Abstract
Sound signals are acquired and digitized in the cochlea by the hair cells that further transmit the coded information to the central auditory pathways. Any defect in hair cell function may induce problems in the auditory system and hearing-based brain function. In the past 2 decades, our understanding of auditory transduction has been substantially deepened because of advances in molecular, structural, and functional studies. Results from these experiments can be perfectly embedded in the previously established profile from anatomical, histological, genetic, and biophysical research. This review aims to summarize the progress on the molecular and cellular mechanisms of the mechano-electrical transduction (MET) channel in the cochlear hair cells, which is involved in the acquisition of sound frequency and intensity—the two major parameters of an acoustic cue. We also discuss recent studies on TMC1, the molecule likely to form the MET channel pore.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
From organization to function
For a thorough description on cochlear structure and function, one can refer to books and reviews from others [1,2,3]. The mammalian cochlea is a tube-like tissue inside a snail-shaped bony capsule that compacts a longitudinal tube into a spiral space (Fig. 1a). The approximately 40-mm-long human cochlea is a 2.5-turn cone, 9 mm in diameter and 4 mm in height [4], which provides an excellent environment and structure for sound decoding. The cochlea is divided into three fluid-filled chambers: scala vestibuli, scala media, and scala tympani (Fig. 1b). The scala vestibuli and the scala tympani are connected as one lumen to support fluid oscillation, while the scala media provides unique physical and chemical conditions (see below) for hair cell function. In the scala media, two types of hair cells, inner hair cells (IHCs) and outer hair cells (OHCs), together with a variety of supporting cell types form an integrated structure, also known as the organ of Corti (Fig. 1c). The tectorial membrane is an extracellular structure that is overlaid on the top of the hair cells, while the basilar membrane forms the bottom of the organ of Corti and displays varied geometrical and mechanical properties (Fig. 1c). Different frequencies induce the best vibration at different places on the basilar membrane, which are amplified locally and actively by the OHCs (a process known as the tonotopy principle; Fig. 1d). The basilar membrane is the first resonator and frequency analyzer to respond when the cochlea encounters sound stimulation (Fig. 1c–e); this is the main concept of the “travelling wave” theory [5, 6]. Recent studies have shown that the tectorial membrane may have an active role in this process [7,8,9], which is not surprising, considering that it is required to support cochlear mechanics. The cochlea exhibits a variety of sophisticated designs that support its role in decoding acoustic parameters. Here, we list several aspects that form the basis for the ability of hair cells to decode acoustic cues.
Organization of hair cells for sound acquisition. a Schematic of the inner ear showing cochlea and vestibule. The conical structure in the center of cochlea is the modiolus, which hosts spiral ganglion neurons to innervate hair cells. b The cochlea contains three chambers: scala vestibuli, scala media, and scala tympani. c In the scala media, the inner hair cells (IHCs) and the outer hair cells (OHCs) are the receptor cells in the organ of Corti. The efferent and afferent nerve fibers are simplified as one tract. d Schematic showing the coiled array of IHCs and OHCs. e Cartoon showing mechanics of the organ of Corti with sequential steps: Basilar membrane vibration (1) induces shear stress of tectorial membrane (2) which further provokes receptor potential to enhance OHC motility (2). This increases the intensity and sharply tunes the frequency resonating at the point of the basilar membrane. The enhanced signals are simultaneously transited to the IHC (2) which encodes the vibrations and transmit the signals to the postsynaptic SGNs (3). f Cartoon showing ionic flux of IHCs and OHCs in a sealed environment providing driving force of voltage. The values of resting membrane potentials of IHCs and OHCs from 2-week mice as described [48, 133]
Organization of hair cells in cochlea
Morphologically, IHCs possess a flask-like cell body and OHCs have a rod-like cell body (Fig. 1c). The hair bundle is the mechanosensitive subcellular structure of the hair cell that protrudes from the apical cell surface and is composed of F-actin-based stereocilia at graded heights (Fig. 1c). Notably, the hair bundles of the OHCs, but not the IHCs, are embedded in the tectorial membrane (Fig. 1c, e). When the sound-induced vibration is converted into shear stress between the tectorial membrane and the hair bundles, the OHCs acquire oscillation of the receptor potential that simultaneously drives the motility of their soma; this is highly dynamic and further enhances the mechanical vibration of the organ of Corti (Fig. 1e). This outstanding electromotility of the OHCs is mediated by the electromotile protein, Prestin, which is abundantly expressed on the lateral wall of the OHC soma. This lays the foundation for the high sensitivity with which cochlea detect the low-intensity sound stimulation, with a measured gain of approximately 60 dB [10, 11]. The OHC-based cochlear mechanics guarantees that the IHCs acquire the wide-range acoustic intensity at graded sensitivity from the force-matched vibration of the tectorial membrane (Fig. 1e).
One row of IHCs and three rows of OHCs are arrayed on the basilar membrane (Fig. 1c–f), and humans are estimated to have approximately 3500 IHCs and 12,000 OHCs in total. The cochlear coil wraps around the modiolus from base to apex (Fig. 1a); IHCs are close to the modiolus, while OHCs are at the rim (Fig. 1d). This highly organized allocation of hair cells lays a general physical foundation for decoding intensities and frequencies of sound: apical hair cells sense low frequencies, basal hair cells detect high frequencies, and middle hair cells sense frequencies in between. Shape and function are strongly correlated in IHCs and OHCs as their shapes change gradually along the cochlear coil [12,13,14]. The apical low-frequency (LF) OHCs have longer soma and longer stereocilia, as well as a smaller opening of their hair bundle in a ‘V’ shape. In contrast, the high-frequency (HF) OHCs at the basal region are much shorter in their soma and stereocilia length, and also have a broader ‘W’-shaped opening of their hair bundle [15, 16]. All these physical parameters perfectly match the mechanical requirement for OHCs to execute their frequency selectivity and mechanical motility [17, 18]. Thus, the mechanical force posed on each IHC contains modulated frequency and intensity information at single-cell resolution along the cochlear coil; from this the tonotopic pattern is initiated at the cochlear level and further projects to every auditory station in the brain.
Extracellular environment of hair cells
A significant characteristic of the scala media is that it contains a high concentration of K + (150 mM) and lower concentration of Ca2+ (20 μM), which in combination power a high endocochlear potential (EP, + 80 ~ + 100 mV) [19, 20]. The scala vestibuli and scala tympani fluid contains ~ 5 mM K+, equal to the concentration in regular perilymph fluid [19, 20]. Because of the tight junction at the top of the organ of Corti, only hair bundles where mechano-electrical transduction (MET) channels are located are immersed in the endolymph fluid, while the hair cell bodies are bathed in the perilymph fluid (Fig. 1f). This design provides a direct driving force of potential (Δ150 ~ 170 mV) and K+ (instead of Na+) is used as the major ion for influx through the MET channel. This makes the hair cell perform transduction more efficiently, without any temporal delay because of ionic exchange between Na+ and K+ [20]. Interestingly, some physical and chemical factors in the scala media also exhibit a tonotopic gradient from apex to base. For example, in guinea pigs (a general mammalian model for auditory study), Ca2+ concentration is graded from 20 to 40 μM [21] and EP is graded from + 65 to + 85 mV [21, 22]. The organ of Corti consumes a great deal of energy to perform auditory transduction, which is supported by stria vascularis, the power plant of scala media, as reviewed elsewhere [20].
MET channel and acoustic acquisition
The development of these well-designed parts in the cochlea provides mammals with excellent hearing capability, and the MET channel is an extremely fascinating aspect of this. To accomplish the sound acquisition, the mammalian MET channel functions as a protein complex and demonstrates complicated biochemical and biophysical properties that are frequency dependent and tunable by many factors.
The MET channel complex
In terms of the MET machinery, OHCs and IHCs likely use identical components. To date, many laboratories have provided sufficient genetic and functional evidence for us to speculate about a working model for the assembly and constitution of the MET channel complex; six transmembrane proteins have been proposed to constitute MET machinery (Fig. 2a) and two cytosol proteins are crucial for MET function. As an integrated protein machine, MET channel assembly, maintenance, and kinetics can be influenced by the genetic variations in MET molecules. In the most serious cases, such variation can cause MET damage that results in deafness.
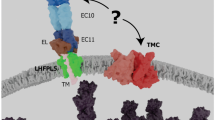
The MET channel complex in hair cells. a Cartoon showing tip link and the characterized molecules at the lower end of the tip link. The MET channel complex is at the lower tip-link density. b Schematic showing molecular coordination at the lower tip-link density, including PCDH15, TMC1, TMC2, LHFPL5/TMHS, TMIE, and CIB2, which are in the same color as in (a). c Top view of putative MET complex with characterized molecules in speculated stoichiometric ratio. The PCDH15 and LHFPL5 are in a 2:2 arrangement, while other molecules (TMC1/2, TMIE, and CIB2) are shown in the dashed boarders as their numbers are not precisely defined
Connecting two neighboring stereocilia, the tip link is an extracellular filament which gates the MET channel (Fig. 2a). When mechanical force is applied to open the MET channel via the tip link, K+ and Ca2+ rapidly flux into the hair bundles to alter receptor potential [23]. The tip link is formed by two unconventional cadherins with a single TM domain: Cadherin 23 (CDH23) and Protocadherin 15 (PCDH15), which form the upper and lower parts, respectively [24,25,26]. CDH23 has 27 EC (extracellular cadherin) domains and PCDH15 has 11 EC domains. These proteins form cis-dimers to connect with each other through their N-terminal EC1 and EC2 in a “handshaking” style [27,28,29] (Fig. 2a). The MET channel complex is localized at the membrane insertion site of PCDH15 (lower tip-link density, LTLD) [30], which makes structural and functional study of PCDH15 important to the understanding of MET channel properties. Importantly, two structural studies both pointed out a collar-like extracellular linker (EL, also called PICA) domain between the EC11 and TM domains (Fig. 2b) [31, 32]; previously overlooked, this domain is worthy of further functional study. PCDH15 was found to undergo dimerization at only two sites (EC2-3 and membrane-proximal EL domain) of the extracellular fragment and with 0.5 helical turns, which suggests that molecular elasticity is important for MET [31]. In addition, PCDH15 elongates under tension and shows reversible EC unfolding, which relies on its lateral thermal undulations at physiological Ca2+ concentration (20 μM) [33]. The intracellular part of PCDH15 contains a common region (CR) enriched with prolines and a cytosol domain (CD), followed by PDZ-binding interface as its C-terminal [34, 35]. Three isoforms of PCDH15 were found based on alternative splicing of CD (CD1, CD2, and CD3) [34]. It was further revealed that CR is critical for MET function [36] and CD2 is essential for kinocilial links [35]. Generally, deficiency of CDH23 or PCDH15 causes problematic tip-link formation (and thus a MET defect) in addition to hair bundle disorganization and degeneration. Mutations of CDH23 and PCDH15 cause Usher syndrome type 1D and 1F, respectively [37,38,39,40,41].
Transmembrane channel-like 1 and 2 proteins (TMC1 and TMC2) are putative MET channel pore proteins, and the first two proteins of an eight-member family [42,43,44]. Defects in TMC1, including dominant DFNA36 and recessive DFNB7/11, in humans cause deafness [42, 45,46,47]. Similarly, mouse models with variable TMC1 mutations show dominant or recessive deafness [44, 48,49,50]. TMC1 and TMC2 have enriched expression in cochlear and vestibular hair cells and knockout of both TMC1 and TMC2 completely abolishes the MET response in mice [51]. In mouse cochlea, TMC1 and TMC2 are expressed in hair cells with an obvious spatiotemporal pattern, which coincides the maturation of MET function. Spatially, the basal coil always shows higher expression level than the apical coil. As for the expression timing, TMC1 and TMC2 are both expressed earlier in basal coil and later in apical coil. However, TMC1 and TMC2 seem to be expressed sequentially; TMC2 expression starts from embryonic stage and disappears until P4, while TMC1 emerges later (from P2) and is sustained in adults [51]. Deletion of TMC1 does not immediately abolish the MET current, which can be partially rescued by forced TMC2 expression, or vice versa, demonstrating that the two proteins are functionally redundant and can compensate for each other [51,52,53]. Similar to the spatiotemporal expression pattern of TMC1/2, the MET currents are graded at different regions of the cochlea to follow the tonotopy [54, 55]. In a transgenic mouse model generated to illuminate TMC1 and TMC2 [56], the number of TMC1/2 shown by quantity of fluorescent signal increases only for OHCs (not IHCs) along the cochlear coil tonotopically, which is considered to represent the MET channel conductance gradient [57]. However, these data were obtained from the transgenic mice under overexpression conditions; the quantitative distribution at endogenous expression level remains an open question. In two independently generated Tmc1-knockin mouse lines (Tmc1-HA and Tmc1-Flag), the immunostaining of tags showed obvious TMC1 molecules in hair bundle [58, 59], and also strong TMC1 expression in cell body [59]. However, neither study looked at the number of TMC1 molecules in OHCs along the cochlear coil.
The unitary MET current can be recorded using a modified MET recording configuration [60, 61]. Normally, the single-channel conductance of MET in basal OHCs would be nearly two fold compared to that of the apical OHCs [61], consistent with the tonotopic principle. If TMC1 is deleted, the gradient of the single MET channel conductance in different locations within the cochlea is nearly the same [62], as is the Ca2+ permeability of OHCs [63]. Mutations in TMC1/2 caused changes in MET channel conductance and dynamics, however, conflicting data have been presented in different reports. Pan et al. observed reduced MET conductance in Tmc1-knockout, Beethoven (M412K mutation of TMC1), and Tmc2-knockout mice compared to control wild-type mice [64]. Beurg et al. also showed reduced MET conductance in the Tmc1-knockout mice, but did not find obvious alteration of the MET conductance in the Beethoven and the Tmc2-knockout mice [63, 65]. It has been proposed that TMC1 mimics the structure of TMEM16A due to sequence similarity [66, 67]. Furthermore, a cysteine substitution test showed that changing certain residues’ electrical properties causes a decrease in macroscopic current and single-channel conductance [67]. Several key amino acids (N404, G411, M412, N447, D528, T532, D569) within the transmembrane domain 4–8 were shown to be critical for MET current, and were proposed to be the pore lining residues [67]. In contrast, Beurg et al. showed that M412K mutation did not change the conductance [49, 65], and that a D569N substitution affects transportation of TMC1 rather than changing conductance [49]. Although these discrepancies may arise from the experimental configurations and preparations, they demonstrate that further studies are required to understand the precise role of TMC1/2 in modulating MET conductance.
The localization of TMC1 expression remains a mystery. Co-immunoprecipitation experiments showed that TMC1 binds PCDH15 via its cytoplasmic and extracellular domains [68, 69], yet a recent structural study failed to detect the complex of PCDH15 and TMC1 by chromatography [32]. When TMC1 was expressed in HEK293T or other cell lines, most of the proteins were trapped in the ER rather than localizing on cytoplasm membrane [51, 70]. It has been speculated that co-expression of other MET components may improve transport through the MET channel; however, no working combinations have been found to localize TMC1 to the cytoplasmic membrane and/or reconstruct MET current in heterogeneous systems (Xiong, unpublished observations). Sequence prediction data suggest that TMC1/2 has ten transmembrane domains [66, 67], and negative stain and cryo-EM data indicate that TMC1 is likely a two-pore structure [67]. More recently, Jia et al. demonstrated that reconstitution of CmTMC1 from the green sea turtle or MuTMC2 from the budgerigar in liposomes exhibits mechanosensitive channel activity [71]. These data are intriguing, but are waiting to be independently confirmed.
LHFPL5 (lipoma HMGIC fusion partner-like 5), also known as TMHS (tetraspan membrane protein of hair cell stereocilia), was characterized from deaf humans [72,73,74] and mice [75, 76]. LHFPL5 has four TM domains and binds with PCDH15′s TM domain and cytosol CR [32, 77]. LHFPL5 is essential for proper transportation and localization of PCDH15 and TMC1 [69, 77]. A previous co-immunoprecipitation experiment showed that LHFPL5 had no detectable interaction with TMC1 [69], but recently a more sensitive single-molecule pull-down assay demonstrated an interaction of LHFPL5 and TMC1, which was disrupted by a deafness mutation (D569N in mouse TMC1) [50]. A Cryo-EM structure of LHFPL5 and PCDH15 fragments (transmembrane domain with 1–4 ECs and CR) clearly showed that the TM region is key for interaction of the two proteins at two copies of each, suggesting a possible assembly core for the MET complex. Lack of LHFPL5 causes a 30% decrease in unitary channel conductance in hair cells [77]. The same mutation caused a greater than 90% reduction of the macroscopic MET current due to the formation of tip links also being affected in Lhfpl5-knockout mice [77]. Intriguingly, an electrophysiological study on unitary MET current revealed that the gradient of MET conductance in OHCs is abolished in Lhfpl5-knockout mice [69], which is reminiscent of the effect of TMC1 [62]. These data suggest that TMC1 and LHFPL5 are essential for establishing a gradient of MET conductance. Mutants of LHFPL5 cause DFNB76/77, which manifests as nonsyndromic hearing loss in humans and both auditory and vestibular dysfunction in mice [72,73,74,75,76, 78].
TMIE (transmembrane inner ear expressed gene) is relatively smaller (153 amino acids) protein with only two putative transmembrane domains. Hair cells with TMIE deletion show no detectable MET current [79]. TMIE mainly binds with the CD of PCDH15-CD2 isoform and LHFPL5, but can also bind CD1 and CD3 isoforms of PCDH15 only with LHFPL5′s participation [79], suggesting that CD2 is more strongly correlated with MET function. TMIE interacts with TMC1 and TMC2 and lack of TMIE severely blocks the proper localization of TMC1 and TMC2 in the hair bundle [59]. However, exogenous overexpression of TMC1 or TMC2 caused them to be found along the stereocilia of Tmie-/- hair cells, but still they were unable to rescue MET, indicating a role for TMIE in MET channel assembly [59]. This observation underlines that physiological stimulation (e.g. hair bundle deflection) and preparation (e.g. hair cell) are important for investigating TMC1-mediated MET, although a liposome study showed that ER-enriched cmTMC1/MuTMC2 could directly provide MET current activated by the membrane stretch [71]. It has been determined that N-terminal part of TMIE affects gating of the MET channel but not binding with TMC1/2, while TMIE can bind TMC1/2 via 80–121 amino acids in its C-terminal [59]. Intriguingly, PIP2 binds to TMIE, at least at sites R82, R85, and R93 [59], suggesting that TMIE is a portal for direct modulation of MET channel kinetics. Deficiency of TMIE causes autosomal recessive nonsyndromic deafness DFNB6 in humans [80,81,82].
Besides these membrane proteins, emerging results have shown that two soluble proteins, CIB2 (Calcium- and integrin-binding protein 2) and TOMT (transmembrane O-methyltransferase, previously named COMT2, catechol-O-methyltransferase 2), functionally participate in the MET machinery, as shown by loss of MET current when either of the two proteins are deleted [83,84,85,86,87]. CIB2 has three EF hand domains that are known for Ca2+-binding capabilities [88]. Co-immunoprecipitation and florescence resonance energy transfer experiments found that CIB2 interacts with TMC1 and TMC2 via an essential sequence of 81–130 amino acids in TMC1 [85]. Mutants in CIB2 cause nonsyndromic hearing loss DFNB48 in humans [86, 89,90,91], and some of these mutants disrupt the interaction between CIB2 and TMC1 [85], however, the precise role of CIB2 in MET is still largely unknown. TOMT does not show stereociliary localization but it interacts with all the LTLD proteins, which have been speculated to transiently transport MET proteins in the cytosol [83, 84]. Surprisingly, the methyltransferase domain is not essential for maintaining TOMT function in hair cells [83]. TOMT deficiency causes nonsyndromic deafness DFNB63 in humans [92,93,94].
MET channel and modulatory factors
Gating kinetics (activation, fast adaptation, and slow adaptation) [95] are functionally modulated by non-genetic factors, including biophysical and pharmacological factors. The roles of Ca2+ and lipids in this process are extremely intriguing due to their potential roles in frequency coding of hair cells. Ca2+ has been recognized as a crucial regulator in MET channel kinetics, although the physiological concentration of Ca2+ is about 0.02–0.1 mM in the scala media. Ca2+ was revealed to modulate the physical status (e.g. stiffness) of the stereocilia [96]. When exposed to a high Ca2+ concentration, the gating compliance in hair bundles is weakened [97]. On the other hand, Ca2+ modulates the MET channel kinetics intracellularly. In one experimental configuration, an influx of Ca2+ modulates the activation and adaptation of the transducer currents in a concentration-dependent way [98,99,100,101,102,103]. It was generally recognized that a higher concentration of Ca2+ induces smaller amplitude waves and increases the amount of adaptation, while the transducer current has larger amplitude and slower adaptation in lower concentrations of Ca2+. In recent years, the role of Ca2+ in fast adaptation has been debated [104,105,106]. The discrepancy regarding fast adaptation may come from the different stimulation configurations—when modified ultrafast stimulation configurations are used to avoid mechanical creep, either stiff probe [104] or fluid jet [106] can elicit MET currents with fast adaptation at a time constant of dozens microseconds.
In recent years, lipids have drawn significant attention for their promising participation in MET. It has been shown that the lipid composition is complicated and compartmentalized [107, 108]. Some lipid-linked proteins, including PTPRQ, Radixin, and Espin, show strong effects on maturation and maintenance of hair bundles [108,109,110]. Some phospholipids, such as PIP2, can bind to ion channels and regulate their kinetics [111]. Hirono et al. proposed that in vestibular hair cells, PIP2 regulates channel dynamics, including adaptation, partially via binding Myo1c IQ domains [107]. However, in cochlear hair cells, the MET channel is located at the low end of the tip link [30]. This may use a different gating mechanism to achieve improved temporal sensitivity as suggested recently by the following evidence: PIP2 directly modulates kinetics of the MET channel (e.g. fast adaptation and open probability) [112, 113], and slow adaptation may come from the Myosin motors close to the MET channel instead of the upper insertion site of the tip link [114]. These apparent discrepancies need to be resolved by further studies.
MET channel modulation and sound acquisition
The sound intensities and frequencies acquired in mammals occur across a very broad range. For example, frequencies audible to humans range from 20 Hz to 20 kHz, yet humans can distinguish intensity at 0 dB SPL (e.g. 20 μPa for 1 kHz pure tone) if ambient noise is low enough. The MET channel properties are modulated by the MET molecules and multiple biochemical factors (e.g. Ca2+ and PIP2) along the cochlear coil, which coincide with requirements of the sound acquisition in many ways.
Compared to other mechanosensitive channels, the hair-cell MET channel has large conductance, ultrafast open and close kinetics, and elastic gating springs (at the extracellular tip link and likely the intracellular link). Based on the amount of stereocilia and tip links, it has been estimated that one tip link connects with two MET channels [61]. From apical OHCs to basal OHCs, the number of tip links does not increase significantly, however, the whole-cell macroscopic current and single MET channel conductance increases drastically (145–210 pS in rats), which is not the case for IHCs [61]. Consistent with this finding, it has been shown in a transgenic mouse line that the number of TMC1 proteins increases along the cochlear coil [57]. The TMC1 puncta were measured at an average of 8–20 molecules per MET site, further suggesting that the MET channels may be more than 2 per tip link [57]. There may be a minimum requirement of MET molecules for assembly of the functional MET channel with baseline conductance. TMC1 and LHFPL5 similarly modulate the gradient of MET conductance and Ca2+ permeability, suggesting that the two proteins have at least one important role in expanding the physical properties of MET complex assembly, such as the number of molecules and space of scaffold (Fig. 2c). Along the cochlear coil, the time constant of fast adaptation becomes shorter from LF OHCs to HF OHCs [100, 115], which explains the correlated responsiveness of OHCs to their representative frequencies. The larger conductance in HF OHCs may also gain enough ionic influx that compensates for the shorter time constant of adaptation to activate the OHC electromotility. This likely results from the integrity of hair bundle organization, at least partly, as TMC1/2 also defines the maturation of the hair bundles, that is, increasing the number of TMC1/2 results in stereocilia forming the three typical rows [57]. Similarly, the typical three-row morphology is missing in Lhfpl5- and Tmie- deficient mice [77, 79]. These results also suggest that normal functionality of the MET channel defines the maturation of hair bundle morphology, including the staircase-like organization [116]. However, the temporal order of MET deficiency and hair bundle disorganization needs to be defined when these key MET molecules are disrupted. There is always a resting tension of stereocilia in OHCs, which induce a background MET current (which can be blocked by aminoglycosides); this is called the resting open probability (Po) of the MET channels [3]. Meanwhile, a leak conductance (which cannot be inhibited by blocking MET channels) exists in OHCs, which is functionally coupled with TMC1 protein [117]. Both Po and leak conductance increase along the cochlear coil, which also follows the frequency-dependent pattern. These two background currents together may set OHCs at an intermediate resting potential to best oscillate with the sound-induced vibration [3]. Ca2+-dependent modulation is also important for MET functionality, including tip-link tension, Ca2+ homeostasis and Ca2+ dependent adaptation. Some of the processes may rely on the Ca2+-binding protein CIB2, a critical binding partner of TMC1/2, however, this needs further investigation (Fig. 2c). As a classic channel modulator, PIP2 is also an indispensable factor for regulation of MET channel kinetics, including channel open probability, channel conductance, and fast adaptation [112, 113]. Reduced PIP2 synthesis impairs high-frequency acquisition, which may be induced by Ca2+ signaling, but its regulation on MET cannot be ruled out [118]. Intriguingly, TMIE modulates MET channel kinetics/properties by interacting with PIP2 [59], which provides a mechanistic insight into the PIP2-dependent MET channel modulation (Fig. 2c). Thus, represented by PIP2, lipid bilayers may generally modulate channel properties in a frequency-dependent way [119]. This concept is worthy of thorough study.
Diverse functions of TMC1/2
As described above, TMC1 and TMC2 play important roles in MET function and maintenance. They have been considered as candidates for the pore-forming subunit of the MET channel in mammals. In parallel, recent studies have showed that TMC1/2 and their orthologues have multiple physiological functions not limited to MET. In this section, by discussing the diverse functions of TMC1/2, we hope to provide a more complete scenario and present a possible “core” principle to unify TMC1′s molecular function that could be responsible for its diverse physiological consequences.
TMC1 and leak conductance
Liu et al. have recently shown that TMC1, but not TMC2, mediates a frequency-dependent leak conductance in cochlear hair cells [117]. This leak coexists with the spontaneous opening of the MET channel at resting status [3]. Interestingly, it has been reported that breakup of tip links enhances a leak current, which may be induced by constantly opened MET channels [120].
The TMC1-mediated leak conductance has different channel properties from the MET channel. Dihydrostreptomycin and other aminoglycosides that block MET current also block the leak channel but at much higher concentration (~ 100 fold) [117]. Non-selective cation channel blockers, such as Gd3+ and La3+, block the leak channel with ~ 100 fold higher KD compare to the MET channel [117]. Most strikingly, the leak channel can be blocked by high concentrations of Ca2+ (IC50 ~ 1.3 mM), which contrasts the high Ca2+ permeability of the MET channel. When extracellular Ca2+ concentration was increased to 10 mM, more than 60% of the leak conductance was blocked [117]. Amino-acid substitution analysis showed that the MET channel and the leak channel use different key amino acids in TMC1 for the two types of conductance [117], which further suggests that TMC1 may not be the pore for the leak channel. The identity of the protein in the channel that confers leak conductance is yet to be defined.
Neonatal IHCs possess strong spontaneous action potentials in the first 2 weeks, which is essential for the wiring of the auditory pathway from IHCs to spiral ganglion neurons before the onset of hearing. IHCs lacking TMC1 showed hyperpolarized resting membrane potential and diminished spontaneous action potential firing, suggesting a role for TMC1 and leak conductance in electrophysiological maturation of hair cells [48, 117].
Correlation of leak conductance and MET
Leak conductance and MET conductance are correlated both molecularly and functionally. The pattern of the leak conductance is synchronous with the MET conductance during the development in the first neonatal week. Similar to the spatiotemporal pattern of MET conductance, leak conductance increases in the OHCs from P0 to P7 and from the apex to the base. However, it is hard to distinguish which conductance emerges first—the leak conductance or the MET conductance. The macroscopic MET current and the unitary MET channel conductance were evaluated when hair cells were treated with 35 mM Ca2+ (a concentration that almost completely blocks leak conductance) or Tmc1 was knocked out [117]; this showed that the tonotopic gradient of the MET channel conductance was removed. This observation is exactly consistent with the evidence that the gradient of the MET channel conductance is lost in Tmc1-knockout mice and Lhfpl5-knockout mice [69, 117]. In parallel, leak conductance was largely abolished when TMC1 or LHFPL5 was deficient (and resulted in further disruption to the resting membrane potential, the excitability, the dynamics of the receptor potential, and the tonotopic gradient of the MET conductance).
How does this happen in hair cells? Removal of the hair bundle resulted in loss of the MET and leak currents [117], suggesting that TMC1 and LHFPL5 mediate the leak conductance in hair bundles. These results formulate a scenario where TMC1 and LHFPL5 may provide quantitative control of both the MET channel and the leak channel. Normally, if there are free TMC1 and LHFPL5 that are not caught by the LTLD, they would assist the formation of the yet-to-be-defined leak channel (Fig. 3a), as the number of TMC1 molecules increases from 8 per apical OHC to 20 per basal OHC [57]. Without either TMC1 or LHFPL5, the MET channel could still be assembled with the key components, such as TMC2, TMIE and CIB2, but the leak channel formation would be disrupted. However, further evidence is needed to support this hypothesis.
TMC1 mediates a leak conductance. a Schematic showing two scenarios in hair cells for generating MET and leak currents, with or without TMC1. The angled line between components indicates an undefined assembly mechanism. b Working model for effector cells in physiological pathways that utilize TMC1 to regulate cell excitability and circuit efficacy
TMCs in animal physiology
The Tmc gene is relatively conserved and is found in multiple species. Recently, TMC1 and its orthologues have been extensively studied in worms and flies, showing a variety of physiological functions in mice beyond the roles in MET and hearing (see review from Yue et al.) [121]. In Caenorhabditis elegans, TMCs participate in at least three physiological processes, including the excitability of muscles in development and sexual behavior [122], the alkaline sensitivity of ASH nociceptive neurons [123], and the excitability of HSN and VM cells for egg laying [124]. While these efforts have failed to demonstrate that TMCs in worms are mechanically gated ion channels, Tang et al. recently pointed out that UNC-44/ankyrin works as an intracellular tether to gain mechanosensitivity for TMCs in OLQ neurons [125]. In Drosophila melanogaster, TMCs are associated with several pathways linked with mechanosensation and motor function, including the class I and class II dendritic arborization neurons and bipolar dendrite neurons that are critical for larval locomotion [126], and md-L neurons that sense food texture [127] and proprioceptor-mediated direction selectivity [128, 129].
These pieces of evidence raise the question of how TMCs mediate such a variety of functions across different species. TMCs likely form and/or associate with channels and modulate the channel properties. For example, mammalian TMC1 and TMC2 may function as integrated components of the MET channel in hair cells, and TMC1 likely mediates a leak conductance to regulate the MET dynamics [117]. This mechanism is consistent with a comparable observation that TMC1-mediated leak conductance regulates the excitability of the HSN neurons and VM muscle cells, and thus influences egg laying behavior in C. elegans [124]. This scenario could also occur in other systems, and it is tempting to propose that the excitability of cells and neural circuits is the target for TMCs to regulate physiological processes from sensory transduction to motor function in organisms (Fig. 3b).
Outlooks
Several lines of evidence have described the ways in which hair-cell MET channel transduces sound-induced vibration and encodes acoustic information. However, there are still a couple of key questions attracting researchers’ attention; these questions are the foundation for us to ultimately understand the full mechanism of auditory transduction.
Structural assembly of MET channel complex
The structure of tip link was first found by classical scanning EM and transmission EM [130, 131], which provided the seminal insight into the auditory MET assembly. A live imaging study affirmed that the MET channel functionally gates at LTLD toward the hair bundle deflection [30]. As a double-helical dimer, PCDH15 gains putative elasticity (potential extension about 35 Å) and enhanced gating force [31, 32]. PCDH15 and LHFPL5 form a robust complex via their transmembrane domains [32]. As the candidate channel protein, TMC1 is likely to fold into a two-pore structure and form dimers [67, 71]. With these pieces of structural evidence, we speculate that TMC1, TMC2, and TMIE are accumulated around the PCDH15-LHFPL5 transmembrane core in high numbers (Fig. 2b). It remains to be discovered whether the proximal-to-membrane ‘collar’-like EL/PICA domain mediates channel assembly. Based on results from the co-immunoprecipitation and single-cell pull-down experiments, it is possible that PCDH15 and LHFPL5 directly binds to the MET channel pore (likely TMC1 or TMC2) [50, 68]. Another possibility is that PCDH15 binds with TMCs through extended handles formed by LHFPL5 and TMIE with a stoichiometric ratio (Fig. 2c), which fits the observed multiple subconductance of MET channel in auditory hair cells [57, 64]. However, both models need further structural study and confirmation, particularly to verify, how TMC1 is embedded in the MET complex. On the other hand, it is equally important to describe the structures of the intracellular spring and the upper tip-link density to understand MET channel assembly in a more integrated way.
Gating mechanisms of MET channel
With the limited structural information available, the gating mechanisms underlying the MET channel remain somewhat unclear. Detailed structure–function analysis is required as this approach has provided us with deep insight into the gating mechanisms of the MET channel. Two aspects in particular need further study: pore lining principle and gating principle. Based on the evidence presented by Pan et al. [67], a more detailed analysis needs to be done by genetically engineering TMC1. The discovery reported by Jia et al., that CmTMC1 could be reconstructed in liposomes with mechanotransduction capability [71], need to be followed by systematic structure–function analysis in cell lines. A recent study on worms suggests that Ankyrin is likely the intracellular spring for the channel gating [125], which mirrors an anticipated tethering model of the MET channel gating mechanism from mammals (Fig. 2a). However, whether mammals use a similar isoform remains to be verified. Another question is how the gating force is transmitted to the channel pore, while maintaining the ultrafast speed of kinetics. The force may be simply conducted by stretch between the extracellular spring (tip link) and intracellular spring. For the well-studied mechanosensitive channels Piezos, they work as a single molecule but with much slower gating kinetics [132]. Thus, the question of how the MET complex uses such a large set of proteins to fulfill the transduction requirement at a microsecond timescale remains to be answered.
References
Kandel ER et al (2012) Principles of neural science. McGraw-Hill Education / Medical; 5th edn (October 26, 2012), p 1760
Robles L, Ruggero MA (2001) Mechanics of the mammalian cochlea. Physiol Rev 81(3):1305–1352
Fettiplace R, Kim KX (2014) The physiology of mechanoelectrical transduction channels in hearing. Physiol Rev 94(3):951–986
Pietsch M et al (2017) Spiral form of the Human Cochlea results from spatial constraints. Sci Rep 7(1):7500
Von Bekesy G (1947) The variation of phase along the basilar membrane with sinusoidal vibrations. J Acoust Soc Am 19(3):452–460
Hubbard A (1993) A traveling-wave amplifier model of the cochlea. Science 259(5091):68–71
Lee HY et al (2015) Noninvasive in vivo imaging reveals differences between tectorial membrane and basilar membrane traveling waves in the mouse cochlea. Proc Natl Acad Sci U S A 112(10):3128–3133
Nankali A et al (2020) A role for tectorial membrane mechanics in activating the cochlear amplifier. Sci Rep 10(1):17620
Sellon JB et al (2015) Longitudinal spread of mechanical excitation through tectorial membrane traveling waves. Proc Natl Acad Sci U S A 112(42):12968–12973
Zheng J et al (2000) Prestin is the motor protein of cochlear outer hair cells. Nature 405(6783):149–155
Liberman MC et al (2002) Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419(6904):300–304
Kaltenbach JA, Falzarano PR (1994) Postnatal development of the hamster cochlea. I. Growth of hair cells and the organ of Corti. J Comp Neurol 340(1):87–97
Kaltenbach JA, Falzarano PR, Simpson TH (1994) Postnatal development of the hamster cochlea. II. Growth and differentiation of stereocilia bundles. J Comp Neurol 350(2):187–198
Soons JA et al (2015) Cytoarchitecture of the mouse organ of corti from base to apex, determined using in situ two-photon imaging. J Assoc Res Otolaryngol 16(1):47–66
Duvall AJ 3rd, Flock A, Wersall J (1966) The ultrastructure of the sensory hairs and associated organelles of the cochlear inner hair cell, with reference to directional sensitivity. J Cell Biol 29(3):497–505
Engstrom H, Engstrom B (1978) Structure of the hairs on cochlear sensory cells. Hear Res 1(1):49–66
Hudspeth AJ (2014) Integrating the active process of hair cells with cochlear function. Nat Rev Neurosci 15(9):600–614
Fettiplace R, Hackney CM (2006) The sensory and motor roles of auditory hair cells. Nat Rev Neurosci 7(1):19–29
Wangemann P (2006) Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 576(Pt 1):11–21
Zdebik AA, Wangemann P, Jentsch TJ (2009) Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology (Bethesda) 24:307–316
Gill SS, Salt AN (1997) Quantitative differences in endolymphatic calcium and endocochlear potential between pigmented and albino guinea pigs. Hear Res 113(1–2):191–197
Conlee JW, Bennett ML (1993) Turn-specific differences in the endocochlear potential between albino and pigmented guinea pigs. Hear Res 65(1–2):141–150
Corey DP, Hudspeth AJ (1979) Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281(5733):675–677
Kazmierczak P et al (2007) Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449(7158):87–91
Siemens J et al (2004) Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428(6986):950–955
Sollner C et al (2004) Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature 428(6986):955–959
Elledge HM et al (2010) Structure of the N terminus of cadherin 23 reveals a new adhesion mechanism for a subset of cadherin superfamily members. Proc Natl Acad Sci U S A 107(23):10708–10712
Sotomayor M et al (2010) Structural determinants of cadherin-23 function in hearing and deafness. Neuron 66(1):85–100
Sotomayor M et al (2012) Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature 492(7427):128–132
Beurg M et al (2009) Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci 12(5):553–558
Dionne G et al (2018) Mechanotransduction by PCDH15 relies on a novel cis -dimeric architecture. Neuron 99(3):480
Ge J et al (2018) Structure of mouse protocadherin 15 of the stereocilia tip link in complex with LHFPL5. Elife. https://doi.org/10.7554/eLife.387707
Bartsch TF et al (2019) Elasticity of individual protocadherin 15 molecules implicates tip links as the gating springs for hearing. Proc Natl Acad Sci U S A 116(22):11048–11056
Ahmed ZM et al (2006) The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci 26(26):7022–7034
Webb SW et al (2011) Regulation of PCDH15 function in mechanosensory hair cells by alternative splicing of the cytoplasmic domain. Development 138(8):1607–1617
Maeda R et al (2017) Functional analysis of the transmembrane and cytoplasmic domains of Pcdh15a in Zebrafish hair cells. J Neurosci 37(12):3231–3245
Alagramam KN et al (2001) The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet 27(1):99–102
Alagramam KN et al (2001) Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet 10(16):1709–1718
Bolz H et al (2001) Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet 27(1):108–112
Bork JM et al (2001) Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet 68(1):26–37
Di Palma F et al (2001) Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet 27(1):103–107
Kurima K et al (2002) Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet 30(3):277–284
Kurima K et al (2003) Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis☆. Genomics 82(3):300–308
Vreugde S et al (2002) Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet 30(3):257–258
de Heer AM et al (2011) Progressive sensorineural hearing loss and normal vestibular function in a Dutch DFNB7/11 family with a novel mutation in TMC1. Audiol Neurootol 16(2):93–105
Kitajiri SI et al (2007) Identities, frequencies and origins of TMC1 mutations causing DFNB7/B11 deafness in Pakistan. Clin Genet 72(6):546–550
Tlili A et al (2008) TMC1 but not TMC2 is responsible for autosomal recessive nonsyndromic hearing impairment in Tunisian families. Audiol Neurootol 13(4):213–218
Marcotti W et al (2006) Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea. J Physiol 574(Pt 3):677–698
Beurg M et al (2019) A Tmc1 mutation reduces calcium permeability and expression of mechanoelectrical transduction channels in cochlear hair cells. Proc Natl Acad Sci U S A 116(41):20743–20749
Yu X et al (2020) Deafness mutation D572N of TMC1 destabilizes TMC1 expression by disrupting LHFPL5 binding. Proc Natl Acad Sci U S A 117(47):29894–29903
Kawashima Y et al (2011) Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest 121(12):4796–4809
Asai Y et al (2018) Transgenic Tmc2 expression preserves inner ear hair cells and vestibular function in mice lacking Tmc1. Sci Rep 8(1):12124
Nakanishi H et al (2018) Tmc2 expression partially restores auditory function in a mouse model of DFNB7/B11 deafness caused by loss of Tmc1 function. Sci Rep 8(1):12125
Lelli A et al (2009) Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophysiol 101(6):2961–2973
Waguespack J et al (2007) Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci 27(50):13890–13902
Kurima K et al (2015) TMC1 and TMC2 localize at the site of mechanotransduction in mammalian inner ear hair cell Stereocilia. Cell Rep 12(10):1606–1617
Beurg M et al (2018) Variable number of TMC1-dependent mechanotransducer channels underlie tonotopic conductance gradients in the cochlea. Nat Commun 9(1):2185
Li X et al (2019) Localization of TMC1 and LHFPL5 in auditory hair cells in neonatal and adult mice. FASEB J 33(6):6838–6851
Cunningham CL et al (2020) tmie defines pore and gating properties of the mechanotransduction channel of mammalian cochlear hair cells. Neuron 107(1):126
Ricci AJ, Crawford AC, Fettiplace R (2003) Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40(5):983–990
Beurg M et al (2006) A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26(43):10992–11000
Beurg M, Kim KX, Fettiplace R (2014) Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J Gen Physiol 144(1):55–69
Kim KX, Fettiplace R (2013) Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel-like proteins. J Gen Physiol 141(1):141–148
Pan B et al (2013) TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79(3):504–515
Beurg M, Goldring AC, Fettiplace R (2015) The effects of Tmc1 Beethoven mutation on mechanotransducer channel function in cochlear hair cells. J Gen Physiol 146(3):233–243
Ballesteros A, Fenollar-Ferrer C, Swartz KJ (2018) Structural relationship between the putative hair cell mechanotransduction channel TMC1 and TMEM16 proteins. Elife. https://doi.org/10.7554/eLife.38433
Pan B et al (2018) TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron 99(4):736-753.e6
Maeda R et al (2014) Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc Natl Acad Sci U S A 111(35):12907–12912
Beurg M et al (2015) Subunit determination of the conductance of hair-cell mechanotransducer channels. Proc Natl Acad Sci U S A 112(5):1589–1594
Labay V et al (2010) Topology of transmembrane channel-like gene 1 protein. Biochemistry 49(39):8592–8598
Jia Y et al (2019) TMC1 and TMC2 proteins are pore-forming subunits of mechanosensitive ion channels. Neuron. https://doi.org/10.1016/j.neuron.2019.10.017
Tlili A et al (2005) A novel autosomal recessive non-syndromic deafness locus, DFNB66, maps to chromosome 6p21.2–22.3 in a large Tunisian consanguineous family. Hum Hered 60(3):123–128
Kalay E et al (2006) Mutations in the lipoma HMGIC fusion partner-like 5 (LHFPL5) gene cause autosomal recessive nonsyndromic hearing loss. Hum Mutat 27(7):633–639
Shabbir MI et al (2006) Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J Med Genet 43(8):634–640
Longo-Guess CM et al (2005) A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci U S A 102(22):7894–7899
Longo-Guess CM et al (2007) Targeted knockout and lacZ reporter expression of the mouse Tmhs deafness gene and characterization of the hscy-2J mutation. Mamm Genome 18(9):646–656
Xiong W et al (2012) TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151(6):1283–1295
Cosetti M et al (2008) Unique transgenic animal model for hereditary hearing loss. Ann Otol Rhinol Laryngol 117(11):827–833
Zhao B et al (2014) TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron 84(5):954–967
Mitchem KL et al (2002) Mutation of the novel gene Tmie results in sensory cell defects in the inner ear of spinner, a mouse model of human hearing loss DFNB6. Hum Mol Genet 11(16):1887–1898
Naz S et al (2002) Mutations in a novel gene, TMIE, are associated with hearing loss linked to the DFNB6 locus. Am J Hum Genet 71(3):632–636
Santos RL et al (2006) Novel sequence variants in the TMIE gene in families with autosomal recessive nonsyndromic hearing impairment. J Mol Med (Berl) 84(3):226–231
Cunningham CL et al (2017) The murine catecholamine methyltransferase mTOMT is essential for mechanotransduction by cochlear hair cells. Elife. https://doi.org/10.7554/eLife.24318
Erickson T et al (2017) Integration of Tmc1/2 into the mechanotransduction complex in zebrafish hair cells is regulated by Transmembrane O-methyltransferase (Tomt). Elife. https://doi.org/10.7554/eLife.28474
Giese APJ et al (2017) CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction in auditory hair cells. Nat Commun 8(1):43
Michel V et al (2017) CIB2, defective in isolated deafness, is key for auditory hair cell mechanotransduction and survival. EMBO Mol Med 9(12):1711–1731
Wang Y et al (2017) Loss of CIB2 causes profound hearing loss and abolishes mechanoelectrical transduction in mice. Front Mol Neurosci 10:401
Blazejczyk M et al (2009) Biochemical characterization and expression analysis of a novel EF-hand Ca2+ binding protein calmyrin2 (Cib2) in brain indicates its function in NMDA receptor mediated Ca2+ signaling. Arch Biochem Biophys 487(1):66–78
Riazuddin S et al (2012) Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet 44(11):1265–1271
Jan A (2013) Mutations in CIB2 calcium and integrin-binding protein disrupt auditory hair cell calcium homeostasis in Usher syndrome type 1J and non-syndromic deafness DFNB48. Clin Genet 83(4):317–318
Booth KT et al (2017) Variants in CIB2 cause DFNB48 and not USH1J. Clin Genet 93(4):812–821
Ahmed ZM et al (2008) Mutations of LRTOMT, a fusion gene with alternative reading frames, cause nonsyndromic deafness in humans. Nat Genet 40(11):1335–1340
Du X et al (2008) A catechol-O-methyltransferase that is essential for auditory function in mice and humans. Proc Natl Acad Sci USA 105(38):14609–14614
Ross CJ et al (2009) Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet 41(12):1345–1349
Maoiléidigh OD, Ricci AJ (2019) A bundle of mechanisms: inner-ear hair-cell mechanotransduction. Trends Neurosci 42(3):221–236
Marquis RE, Hudspeth AJ (1997) Effects of extracellular Ca2+ concentration on hair-bundle stiffness and gating-spring integrity in hair cells. Proc Natl Acad Sci U S A 94(22):11923–11928
Tinevez JY, Julicher F, Martin P (2007) Unifying the various incarnations of active hair-bundle motility by the vertebrate hair cell. Biophys J 93(11):4053–4067
Beurg M et al (2008) The actions of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophys J 94(7):2639–2653
Hacohen N et al (1989) Regulation of tension on hair-cell transduction channels: displacement and calcium dependence. J Neurosci 9(11):3988–3997
Kennedy HJ et al (2003) Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci 6(8):832–836
Kimitsuki T, Ohmori H (1992) The effect of caged calcium release on the adaptation of the transduction current in chick hair cells. J Physiol 458:27–40
Ricci AJ, Fettiplace R (1997) The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells. J Physiol 501(Pt 1):111–124
Ricci AJ, Wu YC, Fettiplace R (1998) The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci 18(20):8261–8277
Peng AW, Effertz T, Ricci AJ (2013) Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron 80(4):960–972
Corns LF et al (2014) Calcium entry into stereocilia drives adaptation of the mechanoelectrical transducer current of mammalian cochlear hair cells. Proc Natl Acad Sci U S A 111(41):14918–14923
Caprara GA et al (2019) Hair bundle stimulation mode modifies manifestations of mechanotransduction adaptation. J Neurosci 39(46):9098–9106
Hirono M et al (2004) Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron 44(2):309–320
Zhao H et al (2012) Large membrane domains in hair bundles specify spatially constricted radixin activation. J Neurosci 32(13):4600–4609
Goodyear RJ et al (2003) A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J Neurosci 23(27):9208–9219
Sekerkova G et al (2006) Espins and the actin cytoskeleton of hair cell stereocilia and sensory cell microvilli. Cell Mol Life Sci 63(19–20):2329–2341
Suh BC, Hille B (2008) PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37:175–195
Peng AW et al (2016) Adaptation independent modulation of auditory hair cell mechanotransduction channel open probability implicates a role for the lipid bilayer. J Neurosci 36(10):2945–2956
Effertz T et al (2017) Phosphoinositol-4,5-bisphosphate regulates auditory hair-cell mechanotransduction-channel pore properties and fast adaptation. J Neurosci 37(48):11632–11646
Caprara GA, Mecca AA, Peng AW (2020) Decades-old model of slow adaptation in sensory hair cells is not supported in mammals. Sci Adv 6(33):eabb4922
Ricci AJ et al (2005) The transduction channel filter in auditory hair cells. J Neurosci 25(34):7831–7839
Krey JF et al (2020) Mechanotransduction-Dependent Control of Stereocilia Dimensions and Row Identity in Inner Hair Cells. Curr Biol 30(3):442-454e7
Liu S et al (2019) TMC1 is an essential component of a leak channel that modulates tonotopy and excitability of auditory hair cells in mice. Elife. https://doi.org/10.7554/eLife.47441
Rodriguez L et al (2012) Reduced phosphatidylinositol 4,5-bisphosphate synthesis impairs inner ear Ca2+ signaling and high-frequency hearing acquisition. Proc Natl Acad Sci U S A 109(35):14013–14018
Gianoli F, Risler T, Kozlov AS (2017) Lipid bilayer mediates ion-channel cooperativity in a model of hair-cell mechanotransduction. Proc Natl Acad Sci U S A 114(51):E11010
Meyer J et al (1998) Evidence for opening of hair-cell transducer channels after tip-link loss. J Neurosci 18(17):6748–6756
Yue X et al (2019) Distinct functions of TMC channels: a comparative overview. Cell Mol Life Sci 76(21):4221–4232
Zhang L et al (2015) TMC-1 attenuates C elegans development and sexual behaviour in a chemically defined food environment. Nat Commun 6:6345
Wang X et al (2016) TMC-1 mediates alkaline sensation in C. elegans through nociceptive neurons. Neuron 91(1):146–154
Yue X et al (2018) TMC proteins modulate egg laying and membrane excitability through a background leak conductance in C. elegans. Neuron 97:1–15
Tang Y-Q et al (2020) Ankyrin is an intracellular tether for TMC mechanotransduction channels. Neuron 107(4):759
Guo Y et al (2016) Transmembrane channel-like (tmc) gene regulates Drosophila larval locomotion. Proc Natl Acad Sci U S A 113(26):7243–7248
Zhang YV et al (2016) The basis of food texture sensation in Drosophila. Neuron 91(4):863–877
He L et al (2019) Direction selectivity in Drosophila proprioceptors requires the mechanosensory channel Tmc. Curr Biol 29(6):945-956e3
Vaadia RD et al (2019) Characterization of proprioceptive system dynamics in behaving drosophila larvae using high-speed volumetric microscopy. Curr Biol 29(6):935–944
Pickles JO, Comis SD, Osborne MP (1984) Cross-links between Stereocilia in the Guinea-Pig organ of corti, and their possible relation to sensory transduction. Hear Res 15(2):103–112
Furness DN, Hackney CM (1985) Cross-Links between Stereocilia in the Guinea-Pig Cochlea. Hear Res 18(2):177–188
Xiao B (2020) Levering mechanically activated piezo channels for potential pharmacological intervention. Annu Rev Pharmacol Toxicol 60:195–218
Marcotti W et al (2003) Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol 548(Pt 2):383–400
Funding
This work was supported by the National Natural Science Foundation of China (31522025, 31571080, 81873703, and 31861163003), Beijing Municipal Science and Technology Commission (Z181100001518001), and a startup fund from the Tsinghua-Peking Center for Life Sciences. W.X. is a CIBR cooperative investigator (2020-NKX-XM-04) funded by the Open Collaborative Research Program of Chinese Institute for Brain Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, S., Wang, S., Zou, L. et al. Mechanisms in cochlear hair cell mechano-electrical transduction for acquisition of sound frequency and intensity. Cell. Mol. Life Sci. 78, 5083–5094 (2021). https://doi.org/10.1007/s00018-021-03840-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03840-8