Abstract
Following the resectional aspect of pancreaticoduodenectomy, three anastomoses are used to reestablish gastrointestinal continuity. The pancreatic–enteric anastomosis is by far the most problematic, and has been considered by many the Achilles heel of the pancreaticoduodenal resection. Multiple clinical trials have been published focusing on improving outcomes of the pancreatic–enteric anastomosis, including elements such as the use of prophylactic octreotide, the use of sealants, stenting of the pancreatic duct, and surgical technique. There are two widely used methods to accomplish an end-to-side pancreaticojejunostomy (PJ) after pancreaticoduodenectomy: either invagination PJ or duct-to-mucosa PJ. Two prospective randomized trials have evaluated these techniques, the first a trial by Bassi and co-authors, and the second a trial by Berger et al. In this article we will focus on our current technique for both invagination pancreaticojejunostomy and duct-to-mucosa pancreaticojejunostomy, recognizing that careful surgical technique, surgeon experience, and surgical volume are factors that are important in yielding the best outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreaticoduodenectomy (PD) is a complex procedure that is commonly performed for both benign and malignant diseases of the pancreas and periampullary region. Once resection of the pancreaticoduodenal specimen has been accomplished, three anastomoses are used to reestablish gastrointestinal continuity: a pancreatic–enteric anastomosis, a biliary–enteric anastomosis, and a gastric or duodenal–enteric anastomosis. The pancreatic–enteric anastomosis is by far the most problematic because it is associated with a significant measurable risk of leakage or failure of healing, resulting in pancreatic fistula (PF). PF then drives the majority of surgical complications associated with PD, including the potential for an intra-abdominal infection, hemorrhage, the occasional need for reoperation, and possible death.
The operative mortality rate for PD is typically less than 5% in high-volume centers. The leading causes of mortality include sepsis (often related to a PF), hemorrhage, cardiac events, and pulmonary embolism. In contrast to this low mortality rate, the morbidity rate remains quite high (ranging from 20 to 60%), with one review showing a rate of 40% [1]. One of the most common causes of morbidity is a leak or PF arising from the pancreatic–enteric anastomosis. A recent review estimated the incidence of this complication to be 10 to 28.5% [2]. Several large single-institution series from the Mannheim, Lahey, and Mayo Clinics have shown leak rates of 11–15% [3–5]. The Mannheim Clinic series demonstrated that 20% of PFs were directly responsible for the patients’ postoperative deaths [5].
The International Study Group on Pancreatic Fistulas (ISGPF) currently defines a PF as “output via an operatively placed drain (or a subsequently placed, percutaneous drain) of any measurable volume of drain fluid on or after postoperative day 3, with an amylase content greater than 3 times the upper normal serum value” [6]. The drain fluid can have a “sinister appearance,” or it can appear milky or like “spring water.” The ISGPF recommendations also include a grading system, grading PFs by severity (A, B, and C), with grade A being least severe and grade C being most severe. This grading system includes the following parameters: clinical condition, treatment used, imaging results, persistent drainage, reoperation, death, signs of infection, and readmission [6].
Multiple clinical trials have been published focused on improving outcomes of pancreaticoenteric anastomoses, including such aspects as the use of prophylactic octreotide, the use of sealants, and stenting of the pancreatic duct. These considerations are outside the scope of this review. There have been, to date, eight published randomized clinical trials focused on surgical technique in pancreaticoenteric anastomoses (Table 1). Four of these compared pancreaticogastrostomy with pancreaticojejunostomy. One compared a traditional, two-layer, end-to-end approach with a novel “binding technique”. The other three compared techniques for the commonly used end-to-side pancreaticojejunal anastomosis.
There are two widely used methods to accomplish an end-to-side pancreaticojejunostomy (PJ) after PD: invagination PJ (or “dunking” the pancreatic remnant into the jejunum) or duct-to-mucosa PJ. In an early single-institution experience using the duct-to-mucosa PJ technique and an internal stent, Strasberg and colleagues [7] reported a PF rate of only 1.6% in 123 patients. In another experience reported by Tani and colleagues [8,] the fistula rate was 11% for a stented duct-to-mucosa PJ technique and 6.5% for a two-layer invaginated end-to-side externally stented technique.
There have been two prospective randomized trials evaluating a duct-to-mucosa PJ versus an end-to-side PJ reported in the literature [9, 10]. In the first trial, Bassi and his coauthors [9] randomized 144 patients undergoing PD to either a two-layer duct-to-mucosa PJ anastomosis or a single-layer end-to-side anastomosis. PFs were seen in 14% of patients—13% in the duct-to-mucosa group and 15% in the end-to-side group, and there was no difference in complications between the groups (overall rate of 54% in both groups). There were no statistically significant differences between the groups with regard to abdominal complications, abdominal fluid collections, or length of stay. The authors concluded that the anastomotic technique did not change the operative risk.
A subsequent dual institution trial by Berger et al. [10] randomized 197 patients to either a two-layer duct-to-mucosa PJ anastomosis or a two-layer end-to-side PJ anastomosis. The majority of cases were performed by five surgeons across two institutions. Patient demographics, including age and gender, were comparable between the two groups. This study revealed an advantage to the two-layer end-to-side invagination technique. The PF rate in the duct-to-mucosa group was 24%, while the rate in the invagination group was only 12% (P < 0.05). There were significant differences in the rates of major complications (Clavien grades 3–5) between the groups (25% duct-to-mucosa versus 12% invagination; P = 0.03) and the need for interventional radiologic procedures (11 vs. 3%; P = 0.03). Length of stay was comparable between the groups. In the multivariate analysis for PF, there were factors that were found to be independent predictors of a fistula. The most powerful predictor was the texture of the pancreatic remnant, with soft or normal glands being associated with a higher PF risk. Patients with a soft pancreas had a much higher likelihood of a PF developing than those with a hard pancreas (odds ratio = 3.7, P = 0.003). The authors also observed a much higher likelihood of PF developing in those patients who underwent a duct-to-mucosa anastomosis (odds ratio = 2.4, P = 0.03).
Technical aspects of pancreaticojejunostomy
One concept that tracks strongly through all trials comparing different PJ techniques is the importance of meticulous technique. Surgeon experience and volume are factors in outcomes in several of the trials, an observation supported by recent publications evaluating the learning curve of attending physicians performing pancreatic surgery [11, 12]. Attention to detail in performing these anastomoses is critical. Therefore, we will present our previously described approach as a guide [13].
Invagination pancreaticojejunostomy
Following removal of the specimen, the pancreatic remnant is mobilized ventrally up out from the retroperitoneum for at least 2 cm, by dividing the tissue located both superiorly and inferiorly along the pancreatic body. The remnant gland is then elevated ventrally, away from the splenic vein. A lacrimal duct probe or a small Bake’s dilator can be placed into the pancreatic duct lumen and used as an atraumatic retractor to help elevate the pancreatic remnant during this dissection. Superiorly, the tissue is typically avascular; however, there may be adjacent lymph nodes present which can bleed if traumatized. Our mobilization typically does not go leftward as far as the splenic artery. Inferiorly, there are often several small veins which may either drain into the inferior mesenteric, splenic, or superior mesenteric vein. Additionally, small arteries originating from the superior mesenteric artery may be found along the inferior border of the gland or posterior to the pancreatic remnant. These should be controlled with silk ties.
Following mobilization of the pancreatic remnant, we commence our pancreaticojejunostomy. This is performed in end-to-side fashion, just distal to the oversewn staple line on the available jejunum. Interrupted 3-0 silk sutures are placed starting at the superior aspect of the remnant pancreas, with a corner stitch placed first through the superior edge of the pancreatic remnant and subsequently as a seromuscular bite on the jejunum. The posterior outer row is then performed in horizontal mattress fashion, incorporating substantial bites of the posterior pancreatic capsule and underlying parenchyma, as well as seromuscular bites of the jejunum. The lacrimal duct probe or Bake’s dilator previously placed in the lumen of the remnant pancreatic duct is kept in place to minimize the chance that any of the posterior stitches will catch and occlude the pancreatic duct. Typically, a total of five to seven posterior outer row sutures are placed and kept well ordered with small clamps, and then tied with minimal tension (Fig. 1). The sutures are then retracted leftwards and electrocautery is used to create a full thickness jejunotomy 2–3 mm from the suture line, typically extending from the penultimate superior silk suture to the penultimate inferior silk suture (Fig. 2). All but the corner sutures are then cut and a vein retractor is placed to hold the jejunal lumen open. The inner posterior aspect of the anastomosis is then created using a running 3-0 polysorb suture. Two sutures are placed at the inferior-most aspect of the jejunal opening. They are placed at essentially the same spot. A continuous running, locking posterior row is then placed by first entering perpendicular to the pancreatic parenchyma and taking a good bite of parenchyma and capsule on the pancreas side. The stitch is continued in a single motion as a full thickness bite of the bowel wall on the jejunal side (Fig. 3). The posterior suture is continued up and around the superior corner of the inner layer of the anastomosis and is then held out of the way under gentle tension with a small clamp. At the completion of the inner posterior row, the probe or Bake’s dilator in the pancreas duct is removed, and a sterile pediatric feeding tube, (size 3.5-, 5.0-, or 8.0-Fr and sized appropriately for the pancreas duct) is placed with one end into the pancreatic duct extending 5 cm into the pancreatic body and the other end through the jejunotomy and downstream into the jejunum. We typically cut the pediatric feeding tube to a length of 20 cm, allowing there to be at least 5 cm within the pancreatic parenchyma and roughly 15 cm into the downstream jejunum. The pediatric feeding tube is not intended as a permanent anastomotic stent but rather as a temporary guide for the placement of the anterior inner row of sutures. Its presence in the lumen of the duct minimizes the chance that any of the anterior inner row sutures will catch the back wall of the duct and occlude the lumen. The pediatric feeding tube is only temporarily left in the lumen through the anastomosis and is removed through the downstream jejunotomy made for the hepaticojejunostomy.
End-to-side pancreaticojejunostomy. The posterior outer row is performed with 3-0 silk, in horizontal mattress fashion (obtained from Ref. [13], with permission)
Invagination pancreaticojejunostomy. The jejunum is opened with electrocautery from the penultimate (next to last) superior silk stitch, to the penultimate inferior silk stitch (obtained from Ref. [13], with permission)
Invagination pancreaticojejunostomy. The posterior inner layer is performed with running locking 3-0 polysorb, taking good bites of the pancreas (parenchyma and capsule) and full thickness bites of the jejunum (obtained from Ref. [13], with permission)
The anterior inner row is then completed by running the second 3-0 polysorb suture from inferior to superior along the anterior aspect of the pancreas. Each bite contains both pancreatic capsule and parenchyma and then a full thickness bite of jejunum. The goal is to invaginate or “dunk” all of the cut edge of the pancreas into the jejunal lumen, thereby allowing apposition of the pancreatic capsule to the jejunal serosa (Fig. 4).
Invagination pancreaticojejunostomy. The anterior inner layer is performed with running 3-0 polysorb, achieving apposition of the pancreatic capsule and the jejunal serosa (obtained from Ref. [13], with permission)
The anterior outer layer is then placed using interrupted 3-0 silk sutures, taking good bites of the pancreatic capsule and parenchyma, and then seromuscular bites of jejunum up to a centimeter away from the inner suture line, allowing the jejunum to roll up over the anterior inner layer (Fig. 5). The sutures are all placed individually and kept well ordered with small clamps and then tied down sequentially. With a gland of soft or normal texture, the first assistant crosses with gentle tension the untied suture adjacent to the one being tied by the operating surgeon, in order to reduce tension and minimize the chance of the suture cutting through the pancreatic parenchyma.
Invagination pancreaticojejunostomy. The anterior outer layer is performed with interrupted 3-0 silk, pulling the mobile jejunum over the immobile suture line, allowing apposition of the jejunal serosa to the pancreatic capsule (obtained from Ref. [13], with permission)
Duct-to-mucosa pancreaticojejunostomy
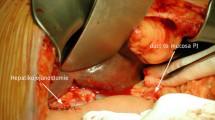
A posterior row of 3-0 silk sutures is placed as described above (see Fig. 1). The pancreatic duct is identified and probed with a lacrimal duct probe or a small Bake’s dilator. A small, full thickness jejunotomy is then created using electrocautery, opposite the pancreatic duct (Fig. 6). The posterior inner layer is then placed using 5-0 polydioxanone (PDS) suture, incorporating good bites of the pancreatic parenchyma and pancreatic duct along with full thickness bites of the jejunum. A total of three to four 5-0 PDS sutures may be adequate along the back row for a small (1–2 mm in diameter) pancreatic duct. With a large pancreatic duct, up to ten 5-0 PDS sutures, spaced no more than 1.5 mm apart, may be required (Fig. 7).
Duct-to-mucosa pancreaticojejunostomy. After the posterior outer row of 3-0 silk sutures is placed, a small hole is created in the jejunum, at the level of the pancreatic duct (obtained from Ref. [13], with permission)
Duct-to-mucosa pancreaticojejunostomy. The posterior inner row of 5-0 polydioxanone (PDS) sutures has been placed into a 5-mm pancreatic duct (obtained from Ref. [13], with permission)
Again, a nice technique to use at this point is to place a sterile pediatric feeding tube, as described above, into the pancreatic duct and the jejunum. The anterior inner row is then placed using 5-0 PDS sutures, taking care to avoid snagging the pediatric feeding tube (Fig. 8). As above, the pediatric feeding tube is only temporary, and is later removed through the downstream jejunotomy made for the hepaticojejunostomy. Once these inner sutures have been tied and cut, the outer anterior row of 3-0 silk sutures is placed. Generous bites of the pancreatic parenchyma and capsule as well good bites of the jejunum up to a centimeter away from the anastomosis are taken, pulling the jejunum up and over the inner suture line (Fig. 9). Again, careful technique is used to avoid tearing the parenchyma of the pancreas when these anterior outer layer sutures are tied.
Duct-to-mucosa pancreaticojejunostomy. A pediatric feeding tube is cut to a length of 20 cm, and 5 cm are placed in the pancreatic duct, and 15 cm are fed into the downstream jejunum (obtained from Ref. [13], with permission)
Duct-to-mucosa pancreaticojejunostomy. The anterior outer row of 3-0 silk sutures is placed, pulling the mobile jejunum over the immobile suture line, allowing apposition of the jejunal serosa to the pancreatic capsule (obtained from Ref. [13], with permission)
Conclusions
Given the importance of a successful pancreaticoenteric anastomosis to the overall outcome in pancreaticoduodenectomy, it is important that the approach used be based upon the best available evidence. The largest randomized trial yet performed comparing end-to-side techniques indicates the superiority of the invagination or “dunking” technique over the duct-to-mucosa approach [10]. These results can be generalized only in the setting of meticulous technique. Any technique, if improperly executed, will perform poorly. The opportunity exists for further investigation, comparing this technique to the newer binding approach, or other techniques in the setting of a prospective randomized trial.
References
Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226(3):248–57 (discussion 257–60).
Bassi C, Butturini G, Molinari E, Mascetta G, Salvia R, Falconi M, et al. Pancreatic fistula rate after pancreatic resection. The importance of definitions. Dig Surg. 2004;21(1):54–9.
Miedema BW, Sarr MG, van Heerden JA, Nagorney DM, McIlrath DC, Ilstrup D. Complications following pancreaticoduodenectomy. Current management. Arch Surg. 1992;127(8):945–9 (discussion 949–50).
Braasch JW, Gray BN. Considerations that lower pancreatoduodenectomy mortality. Am J Surg. 1977;133(4):480–4.
Trede M, Schwall G. The complications of pancreatectomy. Ann Surg. 1988;207(1):39–47.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13.
Strasberg SM, Drebin JA, Mokadam NA, Green DW, Jones KL, Ehlers JP, et al. Prospective trial of a blood supply-based technique of pancreaticojejunostomy: effect on anastomotic failure in the Whipple procedure. J Am Coll Surg. 2002;194(6):746–58 (discussion 759–60).
Tani M, Onishi H, Kinoshita H, Kawai M, Ueno M, Hama T, et al. The evaluation of duct-to-mucosal pancreaticojejunostomy in pancreaticoduodenectomy. World J Surg. 2005;29(1):76–9.
Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs AA, et al. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003;134(5):766–71.
Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S, et al. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg. 2009;208(5):738–47 (discussion 747–9).
Hardacre JM. Is there a learning curve for pancreaticoduodenectomy after fellowship training? HPB Surg. 2010;2010:230287.
Tseng JF, Pisters PW, Lee JE, Wang H, Gomez HF, Sun CC, et al. The learning curve in pancreatic surgery. Surgery. 2007;141(5):694–701.
Kennedy EP, Brumbaugh J, Yeo CJ. Reconstruction following the pylorus preserving Whipple resection: PJ, HJ, and DJ. J Gastrointest Surg. 2010;14(2):408–15.
Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222(4):580–8 (discussion 588–92).
Duffas JP, Suc B, Msika S, Fourtanier G, Muscari F, Hay JM, et al. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. 2005;189(6):720–9.
Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, et al. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. 2005;242(6):767–71 (discussion 771–3).
Langrehr JM, Bahra M, Jacob D, Glanemann M, Neuhaus P. Prospective randomized comparison between a new mattress technique and Cattell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J Surg. 2005;29(9):1111–9 (discussion 1120–1).
Peng SY, Wang JW, Lau WY, Cai XJ, Mou YP, Liu YB, et al. Conventional versus binding pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007;245(5):692–8.
Fernandez-Cruz L, Cosa R, Blanco L, Lopez-Boado MA, Astudillo E. Pancreatogastrostomy with gastric partition after pylorus-preserving pancreatoduodenectomy versus conventional pancreatojejunostomy: a prospective randomized study. Ann Surg. 2008;248(6):930–8.
Acknowledgments
The authors wish to thank Jennifer Brumbaugh, M.A., for the illustrations used in this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kennedy, E.P., Yeo, C.J. Dunking pancreaticojejunostomy versus duct-to-mucosa anastomosis. J Hepatobiliary Pancreat Sci 18, 769–774 (2011). https://doi.org/10.1007/s00534-011-0429-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-011-0429-y