Abstract
Removal of heavy metals by phosphate-solubilizing bacteria (PSB) activating phosphate materials in simulated heavy metal liquid environment was studied. Calcium phosphate was a kind of functional carrier material under action of PSB, accelerating the reaction to form phosphate precipitation of heavy metal. The results indicated that the removal of heavy metals with activated phosphate materials by PSB occurred within 15 days, and achieved high removal rates of Pb2+, Cu2+, Zn2+ and Cd2+ comparing with the phosphate materials inactivated. Therefore the activated phosphate materials by PSB could be a functional material for efficiently removing heavy metal from contaminated soil.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
The soil heavy metal pollution increases rapidly in the past few decades, especially heavy metals in soil serious exceeded the standard near the smelter which referred to lead, zinc, copper, and cadmium etc. Latest survey of soil pollution in country, the exceed the standard rate of national soil is 16.1% and at the same time one eighth of country existence of heavy metal pollution [1]. Heavy metal contamination can affect grain yield and indirectly affect human health.
At present, many different remediation methods can be applied to alleviate the toxic metal pollution, for instant, physical, chemical and biological-chemical ones. The study show biochemical methods remediation method is an effective and environmentally friendly method. A number of activated phosphate materials as hydroxyapatite have been proved to effectively immobilize toxic heavy metals in contaminated soil [2].
The mechanisms of metal immobilization by phosphate material include: (a) ion exchange process at the surface, (b) surface complexation, (c) dissolution of the original phosphate minerals and formation of new metal phosphate minerals, (d) substitution of Ca in phosphate material by other metals during recrystallization (coprecipitation) [3]. According to previous study results, acid pH condition increases phosphate solubility, what is the necessary prerequisite to activated phosphate materials.
At present, the more effective phosphorus is released when 2% citric acid is used as a release agent, which means the greater the amount of soil dissolved in it. The addition of citric acid is exogenous, so that the formation of slightly acidic soil condition [4]. However, there are many microorganisms that can dissolve insoluble phosphate in soil environment, because of secretion of multiple organic acids during metabolism process lowers the environmental pH value [5]. It can maintain pH values in the 4.5–6.5 range and it can convert insoluble forms of phosphorus into an accessible form to promote the immobilization of heavy metal. Eighteen strains of phosphate solubilizing microorganisms were isolated from soil. Named of Pantoea sp. and Enterobacter sp. were used for immobilization in lead contaminated soil. The result showed that they had a great application prospect in soil remediation [6]. Enterobacter sp. did not enhance Pb immobilization in solution because of acidification of bacterial medium, thereby inhibiting the formation of P-induced Pb precipitation. The immobilization of Pb in Pb-spiked soils was attributed to pyromorphite formation as indicated by XRD analysis [7].
The role of microorganisms in the solidification of heavy metals is not very clear, which accelerates the curing process or not. These factors limit the further development of phosphate-solubilizing bacteria activated phosphate materials. Therefore, it is necessary to do further research in this field. The aim of the study was to evaluate the effectiveness of phosphate-solubilizing bacteria activated phosphate material on Cd, Cu, Pb and Zn immobilization in simulation heavy metal contaminated conditions.
Materials and Methods
-
Materials. Phosphate-solubilizing bacteria activated phosphate materials mainly consisted of phosphate-solubilizing bacteria and high pure calcium phosphate. The phosphate-solubilizing bacteria of our research was preserved in Institute of Microbiology, Chinese Academy of Sciences, which derived from soil, obtained after dissolution, dilution and coating. The analytically pure calcium phosphate was purchased at reagent company whose calcium content was between 34 and 40%.
The condition of calcium phosphate was prepared in the laboratory which comprised of 140.41 mg/L Cu2+, 262.32 mg/L Pb2+, 136.88 mg/L Zn2+ and 487.39 mg/L Cd2+. These heavy metal ions were rooted in exogenous heavy metal nitrates. The content of heavy ions was based on molar ratio of phosphate to heavy metal.
-
Methods. Cultivation of phosphate-solubilizing bacteria. phosphate-solubilizing bacteria (PSB) was cultured at 30 ℃ for 3 days in 500 mL Erlenmeyer flask and the culture medium was carried out using National Botanical Research Institute’s phosphate growth (NBRIP) fluid medium, the composition of NBRIP was shown in Table 1. The initial pH value was 7.51. Every three days, we observed PSB by microscope and counted the quantity of bacteria.
Table 1 Solution composition [g/L]
Whether the existence of PSB played a role in the NBRIP medium when the removal rate of heavy metals was studied in this paper. In 300 mL calcium phosphate solution, 3 g of calcium phosphate mentioned above was soaked. The effectiveness of heavy metal ions was compared in the solution with or without bacteria.
-
Analytical Methods. All solid samples are weighed by a scale. The test of solution was filtered through 0.22 μm filter before analyzed. The concentrations of heavy metal ions were determined by ICP-OES. The pH value of the solution was measured with a pH meter. The solid residues after chemical reaction were examined with an X-ray diffractometer.
Results and Discussion
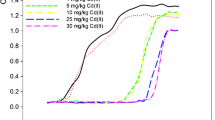
In presence or absence of PSB, during different period the main heavy metal concentration and the value of pH were determined. There were differences of chemical properties between the both solutions, which were shown in Figs. 1, 2, 3, 4, 5, 6, 7 and 8.
From the figure as we could see, the pH value of PSB solution decreased from 7.51 to 3.46 in 21 days, while from 7.51 to 4.47 in sterility solution. The results indicated that nitrates of heavy metals caused decrease of pH value in the solution. Another factor was the produce of acid by PSB, which caused by metabolism of phosphate-solubilizing bacteria.
In the PSB solution, the concentration of bacteria increased first, then decreased. The highest concentration occurred on the sixth day, then decreased rapidly. Maybe bacteria died. But bacteria continued existence in the solution. The bacteria were not well suited to the condition of high concentrations of heavy metals.
In the sterility solution, the concentration of trace P almost stayed in the same, while from 188.32 to 82.91 mg/L in PSB solution. There was a large different between PSB and sterility solution. Because of PSB was the main form to promote the dissolution of phosphorus. These rhizosphere microorganisms could convert insoluble calcium phosphate into soluble form constantly.
The concentration of Pb in PSB solution rapid declined from 1262.32 to 2.71 mg/L, while declined to 140.72 mg/L, increased to 619.91 mg/L. According to solubility product constant of lead phosphate, chloropyromorphite would be formed. However, in the sterility solution, lead may be adsorbed on the surface of phosphate and desorption as pH changed.
The concentration of Cu ion in the solution declined over the time, from 140.41 to 75.33 mg/L in PSB solution and from 140.41 to 118.33 mg/L in sterility solution. By literature we could know it may be caused by decreased of pH value and copper occurred replacement reaction on the surface of phosphate. According to the analysis result, copper sulfide precipitation was formed under the action of PSB. The reason may be that PSB converted sulphate radical into sulfide ion with the process of metabolism.
The concentration of Zn changed little in two different solutions, while PSB played a limited role in removal of zinc. According to the result of measure, the deposition of zinc sulfide was found in solid residues under action of PSB. The reason may be occurred replacement reaction on the surface of phosphate.
The concentration of Cd ion in the solution declined over the time, from 487.39 to 340.53 mg/L in PSB solution and from 487.39 to 426.92 mg/L in sterility solution. The reason was caused by the produce of sulfide ion. Cadmium occurred replacement reaction on the surface of phosphate.
The removal rate of heavy metal was 46.35, 99.79, 22.41 and 30.13% for Cu, Pb, Zn and Cd in the PSB solution, respectively. It was more better than in sterility solution, which value of removal rate was 15.73, 50.89, 16.00 and 12.41% for Cu, Pb, Zn and Cd, respectively. From the figure, we could know that the binding force between metal element were in the order: Pb > Cd > Cu > Zn.
Conclusions
Calcium phosphate is a kind of functional carrier material under action of PSB, the removal efficiency of heavy metal is better with activated phosphate materials than that not activated. Because of high bacterial activity accelerated the reaction to form precipitation, PSB activating phosphate materials is useful to solidified heavy metal.
The binding force between metal element and phosphate ion is in the order: Pb > Cd > Cu > Zn, which the concentration of these elements in the PSB solution will become lower with the generation of phosphate ion.
References
Ministry of environmental protection, Ministry of land and resources, National survey of soil pollution survey. China Environ. Prot. Ind. 5, 10–11 (2014)
S. Mignardi, A. Corami, V. Ferrini, Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 86(4), 354 (2012)
X. Cao, A. Wahbi, L. Ma et al., Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 164(2–3), 555–564 (2009)
R.X. Cao, L.Q. Ma, M. Chen et al., Phosphate-induced metal immobilization in a contaminated site. Environ. Pollut. 122(1) 19–28 (2003)
L. Hui, W.U. Xiao-Qin, J.H. Ren et al., Isolation and identification of phosphobacteria in poplar rhizosphere from different regions of China. Pedosphere 21(1), 90–97 (2011)
H.P. Jin, N. Bolan, M. Megharaj et al., Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. J. Hazard. Mater. 185(2–3), 829–836 (2011)
J.H. Park, N. Bolan, M. Megharaj et al., Concomitant rock phosphate dissolution and lead immobilization by phosphate solubilizing bacteria (Enterobacter sp.). J. Environ. Manage. 92(4), 1115 (2011)
Acknowledgements
This work was supported by the National Natural Science Foundation of China under grant numbers U1402234, 41573074, 50904011, and 31300025; the nation high-level youth talents special support plan; the Guangxi scientific research and technology development plan under grants GuikeAB16380287 and Guikegong14124004-3-1; the public welfare fund of the Ministry of Environmental Protection of People’s Republic of China under grant number 201509049; and Program of International S & T Cooperation S2016G2135.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Li, Y., Zhang, M., liu, X. (2018). Application of Phosphate-Solubilizing Bacteria Activating Phosphate Materials in Solidification of Soil Heavy Metal. In: Han, Y. (eds) Advances in Energy and Environmental Materials. CMC 2017. Springer Proceedings in Energy. Springer, Singapore. https://doi.org/10.1007/978-981-13-0158-2_71
Download citation
DOI: https://doi.org/10.1007/978-981-13-0158-2_71
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0157-5
Online ISBN: 978-981-13-0158-2
eBook Packages: EnergyEnergy (R0)