, including stem cells and cancer cells, respond to mechanical cues. In stem cells, biophysical signaling control stem cell differentiation and self-renewal; and, in cancer cells, these signals lead to tumor invasiveness and metastasis (Lee et al. 2019; Choudhury et al. 2019). All these recent developments have led to the emergence of a new discipline—mechanobiology, which combines physical forces with the biological phenomenon.
The emergence of biomaterials has facilitated to artificially recreate biophysical signals experienced by cells under in vivo conditions. These biomaterials can be employed as a carrier for the transplantation of stem cells or to recruit endogenous progenitor cells at the site to repair and reconstruct damaged tissues or organs. A common hurdle in the use of biomaterials in regenerative medicine is the immune response. After transplantation, the biomaterials are extensively infiltrated by immune cells. These cells facilitate in removing cellular debris caused by injury; however, they can evoke inflammatory responses, which might hinder tissue repair and cell differentiation (Mokarram and Bellamkonda 2014). The development of new strategies has made biomaterials more sophisticated with respect to biocompatibility, biological cues, and the potential to reduce damage by an immune response and facilitate in vivo tissue development and direct repair.
In this chapter, we have explored the mechanical and functional interactions between stem cells and their microenvironment. We begin with a brief overview of the importance of ECM in mechanobiology, along with the fundamental molecular mechanisms and the emerging field of biomaterials for stem cell culture. We touch upon cancer mechanobiology and the implications of stem cell mechanobiology and regenerative medicine. We finally provide a perspective on the use of biomaterials to create a modified 3D microenvironment for stem cell culture, which will provide a model to uncover fundamental aspects of mechanobiology and hold tremendous potential in cell-based therapies.
15.2 Unique Tissue-Specific ECM Stiffness in Normal Physiology
The ECM is composed of fibrous proteins such as collagen, fibronectin, elastin, vitronectin, laminin; proteoglycans, and glycoproteins secreted by cells and matricellular-associated proteins such as CNN family, osteopontin, fibulin, periostin, and secreted protein acidic and rich in cysteine (SPARC); however, the ratios of these proteins vary between tissues (Yue 2014; Mouw et al. 2014). Therefore, each tissue has different stiffness, which is defined as elasticity or Young’s modulus (E) and is measured in a unit called pascal (Pa). For instance, bone ECM is primarily made up of collagen, which makes it stiff, and the estimated stiffness is approximately within the range of 100 kilopascal (kPa)–1 gigapascal (GPa). On the other hand, brain ECM has low fibrous proteins and higher amounts of proteoglycans compared to bone with E of approximately 1 kPa (Fig. 15.1) (Ruoslahti 1996; Wells 2008; Budday et al. 2015). Such variations in tissue ECM have led researchers to develop scaffolds that mimic the biological ECM stiffness and properties.
Our understanding of how mechanical signals direct molecular signaling during embryo development and in in vitro differentiation is constantly evolving. The role of ECM in generating mechanical cues has been explored extensively, as the matrix is crucial in regulating cellular functions (Pelham and Wang 1997; Vining and Mooney 2017). Other than providing physical support for growth attachment, the ECM also regulates cell shape, growth, proliferation, differentiation, and migration. Numerous studies have reported that changing the mechanical properties of the matrix, such as stiffness, affects cell morphology, growth, differentiation, migration, and gene expression (Pelham and Wang 1997; Lo et al. 2000; Justin and Engler 2011; Toh et al. 2012, Ireland and Simmons 2015).
15.3 Biomaterials and Their Types
Traditionally used synthetic scaffolds from 2D polystyrene surfaces to 3D constructs provide only support to the cultured cells. Recent advances in tissue engineering have shown exciting results with various biomaterials of suitable physical and chemical properties in recreating complex in vivo microenvironment in the laboratory. Based on their source and properties, these biocompatible materials can be categorized as natural, semisynthetic, and synthetic biomaterials, with stiffness similar to the stiffness of the biological tissue (Virdi and Pethe 2021).
Natural biomaterials are synthesized using polymers such as chitin, agarose, collagen (Chevallay and Herbage 2000), alginate, and hyaluronic acid hydrogel (Toh et al. 2012) because of their similarity with native ECM. Another advantage is that they are highly biocompatible with binding sites for cells, thereby supporting cell growth. However, natural polymers are not consistent in composition, are not easy to modify, and have limited mechanical properties. To overcome these disadvantages of natural polymers, synthetic substrates have been synthesized using polyacrylamide (PA) gels (Engler et al. 2004), polydimethylsiloxane (PDMS) (Goffin et al. 2006), polyethylene glycol (PEG) hydrogel (Gilbert et al. 2010), and polyvinyl alcohol (Muduli et al. 2017), which provide better mechanical properties than natural biomaterials. The synthetic biomaterials provide a range of various stiffness similar to the stiffness of the biological tissue, have high reproducibility, and are well defined. However, synthetic polymers provide limited cell-ECM interactions as they lack the functional group to allow cells to attach.
To overcome the drawbacks of natural polymers and synthetic biomaterials, a semisynthetic hydrogel, for example, gelatin methyl acrylate (GelMa) (Guilak et al. 2009), was designed, which has the biocompatibility of natural polymer and mechanical properties of synthetic biomaterials. To enhance the clinical application of scaffolds, it is important to achieve a xeno-free, chemically-defined system for stem cell culture other than hydrogels. In this regard, other scaffolds such as artificial nano- and micro-patterned substrates (Théry 2010), flexible micropillars (Halder et al. 2012), and electrospun nanofibers (Maldonado et al. 2015; Zhu et al. 2019) have been synthesized to study the effect of substrate stiffness on stem cell growth, differentiation, and migration.
15.4 Immunomodulatory Biomaterials
As we have introduced above, biomaterials being a foreign material may provoke an immune response, which might hinder tissue repair and regeneration. To address this limitation, researchers are synthesizing new biomaterial designs, which incorporate immunosuppressive molecules or signaling molecules that facilitate activation of the desired phenotype within the host immune cells (Dziki and Badylak 2018). These types of biomaterials are known as immunomodulatory biomaterials. Specific and durable immunomodulation can be achieved by manipulating the surface property of the biomaterials such as topology, surface charge, and ligands; this can induce activation of a desired immune cell phenotype (Stabler et al. 2019). For instance, following the implantation in murine subcutaneous implant and volumetric muscle injury model, flow cytometry analysis identified macrophages (F4/80+), CD11c+ dendritic cells, CD3+ T cells, and CD19+ B cells within the microenvironment of the ECM bioscaffold (Sadtler et al. 2017). The authors have shown that the biomaterial microenvironment changes the polarization of the migrating immune cells upon implantation, causing them to alter the signals generated by microenvironment. This immunomodulatory effect of the biomaterial on the immune cells and the host tissue environment may help in improving the therapeutic capability of the biomaterials. Numerous similar studies that use ECM-based biomaterials show a dynamic interaction between a variety of the immune cells or between stem cells and immune cells, which promotes tissue repair (Brown et al. 2012; Sadtler et al. 2016; Dziki et al. 2018).
15.5 Biomaterials Influence Stem Cell Proliferation and Functionality
In order to design the biomaterial that allows stem cells to be transplanted for clinical use, it is important to study some key aspects such as (1) the traction forces exerted by the cells on the biomaterial, (2) stem cell growth and proliferation, and (3) the changes in the stem cell functionality and differentiation capacity when grown on biomaterial.
The synthetic hydrogel substrates are synthesized using one or more polymers, which forms an interconnecting network with the help of a cross-linking agent. The mechanical properties such as hydrogel substrates can be manipulated by changing concentrations of polymer and cross-linking agent. For example, in PA-gel substrates, altering the ratio of acrylamide to bis-acrylamide cross-linker allows variation in Young’s modulus, which thereby affects cell behavior (Tse and Engler 2010). Human mesenchymal stem cells (hMSCs) cultured on stiff PA substrate with E ~ 25–40 kPa, which resembles bone ECM stiffness, differentiate toward osteoblast lineage as indicated by the gene expression analysis, whereas, on soft PA substrate (E ~ 0.1–1 kPa) resembling brain ECM stiffness, the hMSCs differentiate toward neural lineage (García and Reyes 2005; Engler et al. 2006). Muscle stem cells self-renew when cultured on substrates mimicking the stiffness of muscle tissue (E ~ 12 kPa), and these cells contributed to muscle regeneration when transplanted in mice (Gilbert et al. 2010). Morphologically, stem cells appear flattened on the stiff substrate and spherical with reduced spreading and stress fiber formation on soft substrate (Deroanne et al. 2001; Engler et al. 2004). These studies reveal varying responses of stem cells toward their microenvironment, and substrate stiffness indicates an important role of substrate matrix in regulating cell behavior.
PA-gel substrate functionalized with glycosaminoglycan (GAG) peptides shows better cell attachment. Following this observation, the research group demonstrated that stiff PA-GAG substrate (E ~ 10 kPa) promotes pluripotency of human ESCs as evidently observed from the expression levels of pluripotency marker proteins octamer-binding transcription factor-4 (OCT4) and stage-specific embryonic antigen-4 (SSEA4) (Musah et al. 2012); however soft substrate (E ~ 0.7 kPa) selectively differentiated stem cells toward neuronal lineage. The same research group noted an interesting observation that even in the absence of neuronal inducing factor, hPSCs grown on softer substrate appeared neuronal-like phenotype and expressed high levels of tubulin beta 3 chain (TUJ1) protein, a neuronal specific-marker (Musah et al. 2014). A similar observation was reported by another group that used other biomaterials as well of different stiffness (Chen et al. 2020). These studies indicate that substrate stiffness alone can influence hPSC differentiation when cultured with an optimal mechanical microenvironment, independent of soluble signaling factors. Therefore, it can be said that the mechanical signals have a profound contribution on early embryo development and differentiation.
As explained above, when mimicking various physiological stiffness like neural (E ~ 1 kPa), muscle (E ~ 12 kPa), and bone (E ~ 30 kPa) tissues, substrates can induce respective lineage-specific differentiation of MSCs. In addition to cellular function, substrate stiffness also influence cell migration. Cell migration is important in numerous physiological processes such as wound healing, organogenesis, immune response, tumor metastasis, and morphogenesis; thus, it is crucial in regeneration tissue engineering and cancer therapy. Many studies have demonstrated stem cells migrate toward the stiff substrate, whereas neurons show a preference for softer regions (Tse and Engler 2010; Vincent et al. 2013; Flanagan et al. 2002; Hadden et al. 2017). The mechanical properties of the ECM influence the factors known to regulate cell migration, such as the integrin-cytoskeletal interaction and cytoskeletal stiffness. The cells sense the change in the matrix through an active tactile exploration mechanism and respond by exerting contractile forces (Lo et al. 2000). To understand the migration of stem cells on matrix stiffness, MSCs were treated with focal adhesion kinases (FAK) inhibitor and siRNA targeting transcriptional factor Yes-associated protein (YAP) gene. They observed reduced cellular motility of treated cells compared to untreated cells, indicating that FAK and YAP control the movement of cells from the soft region toward the stiff region (Wang et al. 2001; Hadden et al. 2017; Lachowski et al. 2018).
15.6 Mechanobiology: Mechanism of Interactions (Molecular Mechanisms)
Mechanobiology is the study of the relationship between a cell and its microenvironment. The interactions between the cell and the microenvironment mainly occur at the interface. The properties of biomaterials such as hydrophilicity, surface charge, roughness, softness, and chemical composition affect the transplantation success. To improve the interaction between cell and scaffold, the physical, chemical, and biological properties of the biomaterials need to be optimized according to the cell type. Before seeding the cells onto a scaffold, surface modification is necessary to facilitate cell adhesion and growth. Surface modification can be either coating the surface with extracellular membrane protein or modifying the surface using functional moieties, hydrophobic or hydrophilic molecules (Shi et al. 2015; Elosegui-Artola et al. 2017).
A cell senses its external environment via membrane-bound receptors, focal adhesions to the ECM, adhesion junctions between neighboring cells, and gap junctions. The perturbation of protein conformation by mechanical forces influences the cytoskeletal organization, which triggers a series of intracellular signaling pathway resulting in inactivation or inhibition of gene expression, morphology, and motility (Discher et al. 2005; Guilak et al. 2009). Integrin-based adhesion complexes are one of the key molecular players closely associated with actin filaments. Focal adhesion complex, Ras homologous (Rho) GTPases, myosin light chain kinases, and Rho-associated kinases (ROCK) form a link between integrins and actin filaments. The activated focal adhesion complex comprises talin, vinculin, paxillin, alpha-actinin, p130cas, FAK, and SRC formed near cell surface integrin receptor (Geiger et al. 2009). The cells are able to sense the substrate stiffness, topology, surface area, and dimensionality of the scaffold by means of integrin molecules and focal adhesion complexes (del Rio et al. 2009; Amano et al. 2010; Donato et al. 2010; Ciobanasu et al. 2013; Janoštiak et al. 2014; Elosegui-Artola et al. 2017).
In brief, integrins are transmembrane ECM proteins and mechanoreceptors as they sense the change in the ECM, thereby mediating the mechanotransduction by focal adhesions, which link integrins to cytoskeleton (Hynes 2020). A traction force is generated in the actin cytoskeleton, which activates the downstream signaling and translocates the signal into the nucleus. These traction forces are also exerted on the integrins and focal adhesions, thus maintaining them in the isometric tension (Bershadsky et al. 2003). External stresses generate a mechanics-based positive feedback loop by increasing tension on the cell surface receptor and activating G protein Rho and its target ROCK. Stiff substrate results in an increase in kinase activities of ROCK, FAK, and extracellular signal-related kinases (ERK1/2), causing osteogenic differentiation of MSCs. Inhibition of ROCK and FAK leads to downregulation of osteogenic markers during osteogenic induction (Shih et al. 2011). Taken together, this implies that stiff substrates affect the regulation of ROCK-mediated FAK and ERK1/2, and these signals regulate the transcriptional factors, thereby determining the fate of MSCs.
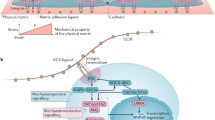
The mechano-sensitive transcriptional coactivators such as myocardin-related transcription factor (MRTF) (Speight et al. 2016), nuclear factor kappa B (NF-κB) (Kumar and Boriek 2003), nuclear factor erythroid 2-related factor 2 (NRF2) (Escoll et al. 2020), YAP, and beta-catenin (Gumbiner 1995; Huber et al. 1996) bind to their respective DNA-binding proteins and activate specific genes. The nuclear or cytoplasmic localization of these transcriptional factors is controlled by nuclear envelope receptor—linker of nucleoskeleton and cytoskeleton (LINC) complex (Guilluy et al. 2014; Driscoll et al. 2015) (Fig. 15.2). Apart from integrin-ligand binding, several studies have suggested that the cells produce nano-length projections that sense the surface for optimum spreading. Thus, different nano-topographical features guide cell migration and spreading on the scaffold with different topographies. The fact that cellular orientation and alignment can be controlled by topographical cues was demonstrated as early as 1911 by Robert Harrison (1911). To date, the biomaterial-based scaffold has undergone many surface modifications and alternations and has emerged as a powerful tool for mimicking in vivo microenvironment.
Schematic representation of the effect of stiff and soft substrate on cell morphology and function via integrin-mediated mechano-signaling. On stiff substrate, a cell receives biophysical cues from integrin-based focal adhesion complex, which increases the cytoskeletal stress via stretching of F-actin filaments. The stretching of LINC complex due to stiff substrate and stretched F-actin causes nuclear localization of transcriptional factors such as YAP. Conversely, on the soft substrate in the absence of less integrin activity, the focal adhesion complex is not formed, leading to less cytoskeletal tension and less stretching of actin filaments, thereby leading to cytoplasmic localization and no substrate-dependent nuclear localization of the transcriptional factors
15.7 Biomaterials as Promising Tools for Tissue Engineering and Regenerative Medicine
From the aforementioned considerations, it can be evident that mechanobiological processes in stem cells will impact the development of innovative therapeutic methods for tissue engineering and, eventually, regenerative medicine applications. The successful outcome of any stem cell-based regenerative medicine critically depends on cell survival after transplantation and to maintain tissue homeostasis mainly by differentiating into the respective lineage. To attain this, it is crucial to maintain optimal physiologically similar culture conditions in vitro for stem cell maintenance, proliferation, and quick differentiation when required. For instance, culturing the resident liver stem cells (RLSC) on polyacrylamide gel substrate having a stiffness of 0.4 kPa has shown to help in differentiation of RLSC into hepatocytes within 24 h, whereas RLSC cultured on a stiff substrate of stiffness 80 kPa resulted in only initial hepatocyte-specific transcriptional activity (Cozzolino et al. 2016). This variation in differentiation potential is due to culturing cells on soft stiffness—which is similar to healthy liver tissue stiffness (0.3–6 kPa)—rather than using normal stiff TCP. Similarly, instead of 2D culture system, Schoonjans and colleagues developed a synthetic 3D culture system using polyethylene glycol (PEG) hydrogels with a matrix stiffness of 1.3 kPa. This 3D culture system mimicking physiological liver stiffness provided better efficiency of live organoid derivation from mouse and human hepatic progenitors (Sorrentino et al. 2020). These studies show that clinically relevant human progenitor/stem cells cultured in physiologically relevant mechanical environments open perspectives for liver organoid-based clinical applications.
An interesting study focused on regenerating complex neural tissue such as motor neurons through modulating substrate stiffness because during embryo development, biophysical cues from the surrounding microenvironment along with soluble morphogens like sonic hedgehog (SHH) and retinoic acid (RA) play an important role in morphogenesis. Sun et al. (2014) and colleagues synthesized a system with PDMS with a stiffness range of E = 1.0–1200 kPa for generating motor neurons (MN) derived from hPSCs. Their findings suggest that soft substrate (E = 1 kPa) support early MN differentiation of hPSCs compared to stiff substrate (E = 1200 kPa). In addition, the yield and purity of functional MNs improve four- to tenfold on soft substrate compared to stiff substrate (Sun et al. 2014). Thus, culturing hPSCs on a synthetic cell culture surface with controlled mechanical properties (such as substrate stiffness) improved the efficiency of hPSC differentiation into motor neurons. Such advances open new doors in the therapeutics of motor neuron-associated neurodegenerative (Sun and Fu 2014).
An electrospun nanofibrous vascular scaffold made up of poly(l-lactide) (PLLA) was embedded within PA hydrogel on the outer surface. This nanofibrous polymer system had stiffer matrix near the polymer and was less stiff away from the polymer and was used as a graft for cell regeneration in vivo. Multipotent neural crest stem cells (NCSCs) generated from hiPSCs were embedded within the graft and implanted in rat carotid arteries. The stiffer matrix of the polymer scaffold with E = 50 kPa or higher supported the differentiation of NCSCs into smooth muscle cells (SMCs). The soft matrix area of the scaffold with E = 15 kPa supported the differentiation into glial cells. The results suggests that the mechanical properties of substrate play a significant role in designing biomaterials for tissue engineering (Zhu et al. 2019).
hiPSCs are traditionally generated by genetic reprogramming of adult somatic cells using biochemical signals (Takahashi and Yamanaka 2006). Fascinatingly, Grãos and colleagues demonstrate that MSCs can be reprogrammed into iPSCs by biophysical cues alone. They showed that human umbilical cord MSCs (huMSCs) exhibit PSC phenotype when cultured on soft PDMS substrate with E = 1.5 kPa and 15 kPa compared to stiff TCP (E ~ 1 GPa). huMSCs undergo chromatin modeling and show enhanced expression of pluripotency-related markers OCT4, SOX2, and NANOG in response to the soft substrate. Soft substrate allowed huMSCs to acquire relaxed nuclei, small FA, fewer stress fibers, and high euchromatic and lower heterochromatic content and expression of pluripotency specific genes. In short, their results suggest that substrate stiffness influences several phenotypic features of iPSCs and colonies and that soft substrate favors iPSC reprogramming (Gerardo et al. 2019). Such milestone studies indicate that substrate stiffness is a critical biophysical cue that influences stem cell differentiation into the specific lineage. Such studies also highlight the importance of biomaterials in tissue engineering and a promising platform for improving tissue engineering and regenerative applications.
15.8 Mechanobiology in Cancer Cells
Mechanobiology is one of the driving forces in guiding cell motility and tissue development during embryonic development. This cellular and tissue mechanobiology approach has been used by many researchers in understanding cancer development and tumor invasion. One of the key mechanisms by which cancer cells evade therapy is metastasis, and it has been hypothesized that the tumor cells might rely on mechanical forces for invasion and migration. The tumor microenvironment is an aggregation of cancer-associated fibroblasts (CAFs), vascular cells, immune cells, an abundance of extracellular matrix proteins, and hypoxic conditions (Choudhury et al. 2019; Sahai et al. 2020). Hypoxia and hypervascularization are directly and indirectly associated with ECM realignment and shear stress (Wang et al. 2017).
The ECM is a fundamentally essential component of the tumor microenvironment that interacts closely with cancer cells. Apart from providing necessary growth factors for tumor growth (Briquez et al. 2015), the ECM also helps in transmitting signals integrins (Canel et al. 2013). Additionally, upregulation of ECM remodeling molecules, such as transforming growth factor-beta (TGF-β), is linked to the development of desmoplasia in tumors (Papageorgis and Stylianopoulos 2015). Desmoplasia is the development of dense fibrous and connective tissue around tumor growth, usually characterized by increased synthesis of total collagen, fibronectin, glycoproteins, mainly tenascin C, proteoglycans, and a sizeable stromal cell population that amasses within the tumor. The increased production of tumorigenic and inflammatory growth factors transforms a large number of fibroblasts into CAFs. It has been proposed that the multifunctional cytokine TGF-β activates the transformation of fibroblasts into CAFs, which produces more ECM fibers, eventually causing desmoplasia (Papageorgis and Stylianopoulos 2015). The ECM stiffness of the fibrotic/cancer tissue is around 1.08–68 kPa (Kawano et al. 2015) and has shown to upregulate alpha-smooth muscle actin (α-SMA) expression, a proven CAF marker. Another known transcriptional factor that facilitates CAF generation and maintenance is YAP/TAZ, which activates only during high actomyosin contractility and high stiffness (Goffin et al. 2006; Calvo et al. 2013). YAP has been shown to regulate the expression of specific cytoskeletal proteins, including anillin actin-binding protein, myosin regulatory light polypeptide 9, and diaphanous related formin 3, which induces CAF (Calvo et al. 2013).
During cancer progression, uncontrolled cell proliferation results in an increase in tumor mass. This leads to a difference between the ECM stiffness of tumorous tissue and normal tissue. For instance, Samani et al. (2007) reported that the mean Young’s modulus of normal breast tissue is 1.9 kPa, whereas that of fibroadenoma was 11.42 kPa and that of invasive ductal carcinoma was 22.55 kPa. Multiple in vitro reports show that the stiffness of the tumor tissue and matrix directly correlates with tumorigenesis and metastasis (Zaman et al. 2006; Tilghman et al. 2010; Gkretsi and Stylianopoulos 2018; Jang et al. 2020). A breakthrough study published by Weaver and colleagues proves the hypothesis that mechanical signals mediate malignant transformation. They showed that culturing non-tumorigenic mammary epithelial cells on stiffness mimicking tumor-like stiffness induces cell proliferation, dysplasia and activates oncogenic epithelial signaling pathways. They also found that transformed cells maintain a functional link between integrins and Rho-dependent cytoskeletal tension, and in the presence of ROCK or integrin adhesion pharmaceutical inhibitors the malignant behavior of tumors was tempered (Paszek et al. 2005).
Cancer stem cells (CSCs) have been shown to reside within the tumor, and these cells have the ability to self-renew and differentiate into several cell types, which proliferate uncontrollably. Thus, CSCs sustain the growth of cancerous mass. The cancer stem cells are hard to eliminate due to their efficient DNA repair mechanisms, relative slow growth rate, and the high number of channel proteins to efflux drugs out (Turdo et al. 2019; Hirschmann et al. 2004; Fujiwara et al. 2021). Cancer stem cells lead to relapse of cancers after treatment (Eyler et al. 2008), and hence, it is necessary to investigate these cells including their mechanobiology machinery. In summary, understanding how cancer cells sense the mechanical signals and converted them into biochemical pathway may usher in new ways to control cancers. Given the similarities between the biology of stem cells and cancer cells (Shackleton 2010; Rahman et al. 2016), researchers are exploring the functional and mechanistic similarities between stem cell mechanobiology and cancer mechanobiology, with the aim of understanding the former using the latter as a guide (Fig. 15.3).
15.9 Concluding Remarks and Perspectives
Many advances in fabricating biomaterials for regenerative medicine have been reported in recent decades. Fundamental properties of biomaterials and of cell responses to biochemical and biophysical cues have been described via structural and functional studies. In this chapter, we have briefly described various properties of biomaterials and their impact on cellular behavior. For detailed information on the physical, chemical, and functional properties of the biomaterials, the authors recommend some extensively detailed reviews by Amani et al. (2019) and Cun and Hosta-Rigau (2020). The existing knowledge on ECM-cell interactions has been mainly derived from 2D in vitro studies. Although the 2D culturing system is convenient and has uncovered several crucial aspects about mechanobiology and biomaterials in cell migration, adhesion, proliferation, and differentiation, it does not mimic the in vivo microenvironment, which is 3D. It is becoming increasingly evident that the cells have a distinct behavior in the 3D microenvironment than that seen in 2D microenvironment. These facts have led to the use of a 3D culture system to mimic the physiological environment required for stem cell differentiation and the generation of organoids (Pepelanova et al. 2018; Bailey et al. 2019). hPSCs cultured on 3D scaffold have already been used to develop neuronal (Levenberg et al. 2003), liver (Baharvand et al. 2004), and cartilage (Hwang et al. 2006; Bai et al. 2010) tissue equivalents, along with rudimentary vascular networks (Ferreira et al. 2007).
Other than 3D culture, 3D bioprinting can be used to fabricate well-organized cell-laden scaffolds, which can be used to repair or regenerate damaged tissue (Antich et al. 2020; Jeong et al. 2020). Further advancement is organ-on-a-chip technology, which helps in generating self-organizing miniature organs from stem cells that replicate the functional and structural characteristics of cells present in in vivo microenvironment (Park et al. 2019). This organ-on-a-chip method has been employed in cancer cells to understand the disease progression and predict drug-induced responses (Sun et al. 2019). The studies discussed herein demonstrate the significance of the extracellular microenvironment in determining cellular behavior. They also highlight the importance of developing novel biomaterials to provide cells with biophysical cues which will help in cell-based therapies and regenerative medicine. Although much is yet to be unraveled about the influence of mechanobiology on stem cells, the newer discoveries give us insight into a promising future but also raised certain fundamental questions, such as the following: How much of the mechanical information is needed for the desired response from stem cells to form complex tissues? Can the biomaterials transplanted cause uncontrolled proliferation of the surrounding tissue? How cells generate their own mechanical forces during embryogenesis? With such diverse materials and methods for synthesizing biomaterials, it becomes crucial to understand how much of the material complexity is required for the desired stem cell response. We envision that the current research will help pave the way in understanding mechanobiological influence on stem cells and have major implications on tissue engineering and regeneration approaches.
References
Amani H, Arzaghi H, Bayandori M, Dezfuli AS, Pazoki-Toroudi H, Shafiee A, Moradi L (2019) Controlling cell behavior through the design of biomaterial surfaces: a focus on surface modification techniques. Adv Mater Interfaces 6(13):1900572
Amano M, Nakayama M, Kaibuchi K (2010) Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67(9):545–554
Antich C, de Vicente J, Jiménez G, Chocarro C, Carrillo E, Montañez E, Gálvez-Martín P, Marchal JA (2020) Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater 106:114–123
Baharvand H, Hashemi SM, Ashtiani SK, Farrokhi A (2004) Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Biol 50(7):645–652
Bai HY, Chen GA, Mao GH, Song TR, Wang YX (2010) Three step derivation of cartilage like tissue from human embryonic stem cells by 2D-3D sequential culture in vitro and further implantation in vivo on alginate/PLGA scaffolds. J Biomed Mater Res A 94(2):539–546
Bailey DD, Zhang Y, van Soldt BJ, Jiang M, Suresh S, Nakagawa H, Rustgi AK, Aceves SS, Cardoso WV, Que J (2019) Use of hPSC-derived 3D organoids and mouse genetics to define the roles of YAP in the development of the esophagus. Development 146(23):dev178855
Bershadsky AD, Balaban NQ, Geiger B (2003) Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 19(1):677–695
Briquez PS, Hubbell JA, Martino MM (2015) Extracellular matrix-inspired growth factor delivery systems for skin wound healing. Adv Wound Care 4:479–489
Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, Daly KA, Reing JE, Badylak SF (2012) Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 8(3):978–987
Budday S, Nay R, de Rooij R, Steinmann P, Wyrobek T, Ovaert TC, Kuhl E (2015) Mechanical properties of grey and white matter brain tissue by indentation. J Mech Behav Biomed Mater 46:318–330
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E (2013) Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15(6):637–646
Canel M, Serrels A, Frame MC, Brunton VG (2013) E-cadherin–integrin crosstalk in cancer invasion and metastasis. J Cell Sci 126(2):393–401
Chen YF, Li YSJ, Chou CH, Chiew MY, Huang HD, Ho JHC, Chien S, Lee OK (2020) Control of matrix stiffness promotes endodermal lineage specification by regulating SMAD2/3 via lncRNA LINC00458. Sci Adv 6(6):eaay0264
Chevallay B, Herbage D (2000) Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. Med Biol Eng Comput 38(2):211–218
Choudhury AR, Gupta S, Chaturvedi PK, Kumar N, Pandey D (2019) Mechanobiology of cancer stem cells and their niche. Cancer Microenviron 12:17–27
Ciobanasu C, Faivre B, Le Clainche C (2013) Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur J Cell Biol 92(10–11):339–348
Cozzolino AM, Noce V, Battistelli C, Marchetti A, Grassi G, Cicchini C, Tripodi M, Amicone L (2016) Modulating the substrate stiffness to manipulate differentiation of resident liver stem cells and to improve the differentiation state of hepatocytes. Stem Cells Int 2016:5481493
Cun X, Hosta-Rigau L (2020) Topography: a biophysical approach to direct the fate of mesenchymal stem cells in tissue engineering applications. Nanomaterials 10(10):2070
Del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP (2009) Stretching single talin rod molecules activates vinculin binding. Science 323(5914):638–641
Deroanne CF, Lapiere CM, Nusgens BV (2001) In vitro tubulogenesis of endothelial cells by relaxation of the coupling extracellular matrix-cytoskeleton. Cardiovasc Res 49(3):647–658
Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310(5751):1139–1143
Donato DM, Ryzhova LM, Meenderink LM, Kaverina I, Hanks SK (2010) Dynamics and mechanism of p130Cas localization to focal adhesions. J Biol Chem 285(27):20769–20779
Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE, Mauck RL (2015) Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys J 108(12):2783–2793
Dziki JL, Badylak SF (2018) Immunomodulatory biomaterials. Curr Opin Biomed Eng 6:51–57
Dziki JL, Giglio RM, Sicari BM, Wang DS, Gandhi RM, Londono R, Dearth CL, Badylak SF (2018) The effect of mechanical loading upon extracellular matrix bioscaffold-mediated skeletal muscle remodeling. Tissue Eng A 24(1–2):34–46
Elosegui-Artola A, Andreu I, Beedle AE, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux AL, Shanahan CM (2017) Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171(6):1397–1410
Engler AJ, Richert L, Wong JY, Picart C, Discher DE (2004) Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: correlations between substrate stiffness and cell adhesion. Surf Sci 570(1–2):142–154
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Escoll M, Lastra D, Pajares M, Robledinos-Antón N, Rojo AI, Fernández-Ginés R, Mendiola M, Martínez-Marín V, Esteban I, López-Larrubia P, Gargini R (2020) Transcription factor NRF2 uses the Hippo pathway effector TAZ to induce tumorigenesis in glioblastomas. Redox Biol 30:101425
Eyler CE, Rich JN (2008) Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol 26(17):2839–2845
Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R (2007) Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials 28(17):2706–2717
Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA (2002) Neurite branching on deformable substrates. NeuroReport 13(18):2411
Fujiwara Y, Tsunedomi R, Yoshimura K, Matsukuma S, Fujiwara N, Nishiyama M, Kanekiyo S, Matsui H, Shindo Y, Tokumitsu Y, Yoshida S, Iida M, Suzuki N, Takeda S, Ioka T, Hazama S, Nagano H (2021) Pancreatic cancer stem-like cells with high calreticulin expression associated with immune surveillance. Pancreas 50(3):405–413
García A, Reyes CD (2005) Bio-adhesive surfaces to promote osteoblast differentiation and bone formation. J Dent Res 84(5):407–413
Geiger B, Spatz JP, Bershadsky AD (2009) Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10(1):21–33
Gerardo H, Lima A, Carvalho J, Ramos JR, Couceiro S, Travasso RD, das Neves RP, Grãos M (2019) Soft culture substrates favor stem-like cellular phenotype and facilitate reprogramming of human mesenchymal stem/stromal cells (hMSCs) through mechanotransduction. Sci Rep 9(1):1–18
Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM (2010) Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329(5995):1078–1081
Gkretsi V, Stylianopoulos T (2018) Cell adhesion and matrix stiffness: coordinating cancer cell invasion and metastasis. Front Oncol 8:145
Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B (2006) Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J Cell Biol 172(2):259–268
Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS (2009) Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5(1):17–26
Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K (2014) Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol 16(4):376–381
Gumbiner BM (1995) Signal transduction by β-catenin. Curr Opin Cell Biol 7(5):634–640
Gungordu HI, Bao M, van Helvert S, Jansen JA, Leeuwenburgh SC, Walboomers XF (2019) Effect of mechanical loading and substrate elasticity on the osteogenic and adipogenic differentiation of mesenchymal stem cells. J Tissue Eng Regen Med 13(12):2279–2290
Hadden WJ, Young JL, Holle AW, McFetridge ML, Kim DY, Wijesinghe P, Taylor-Weiner H, Wen JH, Lee AR, Bieback K, Vo BN (2017) Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc Natl Acad Sci 114(22):5647–5652
Halder G, Dupont S, Piccolo S (2012) Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol 13(9):591–600
Harrison RG (1911) On the stereotropism of embryonic cells. Science 34(870):279–281
Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK (2004) A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci 101(39):14228–14233
Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R (1996) Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev 59(1):3–10
Hwang NS, Kim MS, Sampattavanich S, Baek JH, Zhang Z, Elisseeff J (2006) Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells 24(2):284–291
Hynes RO (2020) Integrins: bidirectional, allosteric signaling machines. Cell 110:673–687
Ireland RG, Simmons CA (2015) Human pluripotent stem cell mechanobiology: manipulating the biophysical microenvironment for regenerative medicine and tissue engineering applications. Stem Cells 33(11):3187–3196
Jang M, An J, Oh SW, Lim JY, Kim J, Choi JK, Cheong JH, Kim P (2020) Matrix stiffness epigenetically regulates the oncogenic activation of the Yes-associated protein in gastric cancer. Nat Biomed Eng 5:114
Janoštiak R, Pataki AC, Brábek J, Rösel D (2014) Mechanosensors in integrin signaling: the emerging role of p130Cas. Eur J Cell Biol 93(10–12):445–454
Jeong HJ, Nam H, Jang J, Lee SJ (2020) 3D bioprinting strategies for the regeneration of functional tubular tissues and organs. Bioengineering 7(2):32
Justin RT, Engler AJ (2011) Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One 6(1):e15978
Kawano S, Kojima M, Higuchi Y, Sugimoto M, Ikeda K, Sakuyama N, Takahashi S, Hayashi R, Ochiai A, Saito N (2015) Assessment of elasticity of colorectal cancer tissue, clinical utility, pathological and phenotypical relevance. Cancer Sci 106(9):1232–1239
Kumar A, Boriek AM (2003) Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J 17(3):386–396
Lachowski D, Cortes E, Robinson B, Rice A, Rombouts K, Del Río Hernández AE (2018) FAK controls the mechanical activation of YAP, a transcriptional regulator required for durotaxis. FASEB J 32(2):1099–1107
Lee G, Han SB, Lee JH, Kim HW, Kim DH (2019) Cancer mechanobiology: microenvironmental sensing and metastasis. ACS Biomater Sci Eng 5(8):3735–3752
Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R (2003) Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci 100(22):12741–12746
Lo CM, Wang HB, Dembo M, Wang YL (2000) Cell movement is guided by the rigidity of the substrate. Biophys J 79(1):144–152
Lodi D, Iannitti T, Palmieri B (2011) Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res 30(1):1–20
Maldonado M, Wong LY, Echeverria C, Ico G, Low K, Fujimoto T, Johnson JK, Nam J (2015) The effects of electrospun substrate-mediated cell colony morphology on the self-renewal of human induced pluripotent stem cells. Biomaterials 50:10–19
Mokarram N, Bellamkonda RV (2014) A perspective on immunomodulation and tissue repair. Ann Biomed Eng 42(2):338–351
Mouw JK, Ou G, Weaver VM (2014) Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol 15(12):771–785
Muduli S, Chen LH, Li MP, Heish ZW, Liu CH, Kumar S, Alarfaj AA, Munusamy MA, Benelli G, Murugan K, Wang HC (2017) Stem cell culture on polyvinyl alcohol hydrogels having different elasticity and immobilized with ECM-derived oligopeptides. J Polym Eng 37(7):647–660
Musah S, Morin SA, Wrighton PJ, Zwick DB, Jin S, Kiessling LL (2012) Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano 6(11):10168–10177
Musah S, Wrighton PJ, Zaltsman Y, Zhong X, Zorn S, Parlato MB, Hsiao C, Palecek SP, Chang Q, Murphy WL, Kiessling LL (2014) Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc Natl Acad Sci 111(38):13805–13810
Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N (2019) Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci 76(17):3323–3348
Papageorgis P, Stylianopoulos T (2015) Role of TGFbeta in regulation of the tumor microenvironment and drug delivery. Int J Oncol 46:933–943
Park SE, Georgescu A, Huh D (2019) Organoids-on-a-chip. Science 364(6444):960–965
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA (2005) Tensional homeostasis and the malignant phenotype. Cancer cell 8(3):241–254
Pelham RJ, Wang YL (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci 94(25):13661–13665
Pepelanova I, Kruppa K, Scheper T, Lavrentieva A (2018) Gelatin-methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering 5(3):55
Rahman M, Jamil HM, Akhtar N, Rahman KMT, Islam R, Asaduzzaman SM (2016) Stem cell and cancer stem cell: a tale of two cells. Progr Stem Cell 3(02):97–108
Ruoslahti E (1996) Brain extracellular matrix. Glycobiology 6(5):489–492
Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD (2016) Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352(6283):366–370
Sadtler K, Sommerfeld SD, Wolf MT, Wang X, Majumdar S, Chung L, Kelkar DS, Pandey A, Elisseeff JH (2017) Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostasis and injury. Semin Immunol 29:14–23
Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO (2020) A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 20:1–13
Samani A, Zubovits J, Plewes D (2007) Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol 52(6):1565
Shackleton M (2010) Normal stem cells and cancer stem cells: similar and different. Semin Cancer Biol 20(2):85–92
Shi C, Yuan W, Khan M, Li Q, Feng Y, Yao F, Zhang W (2015) Hydrophilic PCU scaffolds prepared by grafting PEGMA and immobilizing gelatin to enhance cell adhesion and proliferation. Mater Sci Eng C 50:201–209
Shih YRV, Tseng KF, Lai HY, Lin CH, Lee OK (2011) Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res 26(4):730–738
Sorrentino G, Rezakhani S, Yildiz E, Nuciforo S, Heim MH, Lutolf MP, Schoonjans K (2020) Mechano-modulatory synthetic niches for liver organoid derivation. Nat Commun 11(1):1–10
Speight P, Kofler M, Szászi K, Kapus A (2016) Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFβ-regulated Smad3. Nat Commun 7(1):1–17
Stabler CL, Li Y, Stewart JM, Keselowsky BG (2019) Engineering immunomodulatory biomaterials for type 1 diabetes. Nat Rev Mater 4(6):429–450
Sun Y, Fu J (2014) Harnessing mechanobiology of human pluripotent stem cells for regenerative medicine. ACS Chem Neurosci 5(8):621–623
Sun Y, Yong KMA, Villa-Diaz LG, Zhang X, Chen W, Philson R, Weng S, Xu H, Krebsbach PH, Fu J (2014) Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater 13(6):599–604
Sun W, Luo Z, Lee J, Kim HJ, Lee K, Tebon P, Feng Y, Dokmeci MR, Sengupta S, Khademhosseini A (2019) Organ-on-a-chip for cancer and immune organs modeling. Adv Healthc Mater 8(4):1801363
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Théry M (2010) Micropatterning as a tool to decipher cell morphogenesis and functions. J Cell Sci 123(24):4201–4213
Tilghman RW, Cowan CR, Mih JD, Koryakina Y, Gioeli D, Slack-Davis JK, Blackman BR, Tschumperlin DJ, Parsons JT (2010) Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS One 5(9):e12905
Toh WS, Lim TC, Kurisawa M, Spector M (2012) Modulation of mesenchymal stem cell chondrogenesis in a tuneable hyaluronic acid hydrogel microenvironment. Biomaterials 33(15):3835–3845
Tse JR, Engler AJ (2010) Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol 47(1):10–16
Turdo A, Veschi V, Gaggianesi M, Chinnici A, Bianca P, Todaro M, Stassi G (2019) Meeting the challenge of targeting cancer stem cells. Front Cell Dev Biol 7
Vincent LG, Choi YS, Alonso-Latorre B, Del Álamo JC, Engler AJ (2013) Mesenchymal stem cell durotaxis depends on substrate stiffness gradient strength. Biotechnol J 8(4):472–484
Vining KH, Mooney DJ (2017) Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 18(12):728–742
Virdi JK, Pethe P (2021) Biomaterials regulate mechanosensors YAP/TAZ in stem cell growth and differentiation. Tissue Eng Regen Med 18:199–215
Wang HB, Dembo M, Hanks SK, Wang YL (2001) Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci 98(20):11295–11300
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K, Li X (2017) Role of tumor microenvironment in tumorigenesis. J Cancer 8(5):761
Wells RG (2008) The role of matrix stiffness in regulating cell behavior. Hepatology 47(4):1394–1400
Yue B (2014) Biology of the extracellular matrix: an overview. J Glaucoma 23:S20
Zaman MH, Trapani LM, Sieminski AL, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P (2006) Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci 103(29):10889–10894
Zhu Y, Li X, Janairo RRR, Kwong G, Tsou AD, Chu JS, Wang A, Yu J, Wang D, Li S (2019) Matrix stiffness modulates the differentiation of neural crest stem cells in vivo. J Cell Physiol 234(5):7569–7578
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Virdi, J.K., Pethe, P. (2021). Dynamic Interactions Between Stem Cells and Biomaterials. In: Sheikh, F.A. (eds) Engineering Materials for Stem Cell Regeneration. Springer, Singapore. https://doi.org/10.1007/978-981-16-4420-7_15
Download citation
DOI: https://doi.org/10.1007/978-981-16-4420-7_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4419-1
Online ISBN: 978-981-16-4420-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)