Abstract
Pancreaticoduodenectomy (PD) is a surgical procedure designed to treat benign and malignant diseases of the periampullary region such as chronic pancreatitis and pancreatic cancer. The operation is conducted in two parts, the first of which is the extirpative phase, which includes the removal of the pancreatic head, neck, and uncinate process, distal common bile duct, and duodenum. This is followed by the reconstruction phase where the duodenum, bile duct, and pancreas remnant are anastomosed to the jejunum. The technique for the pancreaticojejunostomy (PJ) anastomosis will be the subject of this chapter.
The PJ is often termed the “Achilles heel” of the operation because it is the aspect associated with the highest occurrence of leakage and the ensuing potential for intra-abdominal sepsis. Leakage of the PJ is also a driver of a variety of other associated complications, such as delayed gastric emptying, intestinal ileus, and wound infection, among others. The two most common techniques in current practice for PJ construction are the invaginated and the duct-to-mucosa anastomoses. Despite a series of prospective, randomized controlled trials, there continues to be a lack of consensus among surgeons as to which method provides the best outcome for patients.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Pancreaticoduodenectomy (PD)
- Pancreaticojejunostomy (PJ)
- Pancreaticogastrostomy (PG)
- Invaginated pancreaticojejunostomy
- Allen Oldfather Whipple
- Pylorus-preserving PJ
- Postoperative pancreatic fistula (POPF)
10.1 Introduction
In the early years of pancreatic surgery, PD was associated with a high rate of mortality. However, with the effort of many surgeons working to improve the operative technique, in addition to advances in postoperative care, there has been a marked improvement in patient outcomes—with a typical perioperative mortality rate in high-volume centers of less than 2% [1].
Developments in the treatment of pancreatic disorders began as early as 1898, when Alessandro Codivilla, an Italian surgeon, performed the first partial resection of the pancreas, duodenum, stomach, and bile duct for treatment of carcinoma of the pancreas. Unfortunately, Codivilla’s patient died from cachexia resulting from steatorrhea 18 days post-op [2]. It was not until 1912 when Walther Kausch, a German surgeon in Berlin, successfully performed a resection of the pancreas, in addition to a partial resection of the duodenum [3]. Like many surgeons operating at the beginning of the twentieth century, Kausch believed that the duodenum was vital for patient survival, and therefore his procedure did not include a complete duodenectomy. However, in 1918 Dragstedt et al. [4] disproved this misconception, demonstrating that dogs could survive following a duodenal resection, thereby setting the stage for Allen Oldfather Whipple to perform the first reported total duodenectomy in 1935 [5].
Building upon the findings of Kausch, Dragstedt, and others, Whipple and his resident John Hawk conducted a series of experiments on dogs, which allowed them to conclude that reimplantation rather than ligation of the pancreatic duct was an important step in reconstruction and could reduce the incidence of pancreatic fistula formation. Additionally, their experimental PJ showed that the connection between the epithelium of the pancreatic duct and the mucosa of the jejunum could heal within a 24-h period [1].
It was in 1935 that Whipple first published a report of three patients who underwent a two-stage procedure at Columbia-Presbyterian Hospital in New York [5]. The operation included the complete resection of the duodenum and a large portion of the pancreas. Unfortunately one of the patients died within 30 h of the procedure due to problems with anastomotic breakdown. The second and third survived for 9 and 24 months when they died of cholangitis and liver metastasis, respectively.
In 1946, Whipple published a second report, which addressed his 10-year experience in radical pancreatic and duodenal resection, and suggested changes to his original report [6]. This publication advocated for a one-stage procedure, following the discovery that vitamin K could correct the hypocoagulability associated with malabsorption of fat-soluble vitamins secondary to chronic biliary obstruction. It was in this second report that Whipple emphasized three central aspects of the operation: (1) the complete resection of the head of the pancreas and duodenum, (2) anastomosis of the pancreatic duct to a jejunal loop, and (3) a choledochoenterostomy in place of a cholecystoenterostomy. Aside from one significant variation that was described by Traverso and Longmire in 1978—the pylorus-preserving modification (PPPD) [7]—the current PD differs relatively little from the procedure described by Whipple in 1946.
The two most common PJ techniques currently in practice around the world are the invaginated and the duct-to-mucosa anastomoses. An advantage of the invaginated technique over the duct-to-mucosa is that the cut edges of the pancreas are invaginated or “dunked” into the jejunal lumen. This allows for the apposition of the pancreatic capsule to the jejunal serosa. Additionally, the technique can be applied even in a patient with a very small pancreatic duct [8] or a soft pancreatic texture [9]. However, despite its advantages, “dunking” requires the entire cut surface of the pancreas to be exposed to bile-activated pancreatic juice, which has the potential to lead to anastomotic breakdown [10]. The duct-to-mucosa technique does not require a large jejunotomy to facilitate pancreatic invagination. However, it can be difficult to perform in a patient with a small pancreatic duct and may leave the patient with an anastomosis that is prone to obstruction [10].
A number of prospective, randomized controlled trials have been conducted to attempt to compare the invaginated and duct-to-mucosa techniques (Table 10.1). The most recent study was performed by Xu et al. in Shanghai, China, in 2015 [10]. The primary variable under consideration was the occurrence of postoperative pancreatic fistula (POPF)—as defined by the International Study Group on Pancreatic Fistula (ISGPF). Although the Xu et al. study observed a slight superiority for the invaginated PJ technique, the authors emphasize that the risk of POPF remains multifactorial. The patient’s BMI, the experience of the surgeon, operating time, and the texture of the pancreas are but some of the variables affecting patient outcomes. The most significant results favoring the invaginated technique were for patients with soft pancreas texture and a non-dilated main pancreatic duct, showing a POPF rate of 9.6% for the “dunking” technique in contrast to 27.3% for the duct-to-mucosa technique (p = 0.001) [10]. This finding is consistent with a dual-institutional prospective randomized controlled trial reported in 2009 by Berger et al. at Thomas Jefferson University and Indiana University, which showed a 12% POPF rate for the invaginated technique vs. a 24% rate (p = 0.04) for the duct-to-mucosa group [11]. A prospective randomized trial by Bassi et al. in 2003 showed no statistically significant differences between the two methods in patients with a soft pancreatic texture—with a 15% POPF rate for the invaginated approach and a 13% POPF rate for the duct-to-mucosa technique [13].
Despite these findings, the most important factors in anastomotic success are generally considered to be the proficiency and experience of the surgeon for the given technique that they favor.
10.2 Technique for Pancreaticoduodenectomy (PD): Resectional Phase [14, 15]
-
1.
The operation is conducted with the patient in the supine position. Following abdominal exploration, a cholecystectomy is performed using the “dome down” technique, and the cystic duct and artery are ligated. The Kocher maneuver is then executed to release the duodenum from its retroperitoneal attachments and mobilize the pancreatic head, leaving the exposed tumor accessible for palpation. Dissection is then carried out within the gastrohepatic ligament. The common hepatic duct is encircled and transected. The gastroduodenal artery (GDA) is test clamped to ensure adequate proper hepatic artery flow before the vessel is controlled with 2-0 silk ties and a 3-0 silk suture ligature. The duodenum is transected 2–3 cm below the pylorus with a stapler. A Penrose drain is passed underneath the pancreatic neck overlying the superior mesenteric vein (SMV). This allows for safe transection of the pancreatic neck with electrocautery.
-
2.
Using the base of the transverse mesocolon as an anatomic landmark, the ligament of Treitz is exposed and lysed. At a distance of 15–20 cm distal to the ligament, the jejunum is divided using a stapler, and the distal jejunal staple line is imbricated with 3-0 silk Lembert sutures. The proximal jejunum is then divided from its mesentery and moved to the right side of the surgical field by passing it under the base of the mesocolon and the superior mesenteric vessels. Working along the lateral border of the superior mesenteric artery (SMA) and SMV, the retroperitoneal margin of the uncinate process is dissected out, and all pancreatic tissue adjacent to the artery is separated from the perivascular plane. The specimen is removed and a hemostatic agent is applied to the retroperitoneal margin to promote clotting. A series of interrupted 3-0 silk sutures is used to close the defect previously created at the ligament of Treitz, with care taken not to injure the inferior mesenteric vein (IMV).
10.3 Technique for the Invaginated PJ [14, 15]
-
1.
Just to the right of the middle colic vessels, a defect is introduced into the transverse mesocolon, and the proximal jejunum is carried through this defect.
-
2.
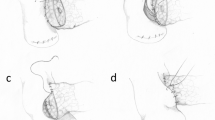
The pancreatic remnant is mobilized for a distance of 2–3 cm from the underlying splenic vein (Fig. 10.1).
10.4 Posterior Outer Row of PJ [14, 15]
-
1.
The PJ is constructed end-to-side, with an interrupted posterior outer row of 3-0 silk mattress sutures placed between the posterior aspect of the pancreatic remnant and the jejunum. In a patient with a characteristic “soft” pancreas, we find that the sutures hold best when placed in a horizontal mattress fashion (Fig. 10.2).
-
2.
Once the sutures are secured and tied down (Fig. 10.3), electrocautery is used to perform the jejunotomy, and a vein retractor is used to expose the jejunal mucosa.
-
3.
Care should be taken to ensure that the jejunotomy is shorter than the width of the cut surface of the pancreas, as the small bowel will stretch during construction of the anastomosis.
The PJ is constructed end-to-side, with an interrupted posterior outer row of 3-0 silk sutures placed between the posterior aspect of the pancreatic remnant and the jejunum. In a patient with a characteristic “soft” pancreas, we find that the sutures hold best when placed in a horizontal mattress fashion
10.5 Inner Rows of PJ [14, 15]
-
1.
A 5 French pediatric feeding tube is placed within the pancreatic duct to ensure that the duct is not inadvertently ligated during the construction of the anastomosis (Fig. 10.4). For larger pancreas ducts, an 8 French pediatric feeding tube can be used.
-
2.
Two 3-0 Polysorb™ sutures are then placed in a running-locking fashion in the inferior corner of the anastomosis. One stitch is used in running-locking fashion to complete the posterior inner layer.
-
3.
The posterior inner layer is joined together and tied to the anterior portion of the anastomosis with the second 3-0 Polysorb™ suture. The anterior inner layer remains unlocked (Fig. 10.5).
10.6 Outer Anterior Row of PJ [14, 15]
-
1.
An outer anterior row of interrupted 3-0 silk sutures is placed in a vertical mattress fashion to complete the pancreatic anastomosis. The vertical sutures are designed to roll the jejunum over the anterior inner layer, and the tension is dispersed by crossing each suture over the preceding suture while they are being tied (Fig. 10.6).
-
2.
Figure 10.7 demonstrates the completed invaginated PJ.
10.9 Drainage and Closure [14, 15]
-
1.
Two Jackson-Pratt drains are positioned on either side of the abdomen as a precaution against the occurrence of fistula. The right drain is positioned within the subhepatic space, posterior to the right upper quadrant (RUQ) jejunal loop, which we term the neoduodenum. The left drain is placed posterior to the stomach through the gastrocolic ligament and superior to the PJ. #2 Nylon suture in a running fashion is used to close the fascia, and the subcutaneous tissue and skin are closed with 3-0 and 4-0 Vicryl™ sutures, respectively.
References
Are C, Dhir M, Ravipati L. History of pancreaticoduodenectomy: early misconceptions, initial milestones and the pioneers. HPB (Oxford). 2011;13(6):377–84.
Sauve L. Des pancreatectomies et spécialment de la pancréatectomie céphalique. Rev Chir. 1908;37:113–52, and 335-85
Kausch W. Das Carcinom der Papilla duodeni und seine radikale Entfernung. Beitrage zur Klinische. Chirurgie. 1912;78:439–86.
Dragstedt LR, Dragstedt C, McClintock JT, Chase CS. Extirpation of the duodenum. Am J Physiol. 1918;46:584–90.
Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg. 1935;102(4):763–79.
Whipple AO. Observations on radical surgery for lesions of the pancreas. Surg Gynecol Obstet. 1946;82:623–31.
Garonzik-Wang J, Majella Doyle M. Pylorus preserving pancreaticoduodenectomy. Clin Liver Dis. 2015;5(3):54–8.
Kennedy E, Yeo CJ. Dunking pancreaticojejunostomy versus duct-to-mucosa anastomosis. J Hepatobiliary Pancreat Sci. 2011;18(6):769–74.
Gómez T. Reconstruction after pancreatoduodenectomy: Pancreatojejunostomy vs pancreatogastrostomy. World J Gastrointest Oncol. 2014;6(9):369.
Xu J, Zhang B, Shi S, Qin Y, Ji S, Xu W, et al. Papillary-like main pancreatic duct invaginated pancreaticojejunostomy versus duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy: A prospective randomized trial. Surgery. 2015;158(5):1211–8.
Berger A, Howard T, Kennedy E, Sauter P, Bower-Cherry M, Dutkevitch S, et al. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg. 2009;208(5):738–47.
Langrehr J, Bahra M, Jacob D, Glanemann M, Neuhaus P. Prospective randomized comparison between a new mattress technique and Cattell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J Surg. 2005;29(9):1111–9.
Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs A, et al. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003;134(5):766–71.
Lavu H, Brumbaugh J, Yeo CJ. Pylorus-preserving pancreaticoduodenectomy with invaginating pancreaticojejunostomy. In: Asbun HJ, Fuchshuber PR, editors. ACS multimedia atlas of surgery: pancreas surgery volume. 1st ed. Woodbury: Cine-Med; 2016.
Lavu H. Invaginated pancreaticojejunostomy (Whipple Reconstruction) by Harish Lavu | CSurgeries [Internet]. Csurgeries.com. 2016 [cited 31 December 2016]. https://www.csurgeries.com/video/invaginated-pancreaticojejunostomy-(whipple-reconstruction)/ouyyb9jyj1
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Walsh, C.D., Yeo, C.J., Lavu, H. (2018). Invaginating Pancreaticojejunostomy: How I Do It. In: Tewari, M. (eds) Surgery for Pancreatic and Periampullary Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-10-7464-6_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-7464-6_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7463-9
Online ISBN: 978-981-10-7464-6
eBook Packages: MedicineMedicine (R0)