Abstract
Designing coated magnetic nanoparticles for nanomedicine applications, such as magnetic resonance imaging contrast enhancement, hyperthermia, and drug-delivery, has been in the focus of scientific interest for the last decade. Biocompatible polymers are used as nanoparticles coating for its physical and chemical properties that are very useful for biomedical applications. The aim of this contribution was to prepare the magnetite nanoparticles stabilized with poly (ethylene glycol) (PEG) and poly (ethylene glycol) methyl ether (mePEG) to elucidate the influence of the molecular weight on the corresponding amount of coating. The X-ray diffraction studies determined inverse spinel structure of magnetite nanoparticles, and field–emission scanning electron microscopy indicated the formation of quasi-spherical nanostructures with the final average particle size of 88–136 nm depending on the type of polymer coating. The bonding status of different polymers on the magnetite nanoparticles was confirmed by the Fourier transform infrared spectroscopy. According to the thermogravimetric analysis polymer amount in nanocomposites is related to molecular weight in the PEG-modified MNPs. The results of this study indicate the possibility of controlling the properties of theranostics nanomaterials, starting from the molecular weight of the polymer used as a coating.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Magnetite nanoparticles (MNPs) can be used for both diagnosis and therapy in the nanomedicine field [1, 2]. However, the naked magnetite nanoparticles are barely suitable for biomedical applications due to the aggregation near the physiological pH in the absence of electrostatic or steric stabilization [3]. Huge efforts are made to find a coating for MNPs, which fulfills the serious criteria of biocompatibility, and the chemical and the colloidal stability as well [4]. An alternative for overcoming such difficulties is to modify the MNPs with biocompatible polymers.
Poly (ethylene glycol) (PEG), is one of the main coatings that is used in nanomedicine because of its effectiveness to inhibit the absorption of proteins in the blood and the capture of nanoparticles by phagocytic cells as well as its long history of safety in humans and classification as Generally Regarded as Safe (GRAS) by the FDA [5]. To date, the synthesis of PEG-modified magnetite, including those derivatives of PEG, have been attempted to investigate the effects of individual parameters on the properties of modified NPs. However, it is difficult to manipulate the amount of polymer adsorbed to the particles. In addition, it is necessary to study the influence of molecular weight on the amount of polymer associated with MNP.
The molecular weight of the polymer used as a coating for nanoparticles is a very important variable because it is directly related to the physical properties of the nanomedicine system. Currently, the molecular weight of the polymer used as a nanoparticle coating is rarely considered within the criteria to control the properties of the final material. In this work, we quantify a critical parameter that influences the efficiency of the nanomedicine systems, the amount of PEG and PEGME associated to MNPs in relation to the molecular weight of the polymer used.
2 Materials and Methods
2.1 Preparation of Magnetite Nanoparticles
The magnetite nanoparticles were synthesized by coprecipitation method as previously reported elsewhere [6]. Briefly, the precursor solutions ferric chloride (10 mM) and ferrous sulfate (5 mM) were mixed with magnetic stirring for 1 h at 60 °C, then a strong base solution (NH4OH) was added dropwise until pH 13. After that, several washes were made until reaching pH 7, the obtained precipitated was repeatedly collected by centrifugation (10 min, 9000 G). The precipitated product was dried in oven at 100 °C and stored to further modification and characterization.
2.2 Preparation of PEG and PEGME-Modified Magnetite
For modification of MNPs, 25 mg of the collected magnetite resuspended in 2% triton. Meanwhile, four different polymer solutions, poly (ethylene glycol) (PEG400 and PEG3350) and poly (ethylene glycol) methyl ether (PEGME400 and PEGME2000), were prepared using 50 mg of the polymer in 30 mL of distilled water. For each coating reaction, the polymer solution was added directly to stirred magnetite. The pH was then adjusted to 9, by addition of NH4OH or HCl, as required, and the reaction mixture was stirred by ultrasound for 5 min. After that, several washes were made with distilled water until reaching neutral pH. Finally, the product was placed in an oven at 80 °C for 24 h and store for further characterization.
2.3 Characterization of Nanoparticles
The magnetite sample was analyzed in PANalytical X’Pert MRD PRO equipment with a Cu-kα source (λ = 1.5406 Å) operated at 40 kV and 30 mA and at a scanning rate of 0.1° 2θs−1 from 10 to 80° 2θ. The morphology of the bared MNPs and surface-modified MNPs were evaluated by Scanning Electronic Microscopy (Hitachi SU5000). To determine the particle size of the nanoparticles diameter of 100 individual nanoparticles was measured directly from the images using the line tool, the results are presented as mean ± SD and statistical comparisons between groups were carried out using one-way ANOVA followed by the Student’s t-test. Infrared spectra were obtained by using Fourier Transform Infrared (FTIR) with attenuated total reflection (ATR) spectrometer (Nicolet 6700/Thermo Electron). The infrared spectra were recorded with a resolution of 4 cm−1, and the scan range was set from 4000 to 600 cm−1. The results are presented as the average of 32 scans. Thermogravimetric analysis (TGA) were performed on a Q600 thermobalance from TA Instruments Inc. The samples were heated from ~25 to 600 °C, at a heating rate of 10 ℃/min. The content of polymer on the modified-MNPs was determined by the weight loss of the samples.
3 Results and Discussion
The X-ray diffraction pattern of MNPs is shown in Fig. 1. Positions and relative peaks intensities point to pure magnetite phase. Positions and relative intensities of all diffraction peaks can be indexed to a Fe3O4 phase. The XRD pattern was indexed in a spinel-like structure and there was no evidence of any extra phases, indicating that pure magnetite had been produced under the experimental conditions.
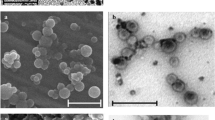
Figure 2 shows selected SEM images with their corresponding particle size distribution histograms for modified-MNPs. SEM images of nanoparticles illustrate that the nanoparticles consist of roughly spherical and almost uniform in size nanoparticles with an average diameter of about 254 ± 169.5 nm for M-PEG400, 58 ± 18.9 nm for M-PEG3350, 85 ± 31.2 nm for M-PEGME550, and 102 ± 35.4 nm for M-PEGME2000. For the bare nanoparticles the main size was 17 nm ± 3.9 nm. The coating with polymeric materials did significantly (P > 0.05) alter mean size. The differences in size indicates that the nanoparticles were not individually coated, but that a nanocomposite was formed in which several magnetite NPs were immobilized in the polymeric matrix. For all four polymers there is a distribution of chain lengths. This could be important for the short-chain ones. PEG is OH end-capped while PEGME is capped with CH3 groups. The OH-terminals in the short PEG chains facilitate the formation of hydrogen bonds between the molecules around the magnetite nanoparticles which causes the formation of larger particles. While the terminal CH3 in PEGME does not form hydrogen bonds with the polymer backbone, this prevent the association of a greater number of molecules, which explains the formation of nanoparticles with smaller size.
The chemical signature of polymer molecules associated to magnetite nanoparticles was demonstrated by IR spectroscopy (Fig. 3). The IR spectra of Poly(ethylene glycol), (PEG, linear formula: H(OCH2CH2)nOH) and Poly(ethylene glycol) methyl ether (PEGME, linear formula: CH3(OCH2CH2)nOH) have very few differences between them. PEG and PEGME consist of the monomer (-O-CH2-CH2-), differing only in the chain length and end groups. It can be noticed that both, PEG and PEGME, exhibit bands around 2877 and 1460 cm−1 attributed to C-H stretching and bending modes of methylene groups, respectively. The absorption peak at 1355 cm−1 is attributed to O-H bending mode and the absorption peak at 1100 cm−1 is due to the C-O stretching vibration mode. It can be noticed that the two bands near to 1100 cm−1 (1147 and 1061 cm−1) in both, PEG and PEGME, are revealed when the molecular weight increases because, when increasing the molecular weight, there are more C-O bonds that contribute to the stretching vibration mode. New absorption peak can be found at about 1730 cm−1 by comparing the IR spectra of the polymers (left) with those of the modified magnetite nanoparticles (right). The new absorption band can be assigned to C = O, this suggests that -CH2–OH group has been oxidized to COO− group during the reaction. The strong absorption band at about 700 cm−1 is due to Fe–O stretching vibration for the polymer-modified magnetite nanoparticles.
In order to confirm the content of polymeric material in the nanocomposites the thermal behavior of the modified-MNPs, M-PEG and M-PEGME nanocomposites were measured via thermogravimetric analysis (Fig. 4). The mass reduction below 100 ℃ corresponding to the loss of residual water. The thermal behavior of the polymeric materials was previously measured, their decomposition starts at around 200 ℃, therefore, the weight loss that occurs after 200 ℃ is attributed to the decomposition of polymeric material in the nanocomposite. The weight loss of the nanocomposites after 200 ℃ was 5.82% for M-PEG400, 8.15% for M-PEG3350, 16.40% for M-PEGME550, and 3.74% for M-PEGME2000. The thermal decomposition of PEGME matrix is presumably due to the breakdown of organic skeleton in a complex degradation process.
4 Conclusion
Magnetite nanoparticles which were synthesized by coprecipitation method were modified with PEG and PEGME to form the nanocomposites. The XRD patterns show pure phase magnetite. According to SEM measurements bare magnetite nanoparticles has a mean particle size of 17 ± 3.9 nm. After coating with PEG and PEGME, the M-polymer nanocomposites have significantly bigger mean particle size with polydispersity attributed to the distribution of chain lengths. The FTIR shows the vibrational modes of the magnetite and the polymeric material onto the nanocomposites and a IR band at 1730 cm−1 indicates the union between the polymer and the magnetite. There is a relationship between the molecular weight of the PEG and the amount of weight loss from 200 ℃; however, this relationship is not observed when the PEGME is used.
References
Golovin, Y. I., Klyachko, N. L., Majouga, A. G., Sokolsky, M., Kabanov, A. V. Theranostic multimodal potential of magnetic nanoparticles actuated by non-heating low frequency magnetic field in the new-generation nanomedicine. J Nanoparticle Res 19:63 (2017)
Bañobre-López, M., Piñeiro, Y., López-Quintela, M. A., Rivas, J. Magnetic Nanoparticles for Biomedical Applications. In: Handbook of Nanomaterials Properties. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 457–493 (2014)
Favela-Camacho, S. E., Pérez-Robles, J. F., García-Casillas, P. E., Godinez-Garcia, A. Stability of magnetite nanoparticles with different coatings in a simulated blood plasma. J Nanoparticle Res 18:176 (2016)
Mandal, S., and Chaudhuri, K. Magnetic Core-Shell Nanoparticles for Biomedical Applications. In: Complex Magnetic Nanostructures. Springer International Publishing, Cham, pp 425–453 (2017)
Suk, J. S., Xu, Q., Kim, N., Hanes, J., Ensign, L. M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 99:28–51 (2016)
Flores-Urquizo, I. A., García-Casillas, P., Chapa-González, C. Development of magnetic nanoparticles Fe +32 X+2O4 (X = Fe, Co y Ni) coated by amino silane. Rev Mex Ing Biomed 38 (2017)
Acknowledgements
The authors would like to thank the CONACYT for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
All authors declare no conflict of interests in this work.
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Chapa, C., Lara, D., García, P. (2019). Study of the Influence of the Molecular Weight of the Polymer Used as a Coating on Magnetite Nanoparticles. In: Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G. (eds) World Congress on Medical Physics and Biomedical Engineering 2018. IFMBE Proceedings, vol 68/3. Springer, Singapore. https://doi.org/10.1007/978-981-10-9023-3_2
Download citation
DOI: https://doi.org/10.1007/978-981-10-9023-3_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-9022-6
Online ISBN: 978-981-10-9023-3
eBook Packages: EngineeringEngineering (R0)