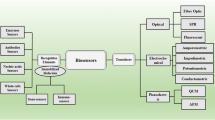
Abstract
Sandwich assay biosensors make it possible to detect bacterial pathogens and cancer cells at extremely low level. In this chapter, we have summarized the recent developments of sandwich assay for pathogen and whole-cell detection using a variety of techniques. In particular, we highlighted some of the most common techniques in sandwich assay biosensors such as optics-based detection, electrochemistry-based detection, and mechanics-based detection.
The original version of this chapter was revised: Foreword has been included and authors’ affiliations have been updated. The erratum to this chapter is available at https://doi.org/10.1007/978-981-10-7835-4_13
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
In the past several years, there are significant developments of diagnostic techniques for public health, food and water safety, and homeland security [1, 2]. In particular, plenty of methods and techniques have vastly advanced the detection of pathogens, cancer cells, and other disorders, namely phenotypic, immunological, molecular, and genotypic protocols [3,4,5]. Nevertheless, many of these techniques are conventional, laboratory-based diagnostic methods, which require long processing time, specialized equipment and are tedious to perform. As such, the demand for sensitive, selective, rapid, and cost-effective detection of bacterial pathogens and cancer cells is highly increasing [6,7,8].
The sandwich assay biosensors can fill this role because they are highly specific and reproducible to a variety of biological structures, organisms, and processes [9,10,11]. Moreover, easy signal amplification of sandwich assay promotes them to be with great sensitivity compared with other platforms. As such, this assay has been extensively applied to a variety of analytes, such as metal ions, small molecules, nucleic acids, proteins, and bacterial pathogens and cells [12, 13].
In terms of pathogen and cancer cell sensing, one of the well-established strategies is the detection of their biomolecule components. These components, including DNA [14, 15], RNA [16, 17], proteins [18], and exotoxins [19], have been successfully detected at exceedingly low levels by sandwich assay using polymerase chain reaction (PCR) or immunoassays techniques. The major disadvantage of this component-detecting strategy is the requirement for sample pre-enrichment, sample processing, expensive instruments, and commercial reagents. To solve this issue, sandwich assay biosensors for whole-cell detection, again, without any sample processing, are much more desirable for accurate, rapid, and cost-effective testing especially for the point-of-care detection. Additionally, whole-cell detection approach also provides the possibility of real-time monitoring of the activities of living pathogens and cancerous cells, which helps in elucidation of their functions in a developmental manner [20].
Significant efforts have been devoted in the development of whole-cell detection based on sandwich assay. In a typical sandwich assay, such as the enzyme-linked immunosorbent assay (ELISA) [21], two antibodies bind to one single target at two distinct sites to form a sandwich complex, which leads to highly specific recognition. Upon the sandwich formation, depending on the enzyme catalytic or amplified signaling mechanism, a measurable change in signals is produced and thus the target whole cell can be detected. The utilization of molecular recognition agents such as antibody, aptamer, polypeptide, and bacteriophage has been employed successfully for specifical detection of whole cells [22]. Likewise, some small molecular compounds, such as antibiotics and carbohydrates have been employed as recognition receptor for whole cells [23, 24]. The signaling mechanism has also been extensively expanded along with the development of nanomaterials. In recent years, many promising techniques have been developed and applied to nondestructive whole-cell sensing, such as optical techniques [including colorimetric analysis, fluorescence, surface plasma resonance (SPR)], electrochemical and mechanical techniques. As the sandwich assays for whole-cell sensing is vast and new works generate constantly, here, we intend to summarize comprehensively the latest advances of this field in general, in support to spur additional ideas in this area.
11.2 Optical Detection
As one of the most popular protocols, the optical whole-cell biosensor combining the nondestructive recognition event with optical measurements is of particular interests due to the highly specific bonding, profound signal amplification, visible radiation, and low detection limit. As such, it has been developed vastly based on a variety of spectroscopic techniques. Herein we discuss the colorimetric analysis, fluorescence, and SPR, which are most commonly used for whole-cell detections.
11.2.1 Colorimetric Analysis
The colorimetric analysis has attracted a lot of interest due to its visible radiation, low cost, quick feedback, and the possibility of avoiding any expensive instrument. Current studies on pathogens, cancer cells sensing by colorimetric methods aim for achieving a more specific, easy to use, more portable, and low-cost analytical system.
Toward this goal, many research works focused on sandwich assay-based biosensor coupled with nanomaterials for signal amplification [25]. For example, Zhang et al. have developed a nanoparticle cluster (NPC)-based amplification biosensor for the detection of Listeria monocytogenes, which is a highly pathogenic foodborne bacterial (Fig. 11.1) [26]. Specifically, they used a glycopeptide antibiotic, vancomycin (Van) as the first recognition agent to capture the cell wall of the pathogen. The aptamer-labeled Fe3O4 NPC was used as the signal amplification probe, which was also recognized to the pathogen. The sandwich recognition showed high specificity, in which the NPC-based method displayed higher sensitivity than the NP-based method due to its improved catalytic activity [27, 28]. Using this new method, the L. monocytogenes cells could be detected within a linear range of 5.4 × 103 to 108 CFU/mL and a visual limit of detection (LOD) of 5.4 × 103 CFU/mL [26]. Likewise, Jain et al. have recently demonstrated a surface aminated polycarbonate membrane (PC)-enhanced sandwich assay for Salmonella typhi detection. A detection limit of 2 × 103 cells/ml of bacteria has been achieved with high immobilization efficiency [29].

(Reprinted with the permission from Ref. [26]. Copyright 2016 Elsevier)
Schematic representation for the preparation of Fe3O4 NPC by cross-linking the individual mother nanoparticle with poly-L-lysine (a), the principle of the Fe3O4 NP-based biosensor (b), and the Fe3O4 NPC-catalyzed signal amplification biosensor (c)
Gold nanoparticles (AuNPs) have been applied as color developing moiety in numerous colorimetric bioassays [30, 31]. The aggregation of AuNPs usually lead to a distinct color change from red to blue and thus promise for target detection including whole cells [32]. Lu and coworkers have developed a modified AuNPs nanoprobe for colorimetric signal amplification in the detection of Salmonella enterica. The optimized LOD is 103 CFU/mL, and their technique has been demonstrated the success of target detection in milk samples with high degree of accuracy (>90%) [33]. Xiong and coworkers have recently established an improved sandwich plasmonic ELISA (pELISA) for determination of L. monocytogenes by combining the sandwich ELISA technique with a novel signal-generation mechanism, the catalase (CAT)-mediated growth of plasmonic AuNPs [34], exhibiting an ultralow LOD value at 8 × 100 CFU/mL (Fig. 11.2) [35].

(Reprinted with the permission from Ref. [35]. Copyright 2015 American Chemical Society)
Schematic of the proposed quantitative immunoassay based on SiO2@PAA@CAT-catalyzed growth of AuNPs. Specifically, the synthetic SiO2@PAA@CAT complexes coupled with the biotin–streptavidin system were used to construct a sandwich assay for naked-eye determination of L. monocytogenes
11.2.2 Fluorescence
Fluorescence detection, in contrast to colorimetric assay, is particularly attractive for bacterial pathogens and cancer cells sensing, due to their high-to-signal ratio and improved sensitivity. The commonly used signal transducers are organic dyes (see Fig. 11.3) [36,37,38] and fluorescent nanoparticles [39].

[Reprinted with the permission from Ref. [38]. Copyright 2017 Multidisciplinary Digital Publishing Institute (MDPI)]
Principle of S. sonnei detection using an aptamer-based fluorescent sandwich-type biosensor platform
One of the objective in this area is to develop high-specific, easily implementable bioassay that can be applied to detection and identification of whole pathogens and cancer cells in complex matrices. Li and coworkers have recently demonstrated a technique for quantitative detection of the Escherichia coli O157:H7 (E. coli O157:H7) in complex media, which is one of the highly pathogenic agents. Hollow silica nanospheres loading with fluorescein (FHSNs) have been applied to the signal amplification in the sandwich-type immunoassays. Under optimized conditions, this platform provided a sensitive detection of E. coli O157:H7 cells with a linear range of 4 to 4 × 108 CFU/mL and a LOD of 3 CFU/mL. Likewise, this architecture has shown high robustness and high sensitivity for whole-cell sensing in complex sample matrices, such as milk, orange juice, and river water [40]. In another study, Dogan et al. have developed a chitosan-coated CdTe quantum dots (CdTe QDs) as the fluorescence label in the sandwich immunoassays for E. coli detection. They achieved a sensitive detection of target in urine matrix and high selectivity over the other four pathogens [41].
Fu and coworkers have recently developed an antibiotic-affinity strategy for fluorimetric detection of Staphylococcus aureus (S. aureus) cell (Fig. 11.4) [42]. Specifically, the targeted cell was sandwiched by vancocin-modified BSA and fluorescein isothiocyanate (FITC)-labeled antibody. They observed a linear detection from 1.0 × 103 to 1.0 × 109 CFU/mL with a LOD of 2.9 × 102 CFU/mL. Their method exhibited 85–130% of recoveries when applied in spiked apple juice for S. aureus detection.

(Reprinted with the permission from Ref. [42]. Copyright 2015 American Chemical Society)
Principle of sandwich fluorimetric detection of S. aureus based on antibiotic-affinity strategy. The target pathogen was captured by vancocin through five-point hydrogen bonds and was further sandwiched by the fluorescein labeled lgG
11.2.3 Surface Plasmon Resonance
During the past two decades, surface plasma resonance (SPR) techniques have been extensively explored for biosensor platforms targeting pathogens and cells detections, because they are sensitive, label-free and particularly enable the real-time detections of biological targets [43].
Pathogen diagnostics using SPR techniques typically involve signal amplification in order to improve the sensitivity. For example, Eum et al. have developed a SPR-sensing platform for E. coli O157:H7 detection. In this study, they immobilized the antibodies onto gold nanorods (GNRs) to enhance the sensitivity of the biosensor. The SPR response with the GNRs labeled antibody was around fourfold improvement of the response than that of from the unlabeled antibody [45]. In another study, Santos et al. have demonstrated the use of SPR to monitor the antibody immobilization protocol for E. coli O157:H7 detection [46]. Recently, Liu et al. proposed a SPR immunosensor coupled with antibody-functionalized magnetic nanoparticles (MNPs) for Salmonella enteritidis detection (see Fig. 11.5) [44]. Specifically, they immobilized capture antibody via EDC/NHS chemistry onto Au chips and anchored the secondary antibody onto Fe3O4 MNPs using the same chemistry. This antibody-functionalized MNPs allowed the selective recognition and separation of S. enteritidis from the sample matrix under an external magnetic field. This MNPs-enhanced sandwich assay exhibited a large improvement in sensitivity as well as the detection range. Charlermroj et al. compared the sensor performance of a direct, sandwich, or subtractive immunoassay for the detection of bacteria Acidovorax avenae subsp. citrulli (Aac) and discovered that the direct assay format exhibited the best sensitivity, while, the sandwich assay provided the best signal enhancement [47].

(Reprinted with the permission from Ref. [44]. Copyright 2016 Elsevier)
Schematic representation for the detection of S. enteritidis by MNPs-enhanced SPR sandwich assay. The antibody-functionalized MNP acts as both the enrichment reagent of the target and the amplification reagent of SPR immunosensor
As it is commonly seen for SPR-based pathogen detections, nanoparticle amplification is widely employed for cancer cell detections using SPR technique. For example, Chen et al. reported a sensitive SPR biosensor coupled with MNPs for the determination of breast cancer cell MCF-7 [48]. The target cancer cells were firstly captured by the aptamer on the surface, followed by the binding event of antibody-labeled MNP to form a sandwich assay. As such, the SPR signal enhanced significantly by MNP immobilization due to the large mass effect and high refractive index of the assays. With such signal enhancement, this platform exhibited a detection limit of 500 cells/mL. In a more recent study, Mousavi et al. have developed a microfluidic chip combined with gold nanoslit SPR for cancer cells detections in human blood [49]. They coupled this platform with magnetic nanoparticles in support for efficient immunomagnetic capturing and separation. At last, a LOD of 13 cells/mL and real-time monitoring of the whole process were achieved (Fig. 11.6).

[Reprinted with the permission from Ref. [49]. Copyright 2015 Multidisciplinary Digital Publishing Institute (MDPI)]
A schematic of the double capturing method. a The first step includes: (i) functionalizing the MNPs with antibody I; (ii) mixing the functionalized MNPs (carrying antibody I) with the sample to capture the target cells. b The second step includes introducing the mixture of blood sample and MNPs to the microfluidic chip and capturing the MNPs-cells to bind to the antibody II on the gold nanoslits. The cell binding on the gold nanoslits was monitored by the wavelength shift of the SPR spectrum
11.3 Electrochemical Detection
The signaling mechanism of electrochemical sandwich assays is based on the electronic communication between the transducer and biomolecules. Because of this unique signaling mechanism, the electrochemical sandwich assays are sensitive, selective, rapid, miniaturizable, and cost-effective, which make them to be of particular interests. They are, for most of cases, more practical for the development of point-of-care devices, especially for the pathogen and cell detections [50].
Electrochemical sandwich-type biosensors for whole-cell detections are typically composed of three components: capture element, target cells, and signal transducer elements. Capture elements are usually DNA/RNA aptamers or antibodies, which are used for anchoring the sandwich scaffold onto electrodes. Meanwhile, transducer elements, which can be small redox labels, metal ion, or other redox-active species, could report the signal change from target cell binding-induction. In order to achieve high sensitivity and selectivity for cell detection, two mainly signal amplification strategies have been explored. One is based on redox tags such as enzymatic catalyst and metal nanoparticles, and the other is adoption of loading substrate where the graphene and carbon nanotube would be widely employed due to their large surface areas.
Conventional culture plating methods for E. coli O157:H7 detection take several days to obtain results, while electrochemical sandwich-type biosensor could provide rapid and sensitive detection [51, 52]. Li et al. have developed a sensitive and efficient electrochemical sandwich assay for detection of E. coli (see Fig. 11.7) [51]. Specifically, they immobilized the capture antibodies, which was pre-assembled onto a SiO2-coated AuNPs via a biotin-avidin interaction, onto chitosan-fullerene (C60) composite nanolayer, and then labeled probe antibodies with glucose oxidase (GOD)-loaded Pt nanochains (PtNCs) which served as tracing tag. With such an immunoreaction, they observed a linear detection from 30 to 106 CFU/mL and a LOD of 15 CFU/mL.

(Reprinted with the permission from Ref. [51]. Copyright 2013 Elsevier)
Schematic description of electrochemical immunoassay for E. coli O157:H7 detection. The procedure of the electrode preparation includes five assembling processes, i.e., immobilization of C60, Fc, CHI–SH, Au–SiO2, SA, and bio-Ab1 on the electrode surface. For pathogen detection and signal amplification, the PtNCs-GOD-Ab2 complex was used
Likewise, in another electrochemical immunosensor study, the polypyrrole (PPy)/AuNP/multi-wall carbon nanotube/chitosan hybrid bionanocomposite was employed to modify pencil graphite electrode (PGE) for signal amplification. As such, this platform exhibited a detection linear range from 10 to 107 CFU/mL and detection limit of 30 CFU/mL in PBS buffer [53]. Dos Santos et al. have developed a label-free immunoassay using electrochemical impedance spectroscopy (EIS). They studied the surface antibody functionalization and morphological features by fluorescence and atomic force microscopy. This label-free platform exhibited a detection limit of 2 CFU/mL and a linear range from 30 to 104 CFU/mL [46]. Wang et al. reported a magnetoimmunoassay for rapid separation and sensitive detection of target cells from broth samples [52]. The electrochemical detection of other foodborne pathogens such as L. monocytogenes, Salmonella pullorum, S. aureus, and Salmonella gallinarum has been also reported [54,55,56,57,58].
Recently, Zhu et al. have developed an aptamer-cell-aptamer assay for MCF-7 cancer cell detection, employing enzyme label HRP as signal amplification [59]. Specifically, they fabricated the sensing platform by firstly immobilizing the capture aptamer on Au electrode surface and then capturing target cells followed by an HRP-labeled aptamer. This platform exhibited a detection range from 100 to 1 × 107 cells/mL, and the detection limit was as low as 100 cells/mL. Likewise, for the detection of the same target cell MCF-7, another study has demonstrated a specific recognition between the aptamer and MUC1 protein that overexpressed on the out surface of the cells [60]. This sensing platform employed aptamer-anchored magnetic beads for cell separations and capture with high selectivity and employed Ag-coated AuNPs as signal amplification. This architecture has achieved a linear detection range between 103 and 105 cells/mL, and the LOD for MCF-7 cell was estimated to be 500 cells/mL. Ge et al. have demonstrated a detection method for the determination of K-562 cells, chronic myelogenous leukemia cells, based on intrinsic peroxidase-like catalytic activity of trimetallic dendritic Au@PdPt nanoparticles, achieving a detection range from 1.0 × 102 to 2.0 × 107 cells/mL and a LOD of 31 cells/mL (see Fig. 11.8) [61].

(Reprinted with the permission from Ref. [61]. Copyright 2013 Elsevier)
Schematic representation of electrochemical sensor of cancer cells by using folic acid functionalized Au@PtPd NPs on paper device. A LOD value of 31 cell/mL has been achieved
As it is commonly seen for aptamer-based sandwich assay, nanoparticles have been reported for cancer cell detection in electrochemical antibody-based sandwich assay. Chandra et al. developed an electrochemical-sensing platform for drug-resistant cancer cells detection based on Permeability glycoprotein (P-gp) antigen–antibody interaction [62]. Employing Au nanoparticles for loading monoclonal P-gp antibody and hydrazine-labeled carbon nanotube as reduction catalyst, this assay exhibited a linear range from 50 to 1.0 × 105 cells/mL with the detection limit of 2000 cells/mL. In a more recent study, the same research group has further developed a similar platform, again, via employing AuNP as loading support and hydrazine as reduction catalyst, for the determination of metastatic cancer cells. This platform, likewise, achieved a wide linear range between 45 and 1.0 × 105 cells/mL [63].
11.4 Mechanical Biosensors
Sandwich assay-based mechanical biosensors are currently underdeveloped area, in contrast to the optical and electrochemical approaches, for the detection of pathogen and whole cell. Quartz crystal microbalance (QCM), a mechanical technique, relies on a mass variation per unit area by measuring the change in frequency of a quartz crystal resonator. Tothill’s group has recently demonstrated a QCM approach based on AuNPs amplified sandwich-type assays for the rapid and real-time detection of bacterial pathogens [64, 65]. For the detection of Salmonella, they observed a LOD value at 10 to 20 CFU/mL, while sensing Campylobacter jejuni, the sensitivity was 150 CFU/mL.
11.5 Conclusion
The applications of sandwich assay biosensors for whole-cell detection are growing rapidly and, as described throughout this chapter, they have been incorporated with different recognition agents and signal transducers. Further improvement of the architecture design, increase bio-receptor selectivity and stability of the assay, and enhancement of transducer sensitivity will pave way for selective, sensitive, rapid, and cost-effective detection of bacterial pathogens and cancer cells at complex sample matrix.
References
Anderson NL, Anderson NG (2002) The human plasma proteome—history, character, and diagnostic prospects. Mol Cell Proteomics 1:845–867
Zhang BH, Pan XP, Cobb GP, Anderson TA (2007) microRNAs as oncogenes and tumor suppressors. Dev Biol 302:1–12
Ahmed A, Rushworth JV, Hirst NA, Millner PA (2014) Biosensors for whole-cell bacterial detection. Clin Microbiol Rev 27:631–646
Li BM, Yu QL, Duan YX (2015) Fluorescent labels in biosensors for pathogen detection. Crit Rev Biotechnol 35:82–93
Burlage RS, Tillmann J (2017) Biosensors of bacterial cells. J Microbiol Methods 138:2–11
Yetisen AK, Akram MS, Lowe CR (2013) Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 13:2210–2251
Kosaka PM, Pini V, Ruz JJ, da Silva RA, Gonzalez MU, Ramos D, Calleja M, Tamayo J (2014) Detection of cancer biomarkers in serum using a hybrid mechanical and optoplasmonic nanosensor. Nat Nanotechnol 9:1047–1053
Hsieh K, Ferguson BS, Eisenstein M, Plaxco KW, Soh HT (2015) Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc Chem Res 48:911–920
Shen JW, Li YB, Gu HS, Xia F, Zuo XL (2014) Recent development of sandwich assay based on the nanobiotechnologies for proteins, nucleic acids, small molecules, and ions. Chem Rev 114:7631–7677
Giri B, Pandey B, Neupane B, Ligler FS (2016) Signal amplification strategies for microfluidic immunoassays. TrAC-Trends Anal Chem 79:326–334
Ye DK, Zuo XL, Fan CH (2017) DNA nanostructure-based engineering of the biosensing interface for biomolecular detection. Prog Chem 29:36–46
Zhu CZ, Yang GH, Li H, Du D, Lin YH (2015) Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal Chem 87:230–249
Liang K, Liu F, Fan J, Sun D, Liu C, Lyon CJ, Bernard DW, Li Y, Yokoi K, Katz MH, Koay EJ, Zhao Z, Hu Y (2017) Nanoplasmonic quantification of tumour-derived extracellular vesicles in plasma microsamples for diagnosis and treatment monitoring. Nat Biomed Eng 1:0021
Blažková M, Javůrková B, Fukal L, Rauch P (2011) Immunochromatographic strip test for detection of genus Cronobacter. Biosens Bioelectron 26:2828–2834
Sharma H, Mutharasan R (2013) hlyA gene-based sensitive detection of listeria monocytogenes using a novel cantilever sensor. Anal Chem 85:3222–3228
Li FY, Peng J, Zheng Q, Guo X, Tang H, Yao SZ (2015) Carbon nanotube-polyamidoamine dendrimer hybrid-modified electrodes for highly sensitive electrochemical detection of microRNA24. Anal Chem 87:4806–4813
Huertas CS, Carrascosa LG, Bonnal S, Valcárcel J, Lechuga LM (2016) Quantitative evaluation of alternatively spliced mRNA isoforms by label-free real-time plasmonic sensing. Biosens Bioelectron 78:118–125
Miranda OR, Li X, Garcia-Gonzalez L, Zhu Z-J, Yan B, Bunz UHF, Rotello VM (2011) Colorimetric bacteria sensing using a supramolecular enzyme–nanoparticle biosensor. J Am Chem Soc 133:9650–9653
Farrow B, Hong SA, Romero EC, Lai B, Coppock MB, Deyle KM, Finch AS, Stratis-Cullum DN, Agnew HD, Yang S, Heath JR (2013) A chemically synthesized capture agent enables the selective, sensitive, and robust electrochemical detection of anthrax protective antigen. ACS Nano 7:9452–9460
Ahmad M, Ameen S, Siddiqi TO, Khan P, Ahmad A (2016) Live cell monitoring of glycine betaine by FRET-based genetically encoded nanosensor. Biosens Bioelectron 86:169–175
Zhu LJ, He J, Cao XH, Huang KL, Luo YB, Xu WT (2016) Development of a double-antibody sandwich ELISA for rapid detection of Bacillus Cereus in food. Sci Rep 6:16092
Vikesland PJ, Wigginton KR (2010) Nanomaterial enabled biosensors for pathogen monitoring—a review. Environ Sci Technol 44:3656–3669
Chen ZH, Liu Y, Wang YZ, Zhao X, Li JH (2013) Dynamic evaluation of cell surface N-glycan expression via an electrogenerated chemiluminescence biosensor based on concanavalin a-integrating gold-nanoparticle-modified Ru(bpy) 2+3 -doped silica nanoprobe. Anal Chem 85:4431–4438
Yang HY, Li ZJ, Shan M, Li CC, Qi HL, Gao Q, Wang JY, Zhang CX (2015) Electrogenerated chemiluminescence biosensing for the detection of prostate PC-3 cancer cells incorporating antibody as capture probe and ruthenium complex-labelled wheat germ agglutinin as signal probe. Anal Chim Acta 863:1–8
Zhang Y, Tan C, Fei RH, Liu XX, Zhou Y, Chen J, Chen HC, Zhou R, Hu YG (2014) Sensitive chemiluminescence immunoassay for E. coli O157:H7 detection with signal dual-amplification using glucose oxidase and laccase. Anal Chem 86:1115–1122
Zhang LS, Huang R, Liu WP, Liu HX, Zhou XM, Xing D (2016) Rapid and visual detection of Listeria monocytogenes based on nanoparticle cluster catalyzed signal amplification. Biosens Bioelectron 86:1–7
Gao LZ, Zhuang J, Nie L, Zhang JB, Zhang Y, Gu N, Wang TH, Feng J, Yang DL, Perrett S, Yan XY (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583
Wei H, Wang E (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 42:6060–6093
Jain S, Chattopadhyay S, Jackeray R, Abid C, Kohli GS, Singh H (2012) Highly sensitive detection of Salmonella typhi using surface aminated polycarbonate membrane enhanced-ELISA. Biosens Bioelectron 31:37–43
Dykman L, Khlebtsov N (2012) Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev 41:2256–2282
Saha K, Agasti SS, Kim C, Li XN, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779
Lu WT, Arumugam SR, Senapati D, Singh AK, Arbneshi T, Khan SA, Yu HT, Ray PC (2010) Multifunctional oval-shaped gold-nanoparticle-based selective detection of breast cancer cells using simple colorimetric and highly sensitive two-photon scattering assay. ACS Nano 4:1739–1749
Wu WH, Li J, Pan D, Li J, Song SP, Rong MG, Zi Li, Gao JM, Lu JX (2014) Gold nanoparticle-based enzyme-linked antibody-aptamer sandwich assay for detection of Salmonella Typhimurium. ACS Appl Mater Interfaces 6:16974–16981
de la Rica R, Stevens MM (2012) Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nanotechnol 7:821–824
Chen R, Huang XL, Xu HY, Xiong YH, Li YB (2015) Plasmonic enzyme-linked immunosorbent assay using nanospherical brushes as a catalase container for colorimetric detection of ultralow concentrations of Listeria monocytogenes. ACS Appl Mater Interfaces 7:28632–28639
Sung D, Yang S (2014) Facile method for constructing an effective electron transfer mediating layer using ferrocene-containing multifunctional redox copolymer. Electrochim Acta 133:40–48
Gehring AG, Brewster JD, He YP, Irwin PL, Paoli GC, Simons T, Tu SI, Uknalis J (2015) Antibody microarray for E. coli O157:H7 and shiga toxin in microtiter plates. Sensors 15:30429–30442
Song MS, Sekhon SS, Shin WR, Kim HC, Min J, Ahn JY, Kim YH (2017) Detecting and discriminating Shigella sonnei using an aptamer-based fluorescent biosensor platform. Molecules 22:825
Demirkol DO, Timur S (2016) A sandwich-type assay based on quantum dot/aptamer bioconjugates for analysis of E. coli O157:H7 in microtiter plate format. Int J Polym Mater Polym Biomater 65:85–90
Hu RR, Yin ZZ, Zeng YB, Zhang J, Liu HQ, Shao Y, Ren SB, Li L (2016) A novel biosensor for Escherichia coli O157:H7 based on fluorescein-releasable biolabels. Biosens Bioelectron 78:31–36
Dogan U, Kasap E, Cetin D, Suludere Z, Boyaci IH, Turkyilmaz C, Ertas N, Tamer U (2016) Rapid detection of bacteria based on homogenous immunoassay using chitosan modified quantum dots. Sens Actuators B-Chem 233:369–378
Kong W, Xiong J, Yue H, Fu Z (2015) Sandwich fluorimetric method for specific detection of Staphylococcus aureus based on antibiotic-affinity strategy. Anal Chem 87:9864–9868
Yanase Y, Hiragun T, Ishii K, Kawaguchi T, Yanase T, Kawai M, Sakamoto K, Hide M (2014) Surface plasmon resonance for cell-based clinical diagnosis. Sensors 14:4948–4959
Liu X, Hu YX, Zheng S, Liu Y, He Z, Luo F (2016) Surface plasmon resonance immunosensor for fast, highly sensitive, and in situ detection of the magnetic nanoparticles-enriched Salmonella enteritidis. Sens Actuators B-Chem 230:191–198
Eum NS, Yeom SH, Kwon DH, Kim HR, Kang SW (2010) Enhancement of sensitivity using gold nanorods-antibody conjugator for detection of E. coli O157:H7. Sens Actuators B-Chem 143:784–788
Barreiros dos Santos M, Agusil JP, Prieto-Simón B, Sporer C, Teixeira V, Samitier J (2013) Highly sensitive detection of pathogen Escherichia coli O157:H7 by electrochemical impedance spectroscopy. Biosens Bioelectron 45:174–180
Charlermroj R, Oplatowska M, Gajanandana O, Himananto O, Grant IR, Karoonuthaisiri N, Elliott CT (2013) Strategies to improve the surface plasmon resonance-based immmunodetection of bacterial cells. Microchim Acta 180:643–650
Chen HX, Hou YF, Ye ZH, Wang HY, Koh K, Shen ZM, Shu YQ (2014) Label-free surface plasmon resonance cytosensor for breast cancer cell detection based on nano-conjugation of monodisperse magnetic nanoparticle and folic acid. Sens Actuators B-Chem 201:433–438
Mousavi M, Chen HY, Hou HS, Chang CYY, Roffler S, Wei PK, Cheng JY (2015) Label-free detection of rare cell in human blood using gold nano slit surface plasmon resonance. Biosensors 5:98–117
Akanda MR, Tamilavan V, Park S, Jo K, Hyun MH, Yang H (2013) Hydroquinone diphosphate as a phosphatase substrate in enzymatic amplification combined with electrochemical–chemical–chemical redox cycling for the detection of E. coli O157:H7. Anal Chem 85:1631–1636
Li Y, Fang LC, Cheng P, Deng J, Jiang LL, Huang H, Zheng JS (2013) An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 using C-60 based biocompatible platform and enzyme functionalized Pt nanochains tracing tag. Biosens Bioelectron 49:485–491
Wang Y, Alocilja EC (2015) Gold nanoparticle-labeled biosensor for rapid and sensitive detection of bacterial pathogens. J Biol Eng 9:16
Guner A, Cevik E, Senel M, Alpsoy L (2017) An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 by using chitosan, MWCNT, polypyrrole with gold nanoparticles hybrid sensing platform. Food Chem 229:358–365
Cheng CN, Peng Y, Bai JL, Zhang XY, Liu YY, Fan XJ, Ning BA, Gao ZX (2014) Rapid detection of Listeria monocytogenes in milk by self-assembled electrochemical immunosensor. Sens Actuators B-Chem 190:900–906
Abbaspour A, Norouz-Sarvestani F, Noon A, Soltani N (2015) Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of staphylococcus aureus. Biosens Bioelectron 68:149–155
Chen Q, Lin JH, Gan CQ, Wang YH, Wang D, Xiong YH, Lai WH, Li YT, Wang MH (2015) A sensitive impedance biosensor based on immunomagnetic separation and urease catalysis for rapid detection of Listeria monocytogenes using an immobilization-free interdigitated array microelectrode. Biosens Bioelectron 74:504–511
Fei JF, Dou WC, Zhao GY (2015) A sandwich electrochemical immunosensor for Salmonella pullorum and Salmonella gallinarum based on a screen-printed carbon electrode modified with an ionic liquid and electrodeposited gold nanoparticles. Microchim Acta 182:2267–2275
Chen Q, Wang D, Cai GZ, Xiong YH, Li YT, Wang MH, Huo HL, Lin JH (2016) Fast and sensitive detection of foodborne pathogen using electrochemical impedance analysis, urease catalysis and microfluidics. Biosens Bioelectron 86:770–776
Zhu XL, Yang JH, Liu M, Wu Y, Shen ZM, Li GX (2013) Sensitive detection of human breast cancer cells based on aptamer-cell-aptamer sandwich architecture. Anal Chim Acta 764:59–63
Zhang JJ, Cheng FF, Zheng TT, Zhu JJ (2017) Versatile aptasensor for electrochemical quantification of cell surface glycan and naked-eye tracking glycolytic inhibition in living cells. Biosens Bioelectron 89:937–945
Ge SG, Zhang Y, Zhang L, Liang LL, Liu HY, Yan M, Huang JD, Yu JH (2015) Ultrasensitive electrochemical cancer cells sensor based on trimetallic dendritic Au@PtPd nanoparticles for signal amplification on lab-on-paper device. Sens Actuators B-Chem 220:665–672
Chandra P, Noh HB, Pallela R, Shim YB (2015) Ultrasensitive detection of drug resistant cancer cells in biological matrixes using an amperometric nanobiosensor. Biosens Bioelectron 70:418–425
Pallela R, Chandra P, Noh HB, Shim YB (2016) An amperometric nanobiosensor using a biocompatible conjugate for early detection of metastatic cancer cells in biological fluid. Biosens Bioelectron 85:883–890
Salam F, Uludag Y, Tothill IE (2013) Real-time and sensitive detection of Salmonella Typhimurium using an automated quartz crystal microbalance (QCM) instrument with nanoparticles amplification. Talanta 115:761–767
Masdor NA, Altintas Z, Tothill IE (2016) Sensitive detection of Campylobacter jejuni using nanoparticles enhanced QCM sensor. Biosens Bioelectron 78:328–336
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Li, S., Li, H., Xia, F. (2018). Sandwich Assay for Pathogen and Cells Detection. In: Xia, F., Zhang, X., Lou, X., Yuan, Q. (eds) Biosensors Based on Sandwich Assays. Springer, Singapore. https://doi.org/10.1007/978-981-10-7835-4_11
Download citation
DOI: https://doi.org/10.1007/978-981-10-7835-4_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7834-7
Online ISBN: 978-981-10-7835-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)