Abstract
Poly γ-glutamic acid is a promising biodegradable polymer from bacterial sources with intense applications in the field of medicine, wastewater treatment, food and cosmetics, etc. In the production process, the yield of polygamma glutamic acid varies with the various parameters like nutrient requirements, culture conditions, and the bacterial strains used for the production and the mode of fermentation. Industrial scale production of this high value-added product is not still feasible because of its high production cost. Due to its commercial interest, the cost-effective process for production, efficient recovery, and purification are the current area of interest. Here, main focus is on the production, purification, characterization, and applications of PGA from bacterial sources.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
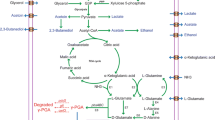
The naturally occurring poly amino acids are poly-γ-glutamic acid, poly-ε-lysine, and cyanophycin (Obst and Steinbüchel 2004). Poly-γ-glutamic acid is an extracellular viscous material produced by several bacteria belonging to Bacillus genus. It is an anionic, water-soluble, biodegradable polyamide which is nontoxic to human and environment. Poly gamma glutamic acid is a polymer of glutamic acid units and is linked by γ-amide linkages (α-amino and γ-carboxylic acid units). It is produced in a ribosome-independent manner and polymerization by the enzyme system present on the membrane of the organism (Hajdu et al. 2008). It is free from protease attack because of the γ-amide linkages. Usually proteins and other peptides are made up of α-amino and α-carboxylic acid units which are susceptible to protease attack. It is an optically active polymer with chiral center in every glutamate residues. Stereochemically, three different forms of PGA have been found: a homopolymer composed of d-glutamate units, a homopolymer of l-glutamate units and a copolymer of d- and l-glutamate units.
PGA was first discovered by Ivanovics and Bruckner as a component of capsules of Bacillus anthracis and they found that the polymer was released into the medium on autoclaving, or on aging and autolysis of the cells. It was also present in the mucilage of “natto” (fermented soybeans, a traditional food in Japan) and later found that γ-PGA was freely secreted into the growth medium of Bacillus subtilis as a product on fermentation. Several Bacillus species have now been shown to produce γ-PGA extracellularly (Kunioka 1997). These include B. licheniformis, B. subtilis, B. megaterium, B. pumilus, B. mojavensis, and B. amyloliquefaciens. A halophilic archaebacterium, Natrialba aegyptiaca sp. is also known to produce PGA, but its difficulty in cultivation makes it unsuitable for fermentative production of PGA. Bacillus licheniformis and Bacillus subtilis are the most commonly used strains for fermentative production of PGA.
PGA has various physiological functions that differ according to the species synthesizing it and its environment. Soil bacteria mainly from the genus Bacillus except B. anthracis use the released PGA for sequestration of metal ions, thus increasing their resistance to adverse environments. PGA produced extracellularly by Bacillus also acts as the source of glutamate during starvation in the late stationary phase. Two highly pathogenic bacteria B. anthracis and Staphylococcus epidermidis synthesize surface-associated PGA, which helps them to escape phagocytosis and thus acts as a virulence factor. B. anthracis capsule is composed exclusively of the D-enantiomer, making it nonimmunogenic and prevents antibodies from gaining access to the bacterium. It protects B. anthracis from phage infections and S. epidermidis against antimicrobial peptides. Thus, anchored PGA protects the pathogen from the immune system and acts as a virulence factor, whereas released PGA may be a persistence factor that protects the bacterium from its environment. It is highly hygroscopic in nature. It can exist either in water-insoluble free acid form or as its salt with a variety of cations (Na+, Mg2+, K+, NH4 + or Ca2+), which is completely soluble (Ogunleye et al. 2015).
Here, the production aspects of PGA, purification, and its characterization by different techniques are mainly discussed. It also explains the applications of PGA in various fields, on which much attention has been recently focused.
2 Fermentative Production of PGA
2.1 Submerged Fermentation
2.1.1 Effects of Nutrients on PGA Production
PGA production by submerged fermentation is influenced by various media components. The yield, molecular weight, and stereochemical composition of γ-PGA are mainly influenced by nutrient composition. Researchers have focused on the nutritional requirements for the improved production of PGA and found that the nutrient requirements varied according to the strain used. Based on the glutamate obligation, PGA-producing strains are classified into two types: glutamic acid dependent and glutamic acid independent strains for PGA production. Exogenous glutamic acid dependent strains include B. licheniformis ATCC 9945 (Troy 1973). B. subtilis IFO 3335 (Kunioka 1995), and B. subtilis F-2-01 (Kubota et al. 1993). The exogenous glutamic acid independent strains include Bacillus licheniformis A35 (Chang et al. 2009), Bacillus subtilis TAM-4 (Ito et al. 1996), Bacillus sp. SAB-26 (Soliman et al. 2005), and Bacillus amyloliquefaciens LL3 (Cao et al. 2011). Glutamic acid dependent strains cannot produce PGA without the addition of glutamate in the medium. The amount of glutamate added to the medium depends on the strain used and also the other medium components. Glutamate showed positive interactions with other medium components during PGA production. As the L-glutamate concentration increases, an increase in PGA production was observed with Bacillus licheniformis ATCC9945 but there was no effect on the molecular size of PGA produced (Kongklom et al. 2015). Glutamate-dependent strains and their yield are shown in Table 1.
The glutamate-independent strain Bacillus subtilis C10 utilizes citric acid and oxalic acid as the substrate for PGA production. It produces PGA by de novo pathway, and oxalic acid enhances the activity of pyruvate dehydrogenase essential for the synthesis of glutamate from glucose. The PGA yield of 27.7 g/l was obtained from this strain. High production of 33.84–35 g/l of PGA was derived from glutamate-independent strain Bacillus methylotrophicus SK19.001 using glycerol, sodium citrate, and peptone as the substrates (Peng et al. 2015). Bacillus licheniformis TISTR 1010 was found to be glutamate-independent strain, and it uses NH4Cl as the main nitrogen source in a modified B medium. High yield of PGA was obtained by fed-batch fermentation, with the continuous supply of glucose and NH4Cl (Kongklom et al. 2015). PGA yield by glutamate-independent strains is low compared to the glutamate-dependent strains. Therefore, the industrial production of PGA mainly focuses on glutamate-dependent strains.
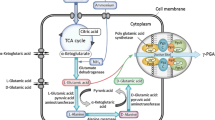
The intracellular glutamate was produced by tricarboxylic acid cycle, and citrate acts as the main precursor for PGA production (Kunioka and Goto 1994). Bacillus uses extracellular ammonium to convert α-keto glutaric acid into glutamic acid. Thus, citrate acts as the main substrate for PGA production.
Bacillus methylotrophicus strain SK 19.001 was glutamate independent and found to produce 33.84 g/l of PGA produced within 36 h of fermentation in the medium containing glycerol, sodium citrate, and peptone as substrate. Intracellular l-glutamate was synthesized in this strain by the increased activity of l-aspartate amino transferase, glutamate synthase, and l-glutaminase enzymes. Organic nitrogen sources were used for the synthesis of intracellular glutamate (Peng et al. 2015). Bacillus subtilis MJ80 was found to produce PGA with or without the addition of glutamate and found to be facultative glutamic acid metabolizing bacterium. It could produce PGA from glutamate and also from soybean powder. Bacillus subtilis MJ80 was found to be glutamate-dependent and glutamate-independent strain. It produced 75.5 and 68.7 g/l of PGA from glutamate and soybean powder, respectively (Ju et al. 2014). Bacillus licheniformis NRC20 was found to be glutamate independent but enhanced the PGA production with the addition of glutamate (Tork et al. 2015).
Glycerol enhances the poly gamma glutamic acid production by increasing the metabolism of known precursors such as citrate and glutamate. It enhances the activity of poly glutamyl synthetase complex which involved in the racemization and polymerization of glutamic acid and thus increasing the PGA production (Bajaj et al. 2009). Addition of glycerol increased the secretion of PGA by changing the phospholipid composition on the cell wall and thus increases the permeability of the cell wall. Since PGA polymerization happens intracellularly and secreted extracellularly, the increase in permeability of the cell membrane increases the secretion of PGA (Du et al. 2005). In Bacillus subtilis CGMCC 0833, the carbon metabolic pathway was found to be regulated by glycerol, tween 80, and dimethyl sulfoxide. By the addition of glycerol, the activity of 2-oxoglutarate dehydrogenase depressed and carbon flux distribution for the synthesis of glutamate was enhanced. Tween 80 and DMSO stimulate the isocitrate dehydrogenase and the carbon flux enhances from 2-oxoglutarate to glutamate. Hence, addition of glycerol, tween 80, and DMSO enhanced the synthesis of intracellular glutamate. In addition, DMSO and tween 80 increase the cell permeability and thus enhances the intracellular uptake of substrate and secretion of PGA (Wu et al. 2008).
In Bacillus licheniformis NCIM 2324, l-glutamine and α-keto glutaric acid are found to be the metabolic precursor for the production of PGA. By the addition of these precursors, the yield and molecular weight of PGA increased (Bajaj and Singhal 2009). The addition of TCA cycle intermediates like glutamine and α-keto glutaric acid to the fermentation media leads to increase in PGA production by enhancing the activity of the enzyme, poly glutamyl synthetase complex. The addition of these precursors enhances the utilization of glutamic acid by the organism for PGA production (Bajaj et al. 2009).
Glycerol enhances the poly gamma glutamic acid production by increasing the metabolism of known precursors such as citrate and glutamate. It enhances the activity of poly glutamyl synthetase complex which involved in the racemization and polymerization of glutamic acid and thus increasing the PGA production. The addition of TCA cycle intermediates like glutamine and α-keto glutaric acid to the fermentation media leads to increase in PGA production by enhancing the activity of the enzyme, poly glutamyl synthetase complex (Bajaj et al. 2009). Addition of glycerol increased the secretion of PGA by changing the phospholipid composition on the cell wall and thus increases the permeability of the cell wall. Glycerol addition leads to decrease in C 16:1 and C 18:1 fatty acids and increase in C 10:0 and C 12:0 fatty acids which may improve the fluidity of the cell membrane. Since PGA polymerization happens intracellularly and secreted extracellularly, the increase in fluidity of the cell membrane increases the secretion of PGA (Du et al. 2005). In Bacillus subtilis CGMCC 0833, the carbon metabolic pathway was found to be regulated by glycerol, tween 80, and dimethyl sulfoxide. The addition of glycerol leads to depression of the activity of 2-oxoglutarate dehydrogenase and carbon flux distribution will be directed for the synthesis of glutamate. Tween 80 and DMSO stimulate the isocitrate dehydrogenase and the carbon flux enhances from 2-oxoglutarate to glutamate. Hence, addition of glycerol, tween 80, and DMSO enhances the synthesis of intracellular glutamate. In addition, DMSO and tween 80 increase the cell membrane permeability and thus enhance the intracellular uptake of substrates and secretion of PGA.
The stereochemical composition of PGA mainly affected by the Mn2+ supply to the production media. In B. licheniformis, Mn2+ enhances both biomass and PGA production by increasing the carbon source utilization such as citrate and glycerol. In B. subtilis, Mn2+ influences the glutamate racemase activity and thus increases the d-glutamate percentage in PGA.
PGA yield, molecular weight, stereochemistry, and the viscosity of the fermentation broth etc mainly influenced by the ions in the PGA production media. The KCl addition to the fermentation medium plays a vital role in controlling PGA production because K+ induces its production. Addition of KCl to the fermentation medium effectively reduces broth viscosity and increases the productivity and molecular weight. K+ regulates the d/l-glutamate ratio by reducing the l-Glutamate percentage in B. subtilis GXA-28 (Zeng et al. 2016) which showed a similar effect with Mn2+ in B. licheniformis ATCC9945A. Mn2+ influences the glutamate racemase activity and thus increases the d-glutamate percentage in PGA (Wu et al. 2006). The yield and the molecular weight of PGA produced can be controlled by verifying the NaCl concentration in the medium, but it mainly depends on the strain used. The high yield of PGA obtained when the NaCl concentration was 8%, but as the NaCl concentration increases in modified E medium, there is a decrease in γ-PGA molecular size (Wei et al. 2010).
High ferric ion (Fe3+) concentrations lead to low-molecular-weight γ-PGA with enhanced production. High FeCl3 concentrations could enhance the expression of γ-PGA synthetase genes (pgsA, pgsB, and pgsC), which leads to the improved production of γ-PGA. In addition, γ-PGA degradation genes contribute to the low-molecular-weight γ-PGA (Feng et al. 2017). PGA could be produced from inorganic nitrogen sources which are cost-effective than glutamate. Inorganic nitrogen sources and α-keto glutaric acid metabolized to form glutamate which further involved in polymerization of PGA in Bacillus subtilis HSF1410 (Ren et al. 2015). And also nitrate enhances the PGA production in Bacillus licheniformis WX-02 by increasing the glutamate assimilation and also by increasing the biosynthesis of glutamic acid inside the cell. Nitrate reduction leads to accumulation of ammonium and thus increases the glutamate synthesis inside the cell. Nitrate acts as a positive inducer of PGA production (Li et al. 2014).
Addition of CaCl2 in fermentation medium leads to decrease in viscosity of the media and enhancing the consumption of extracellular glutamic acid and thus helps in increased production of PGA. It also enhances the activity of three enzymes such as isocitrate dehydrogenase (ICDH), glutamate dehydrogenase (GDH) and 2-oxoglutarate dehydrogenase complex (ODHC), which are involved in conversion of 2-oxoglutarate to glutamate (Meng et al. 2016).
Due to its extensive industrial applications, the cost-effective production of PGA using cheap nutritional sources is one of the prime requirements. Several researchers tried to produce PGA from cheaply available carbon sources. Zhang et al. (2012) used cane molasses and monosodium glutamate waste liquor (contain 1–2% glutamic acid) by Bacillus subtilis NX-2 for the production of PGA and observed 52.1 ± 0.52 g/l of PGA under fed-batch fermentation. Instead of glucose, citrate, sucrose, fructose, and glycerol, the C5 sugar xylose was used as substrate (Zhang et al. 2012). The production of 23.62 g/l PGA was obtained with xylose by Bacillus subtilis HB-1. Based on this, the multiple sugars containing substrate such as corncob fibers hydrolysate were used as an alternative carbon source for the production of PGA. The PGA obtained was high (24.92 g/l) compared to xylose, so that this low cost lignocellulosic biomass can be used for the industrial production of PGA (Zhu et al. 2014).
2.1.2 Effect of Culture Conditions on PGA Production
The PGA is produced as a capsular component or accumulated as highly viscous material in the fermentation medium. The increased viscosity of the fermentation broth leads to decrease in volumetric oxygen transfer and thus oxygen limitation. This affects the cell growth and PGA production (Kongklom et al. 2015). Oxygen plays an important role in the highly aerobic process like the production of PGA. Aeration of the fermentation broth can be increased by increasing the agitation speed. Increased aeration and agitation leads to increased glutamate utilization and thus increased the PGA production (Cromwick et al. 1996). Increased agitation speed enhances the substrate utilization by increasing the activity of enzymes involved in metabolism and results in enhanced PGA production. Oxygen vectors such as n-hexane, n-heptane, n-hexadecane increases the solubility of oxygen in the fermentation broth and thus enhanced the PGA concentration and its molecular weight. However, n-dodecane addition leads to rapid cell growth, which results in loss of nutrients in the fermentation broth and thus decreased the PGA production and its molecular weight. Polydimethylsiloxane (PDMS) is an oxygen carrier that increases the production of PGA in Bacillus subtilis BL53 along with the addition of metabolic precursors such as glutamine and α-keto glutamic acid. By the addition of polydimethylsiloxane to the fermentation medium, there is an increase in oxygen mass transfer rate and thus increases PGA yield.
Physiochemical stress strategy could achieve enhanced γ-PGA production in Bacillus licheniformis WX-2. There was an increase in PGA production by induced osmotic stress by 3% KCl, induced heat stress by 50 °C, and induced alkali stress by pH 8.5. The stress mediated strategy leads to the upregulation of the PGA synthetase genes pgsB and pgsC. This strategy could be used as an efficient method for the production of PGA in future. The growth of microorganism and PGA production are influenced by pH of the fermentation medium. Citrate acts as the main substrate for the production of PGA by Bacillus species and at pH 6.5, there is an enhanced citrate utilization and improved production of PGA (Cromwick et al. 1996). Two-stage pH control strategy was employed in Bacillus subtilis CGMCC 0833 as it is an important parameter for cell growth and for the utilization of extracellular glutamate. During first 24 h, the medium pH was controlled at 7, to obtain maximum cell growth and then to obtain improved production of PGA, pH was maintained at 6.5. The pH shift control strategy leads to increased utilization of glutamate and thus increased the PGA production (Wu et al. 2010).
The molecular weight of PGA depends on the strain used for PGA production and culture conditions. As there is increase in fermentation time, molecular weight of PGA decreases due to the action of the enzyme depolymerase. The PGA acts as a glutamate source for the microorganism during the late stationary phase. High molecular weight PGA is desirable for various applications such as viscosity adding agent and flocculent. The flocculating activity of PGA increases with the increase in molecular weight. Low-molecular-weight PGA required for the drug delivery and the antifreeze activity of PGA increases with the decrease in its molecular weight (Tork et al. 2015).
2.1.3 Modes of Submerged Fermentation
For industrial scale production of poly gamma glutamic acid, various fermentation modes have been tested such as batch, fed-batch and continuous culture, cell recycling and immobilization. Batch and fed-batch fermentation are most common fermentation strategies for PGA production.
To improve PGA production, the aerobic plant fibrous bed bioreactor was constructed and Bacillus subtilis NX-2 was immobilized in the bioreactor. Fed-batch fermentation was done to improve production of highly viscous PGA. Sugarcane bagasse, the cheap lignocellulosic biomass acts as the supporting material for immobilization of cells and to further increase the yield of PGA (Xu et al. 2014a). The cost-effective production of PGA was obtained with lower concentrations of yeast extract (40 g/l) and l-glutamate (30 g/l) in the formulated medium. It was done by fed-batch fermentation by maintaining the glucose concentration to 2–10 g/l. The PGA yield of 101.1 g/l was obtained by this method which was higher than the batch fermentation (Huang et al. 2011). Due to low-cost media formulation, this approach can be used for the large-scale PGA production.
2.1.4 Recovery and Purification of Poly Gamma Glutamic Acid
The recovery and purification of PGA is mainly done by solvent precipitation. The addition of miscible solvents to water reduces the dielectric constant of water, which may cause the PGA in the solution to precipitate. Various solvents such as acetone, methanol, ethanol, 1-propanol, etc., could precipitate of PGA. Among these solvents, maximum recovery is obtained by the addition of ethanol. Cold ethanol provides better recovery than ethanol at room temperature. Recovery of PGA from the fermentation broth involves the following steps. First, remove the biomass from the fermentation broth by centrifugation or filtration. Then add four volumes of the particular solvent to the culture supernatant, and allow to precipitate for 12 h at 4 °C and centrifuge to recover PGA. Repeated precipitation will help to further purification of PGA. Finally, the pellet can be dialyzed to remove the salts and then lyophilized.
The drawback of ethanol precipitation involves the precipitation of proteins and other unwanted products from the fermentation broth and also the need of high amount of ethanol required for precipitation. Ultracentrifugation can be done to concentrate the cell-free broth. During ultracentrifugation, low-molecular-weight particles are rejected and usually retain macromolecules and colloids in a system. PGA with <100 KDa rejected and retain PGA with >100 KDa, and it helps to separate and recover PGA with desired molecular weight. Ultracentrifugation contributes to reduce the amount of ethanol required for precipitation of PGA.
Compared to ethanol precipitation, CuSO4 precipitation is most efficient for poly gamma glutamic acid recovery. By comparing the efficiency and recovery of PGA obtained by CuSO4 precipitation and ethanol precipitation, 85% of the PGA obtained by CuSO4 induced precipitation whereas 82% recovery by ethanol precipitation. CuSO4 precipitation offers better selectivity than ethanol precipitation because 48% of proteins get precipitated by ethanol precipitation but in case of CuSO4 precipitation only 3% of proteins gets precipitated.
2.2 Solid State Fermentation
Solid state fermentation is defined as the fermentation of solids in the absence (or near absence) of free water in which substrate possesses the moisture to maintain the growth and metabolism of microorganism. Agro-industrial waste residues can be used as a solid substrate so that it acts as an environmentally friendly approach for the production of value-added products. Solid state fermentation offers a cheap strategy for the manufacture of poly gamma glutamic acid. Soybean cake powder, wheat bran, dairy manure compost, swine manure, sweet potato, monosodium glutamate production residues, corn flour, etc., alone or in combination have been used as solid substrate for the production (Bajaj and Singhal 2011b; Yong et al. 2011a). SSF acts as an alternative to submerged fermentation. During submerged fermentation, the viscosity of culture broth increases as PGA production increases and thus decreases the oxygen mass transfer. The oxygen limitation affects the growth of microorganism and yield of PGA. Also SmF could be an expensive process, due to the high coast of media components. SSF offers a cheap strategy for the production of industrially important compound and also limits the problems encountered during SmF. The cost-effective production of poly gamma glutamic acid could be achieved by solid state fermentation.
For the production of PGA under SSF by Bacillus amyloliquefaciens C1, dairy manure compost rapeseed cake, corn flour, and monosodium glutamate production residues were used as the solid substrate. Nutrient requirements found to vary with the strain used. In this strain, the 43.7 mg gds−1 PGA was produced with the addition of citrate, MnSO4 and MgSO4 (Yong et al. 2011b). The average of 60 mg gds−1 was reported on Bacillus subtilis using swine manure as the solid substrate (Chen et al. 2005). The PGA yield of 83.61 mg gds−1 was obtained by the strain Bacillus subtilis CCTCC202048 with the mixed substrates of soybean cake powder and wheat bran supplemented with glutamate, citric acid, and NH4NO3 with initial moisture content of 65%, at 40 °C incubated for 42 h (Jian et al. 2005). From Bacillus licheniformis NCIM 2324 98.64 ± 1.61 mg gds−1 was obtained by using the soybean meal as substrate (Bajaj et al. 2008). Solid state fermentation was carried out with Bacillus subtilis NX-2 by using the dry mushroom residues and monosodium glutamate production residues (as the substitute of glutamate). Among dry mushroom residues, the Dry Shiitake Mushroom Residue (DSMR) was found to be the most suitable solid substrate. DSMR and monosodium glutamate production residues ratio was optimized to 12:8 and addition of industrial waste glycerol increased the PGA production under SSF to 107.7 mg gds−1, which was the highest production till date under SSF (Tang et al. 2015).
2.2.1 Recovery After SSF
The recovery of poly gamma glutamic acid from the fermented substrate can be done by the following method. Treat the fresh fermented substrate with distilled water (1:10 w/v, based on initial dry weight of the substrate) in flasks, and mixed at room temperature on a rotary shaker 200 rpm for 2 h and then filtered through two-layer muslin cloth. The filtrate so obtained was centrifuged at 10,000 rpm for 15 min. Clarified supernatants were used for γ-PGA purification (Bajaj et al. 2008). Purification of PGA can be done by the addition of 4 volumes of alcohols such as methanol, ethanol, or other solvents for PGA precipitation. The precipitate was centrifuged at 12000 rpm for 20 min. The other impurities were removed by dialysis and finally lyophilized (Bajaj et al. 2008).
3 Characterization of Poly Gamma Glutamic Acid
The stereochemical composition of PGA can be done by circular dichroism (CD) and also by RT-HPLC. By circular dichroism determination of % of d/l-glutamate unit in pure PGA can be estimated from the standard graph of the same. By RT-HPLC, the d/l amino acids can be measured by Marfey’s reagent, 1-fluoro-2,4-dinitrophenyl-5-l-alanine amide (FDAA). FDAA reagent reacts specifically to d and l amino acid to produce stable derivative and can be determined by RT-HPLC. The poly gamma glutamic acid obtained by fermentation was purified and hydrolyzed with 6 N HCl at 110 °C in a sealed and evacuated tube and hydrolysate neutralized and allowed to react with FDAA. RT-HPLC analyzed samples by using Eclipse XDB-18 column. Commercial d and l-glutamic acid derivatized with FDAA and stereochemical composition of the sample can be determined from the corresponding peaks. For amino acid analysis, hydrolysis of PGA can be done by the method mentioned above. Then thin layer chromatography can be done on a cellulose plate with solvent systems of butanol–acetic acid–water (3:1:1, w/w) and 96% ethanol–water (63:37, w/w) (Stewart and Young 1984; Yokoi et al. 1995). The purity of PGA can be determined by the presence of only of glutamic acid. Amino acids were detected by spraying with 0.2% ninhydrin in acetone. The total carbohydrate content can be determined by phenol sulfuric acid method montreuil (Montreuil et al. 1986). Bradford assay can determine the protein content by keeping bovine serum albumin as standard (Bradford 1976). The viscosity of the culture broth, after cell removal, can be measured using a conventional Ostwald viscometer at 30 °C and ratio between polymer viscosity and solvent viscosity gives the relative viscosity (Tork et al. 2015).
Gel permeation chromatography could be used to measure the number average molecular weight of the PGA. The number average molecular weight of PGA will be varied with the strain and the culture conditions. GPC can be done with Shodex KB800 series columns with a refractive index (RI) detector using deionized water with flow rate 1 ml/min as the mobile phase. Dextran standards with varying molecular weight can be used to construct a calibration curve (Shih et al. 2005). Molecular weight can also be determined by the measuring the diffusion distance of the concentric zone on neutral red plates. By this method, the molecular weight of PGA produced by Bacillus licheniformis NRC20 is determined as 1266 KD (Tork et al. 2015).
Structural elucidation of PGA can be done by H1 NMR and C13 NMR spectroscopy. The peak shift of PGA has been reported by many researchers. Samples for NMR spectroscopy have to be dissolved in D2O. Liquid chromatography can be done to determine the d-/l-glutamate composition of PGA with Crownpak CR (+) column (Goto and Kunioka 1992). Functional groups in PGA can be analyzed by Fourier Transform Infrared Spectroscopy (FT-IR).
4 Applications of PGA
Microbial PGA is produced by ribosome independent manner and has attractive properties such as water soluble, biodegradable, edible, and anionic in nature. It is nontoxic to human and environment. Hence, it could be used for extensive applications. Applications of γ-PGA are shown in Fig. 1.
4.1 Flocculation
It has extensive properties such as solubility in water, edible, biodegradable, and nontoxic to human and environment. These properties lead to the wide applications of PGA. It can be used as a bio-flocculent in wastewater treatment, downstream processing of food industries, and fermentation industries. In wastewater treatment, PGA is used for the flocculation of solid waste and metals (Deng et al. 2003). As the molecular weight increases, the flocculation efficiency increases. Ultra-high molecular weight PGA produced by the bacterial strains such as Bacillus subtilis P-104 shows high flocculating activity (Zhao et al. 2013). Cations, pH, and temperature are the main factors influencing the flocculation efficiency of PGA. Cations stimulate the flocculating activity of PGA by neutralizing and stabilizing the negative charge on the functional groups of the bio-flocculent by forming bridging between particles. Synergistic effects on flocculation occurred at different pH based on the cations and the extent of synergistic effect on flocculation decreased in the order, trivalent (Al3+ and Fe3+), bivalent (Ca2+and Mg2+), and monovalent ions (Na+ and K+). pH between 6 and 7 and temperature of 30 °C was found to be effective for flocculation by PGA. The flocculation activity of PGA decreased with increasing the incubation temperature and completely lost by heating up to 120 °C due to the destruction of polyamide structure of PGA.
PGA could efficiently remove basic dyes from aqueous solution. The electrostatic interaction of PGA and dyes leads to its sorption at pH above 5 and the removal of dyes from PGA takes place at pH 1, which helps to reuse the spent γ-PGA (Inbaraj et al. 2006). During wastewater treatment PGA could be used as a good adsorbent for the removal of mercury (II). The adsorption of mercury by PGA takes place at pH above 3 and reached maximum at pH 6. Desorption of mercury from PGA can be possible by treating it with the distilled water with pH 2 (pH adjusted to 2 by the addition of HCl) to reuse the spend PGA (Inbaraj et al. 2009). Presence of lead in soil inhibits plant growth. Lead removal from the soil can be made possible by PGA. Studies were carried out on Brassica chinensis L. seedlings in laboratory conditions and found that lead inhibited the growth of this plant. PGA acts as a good adsorbent for the lead at pH 5 (Chunhachart et al. 2014).
The synthetic flocculants can be replaced with PGA because it can be used as the flocculant in waste water treatment, downstream processing of food, pharmaceutical, and medicine industries. In food and fermentation industries, PGA can be used as a bio-flocculent to harvest microalgae. Lipid producing microalgae has commercial interest in biodiesel producing industries. Harvesting of the microalgae with PGA is cost-effective, and it avoids the loss of lipid due to the breakage of the algal cell during centrifugation and other harvesting techniques (Zheng et al. 2012). The γ-PGA produced from Bacillus licheniformis CGMCC 2876 was found to be an efficient bio-flocculent in the sugar cane industry. The color and turbidity of the sugarcane juice was IU 1877.36 and IU 341.41 with 0.8 ppm of γ-PGA, respectively. This was good as the sugar cane juice obtained by the most widely is used chemically synthesized flocculent in the sugarcane industry such as poly acrylamide with 1 ppm. Thus, PGA could be used as a potential substitute for polyacrylamide in the sugar refinery process (Yan et al. 2015).
4.2 Fertilizer
Plant growth and development are improved by the addition of fertilizers to the soil. PGA can be used as a synergist to chemical fertilizers to avoid the environmental pollution. PGA helps to improve growth by increasing the nutrient utilization even in depleted nutrient condition. The activity of soil enzymes such as urease, sucrose, and catalase increased and the total nitrogen accumulation in soil increased by the nitrogen immobilized microbes after the supplementation of PGA (Xu et al. 2013). PGA promoted the growth of Chinese cabbage and increased the content of total nitrogen, soluble protein, and soluble amino acids in leaves. The activity of enzymes involved in nitrogen metabolism and assimilation are increased by the addition of PGA. PGA facilitates the influx of Ca in the cytoplasm and Ca acts as a positive signal for nitrogen metabolism and thus promoted the growth of plants (Xu et al. 2014b). The protease-producing bacteria Bacillus subtilis strain NX-2 is used for the fermentative production of water-soluble fertilizer by using soybean meal as a substrate under SSF. The protease will degrade the proteins present in the soybean meal and Bacillus subtilis strain NX-2 used these amino acids for the fermentative production of water-soluble fertilizer. The fermented product can be directly used as a fertilizer and showed improved effect on rapeseed growth (Wang et al. 2014).
4.3 Cryoprotectant
PGA is having antifreeze activity because of its high anionic amino acid composition (Shih et al. 2003). Polymers with acidic amino acids have high antifreeze activity compared to other polymers. PGA with molecular weight below 20,000 had antifreeze activity greater than that of glucose and the antifreeze activity decreased in the order Na salt = K salt > Ca salt > acidic form (Shih et al. 2003). PGA produced from Bacillus subtilis natto has the ability to protect Lactobacillus paracasei during freeze drying. PGA was found to protect the probiotic bacteria Lactobacillus paracasei significantly better than sucrose. The probiotic Bifidobacteria strains (Bifidobacteria longum, Bifidobacteria breve) were necessary for proper functioning of the gastrointestinal tract (Bhat et al. 2015). PGA protects these cells in fruit juices and helps to survive the cells in the harsh environments of the digestive tract.
4.4 Applications in Food and Cosmetics
PGA is a promising food ingredient because of its physiological and physic-chemical characteristics. Ca supplementation along with PGA, increases Ca absorption by increasing the solubility of Ca in the intestinal tract and also PGA is not susceptible to intestinal enzymes (Tanimoto et al. 2007). PGA has an antidiabetic effect because it reduces the rate of intestinal absorption of sugar. The absorption of insulin-mimetic inorganic salt vanadyl sulfate increases by conjugating with PGA. γ-PGA vanadyl complex having high insulin-mimetic activity than free vanadyl sulfate and thus reduces the intestinal absorption of glucose. Food supplement with K-γ-PGA composition prevents the increase of blood pressure by reducing sodium absorption and thus helps in controlling hypertension (Kishimoto et al. 2008).
Supplementation of PGA can prevent osteoporosis of bones because it increases the solubility of Ca in invitro and also in vivo. Thus, it helps in increasing the absorption of Ca in rats and postmenopausal women and thus increases Ca absorption in bones (Tanimoto et al. 2001, 2007). PGA is having antifreeze activity so that it acts as a cryoprotectant for frozen foods. PGA also used as a thickener in foods/beverages. And it prevents aging and improves the texture of foods. During deep frying of foods, PGA can be added, and it reduces the oil uptake and moisture loss. Due to its water retention capacity PGA helps to control water evaporation and produce a dense matrix with improved integrity. Thus, PGA can be used as a functional oil reducing agent in deep-fat fried foods (Lim et al. 2012).
PGA is having a vital role in cosmetics because PGA-vitamin C complex increases the solubility of Vitamin C. Vitamin C is important for collagen formation and collagen helps in skin repair. Vitamin C also having antioxidation activity leads to antiaging. The properties of PGA such as hygroscopicity and skin compatibility make it an active ingredient in cosmetic compositions. In cosmetic compositions, PGA helps in maintaining the skin moisturization and elasticity by inhibiting the hyaluronidase enzyme that degrades the hyaluronic acid present in the skin dermis. And it helps in relieving allergic symptoms by inhibiting the permeability of inflammatory cells. The cosmetic ingredient with PGA-vitamin complex shows increased stability, improved absorption, and sustained release of vitamins from the complex (Sung et al. 2008, 2014).
4.5 Biomedical Applications
4.5.1 As Hydrogels
Hydrogel is a biocompatible material that can swell in water and have the capacity to hold water inside its structure. It has extensive applications in the field of drug delivery and as a scaffold in tissue engineering. Hydrogels can be prepared by γ-irradiation, chemical, or physical cross-linking. Biodegradable hydrogels were prepared by cross-linking of microbial PGA and l-lysine by amide bond in the presence of DMT-MM in water and form ester bond in the presence of WSC in DMSO. The use of chemical cross-linkers may be unfavorable for biological applications. The noncovalent interactions (physical cross-links) avoid the need for irradiation and toxic chemical cross-linkers (Murakami et al. 2011).
PGA reacted with PVA in aqueous solution to form hydrogel without any chemical treatment. Increasing the PGA concentration increases the water retention capacity and elongation of hydrogel but decreases the tensile strength. Adsorption of proteins and platelet adhesion on hydrogel decreases with the rise of PGA concentration and thus improved the blood compatibility of the hydrogel. Due to water resistance, blood compatibility, and mechanical properties, PGA-PVA hydrogel became good biomaterial for blood containing medical devices (Lin et al. 2006).
Chitosan-PGA PEC hydrogels are prepared by simple ionic gelation method in which amino group of chitosan and the carboxylic group of PGA interact to form hydrogels. The ionic interaction was confirmed by FT-IR. Due to the presence of PGA, the hydrophilic nature of hydrogel increased and thus having high affinity to cell and attracts serum proteins essential for cell attachment and proliferation. It shows antibacterial activity against E. coli and S. aureus and was biocompatible for cell culture and proliferation (Tsao et al. 2010).
4.5.2 Nanoparticles
Drug and gene delivery are made possible by nanoparticles. Due to the small size of the nanoparticle, it could escape from the recticulo endothelium system and thus the circulation time in blood increases. PGA is hydrophilic and water soluble and used to deliver anticancer drugs. Nanoparticles formed from γ-PGA and chitosan have been used for the oral delivery of hydrophobic drugs and proteins. PGA-chitosan nanoparticles were found to be the efficient system for the delivery of insulin for the treatment of diabetics (Lin et al. 2005, 2007; Mukhopadhyay et al. 2012). The ζ-potential and the particle size can be modified by varying the composition of the reaction mixture. Particles of a mean diameter of 218 nm and ζ-potential of +21 mV could able to penetrate a monolayer of Caco-2 cells, which are an in vitro model system for the small intestine. It also functions as an effective carrier for DNA and siRNA delivery because of its preparation by simple ionic gelation method in aqueous solution without any solvents (Lee et al. 2008). The heavy metal such as lead and cadmium can be removed with superparamagnetic iron oxide nanoparticle coated with PGA. This could be prepared by coprecipitation method (Rajan et al. 2014). In vitro removal of lead and cadmium was done in simulated gastrointestinal fluid and metal solution and found that SPIONs-PGA nanoparticle could be used as a metal chelator for the clinical treatment of metal poisoning (Inbaraj and Chen 2012). PGA can be modified with phenylalanine ethyl ester to make the material amphiphilic in nature. It acts as a good carrier for the poorly water-soluble drugs (Akagi et al. 2005) and also acts as a carrier for protein delivery. γ-PGA-l-phenylalanine esters were non-cytotoxic material and shown not to release proteins entrapped inside the particle for 10 days under physiological conditions.
Targeted drug delivery helps to deliver the chemotherapeutic agents and other drugs to the specific sites without any side effects. Active targeting enhances the interaction between NP and the target site and thus increased internalization of drugs through receptor-mediated endocytosis. The targeting ligands mainly used are antibodies and antibody fragments, aptamers, peptides, sugars, and small molecules. Asialoglycoprotein receptors are overexpressed in hepatoma cells and galactosamine used as a ligand to target hepatoma cells. The anticancer drug, doxorubicin, and galactosamine as a targeting moiety were conjugated to the carrier γ-PGA. Conjugation of DOX to γ-PGA decreased its cytotoxicity on hepatoma cells, but the conjugation of galactosamine to γ-PGA-DOX restored the cytotoxicity of the conjugate to hepatoma cells. Low-intensity ultrasound treatment increased the cytotoxicity of γ-PGA-DOX conjugated.
4.5.3 Tissue Engineering
Tissue engineering is the process of developing biological substitutes to restore and maintain the functions of tissue. In tissue, engineering cells are cultured on a scaffold to form tissue and then implanted in patients body or cells with scaffold implanted directly into patients and the tissue will develop inside the body. This helps to overcome immune response between donors and acceptors. The anionic nature of poly glutamic acid is highly hydrophilic, this reduces its use as scaffold but the esterification of –COO– to γ-PGA ethyl, γ-PGA-propyl and γ-PGA-benzyl enhances its water resistance and thus become the versatile material for tissue engineering. γ-PGA-Bn showed better cell adhesion and viability compared to others and the attachment of integrin-binding RGD peptide to unmodified –COO– groups making the scaffold target integrin-binding mechanisms in cells. Thus, the electrospun γ-PGA-Bn scaffolds were developed as a good biomaterial for in situ human mesenchymal stem cell differentiation. For bone tissue engineering, PGA/hydroxyapatite monolith (PGA/HAp) was prepared by biomineralization and used as a scaffold. PGA/HAp monolith was found to be nontoxic to cells, and it absorbed bone morphogenetic protein-2 (BMP-2) and released into the medium during cell culture. PGA/HAp monolith enhances the BMP-induced alkaline phosphatase activity (which is an early stage marker for osteogenic differentiation) compared to PGA monolith itself. PGA/HAp acts as a good biomaterial for bone regeneration. Poly γ-glutamic acid graft chondroitin sulfate/polycaprolactone scaffolds could be used for cartilage tissue engineering. The extracellular matrix gets replaced by this scaffold because it provides the structure for the cells to be attached and proliferate. Polycaprolactone provides the mechanical strength and γ-PGA provides high water binding capacity and signal polysaccharides from chitosan. Thus, it helps to repair and regenerate articulate cartilage. The scaffold provides an ideal environment for cellular adhesion and the induction of differentiation.
Tubular scaffolds of gelatin and poly ε-(caprolactone) block poly(γ-glutamic acid) blending hydrogel act as the good biomaterial for cell proliferation. It has properties such as biocompatible, high elasticity, and the cell proliferation was in time-dependent manner. This scaffold was suitable for the proliferation of smooth intestinal muscle cells of rats. The in vitro degradation of the scaffold was fast but no degradation occurred when cells are present.
Human mesenchymal stem cells have great potential for tissue engineering and cell-based therapies. These stem cells have many roles in tissue remodeling and regeneration by differentiation into multiple lineages and immune suppression. Chitosan/PGA has good biocompatibility, and it assembled into polyelectrolyte multilayer films by layer by layer method. Chemokine stromal derived factor-1 into these complexes and are released periodically. CS/PGA PEMS with SDF-1 recruit hMSCs and thus promote the cell proliferation.
4.5.4 Biological Adhesive
Wound healing involves blood coagulation, inflammation, fibroplasia, collagen deposition, and wound contraction. PGA along with other components such as gelatin or collagen acts as an alternative to conventional surgical glue. Conventional surgical glue may lead to the low rate of degradation and chronic inflammation. The most common poly gamma glutamic acid based biological adhesive was water-soluble carbodiimide cross-linked gelatin and poly gamma glutamic acid hydrogel. This hydrogel glue was found to be effective in sealing the lung air leakage (Bajaj and Singhal 2011a). It acts as a potential replacement to the widely used surgical glue, fibrin. In diabetic rat models, wound healing was found to be improved by a novel layered hydrogel composed of alginate, chitosan, poly gamma glutamic acid. The swelling behavior of the hydrogel increases with the increase of the concentration of PGA and water vapor evaporation rate maintained at the ideal level for wound healing in this hydrogel. Ca release rate is high and Ca helps in releasing coagulation factors. Thus, AL-CS-PGA hydrogel acts as effective glue for wound healing in rat models (Lee et al. 2012).
5 Future Perspectives
PGA is an interesting polymer because of its wide applications in the field of medicine, agriculture, wastewater treatment and food industries, etc. Extensive research has been carried out on the economical production of PGA by many researchers. Culture conditions and the nutrient requirements vary with the strains used for PGA production. Different fermentation strategies are carried out for the effective production of PGA. The high production cost is the main drawback for the industrial scale production of PGA. The productions of PGA from economically feasible materials are the extensive area of research for the cost-effective production of PGA. The extensive studies are carried out on the economical production of the eco-friendly material.
Abbreviations
- γ-PGA:
-
Poly-γ-glutamic acid
- DMSO:
-
Dimethyl sulfoxide
- TCA:
-
Tricarboxylic acid
- ICDH:
-
Isocitrate dehydrogenase
- GDH:
-
Glutamate dehydrogenase
- ODHC:
-
2-oxoglutarate dehydrogenase complex
- PDMS:
-
Polydimethylsiloxane
- DSMR:
-
Dry shiitake mushroom residue
- SSF:
-
Solid state fermentation
- FDAA:
-
1-fluoro-2,4-dinitrophenyl-5-l-alanine amide
- GPC:
-
Gel permeation chromatography
- KD:
-
Kilodalton
- RI:
-
Refractive index
- NMR:
-
Nuclear magnetic resonance spectroscopy
- FT-IR:
-
Fourier transform infrared spectroscopy
- WSC:
-
Water-soluble carbodiimide
- PVA:
-
Poly vinyl alcohol
- NP:
-
Nanoparticle
References
Akagi T, Kaneko T, Kida T, Akashi M (2005) Preparation and characterization of biodegradable nanoparticles based on poly(γ-glutamic acid) with l-phenylalanine as a protein carrier. J Controlled Release 108:226–236
Ashiuchi M, Kamei T, Baek D-H, Shin S-Y, Sung M-H, Soda K, Yagi T, Misono H (2001) Isolation of Bacillus subtilis (chungkookjang), a poly-γ-glutamate producer with high genetic competence. Appl Microbiol Biotechnol 57:764–769
Bajaj IB, Singhal RS (2009) Enhanced production of poly(γ-glutamic acid) from Bacillus licheniformis NCIM 2324 by using metabolic precursors. Appl Biochem Biotechnol 159:133–141
Bajaj I, Singhal R (2011a) Poly(glutamic acid)—an emerging biopolymer of commercial interest. Bioresour Technol 102:5551–5561
Bajaj I, Singhal R (2011b) Poly(glutamic acid)—an emerging biopolymer of commercial interest. Bioresour Technol 102(10): 5551–5561
Bajaj I, Lele S, Singhal R (2008) Enhanced production of poly(γ-glutamic acid) from Bacillus licheniformis NCIM 2324 in solid state fermentation. J Ind Microbiol Biotechnol 35:1581–1586
Bajaj IB, Lele SS, Singhal RS (2009) A statistical approach to optimization of fermentative production of poly(γ-glutamic acid) from Bacillus licheniformis NCIM 2324. Bioresour Technol 100(2):826–832
Bhat A, Irorere V, Bartlett T, Hill D, Kedia G, Charalampopoulos D, Nualkaekul S, Radecka I (2015) Improving survival of probiotic bacteria using bacterial poly-γ-glutamic acid. Int J Food Microbiol 196:24–31
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao M, Geng W, Liu L, Song C, Xie H, Guo W, Jin Y, Wang S (2011) Glutamic acid independent production of poly-γ-glutamic acid by Bacillus amyloliquefaciens LL3 and cloning of pgsBCA genes. Biores Technol 102:4251–4257
Chang K-Y, Cheng L-W, Ho G-H, Huang Y-P, Lee Y-D (2009) Fabrication and characterization of poly(γ-glutamic acid)-graft-chondroitin sulfate/polycaprolactone porous scaffolds for cartilage tissue engineering. Acta Biomater 5:1937–1947
Chen X, Chen S, Sun M, Yu Z (2005) High yield of poly-γ-glutamic acid from Bacillus subtilis by solid-state fermentation using swine manure as the basis of a solid substrate. Biores Technol 96:1872–1879
Chunhachart O, Kotabin N, Yadee N, Tahara Y, Issakul K (2014) Effect of lead and γ-polyglutamic acid produced from Bacillus subtilis on growth of brassica chinensis L. APCBEE Procedia 10:269–274
Cromwick AM, Birrer GA, Gross RA (1996) Effects of pH and aeration on γ-poly(glutamic acid) formation by Bacillus licheniformis in controlled batch fermentor cultures. Biotechnol Bioeng 50:222–227
Deng S, Bai R, Hu X, Luo Q (2003) Characteristics of a bioflocculant produced by Bacillus mucilaginosus and its use in starch wastewater treatment. Appl Microbiol Biotechnol 60:588–593
Du G, Yang G, Qu Y, Chen J, Lun S (2005) Effects of glycerol on the production of poly(γ-glutamic acid) by Bacillus licheniformis. Process Biochem 40:2143–2147
Feng J, Shi Q, Zhou G, Wang L, Chen A, Xie X, Huang X, Hu W (2017) Improved production of poly-γ-glutamic acid with low molecular weight under high ferric ion concentration stress in Bacillus licheniformis ATCC 9945a. Process Biochem 56:30–36
Goto A, Kunioka M (1992) Biosynthesis and hydrolysis of poly(γ-glutamic acid) from Bacillus subtilis IF03335. Biosci Biotechnol Biochem 56:1031–1035
Hajdu I, Bodnár M, Filipcsei G, Hartmann JF, Daróczi L, Zrínyi M, Borbély J (2008) Nanoparticles prepared by self-assembly of Chitosan and poly-γ-glutamic acid. Colloid Polym Sci 286:343–350
Huang J, Du Y, Xu G, Zhang H, Zhu F, Huang L, Xu Z (2011) High yield and cost-effective production of poly(γ-glutamic acid) with Bacillus subtilis. Eng Life Sci 11:291–297
Inbaraj BS, Chen B-H (2012) In vitro removal of toxic heavy metals by poly(γ-glutamic acid)-coated superparamagnetic nanoparticles. Int J Nanomed 7:4419
Inbaraj BS, Chiu C, Ho G, Yang J, Chen B (2006) Removal of cationic dyes from aqueous solution using an anionic poly-γ-glutamic acid-based adsorbent. J Hazard Mater 137:226–234
Inbaraj BS, Wang J, Lu J, Siao F, Chen B (2009) Adsorption of toxic mercury (II) by an extracellular biopolymer poly(γ-glutamic acid). Bioresour Technol 100:200–207
Ito Y, Tanaka T, Ohmachi T, Asada Y (1996) Glutamic acid independent production of poly(γ-glutamic acid) by Bacillus subtilis TAM-4. Biosci Biotechnol Biochem 60:1239–1242
Jian X, Shouwen C, Ziniu Y (2005) Optimization of process parameters for poly γ-glutamate production under solid state fermentation from Bacillus subtilis CCTCC202048. Process Biochem 40:3075–3081
Ju W-T, Song Y-S, Jung W-J, Park R-D (2014) Enhanced production of poly-γ-glutamic acid by a newly-isolated Bacillus subtilis. Biotech Lett 36:2319–2324
Kishimoto N, Morishima H, Uotani K (2008) Compositions for prevention of increase of blood pressure Japanese patent application. Publication(2008-255063)
Kongklom N, Luo H, Shi Z, Pechyen C, Chisti Y, Sirisansaneeyakul S (2015) Production of poly-γ-glutamic acid by glutamic acid-independent Bacillus licheniformis TISTR 1010 using different feeding strategies. Biochem Eng J 100:67–75
Kubota H, Matsunobu T, Uotani K, Takebe H, Satoh A, Tanaka T, Taniguchi M (1993) Production of poly(γ-glutamic acid) by Bacillus subtilis F-2-01. Biosci Biotechnol Biochem 57:1212–1213
Kunioka M (1995) Biosynthesis of poly(γ-glutamic acid) from l-glutamine, citric acid and ammonium sulfate in Bacillus subtilis IFO3335. Appl Microbiol Biotechnol 44:501–506
Kunioka M (1997) Biosynthesis and chemical reactions of poly(amino acid) s from microorganisms. Appl Microbiol Biotechnol 47:469–475
Kunioka M, Goto A (1994) Biosynthesis of poly(γ-glutamic acid) from l-glutamic acid, citric acid, and ammonium sulfate in Bacillus subtilis IFO3335. Appl Microbiol Biotechnol 40:867–872
Lee P-W, Peng S-F, Su C-J, Mi F-L, Chen H-L, Wei M-C, Lin H-J, Sung H-W (2008) The use of biodegradable polymeric nanoparticles in combination with a low-pressure gene gun for transdermal DNA delivery. Biomaterials 29:742–751
Lee Y-H, Chang J-J, Yang M-C, Chien C-T, Lai W-F (2012) Acceleration of wound healing in diabetic rats by layered hydrogel dressing. Carbohydr Polym 88:809–819
Li X, Gou X, Long D, Ji Z, Hu L, Xu D, Liu J, Chen S (2014) Physiological and metabolic analysis of nitrate reduction on poly-gamma-glutamic acid synthesis in Bacillus licheniformis WX-02. Arch Microbiol 196:791–799
Lim S-M, Kim J, Shim J-Y, Imm B-Y, Sung M-H, Imm J-Y (2012) Effect of poly-γ-glutamic acids (PGA) on oil uptake and sensory quality in doughnuts. Food Sci Biotechnol 21:247–252
Lin Y-H, Chung C-K, Chen C-T, Liang H-F, Chen S-C, Sung H-W (2005) Preparation of nanoparticles composed of chitosan/poly-γ-glutamic acid and evaluation of their permeability through Caco-2 cells. Biomacromol 6:1104–1112
Lin W-C, Yu D-G, Yang M-C (2006) Blood compatibility of novel poly(γ-glutamic acid)/polyvinyl alcohol hydrogels. Colloids Surf B 47:43–49
Lin Y-H, Chen C-T, Liang H-F, Kulkarni AR, Lee P-W, Chen C-H, Sung H-W (2007) Novel nanoparticles for oral insulin delivery via the paracellular pathway. Nanotechnology 18:105102
Meng Y, Dong G, Zhang C, Ren Y, Qu Y, Chen W (2016) Calcium regulates glutamate dehydrogenase and poly-γ-glutamic acid synthesis in Bacillus natto. Biotech Lett 38:673–679
Montreuil J, Bouquelet S, Debray H, Fournet B, Spik G, Strecker G, Chaplin M, Kennedy J (1986) Carbohydrate analysis: a practical approach carbohydrate analysis: a practical approach. IRL Press, Oxford, pp 143–204
Mukhopadhyay P, Mishra R, Rana D, Kundu PP (2012) Strategies for effective oral insulin delivery with modified chitosan nanoparticles: a review. Prog Polym Sci 37:1457–1475
Murakami S, Aoki N, Matsumura S (2011) Bio-based biodegradable hydrogels prepared by crosslinking of microbial poly(γ-glutamic acid) with l-lysine in aqueous solution. Polym J 43:414–420
Obst M, Steinbüchel A (2004) Microbial degradation of poly(amino acid) s. Biomacromolecules 5:1166–1176
Ogawa Y, Yamaguchi F, Yuasa K, Tahara Y (1997) Efficient production of γ-polyglutamic acid by Bacillus subtilis (natto) in jar fermenters. Biosci Biotechnol Biochem 61:1684–1687
Ogunleye A, Bhat A, Irorere VU, Hill D, Williams C, Radecka I (2015) Poly-γ-glutamic acid: production, properties and applications. Microbiology 161:1–17
Peng Y, Jiang B, Zhang T, Mu W, Miao M, Hua Y (2015) High-level production of poly(γ-glutamic acid) by a newly isolated glutamate-independent strain, bacillus methylotrophicus. Process Biochem 50:329–335
Rajan YC, Inbaraj BS, Chen BH (2014) In vitro adsorption of aluminum by an edible biopolymer poly(γ-glutamic acid). J Agric Food Chem 62:4803–4811
Ren Y, Huang B, Meng Y, Wei L, Zhang C (2015) Metabolic and phylogenetic analyses based on nitrogen in a new poly-γ-glutamic acid-producing strain of Bacillus subtilis. Biotech Lett 37:1221–1226
Shih I, Van Y, Chang Y (2002) Application of statistical experimental methods to optimize production of poly(γ-glutamic acid) by Bacillus licheniformis CCRC 12826. Enzym Microb Technol 31:213–220
Shih L, Van Y-T, Sau Y-Y (2003) Antifreeze activities of poly(γ-glutamic acid) produced by Bacillus licheniformis. Biotech Lett 25:1709–1712
Shih L, Wu P-J, Shieh C-J (2005) Microbial production of a poly(γ-glutamic acid) derivative by Bacillus subtilis. Process Biochem 40:2827–2832
Soliman NA, Berekaa MM, Abdel-Fattah YR (2005) Polyglutamic acid (PGA) production by Bacillus sp. SAB-26: application of Plackett-Burman experimental design to evaluate culture requirements. Appl Microbiol Biotechnol 69:259–267
Stewart JM, Young JD (1984) Solid phase peptide synthesis. Pierce Chemical Company, USA
Tang B, Xu H, Xu Z, Xu C, Xu Z, Lei P, Qiu Y, Liang J, Feng X (2015) Conversion of agroindustrial residues for high poly(γ-glutamic acid) production by Bacillus subtilis NX-2 via solid-state fermentation. Bioresour Technol 181:351–354
Tanimoto H, Mori M, Motoki M, Torii K, Kadowaki M, Noguchi T (2001) Natto mucilage containing poly-γ-glutamic acid increases soluble calcium in the rat small intestine. Biosci Biotechnol Biochem 65:516–521
Tanimoto H, Fox T, Eagles J, Satoh H, Nozawa H, Okiyama A, Morinaga Y, Fairweather-Tait SJ (2007) Acute effect of poly-γ-glutamic acid on calcium absorption in post-menopausal women. J Am Coll Nutr 26:645–649
Tork SE, Aly MM, Alakilli SY, Al-Seeni MN (2015) Purification and characterization of gamma poly glutamic acid from newly Bacillus licheniformis NRC20. Int J Biol Macromol 74:382–391
Troy FA (1973) Chemistry and Biosynthesis of the poly(γ-d-glutamyl) capsule in Bacillus licheniformis I. Properties of the membrane-mediated biosynthetic reaction. J Biol Chem 248:305–315
Tsao CT, Chang CH, Lin YY, Wu MF, Wang J-L, Han JL, Hsieh KH (2010) Antibacterial activity and biocompatibility of a chitosan–γ-poly(glutamic acid) polyelectrolyte complex hydrogel. Carbohydr Res 345:1774–1780
Wang J, Liu Z, Wang Y, Cheng W, Mou H (2014) Production of a water-soluble fertilizer containing amino acids by solid-state fermentation of soybean meal and evaluation of its efficacy on the rapeseed growth. J Biotechnol 187:34–42
Wei X, Ji Z, Chen S (2010) Isolation of halotolerant Bacillus licheniformis WX-02 and regulatory effects of sodium chloride on yield and molecular sizes of poly-γ-glutamic acid. Appl Biochem Biotechnol 160:1332–1340
Wu Q, Xu H, Xu L, Ouyang P (2006) Biosynthesis of poly(γ-glutamic acid) in Bacillus subtilis NX-2: regulation of stereochemical composition of poly(γ-glutamic acid). Process Biochem 41:1650–1655
Wu Q, Xu H, Shi N, Yao J, Li S, Ouyang P (2008) Improvement of poly(γ-glutamic acid) biosynthesis and redistribution of metabolic flux with the presence of different additives in Bacillus subtilis CGMCC 0833. Appl Microbiol Biotechnol 79:527–535
Xu H, Jiang M, Li H, Lu D, Ouyang P (2005) Efficient production of poly(γ-glutamic acid) by newly isolated Bacillus subtilis NX-2. Process Biochem 40:519–523
Xu Z, Wan C, Xu X, Feng X, Xu H (2013) Effect of poly(γ-glutamic acid) on wheat productivity, nitrogen use efficiency and soil microbes. J Soil Sci Plant Nutr 13:744–755
Xu Z, Feng X, Zhang D, Tang B, Lei P, Liang J, Xu H (2014a) Enhanced poly(γ-glutamic acid) fermentation by Bacillus subtilis NX-2 immobilized in an aerobic plant fibrous-bed bioreactor. Biores Technol 155:8–14
Xu Z, Lei P, Feng X, Xu X, Liang J, Chi B, Xu H (2014b) Calcium involved in the poly(γ-glutamic acid)-mediated promotion of Chinese cabbage nitrogen metabolism. Plant Physiol Biochem 80:144–152
Yan S, Yao H, Chen Z, Zeng S, Xi X, Wang Y, He N, Li Q (2015) Poly‐c‐glutamic acid produced from Bacillus licheniformis CGMCC 2876 as a potential substitute for polyacrylamide in the sugarcane industry. Biotechnol Prog 31(5):1287–1294
Yokoi H, Natsuda O, Hirose J, Hayashi S, Takasaki Y (1995) Characteristics of a biopolymer flocculant produced by Bacillus sp. PY-90 J Ferment Bioeng 79:378–380
Yong X, Cui Y, Chen L, Ran W, Shen Q, Yang X (2011a) Dynamics of bacterial communities during solid-state fermentation using agro-industrial wastes to produce poly-γ-glutamic acid, revealed by real-time PCR and denaturing gradient gel electrophoresis (DGGE). Appl Microbiol Biotechnol 92:717–725
Yong X, Raza W, Yu G, Ran W, Shen Q, Yang X (2011b) Optimization of the production of poly-γ-glutamic acid by Bacillus amyloliquefaciens C1 in solid-state fermentation using dairy manure compost and monosodium glutamate production residues as basic substrates. Biores Technol 102:7548–7554
Yoon SH, Do JH, Lee SY, Chang HN (2000) Production of poly-γ-glutamic acid by fed-batch culture of Bacillus licheniformis. Biotech Lett 22:585–588
Zeng W, Liang Z, Li Z, Bian Y, Li Z, Tang Z, Chen G (2016) Regulation of poly-γ-glutamic acid production in Bacillus subtilis GXA-28 by potassium. J Taiwan Inst Chem Eng 61:83–89
Zhang D, Feng X, Zhou Z, Zhang Y, Xu H (2012) Economical production of poly(γ-glutamic acid) using untreated cane molasses and monosodium glutamate waste liquor by Bacillus subtilis NX-2. Bioresour Technol 114:583–588
Zhao C, Zhang Y, Wei X, Hu Z, Zhu F, Xu L, Luo M, Liu H (2013) Production of ultra-high molecular weight poly-γ-glutamic acid with Bacillus licheniformis P-104 and characterization of its flocculation properties. Appl Biochem Biotechnol 170:562–572
Zheng H, Gao Z, Yin J, Tang X, Ji X, Huang H (2012) Harvesting of microalgae by flocculation with poly(γ-glutamic acid). Bioresour Technol 112:212–220
Zhu F, Cai J, Zheng Q, Zhu X, Cen P, Xu Z (2014) A novel approach for poly-γ-glutamic acid production using xylose and corncob fibres hydrolysate in Bacillus subtilis HB-1. J Chem Technol Biotechnol 89:616–622
Acknowledgements
We acknowledge the financial support provided by University Grant Commission (UGC).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jose Anju, A., Sindhu, R., Parameswaran, B., Pandey, A. (2018). Production, Characterization, and Applications of Microbial Poly-γ-Glutamic Acid. In: Varjani, S., Parameswaran, B., Kumar, S., Khare, S. (eds) Biosynthetic Technology and Environmental Challenges. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-10-7434-9_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-7434-9_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7433-2
Online ISBN: 978-981-10-7434-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)