Abstract
In this chapter, we will introduce the cellular structure that is responsible for hair-cell mechanotransduction (MET). Auditory hair cells reside in the inner ear, where they are interlaced in a precise pattern with various supporting cells. Hair cells got their name because each one has hundreds of hairy-looking membrane protrusions, namely, stereocilia, extending from its apical surface. Within each hair cell, various types of extracellular links couple different stereocilia with one another. Tip links connect the tips of shorter stereocilia to the lateral shaft of its taller neighbouring stereocilia, and the yet-unidentified MET channels localize at the tips of shorter stereocilia, near the lower end of tip links. When the stereocilia are deflected positively, tension of tip links increases, which changes the conformation of MET channels and increases their open probability. Driven by the potential difference and ion concentration difference, cations flux into hair cells through the MET channels and cause membrane depolarization.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Inner Ear and Hair Cells
The auditory system consists of three parts: the outer ear, the middle ear, and the inner ear (Fig. 2.1a). The outer ear funnels sound into the middle ear, which converts the airborne vibration into mechanical vibration and then transfers it to the cochlea of the inner ear. Besides the cochlea, in the inner ear, there is also the labyrinth, which belongs to the vestibular system (Fig. 2.1a). Here we are only concerned with the auditory hair cells in the cochlea, but we want to point out that the vestibular hair cells in the labyrinth have similar structure as auditory hair cells and that the molecular MET mechanism in auditory hair cells and vestibular hair cells is believed to be similar, if not the same.
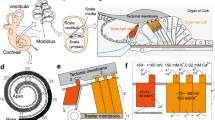
Auditory pathway: the ear, organ of Corti, and hair cells. (a) The outer, middle, and inner ear. (b) The cross-section view of the cochlea. The cochlea contains three chambers: scala vestibuli, scala media, and scala tympani, which are separated by Reissner’s membrane and the basilar membrane. The organ of Corti resides upon the basilar membrane. (c) A traveling wave in the basilar membrane. For the purpose of simplicity, the scala media and Reissner’s membrane are not illustrated here. (d) The cross-section view of the organ of Corti. The organ of Corti sits upon the basilar membrane and is covered by the tectorial membrane. (e) Top view of the hair bundles of P8 mouse IHC and OHC. Scale bars, 1 μm
(a) through (d): Reprinted with permission from Bear MF. et al., Neuroscience: Exploring the brain (4th edition, 2015). Publisher: Wolters Kluwer. (e) Reprinted with permission from Wang Y. et al., Front. Mol. Neurosci., 2017, 10: 401
The cochlea has a spiral shape, like a snail’s shell. The inside of the cochlea is divided into three fluid-filled chambers: scala vestibuli, scala media, and scala tympani. Scala vestibuli is separated from scale media by Reissner’s membrane, and scala media is separated from scala tympani by basilar membrane (Fig. 2.1b). Scala vestibuli and scala tympani are connected to each other at the apex of the cochlea through a hole called helicotrema, and these two chambers are filled with perilymph, which contains relatively low K+ (7 mm) and high Na+ (140 mm), similar to regular cerebrospinal fluid. In contrast, scala media is filled with endolymph, which contains high K+ (150 mm) and low Na+ (1 mm), similar to intracellular fluid. The ionic concentration differences and the selective permeability of Reissner’s membrane result in the so-called endocochlear potential (EP): the electrical potential of endolymph is about 80 mV more positive than that of the perilymph.
The mechanical vibration transmitted from the middle ear moves the membrane that covers the oval window, an opening at the base of scala vestibuli. This movement displaces the perilymph and endolymph inside the cochlea and eventually causes the basilar membrane to move up and down. The basilar membrane starts its movement by bending near its base, which then propagates towards the apex as a “travelling wave” (Fig. 2.1c). Sitting on the basilar membrane is the organ of Corti, which harbours the auditory hair cells as well as various supporting cells (Fig. 2.1d). From base to apex along the cochlear duct, hair cells at different positions are tuned to different frequencies, which are known as characteristic frequencies. Hair cells at the base respond to high frequencies, and those at the apex respond to low frequencies [1, 2].
In mammals, there are two types of auditory hair cells: inner hair cells (IHCs) and outer hair cells (OHCs). Along the spiral cochlea, there are one single row of IHCs (about 3500 in human) and three rows of OHCs (about 12,000 in human). Hair cells got their name because each one has hundreds of hairy-looking membrane protrusions, namely, stereocilia, extending from its apical surface. IHCs have pear-shaped cell body and contain flattened U-shaped stereocilia. OHCs have cylinder-shaped cell body and contain V-shaped stereocilia (Fig. 2.1e). The tips of the OHC stereocilia are embedded in the overlying tectorial membrane.
IHCs form synapses with the spiral ganglion cells, which are afferent neurons. Spiral ganglion cells relay the auditory information to the cochlear nuclei in the medulla and eventually to the auditory cortex. OHCs, on the other hand, usually do not form synapses with the spiral ganglion. Instead, they mainly form synapses with efferent neurons and work as cochlear amplifier and play an important role in frequency tuning [3, 4]. Nevertheless, the molecular MET mechanisms in these two types of auditory hair cells are considered to be the same.
2.2 Hair Bundle: Stereocilia and Kinocilia
Hair cells are characteristic of their hairy-looking, F-actin-based stereocilia on the apical surface (Fig. 2.2a). One hair cell has dozens to hundreds of stereocilia, which are organized into several rows of increasing heights, forming a staircase-like pattern. The actin core in each stereocilium is tightly packed in a paracrystalline array, with the barbed (plus) ends pointing towards the stereociliary tips [5]. The actin core is dynamic in developing stereocilia but stable in mature stereocilia [6, 7]. The stereocilium tapers at its base, and a few actin filaments (referred as rootlet) continue to insert into the F-actin matrix of the cuticular plate underneath the apical surface. This makes stereocilia easy to bend at the base when basilar membrane moves up and down (see below). Stereocilia are organized in a flattened U-shaped (IHCs) or V-shaped (OHCs) pattern, with the tallest stereocilia positioning at the vertices. The vertices of all stereocilia point away from the centre of the cochlea, establishing the planar cell polarity (PCP) in the cochlear epithelia.
Hair bundles: structure and function. (a) Schematic drawing of a hair cell. (b) Schematic drawing of extracellular links that connect stereocilia. (c) The model of sound-induced hair bundle deflection (Reprinted with permission from Bear MF. et al., Neuroscience: Exploring the brain (4th edition, 2015). Publisher: Wolters Kluwer)
Besides stereocilia, there is a single microtubule-based kinocilium on each hair cell’s apical surface. Stereocilia and kinocilium together constitute the so-called hair bundle. Kinocilium localizes at the vertex of stereocilia, juxtaposed next to the tallest stereocilia. Kinocilium is degenerated at late developmental stage in cochlear hair cells (but persists in vestibular hair cells), suggesting that it is not necessary for MET [8,9,10]. Nevertheless, kinocilium is believed to play pivotal roles in stereocilia development and cochlear PCP establishment.
There are various types of extracellular links that couple different stereocilia with one another as well as with kinocilium [11, 12]. Four types of links connect stereocilia, namely, tip links, horizontal top connectors, lateral links, and ankle links. Among them, tip links and horizontal top connectors are found in the mature cochlear stereocilia, whereas lateral links and ankle links only temporally exist in developing cochlear stereocilia (Fig. 2.2b). The tallest stereocilia are connected to kinocilium by kinociliary links. As kinocilium only exist in developing auditory hair cells, kinociliary links in auditory hair cells are also transient. These links play important roles in the development and function of hair bundle.
The morphogenesis of hair bundle was first thoroughly studied in avian hair cells [5, 13]. At the onset of hair bundle morphogenesis, the apical surface of hair cell is covered uniformly with multiple microvilli, and a single kinocilium is localized in the centre of the apical surface. The microvilli elongate and form stereocilia of similar height then stop elongation and start to increase the width by adding more actin filaments. Meanwhile, the kinocilium moves from the centre to the bundle periphery at a random direction then reorients so that the kinocilia of all the hair cells are on approximately the same side of the cell. The stereocilia next to the kinocilium then start to elongate again, followed by elongation of the adjacent rows of stereocilia, forming a staircase-like pattern. After that, stereocilia stop elongation and increase their width. Kinocilium is lost during this period, and the unelongated stereocilia are eventually resorbed. The filaments in the central core of stereocilia extend basally to form rootlets. At last, stereocilia reinitiate elongation and grow to their final lengths. Development of mammalian auditory hair bundles generally follows the same theme, although the stages are less distinct [14, 15].
When sound-induced mechanical energy is transferred to the cochlea, the basilar membrane moves up and down. The tips of the OHC stereocilia are embedded in the tectorial membrane; hence, the movement of the basilar membrane relative to the tectorial membrane causes the deflection of the stereocilia of OHCs. Although the tips of IHC stereocilia are not embedded in the tectorial membrane, IHC stereocilia are also deflected, possibly by the moving endolymph. The extracellular links hold the stereocilia together, so all the stereocilia move as a unit (Fig. 2.2c). The movement of hair bundle provides the basis for hair-cell MET. The response of hair cells to stereocilia movement is direction-sensitive: movement towards the tallest stereocilia produces depolarized receptor potential, whereas opposite movement produces hyperpolarized receptor potential. There is a cosine relationship for responses to stereocilia movement of any directions, and the sensitive direction is referred as “axis of mechanical sensitivity” [16, 17].
2.3 Tip Links and Mechanotransduction
Among all the extracellular links that connect the stereocilia, tip links probably attract the most attention. Tip links connect the tips of shorter stereocilia to the lateral shaft of its taller neighbouring stereocilia [18]. Ultrastructural studies showed that they are 150–200 nm long and 8–11 nm thick, adopting a double-helical conformation with a 20–25 nm periodicity [19, 20] (Fig. 2.3a–c). Each filament in the double helix is composed of multiple globular structures that are 4 nm in diameter. Tip links are separated into two or three strands at both ends, which insert into the plasma membrane of stereocilia. Electron microscopy revealed that the upper and lower tip-link insertion sites display electron-dense plaques [21], which were named later as upper tip-link density (UTLD) and lower tip-link density (LTLD). Ca2+ chelators such as BAPTA cause disruption of tip links, suggesting that the structural integrity of tip links is Ca2+-dependent [22]. Tip links were also shown to be asymmetric: the upper part and lower part are formed by different proteins [23], and the MET channels are placed near the lower end of tip links [24].
Tip links: the pivotal linkers that gate the MET channels. (a) Model of tip-link structure. (b) Freeze-etch image of tip link from guinea pig hair cells. (c) Higher magnification view of the tip link in (b). (d) Model of MET mechanism (Scale bars: 50 nm in b; 10 nm in c)
(a) through (c): Reprinted with permission from Kachar B, et al. Proc. Natl. Acad. Sci. U. S. A., 2000, 97(24):13336–41. Copyright (2000) National Academy of Sciences, U.S.A. (d) Reprinted with permission from Bear MF. et al., Neuroscience: Exploring the brain (4th edition, 2015). Publisher: Wolters Kluwer
Several lines of evidence indicate that tip links are directly involved in the MET process. First, the direction of tip links is parallel to the bundle’s axis of mechanical sensitivity [18]. Second, high-speed calcium imaging revealed that the MET channels are localized at the tips of shorter stereocilia, near the lower end of tip links [24]. Third, when tip links are disrupted with calcium chelators, MET is also abolished [22]. Fourth, hair-cell MET is interrupted in mutant mice that lose tip links [25,26,27].
Figure 2.3d summarizes our present understanding how MET happens in hair cells. When there is no sound stimuli, the stereocilia are not deflected and stand straight up. The resting tension of the tip links causes the MET channels to spend part of the time in the open state. Driven by the EP and the ionic concentration differences, a small amount of cations (K+ and Ca2+) enter hair cells through the channels (Fig. 2.3d1). When the stereocilia are deflected towards the taller edge, tension of the tip links increases, which in turn increases the open probability of the channels, and allows more cations to enter hair cells. The flux of cations causes membrane depolarization in hair cells (Fig. 2.3d2). On the other hand, when the stereocilia are deflected towards the shorter edge, tension of tip links decreases, less cations enter hair cells, and cell membrane is hyperpolarized (Fig. 2.3d3).
2.4 Discussion
Cochlear hair cells are the auditory receptor cells, and their characteristic stereocilia are indispensable for MET. In the present model, tip links, which connect the tips of shorter stereocilia to the lateral shaft of its taller neighbouring stereocilia, are placed in a central position next to the MET channels. Deflection of stereocilia changes tension of tip links, opens the MET channels, and produces receptor potential.
Given the importance of stereocilia in hair-cell MET, it is not surprising that deficits in stereocilia development or maintenance could lead to profound hearing loss. Indeed, mutations that affect stereociliary F-actin polymerization, bundling, or even actin itself have been shown to associate with syndromic or nonsyndromic deafness [28]. Kinocilia are not present in mature mammalian cochlear hair cells and hence are not necessary for MET. Nevertheless, kinocilia are essential for hair bundle development, as mutations that affect kinocilia are associated with hair bundle polarity deficits [29, 30].
The small numbers of hair cells and the scarcity of functional MET channels in each hair cell hindered the identification of the channel. We now know that the MET channels localize at the tips of shorter stereocilia, close to the lower insertion site of tip links. The property and molecular composition of the MET machinery are discussed in the following chapters.
References
Liberman, M.C. 1982. The cochlear frequency map for the cat: labeling auditory-nerve fibers of known characteristic frequency. The Journal of the Acoustical Society of America 72 (5): 1441–1449.
Muller, M. 1991. Frequency representation in the rat cochlea. Hearing Research 51 (2): 247–254.
Kiang, N.Y., et al. 1986. Single unit clues to cochlear mechanisms. Hearing Research 22: 171–182.
Dallos, P. 1992. The active cochlea. The Journal of Neuroscience 12 (12): 4575–4585.
Tilney, L.G., M.S. Tilney, and D.J. DeRosier. 1992. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annual Review of Cell Biology 8: 257–274.
Zhang, D.S., et al. 2012. Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature 481 (7382): 520–524.
Drummond, M.C., et al. 2015. Live-cell imaging of actin dynamics reveals mechanisms of stereocilia length regulation in the inner ear. Nature Communications 6: 6873.
Lindeman, H.H., et al. 1971. The sensory hairs and the tectorial membrane in the development of the cat s organ of Corti. A scanning electron microscopic study. Acta Oto-Laryngologica 72 (4): 229–242.
Tanaka, K., and C.A. Smith. 1978. Structure of the chicken’s inner ear: SEM and TEM study. The American Journal of Anatomy 153 (2): 251–271.
Denman-Johnson, K., and A. Forge. 1999. Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. Journal of Neurocytology 28 (10–11): 821–835.
Goodyear, R., and G. Richardson. 1992. Distribution of the 275 kD hair cell antigen and cell surface specialisations on auditory and vestibular hair bundles in the chicken inner ear. The Journal of Comparative Neurology 325 (2): 243–256.
Goodyear, R.J., et al. 2005. Development and properties of stereociliary link types in hair cells of the mouse cochlea. The Journal of Comparative Neurology 485 (1): 75–85.
Tilney, L.G., and M.S. Tilney. 1986. Functional organization of the cytoskeleton. Hearing Research 22: 55–77.
Kaltenbach, J.A., P.R. Falzarano, and T.H. Simpson. 1994. Postnatal development of the hamster cochlea. II. Growth and differentiation of stereocilia bundles. the Journal of Comparative Neurology 350 (2): 187–198.
Zine, A., and R. Romand. 1996. Development of the auditory receptors of the rat: a SEM study. Brain Research 721 (1–2): 49–58.
Flock, A. 1964. Electron microscopic and electrophysiological studies on the lateral line canal organ. Acta Oto-Laryngologica. Supplementum SUPPL 199: 1–90.
Shotwell, S.L., R. Jacobs, and A.J. Hudspeth. 1981. Directional sensitivity of individual vertebrate hair cells to controlled deflection of their hair bundles. Annals of the New York Academy of Sciences 374: 1–10.
Pickles, J.O., S.D. Comis, and M.P. Osborne. 1984. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hearing Research 15 (2): 103–112.
Kachar, B., et al. 2000. High-resolution structure of hair-cell tip links. Proceedings of the National Academy of Sciences of the United States of America 97 (24): 13336–13341.
Tsuprun, V., R.J. Goodyear, and G.P. Richardson. 2004. The structure of tip links and kinocilial links in avian sensory hair bundles. Biophysical Journal 87 (6): 4106–4112.
Furness, D.N., and C.M. Hackney. 1985. Cross-links between stereocilia in the guinea pig cochlea. Hearing Research 18 (2): 177–188.
Assad, J.A., G.M. Shepherd, and D.P. Corey. 1991. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7 (6): 985–994.
Kazmierczak, P., et al. 2007. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449 (7158): 87–91.
Beurg, M., et al. 2009. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nature Neuroscience 12 (5): 553–558.
Schwander, M., et al. 2009. A mouse model for nonsyndromic deafness (DFNB12) links hearing loss to defects in tip links of mechanosensory hair cells. Proceedings of the National Academy of Sciences of the United States of America 106 (13): 5252–5257.
Alagramam, K.N., et al. 2011. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS One 6 (4): e19183.
Geng, R., et al. 2013. Noddy, a mouse harboring a missense mutation in protocadherin-15, reveals the impact of disrupting a critical interaction site between tip-link cadherins in inner ear hair cells. The Journal of Neuroscience 33 (10): 4395–4404.
Barr-Gillespie, P.G. 2015. Assembly of hair bundles, an amazing problem for cell biology. Molecular Biology of the Cell 26 (15): 2727–2732.
Ross, A.J., et al. 2005. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nature Genetics 37 (10): 1135–1140.
Jones, C., et al. 2008. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nature Genetics 40 (1): 69–77.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Xu, Z. (2018). Cellular Structure for Hair-Cell Mechanotransduction. In: Mechanotransduction of the Hair Cell. SpringerBriefs in Biochemistry and Molecular Biology. Springer, Singapore. https://doi.org/10.1007/978-981-10-8557-4_2
Download citation
DOI: https://doi.org/10.1007/978-981-10-8557-4_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8556-7
Online ISBN: 978-981-10-8557-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)