Abstract
Infrared neural stimulation (INS) gains growing interest both in electrophysiological research and for potential clinical applications, promising advantages like contactless operation, superior focality and hence spatial selectivity, and lack of electrical stimulation artifacts for nerve stimulation. We established an experimental setup for systematic investigation of relevant INS parameters, since little quantitative research for deeper understanding has been performed yet. Our customized setup facilitates the use of multiple fiber-based infrared laser systems of different wavelengths for remote stimulation, multi-site low-noise EMG recording and automated laser beam characterization. Hence, this setup simplifies upcoming systematic studies on both technical and physiological conditions for laser-induced neural activation. Determination of safe margins for reliable stimulation will help to understand the underlying physiological mechanisms and establish INS as alternative method for neural activation. Here, we present our experimental setup and preliminary results of our ongoing work.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Infrared neural stimulation (INS) was introduced in 2005 by Wells in order to investigate alternative methods for neural activation [1, 2]. It found with work by Izzo [3] and Teudt [4] its path into auditory respectively facial nerve stimulation, yet little was published for stimulation of the central nervous system. Three different theories compete to elucidate the modus operandi of INS as there are: transient photothermal gradients [1], change of cell membrane capacitance due to heating [5] or nanoporation meaning short-time opening of the cell membrane [6] leading to depolarization of neurons. However, to the best of our knowledge no final conclusion was presented and the existing publications are hard to independently reproduce due to a lack of technical details reported. The underlying bio-physiological mechanisms remain unclear. We therefore reached out to re-evaluate infrared nerve stimulation with our experimental approach, which is not only to qualitatively reproduce INS, but also to enable novel explanation based on quantitative observations in the animal model. In contrast to literature, we try to maximize the working distance between laser fiber and illuminated tissue to maintain the touch-free properties of laser stimulation with regard to potential clinical application. Here, we depict our experimental approach to explore the promising field of INS.
2 Methods
2.1 Infrared Laser Stimulation
Laser devices for INS stimulation are commonly being operated in pulsed rather than continuous mode [1, 2, 7,8,9]. Illuminating a target with a pulsed laser, the radiant exposure \( H_{p} \) caused by one pulse is defined as
where \( E_{p} \) is the radiant energy of the pulse and A is the effective illuminated surface area assuming a circular laser spot of diameter d, commonly referred to as spot size. The pulse energy \( E_{p} \) depends on both average laser power and the pulsewidth \( \tau_{p} \). Although reported laser pulses for INS stimulation in the current literature are mainly described by means of the radiant exposure H, however, this does not account for the exposure time at all [1, 2, 7,8,9]. Considering the radiant energy \( E_{p} \) of a single pulse being delivered to a target within a spot of area A and a discrete exposure time \( \tau = \tau_{p} \), we describe the radiant load using the irradiance I, which is given by
For a pulse train of duration \( \tau_{tr} \) containing a fast burst of n identical pulses, the radiant exposure and irradiance have to be modified to be \( H_{tr} \) and \( I_{tr} \) respectively:
For the application of laser radiation into the rat sciatic nerve, we use an infrared diode laser system (1470 nm, 12 W, DILAS Diodenlaser GmbH, Mainz, Germany), coupled into a multi-mode fiber of 200 μm core diameter and a numerical aperture NA = 0.22. The laser fiber is connected to a customized focusing optics, composed of a matched set of five spherical lenses mounted on a vertical C-mount rail (Fig. 1a). Shifting of the last lens allows the variation of the laser spot size diameter down to \( d = 0.9\;{\text{mm}} \) while maintaining minimum \( 15\;{\text{mm}} \) depth of field and a fixed focus position of about \( 50\;{\text{mm}} \) below the rail mount. The optical assembly supports the use of any other fiber-coupled laser system for future studies with different (infrared) wavelengths.
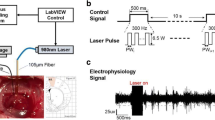
Experimental setup. a Vertically mounted focusing optics for INS studies. Automated beam characterization (knife-edge) with micromanipulator and power meter. b Rat sciatic nerve under stimulation with laser pulses (white arrow). EMG is recorded by means of intramuscular needle electrodes (Color figure online)
2.2 Laser Characterization
To determine the radiant energy \( E_{p} \) of single laser pulses and fast pulse trains of short (<2 ms) duration, we use a pyroelectric energy meter (ES145C, Thorlabs, Newton, New Jersey, US). For beam profiling, we employ the commonly known knife-edge method [10]: a razor blade is moved orthogonally into the continuous beam by a motorized micromanipulator (Luigs und Neumann, Ratingen, Germany), while measuring the downstream radiant power (S310C, Thorlabs) with regard to the blade position (Fig. 1a). Beam diameter \( d(z) \) as a function of the position z along the optical axis can be extracted, the beam profile characterized. We use a laser beam profiler (LBP-1-usb, Newport Corporation, Irvine, California, US) to rapidly determine the beam diameter or spot size during experiments.
2.3 Parametric Laser Control
The ability of our setup to cover a wide range of defined stimulation conditions like pulse energy \( E_{p} \), exposure time \( \tau \) and the irradiated area A (spot size), holds great potential for detailed research of INS. To this end, we use a set of parameters for pulse train definition, which are depicted in Fig. 2. Assuming a fixed average laser power \( \bar{P} \), the pulse energy \( E_{p} \) scales linearly with the pulsewidth \( \tau_{p} \). Considering n identical pulses as shown in Fig. 2, the period between two pulses is set by the Interval time \( \tau_{i} \). Hence, the pulse train is defined and can be iterated specified by the Repeat time and number of Runs to execute.
The free choice of those parameters allows arbitrary laser stimulation sequences and ensures maximum versatility for upcoming studies. With regard to Eq. (3), differently composed pulse trains that result in identical irradiance \( I_{tr} \), however, may still feature different internal patterns, which might be physiologically relevant when optically activating nerves.
The inherent constraints of the laser’s proprietary control software require a more sophisticated approach for external laser control to facilitate advanced flexibility. Hence, a customized LabVIEW user interface operates the entire experiment. All parameters and commands are passed to an Arduino Nano microcontroller and electronic circuit (Fig. 3). On the Arduino, a local program interprets the parameters to render pulse trains and—after a firing command—accordingly generates pin output voltages, which are then fed into the circuitry and drive the laser gate. On the left side in Fig. 3, a PWM output voltage is stabilized using a combination of two integrating capacitors and an operational amplifier to provide a steady analog voltage (\( 0 \ldots 10 \) V) for setting the power value \( \bar{P} \) as a fraction of full laser power (\( 12{\kern 1pt} \,{\text{W}} \)). Analog inputs constantly monitor the laser status for safety and prevent unintended firing. On the right side, the laser’s internal gate voltage is controlled by means of a digital output controlling a set of two serial fast switching transistors (Darlington) to supply a gate voltage above 18 V. Further, a trigger signal envelopes each pulse train according to Fig. 2 for data synchronization.
2.4 Data Acquisition
Laser-induced nerve activation by means of INS can result in visible muscle activity like twitching [11]. The elicited neural signal travels along the nerve leading to muscle contraction, termed compound muscle action potential (CMAP). Depending on the strength of the neural signal, limb or muscle movement can be weak and therefore remain unobserved. For the reliable detection of any emerging muscle activity, we record the electromyogram (EMG) by means of intramuscular needle electrodes attached to a commercial EMG recording system (ISIS, inomed Medizintechnik GmbH, Emmendingen, Germany). For each experiment, all laser parameters are saved into a text file.
2.5 Animal Preparation and Electrode Placement
For preliminary studies of INS on the rat sciatic nerve in vivo, we used female Sprague-Dawley rats (n = 11, 240–350 g). All animal experiments conducted in this study were performed with approval from the locally responsible Animal Welfare Committee with the Regierungspraesidium Freiburg in accordance with the guidelines of the European Union Directive 2010/63/UE (permit G17/80). Rats were initially anesthetized with Isofluran prior to intraperitoneal injection of a mixture of ketamine (100 mg/kg) with xylazine (8 mg/kg) and accordingly readministered depending on the depth of anesthesia. The skin was shaved and removed over both thighs. The sciatic nerves were then exposed by incision of the muscular fascia and mostly blunt dissection of m. gluteus superficialis and m. biceps femoris. For INS, the rat was placed under the laser setup and the wound was held open using a surgical retractor and moistened frequently with saline solution. A reference electrode was placed close to the tail and a pair of EMG electrodes was inserted into m. biceps femoris for preliminary studies. Proper nerve function was verified using an electrical nerve stimulation device (Neurostimulator, inomed Medizintechnik GmbH, Emmendingen, Germany). After experiment termination, both sciatic nerves were extracted for histological analysis.
2.6 Experimental Procedure
For proof of concept, the laser was focused at various locations on the main trunk of the sciatic nerve, up to about 15 mm proximal to the first branching point (Fig. 1b). Data recording was started and the laser was fired according to the pulse parameters (see Fig. 2). Overall radiant exposure per pulse train \( H_{tr} \) was then successively increased with the number of pulses n.
3 Results
In all of \( \;n = 11 \) preliminary experiments, we were able to evoke muscle activity from the rat sciatic nerve. However, successful stimulation occurred primarily at specific locations susceptible for INS and is therefore considered to be highly location-dependent. At locations that did not reveal a clear response to INS at low to medium radiation levels, the increase of radiant exposure for stimulation partially led to visible thermal damage. Tissue damage by INS will therefore be addressed in upcoming dosimetry histo-pathological studies.
In Fig. 4, an exemplary data set is shown. The top graph depicts a recorded EMG under INS. The laser trigger signals are shown in yellow. The bottom left graph shows one selected pulse train with laser-evoked CMAP signals, in the right graph an overlay plot is depicted. In general, the majority of the elicited CMAP responses rated “successful” occurred in a synchronous, rhythmical manner according to the laser stimulus, within 3–20 ms after stimulus onset (\( t = 0 \)). Also, muscle twitching was observed at higher CMAP amplitudes. Apart from few outliers, the shape of the evoked responses was highly consistent. No stimulation artifact was observed through all experiments.
Preliminary results. Top: long-term EMG recording under INS with increasing number of stimulation pulses. Left: Detailed extract (top green arrow, 8 pulses) of distinct response to laser stimulation with parameters according to Fig. 2. Right: Overlay of the 10 laser-evoked CMAPs with regard to the stimulation onset (trigger). CMAPs were highly consistent and do not show stimulation artifacts (Color figure online)
When considering the approximation for the train time \( \tau_{tr} \approx n \cdot \tau_{i} \), we note that the irradiance \( I_{tr} \) remains constant with fixed single pulse energy which was determined to be \( E_{p} = 1.9 - 2\;{\text{mJ}} \). In upcoming studies, different stimulation patterns, and therefore alteration of the irradiance, will be performed by variation of laser power \( \bar{P}_{i} \), exposure times \( \tau \) (\( \tau_{p} ,\tau_{i} \)) and different spot sizes.
4 Conclusion
In this paper, we presented our novel experimental setup, that allowed us to consistently reproduce infrared nerve stimulation in all preliminary experiments on the rat sciatic nerve. In the future we will continue our research on different optical stimulation parameters and wavelengths, exposure thresholds and limits for reliable and safe stimulation, and bio-physiological aspects of INS.
References
Wells, Jonathon; Kao, Chris; Mariappan, Karthik; Albea, Jeffrey; Jansen, E D.; Konrad, Peter; Mahadevan-Jansen, Anita: Optical stimulation of neural tissue in vivo. In: Optics letters 30 (2005), Nr. 5, S. 504–506
Wells, Jonathon; Kao, Chris; Jansen, E D.; Konrad, Peter; Mahadevan-Jansen, Anita: Application of infrared light for in vivo neural stimulation. In: Journal of biomedical optics 10 (2005), Nr. 6, S. 064003–064003
Izzo, Agnella D.; Richter, Claus-Peter; Jansen, E D.; Walsh, Joseph T.: Laser stimulation of the auditory nerve. In: Lasers in surgery and medicine 38 (2006), Nr. 8, S. 745–753
Teudt, Ingo U.; Nevel, Adam E.; Izzo, Agnella D.; Walsh, Joseph T.; Richter, Claus-Peter: Optical stimulation of the facial nerve: a new monitoring technique? In: The Laryngoscope 117 (2007), Nr. 9, S. 1641–1647
Shapiro, Mikhail G.; Homma, Kazuaki; Villarreal, Sebastian; Richter, Claus-Peter; Bezanilla, Francisco: Infrared light excites cells by changing their electrical capacitance. In: Nature communications 3 (2012), S. 736
Beier, Hope T.; Tolstykh, Gleb P.; Musick, Joshua D.; Thomas, Robert J.; Ibey, Bennett L.: Plasma membrane nanoporation as a possible mechanism behind infrared excitation of cells. In: Journal of neural engineering 11 (2014), Nr. 6, S. 066006
Wells, Jonathon D.; Thomsen, Sharon; Whitaker, Peter; Jansen, E D.; Kao, Chris C.; Konrad, Peter E.; Mahadevan-Jansen, Anita: Optically mediated nerve stimulation: Identification of injury thresholds. In: Lasers in surgery and medicine 39 (2007), Nr. 6, S. 513–526
Izzo, Agnella D.; Walsh, Joseph T.; Ralph, Heather; Webb, Jim; Bendett, Mark; Wells, Jonathon; Richter, Claus-Peter: Laser stimulation of auditory neurons: effect of shorter pulse duration and penetration depth. In: Biophysical journal 94 (2008), Nr. 8, S. 3159–3166
Richter, C.-P.; Matic, A.I.; Wells, J.D.; Jansen, E.D.; Walsh, J.T.: Neural stimulation with optical radiation. In: Laser & Photonics Reviews 5 (2011), Nr. 1, 68–80. http://dx.doi.org/10.1002/lpor.200900044. – https://doi.org/10.1002/lpor.200900044. – ISSN 1863–8899
Khosrofian, John M.; Garetz, Bruce A.: Measurement of a Gaussian laser beam diameter through the direct inversion of knife-edge data. In: Applied Optics 22 (1983), Nr. 21, S. 3406–3410
McCaughey, Ryan G.; Chlebicki, Cara; Wong, Brian J.: Novel wavelengths for laser nerve stimulation. In: Lasers in surgery and medicine 42 (2010), Nr. 1, S. 69
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare that they have no conflict of interests. This work was funded by the German Federal Ministry of Education and Research (BMBF), project NeuroPhos, FKZ 13GW0155C.
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Schlett, P., Wegner, C., Krueger, T., Buckert, T., Klotzbuecher, T., Hofmann, U.G. (2019). Experimental Setup for the Systematic Investigation of Infrared Neural Stimulation (INS). In: Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G. (eds) World Congress on Medical Physics and Biomedical Engineering 2018. IFMBE Proceedings, vol 68/3. Springer, Singapore. https://doi.org/10.1007/978-981-10-9023-3_14
Download citation
DOI: https://doi.org/10.1007/978-981-10-9023-3_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-9022-6
Online ISBN: 978-981-10-9023-3
eBook Packages: EngineeringEngineering (R0)