Abstract
Production of recombinant pharmaceutical protein is a multibillion-dollar industry and plays a crucial role in treatment of many diseases. Among the several potent microbial production systems, yeast offers several advantages in production of pharmaceutically important proteins due to its unicellular nature, easy gene manipulation, cost-effectiveness and fast growth and incorporates post-translational modifications in heterologous proteins. Saccharomyces cerevisiae is the widely used heterologous host for the production of medically important proteins and drugs; however, several non-conventional yeast species including Hansenula polymorpha, Pichia pastoris, and Yarrowia lipolytica are also gaining much attention as alternative heterologous hosts for the industrial production of therapeutic proteins. In this chapter, most recent advances in glycoengineering of yeast for successful therapeutic pharmaceutical production, current progress in humanization of yeast and various interventions in the secretory mechanisms and pathways in yeast for the improvement of the production of pharmaceutical proteins are overviewed. In addition, emerging genetic, omics, systems and synthetic biology tools and other technologies to enhance the efficiency of yeast pharmaceutical proteins are precisely discussed. The use of synthetic biology tools in yeast for the production of pharmaceuticals is clearly entering a new phase right now. Combination of yeast systems biology data with synthetic biology will open new vistas to better production, improved glycosylation and secretory mechanism. The application of currently available synthetic biology tools like CRISPR/Cas9 in yeast pathway engineering is also discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Biopharmaceuticals are important part of modern medical biotechnology and current commercial market of pharmaceutics are $70–80 billion and annual growth rates between 7 and 15% are expected (Walsh 2010; Goodman 2009). As a heterologous protein production host, yeast has been used to synthesize a wide range of products including terpenoids, sterols, organic acids, biofuels, derivatives of sugars, fatty acids, terpenes, different aromatics, peptides and other engineered therapeutic proteins. Above all, the heterologously produced biopharmaceutical proteins are one of the rapid-growing and attractive classes of biomedicine. Recombinant vaccines, interferons, interleukins, hormones like insulin, glucagon, human growth hormone (hGH) and erythropoietin (EPO) and other therapeutic proteins are examples for protein biopharmaceuticals. Antibodies represent the large group of protein pharmaceuticals (Wriessnegger and Pichler 2013; Liu et al. 2013).
Unlike the traditional E. coli, yeast (Saccharomyces cerevisiae, Pichia pastoris) based expression systems offer the advantages of inexpensive cultivation, easy induction, efficient secretion and a protein synthetic machinery closer in functioning to the higher order animals which has advantages in production of several proteins of interest in pharma and diagnostic industries and requires efficient post-translational modification. Mammalian cell lines like Chinese Hamster Ovarian cell line offers the advantages of human-like N-glycosylation and gave better pharmacokinetics (Mattanovich et al. 2012). However, as compared to other widely used expression hosts, they need complex growth media and are more sensitive to growth conditions than E. coli or yeast. Their growth rate is relatively slow and is highly susceptible to viral contamination (Kim et al. 2014). The prokaryotic protein production host E. coli uses simple media components, easy cultivation conditions and attains high growth density in minimum time with high biomass production rate and protein yield. However, E. coli has the drawbacks of inefficient folding of proteins, low protein secretion capacity and lack of post-translational modifications (Swartz 2001). Yeast is generally recognized as safe (GRAS) organism and has a good tolerance to acidic pH conditions and other fermentation inhibitors (Nielsen 2013). The complete genome sequence of S. cerevisiae was the first-sequenced eukaryotic genome (Goffeau et al. 1996), currently the complete genome sequences of many industrially important yeast species are available in the public domain (Dujon et al. 2004; De Schutter et al. 2009; Ravin et al. 2013), facilitating the function level genomics approaches based on various genomics and proteomics technologies. Systems biology-based approaches could enhance our knowledge of yeast metabolic and physiological networks, which can help to identify current bottlenecks in recombinant protein production. The advent of novel technologies and tools in synthetic biology facilitated quick and efficient engineering metabolic pathway and secretion machinery to improve heterologous protein production, based on the data generated from systems-level approaches. Thus, synthetic biology and other genome engineering tools are expected to revolutionize the construction of ‘yeast cell factories’ for the production of biopharmaceuticals with improved yield and quality (Vogl and Glieder 2013). The characteristics of various yeast cell factories used for recombinant protein production and some biopharmaceuticals produced in them are described in Table 1.
In this chapter, we focus on new developments in the area of pharmaceutical production by reengineered yeast species by employing new metabolic system and synthetic biology tools, secretion pathway engineering, etc.
2 Secretory Pathways in Yeast and Engineering
Bacteria are known for their efficiency in producing pharmaceutically important heterologous proteins; however, they lack machinery to perform folding, trafficking and secretion of eukaryotic proteins as these are the main post-translational modifications carried out in a eukaryotic system. Here comes the importance of ‘Yeast’ as it is able to produce and do the post-translational modifications and release heterologous eukaryotic proteins in their indigenous and biologically efficient form. Yeast cells are generally recognized as safe organisms and are predominant hosts for biopharmaceutical production. S. cerevisiae is one of the well-described and commonly used eukaryotic host systems since from the budding of recombinant protein production (Martinez et al. 2012). Hansenula polymorpha, Pichia pastoris, Yarrowia lipolytica, Schizosaccharomyces pombe and Kluyveromyces lactis are also used as unconventional source for recombinant protein manufacturing (Kim et al. 2015).
The secretory expression is one of the important criteria for facilitating the subsequent protein purification process. On the other hand, there are several barriers that obstruct the secretory expression of heterologous proteins in yeast. Since the protein expression level is low in eukaryotic systems, several experiments have been done to engineer yeast for enhanced protein production, which mainly includes optimization of fermentation process (Caspeta et al. 2015), selection of the expression vectors systems (Partow et al. 2010), identifying the signal sequence for extracellular targeting (De Pourcq et al. 2012a, b, c) and engineering host strains for better protein-folding and post-translational modification (Idiris et al. 2010a, b). Nonetheless, nearly all of these efforts effectively work only for one or a few proteins which could not be extrapolated as a common way for manufacturing diverse collection of recombinant proteins (Hou et al. 2012). Apart from this, the quantity of protein produced by yeast is found to be lower (100–1000 fold) than the theoretically estimated range (Robson 2007; Schroder 2007). Hence, there is an urgent prerequisite of improving the recombinant protein expression in yeast, especially focusing the secretion system. One of the effective strategies to overwhelm the defects in yeast secretion pathways is genetic modification of yeast strains. This strategy is aided by prevailing postgenomic and system biology technology. As evident, there are many cross-linked factors that complicate the process of modulation of the yeast secretion system (Idiris et al. 2010a, b). This section focuses on latest approaches and their leverage for efficient engineering of yeast strains for potent protein secretion.
2.1 The Secretory Pathway
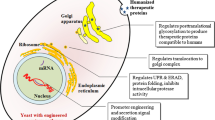
Before maturation and targeting to specific location, secreted proteins undergo many post-translational modifications through a common corridor called ‘the secretory pathway’. A simple schematic representation of secretory pathway implemented by yeast is shown in Fig. 1 with major bottlenecks involved in heterologous protein expression. However, the pathway is somewhat complex and is estimated that more than 160 proteins that are responsible for different post-translational processes like folding, glycosylation, etc., are involved in the accomplishment of the pathway (Nielsen 2013). The protein folding is mainly controlled by endoplasmic reticulum (ER) through the ER-resident protein-folding machinery (Anelli and Sitia 2008). Just after synthesis from ribosome, the secretory proteins are translocated to ER through translocon protein Sec61protein seen on the ER membrane. Thereafter, the incipient proteins are obliged to the BiP or calnexin proteins for their correct folding. The folding usually involves signal sequence processing, disulfide bond formation, N-glycosylation, glycosyl-phosphatidyl-inositol addition, degradation and sorting.
An illustrative diagram showing yeast secretory pathway and distinctive barriers in the secretory pathway of heterologous proteins in yeast. The barriers are denoted with numbers 1–6. 1 Ineffective translocation, 2 Misfolding, aggregation, UPR and ERAD, 3 Intracellular degradation, 4 ALP pathway and missorting, to vacuole, 5 CPY pathway and missorting, to vacuole and 6 Degradation of extracellular secreted proteins
Only accurately folded and compiled proteins are transported to Golgi complex through vesicles, where it undergoes further modifications to being shifted to extracellular matrix, vacuoles or other organelles. In the mean time, misfolded proteins are identified by the quality control system in ER and are redirected to degradation process via BiP complex (Yoshida 2007). Eventually, if the misfolded protein binds to the BiP complex for a prolonged time, the unfolded protein response (UPR) gets induced, this activates proteolysis and inhibits the transcription and translocation of the target protein. Due to the stringent quality control mechanisms in the ER, protein folding often has an inclination to become the most rate-limiting obstacle in heterologous protein secretion. Thus, genetic alteration of the ER protein folding and control mechanism in ER has become the most useful approach in the existing strain engineering technique.
2.2 Endoplasmic Reticulum as a Target for Enhancing Recombinant Protein Secretion
The efficiency of protein expression and quality of protein can be enhanced through engineering of the protein secretory machinery. The initial stage of secretion mechanism is the relocation of target protein through ER (as explained earlier) into the secretion pathway, depends upon secretion signal peptides for the translocation of proteins into ER (Kim et al. 2015). Therefore, the capability of leader sequence portrays a significant part in the secreted protein production. The synthetic prepro-leader sequence exhibited more efficient protein secretion when correlated to the prepro-leader sequences of native and alpha-mating factor (Swartz 2001). Misfolded proteins cause a luminal burden in ER which further induces UPR that accordingly reduces elevated stress in ER. Hence, by ER luminal environment manoeuvring, betterment of the secretion efficiency can be enhanced by the overexpression of chaperones and redox enzymes in ER (Payne et al. 2008). For instance, the overexpression of the activated mutant of heat shock factor 1 initiates heat shock response (Hou et al. 2012) and Hac1p (Guerfal et al. 2010) contributes to enriched protein secretion mechanism. A few studies have reported a 5-fold increase in secretion of human erythropoietin (Robinson et al. 1994) and a 26-fold increase in bovine prochymosin (Harmsen et al. 1996) in S. cerevisiae, through the overexpression of the chaperone BiP; and a 7-fold increase in human leukocyte elastase inhibitor secretion by overexpressing polyubiquitin gene UBI4 in S. cerevisiae. However, overexpression of BiP halts the action of other chaperone mechanisms, as it increases the strength of binding between BiP and its target proteins, which drives to endoplasmic associated degradation (ERAD) rather than discharge of protein (Kauffman et al. 2002).
A different concept to encourage the secretory pathway effectively is by manipulating the UPR pathway which involves Hac1p, PDI, PPI and ERAD genes. On the basis of different studies, many targets in the UPR pathway have been identified for improving protein secretion (Hou et al. 2012) and are achieved by reducing ER stress and its subsequent impairment caused by heterologous protein production results in augmented secretion of the protein. Heterologous overexpression of Hac1 improves protein secretion of Bacillus amylase (2.4-fold), endogenous invertase (2-fold) and recombinant α-amylase (70%) (Valkonen et al. 2003). The production of recombinant human transferrin (rTf) was enhanced by introducing deletion of YPS1 and HSP150 and PDI1 overexpression in S. cerevisiae which in turn results in improved yield and quality (Finnis et al. 2010). The overexpression of UPR pathway genes is protein or host-specific and regulatory effects between chaperones, hence Schröder (2008) recommended that co-expression of multiple chaperones and folding partners in the ER lumen may improve the expression of different heterologous proteins. Single and multiple gene introductions of S. cerevisiae chaperones to P. pastoris have improved recombinant protein secretion. However, a synergistic effect was seen in multiple gene introductions as the protein secretion was much higher in multiple genes than the single gene introduction (Zhang et al. 2006).
2.3 Glycoengineering of Yeast
Glycoengineering modifications play significant roles in proper folding, half-life, and pharmacokinetic properties of recombinant proteins (Fidan and Zhan 2015). Initial glycosylations occur during translocation that helps with proper protein folding, protects from proteases and provides beckon for quality control. Yeast is able to carry out N-linked and O-linked glycosylation. N-linked glycosylation is accomplished by adding a 14 sugar glycan tree to the asparagine residue of the recognition sequence (Bause 1983). The N-linked glycosylation is completed by the ER-resident oligosaccharyl transferase (Burda and Aebi 1998). O-mannosyltransferases catalyse O-linked glycosylation at the hydroxyl groups of serine and threonine (Strahl-Bolsinger et al. 1999) and this O-linked glycosylation happens prior to N-linked glycosylation, showing that N-linked asparagine glycosylation and O-linked serine/threonine glycosylation possibly will be in competition (Ecker et al. 2003). Interestingly, as indigenous high-mannose yeast N-glycans interact with components of immune system especially with human C-type lectins, it is not appropriate for medical use without modifications (Hamilton and Gerngross 2007).
The pharmacokinetic characteristics, potency, immunogenicity and half-life of therapeutic proteins are greatly affected by the structure and the number of mannose glycans present (Rabinovich et al. 2012; Piirainen et al. 2014). Hence, the so-called glycoengineered yeast strains have been developed with the capability to produce human-like glycans rather than yeast-specific ones. In glycoengineered yeasts, N-glycan humanization generally occurs in three steps. Eliminating the yeast hypermannolysation is the first stage which is achieved by disrupting or deleting glycotransferase genes while expressing mannosidase genes, ensuing human-like Man5GlcNAc2 glycoform (De Pourcq et al. 2012a) or deletion of ALG3 gene to obtain human-like Man3GlcNAc2 glycoform (De Pourcq et al. 2012b). In the second stage, a glycoform with GlcNAc terminal was generated using N-acetylglucosamine transferase, followed by the addition of second GlcNAc sugar to mannose resulting in GlcNAc2–Man3GlcNAc2 (Fidan and Zhan 2015). In the last step, heterologous synthetic genes are introduced to produce sialylated glycoproteins (Hamilton et al. 2006).
In the same way, O-linked glycosylation reduces the pharmacokinetic properties of proteins as these proteins interact with human mannose lectins (Rabinovich et al. 2012). To overcome this issue, the PMT gene was disrupted to reduce the possession of O-glycan, however this technique will not eliminate all O-glycans as this disruption is usually interrupted by the O-mannosyltransferases available in yeast. As an alternative, O-glycans were reengineered of in yeast through mimicking mammalian O-glycosylation which mainly involves the action of a-1,2-mannosidase and b-1,2-N-acetylglucosaminyltransferase enzymes to produce the human-like sialylated glycoforms (Hamilton et al. 2013) or through including genes encoding Bacillus subtilis UDP-Gal/GalNAc 4-epimerase, human UDP-Gal/GalNAc transporter, human ppGalNAc-T1 and Drosophila melanogaster core 1 b1–3 GalT (Amano et al. 2008) which introduces mucin-type O-glycosylation in S. Cerevisiae. Even though, glycoengineering of yeast to remodel N- and O-glycosylation helps to optimize the pharmacokinetic characteristics of recombinant proteins, more studies are necessary to completely humanize N- and O-glycosylation pathways in yeast and retain the consistency of glycosylation (Lepenies and Seeberger 2014).
3 Protein Trafficking as a Target for Enhancing Recombinant Protein Secretion
Through the engineering of protein expression and folding mechanisms in yeast, the recombinant proteins can be folded precisely in ER, even then the secretion of the required protein is regularly deprived because the protein secretion machinery takes place in more than a few cell compartments (Fig. 1). ER, Golgi, trans-Golgi network, endosome and either cell membrane or vacuole are the cell compartments that are involved in the secretory machinery of yeast for protein trafficking (Ellgaard and Helenius 2003). Sec1p, Sly1p, Vps45p and Vps33p are some of the responsible proteins at each vesicle trafficking steps and overexpression of these proteins resulted in 70% rise in on the whole α-amylase production (Hou et al. 2012). After exact folding of newly synthesized proteins in the ER lumen, they are gathered into discrete vesicles, which are further targeted to a precise acceptor cubicle. On the whole, secretory effects are secured by several intracellular membrane proteins that determine each traffic step and correct vesicular destination. As a result, genetic augmentation of traffic pathway is needed mainly on the terms of disorganized traffic or missorting, which frequently marks in intracellular build-up of the target proteins for secretion.
Co-translational and post-translational pathways are availed by nascent proteins for cytosol to ER targeting. The indigenous ribosomal protein usually utilizes signal recognition particle (SRP) and its receptor in co-translational translocation pathway (Kida et al. 2007); however proteins have reasonable hydrophobic signal sequences which helps them to flee from detection by the SRP during their production in cytosol (Rapoport 2007). In both translocation secretory pathways, protein targeting specificity is predominantly accorded by the hydrophobic core of the secretory signal sequences and its synergy with SRP in cytosol (Kida et al. 2009). Hence, several types of N-terminal secretory signal sequences have been established for each host system and yeast prepro-sequences like mating pheromone α- factor signal MFα1 are used regularly (Fuller et al. 1989). The yield of heterologous proteins of Escherichia coli phytase, human lysosomal acid lipase and interleukin-6 was enhanced in fission yeast S. pombe using a prepro-secretory signal peptide P4 (Giga-Hama 1997). Preprotoxin signal peptide—viral K28 has been reported to be used as signal peptide for the secretion of GFP in four yeast species, Candida glabrata, P. pastoris, S. cerevisiae and S. pombe, indicating the possibility of the viral signal peptides as exceptional gears to speed up the recombinant protein production (Eiden-Plach et al. 2004).
Even though the proteins intended for secretion are folded correctly into their indigenous structure inside the ER lumen, they failed to secrete completely in some cases. This vacuolar missorting is mainly caused by the vacuolar protein sorting (vps) receptor Vps10p, which recognize and targets vacuolar carboxypeptidase Y to the vacuole (Iwaki et al. 2006; Idiris et al. 2010a, b). A single vps10 deletion is not sufficient to block completely the vacuolar missorting pathway due to the presence of the alkaline phosphatase pathway (Conibear and Stevens 1998). Therefore further systematic analysis of gene functions of the vacuolar missorting pathway is necessary. Even though, some studies have showed the possible traffic modification in yeast engineering, it seems that complete blocking of genes is because of the complexity of membrane trafficking mechanisms affecting the viability of cells.
4 Protein Degradation as a Target for Enhancing Recombinant Protein Secretion
Yeast produces abundant number of proteases that disintegrate the expressed protein thereby reducing the yield and virtue of the interested protein. The disintegration occurs during the pathways involved in endocytosis to vacuole and from post-Golgi sorting to vacuole (Tyo et al. 2014). Various techniques have been adapted to avoid disintegration which mainly includes modification of growth conditions and addition of protease inhibitors (Gonzalez-Lopez et al. 2002; Kang et al. 2000). However, these techniques are of host specific and have limitations. Accordingly, many protease-deficient yeast strain, as genetic alteration of the host proteases minimizes host-specific degradation (Chung and Park 1998; Komeda et al. 2002; Jønson et al. 2004). Multiple deletions of YPS1, YPS2, YPD3, YPS6 and YPS7 genes have done in S. cerevisiae for the efficient secretory production of recombinant proteins (Kim et al. 2014). This technique efficiently reduces the cleavage of recombinant parathyroid hormone protein (Cho et al. 2010) proving that the removal of proteases potentially increases the efficiency of protein secretion.
5 Synthetic Biology Tools for Improving Pharmaceutical Production in Yeast
Different pathways for alcohols, lipids, terpenoids and polyketides have been constructed synthetically by introducing individual gene parts from different organisms and transferred into E. coli host (Singh 2014). This approach can be implemented for several other molecules. The atremisin production is a classic example for the success of synthetic biology approach (Paddon et al. 2013). Another pioneering work in the area of synthetic biology was the synthetic pathway design for d-hydroxy phenyl glycine—the essential building block for the penicillins and cephalosporins (Müller et al. 2006). They constructed the synthetic pathway by incorporating hydoxy mandelate synthase and a hydroxyl mandelate oxidase from Streptomyces coilicolor and Amycolatopsis orientalis and hydroxyl phenyl glycine aminotransferase from Pseudomonas putida.
The widely accepted employment of synthetic biology in health sector is the microbial synthesis of different pharmaceutical like artemisinin, the antimalarial drug. This drug is now produced large quantities in several heterologous hosts like E. coli and S. cerevisiae (Paddon et al. 2013). Last few years witnessed dramatic increase in the production of pharmaceuticals with the help of synthetic biology tools like taxol (Jiang et al. 2012), farnesene (Zhu et al. 2014) and many others.
6 Role of System Biology in Synthetic Biology of Yeast
System biology is the union of theoretical and experimental findings. The experimental concepts include high throughput analysis of various omics technologies. All these omics approaches help to improve the understanding of the complex cellular networks and circuits (Lee et al. 2011). Information obtained from these approaches can be integrated into a biological database for further use. This database can be further used for several computational modelling and simulation—the theoretical system biology (Jung et al. 2010). In summary, the massive volume of biological data generated allows the theoretical system biology for the mathematic modelling and computer simulation. System biology and synthetic biology have already successfully been applied to metabolic engineering for strain development and increasing the production of target molecule (Stephanopoulos 2012). The system biology tools can be used to identify the problems to increase the target molecule. Sanchez and Nielsen (2015) discussed how we can integrate different omics data into genome-scale metabolic models. Recently, full proteome of S. cerevisiae has been characterized by Picotti et al. (2013) and also structural alterations in proteins also have been profiled (Feng et al. 2014) and both will aid in the future developments of metabolic models and synthetic regulatory network construction. Recently targeted mutation for enhancing succinic acid in S. cerevisiae has been identified (Otero et al. 2013). The genes encoding succinate dehydrogenase (SDH3) and glycine biosynthetic genes were deleted and this led to the forced production of glycine from glyoxylate, and as a result cells will produce succinate. Further conversion of succinate is not possible due to the deletion of SDH3 and succinate starts accumulates. The growth rate of recombinant stain was improved by adaptive laboratory evolution. Transcriptome analysis of this strain identified a possible target isocitrate lyase (coding gene is ICL1). The overexpression of this ICL1 resulted in the increased production of succinate and resulted in the improved production (80 fold) of succinate. This provided a real insight into the importance of system and synthetic biology for the production of biochemicals. The transcriptional level regulatory systems in yeast is highly complex and more than 80% of the transcriptional control elements are interacting (Osterlund et al. 2015).
6.1 Various Steps Synthetic Biology Technique
6.1.1 Pathway Design
Because of the difficulties in marketing new chemicals, most biotechnological production has centered on molecules with well established market. Initially, most of the microbial production system has been focussed on the high-value low-volume products like drugs and pharmaceuticals, in which the value in the commercial market could produce an immediate impact (Baeshen et al. 2014). Later the scenario has been changed to low-value high-volume products like biofuels, industrial enzymes, etc. The selection of a molecule is based on economic consideration and commercial market.
6.1.1.1 Target Gene Identification and Metabolic Pathway Construction
After the selection of a target molecule, the best production pathway for that molecule has to be identified. Previously, the pathway identification involves manual selection and was based on very little gene protein expression data. Advanced technologies in next-generation sequencing and recent developments in bioinformatics analyses have changed the scenario of gene discovery for synthetic biology and metabolic engineering by enabling a large genome data. Many computational tools are in public domain to use this genome data for gene mining, identification of gene clusters, etc. The recent introduction of computer-assisted programs and database based on biological reaction and availability of huge genome data has automated the identification process and implemented several scoring methods for pathway selection (Medema et al. 2014).
Biosynthetic gene cluster consists of several genes arranged in an operon or more than one operon and their expression is tightly regulated by several regulators at transcriptional and translational level. Most of these regulators are overlapping with or situated in neighbouring genes make the individual engineering of each genes difficult and they are under the tight control of several transcription factors and regulators (Temme et al. 2012). The concept of genetic parts implies that they are modular parts with well-defined function. This can be applied to biosynthetic gene cluster by ‘refactoring’ in which the natural genetic information are modified and rewritten in a way to make the gene manipulation more easy (Chan et al. 2005). In this process, several non-essential genes, UTR regions, regulatory elements like promoter, RBS and transcription regulators are eliminated and several crucial genes are codon-randomized to remove any undercharacterized regulatory gene (Carrera and Jaramillo 2013). The newly designed coding DNA sequences are synthesized in vitro and introduce to regulate the transcription and protein biosynthesis. This technique was tested in phage and in bacteria (Durante Rodríguez et al. 2016). Another example for refactoring is the replacement of natural control element (promoter) in the spectinabilin gene cluster of Streptomyces orinoci with highly characterized and strong promoter increased the heterologous production of spectinabilin to an amount of 105 μg/l (Shao et al. 2013).
The key importance of synthetic biological approach to pathway design is several possible roots of different pathways considered for the production of a single compound. Some computational tools are designed for the alteration of the metabolic regulatory pathway by using gene knockouts or by the introduction of new catalytically active enzymes. Others involved in finding entirely new metabolic pathway other than the first one (Prather and Martin 2008). One important tool for metabolic pathway prediction is From Metabolite to Metabolite (FMM)—freely available software (Medema et al. 2012). This includes the compilation of the KEGG map and KEGG ligand data to obtain a combined pathway. The list of softwares available for pathway design is enlisted in Table 2.
6.1.1.2 Synthetic Genetic Circuits
Synthetic genetic circuits are essential for controlling the target molecule production (Kalir et al. 2001). In natural condition, the natural molecule production is controlled by different regulatory networks containing DNA, RNA, interacting proteins, transcription factor, all these components work together for the production of a target compound. In this context, synthetic genetic elements circuits help to regulate the target molecule production by artificially connecting different inputs and outputs of different regulatory factors to control the cell mechanism to produce target molecule. Synthetic genetic circuits can be used to create different cascades, genetic on/off switches, logic gates, oscillators and different feedback motifs to control a synthetic pathway. Most of the natural biosynthetic clusters involved in the production of natural molecules and their natural regulation are not enough to produce the desired molecule at high titres (Brophy and Voigt 2014).
6.1.2 Pathway Construction
Combinational prospecting of new natural products from genome requires information about biosynthetic gene cluster. The derived knowledge about biosynthetic gene clusters has to be converted into gene constructs for increasing the production capability. This requires simultaneous screening of different designed DNA constructs and then increasing the titre of the production (Kakule et al. 2014).
Most of these are achieved by combinatorial biosynthesis. That is a pathway can be engineered by changing the combination of genes involved in a biosynthetic gene cluster to increase the product yield (Sánchez et al. 2005). Currently, there are two types of pathway construction strategies: (a) De novo synthesis: here the whole gene clusters are chemically synthesized (Kosuri and Church 2014). (b) DNA assembly: here the different genes are assembled together to construct large cassette. Several gene assembly methods are currently available like Gibson assembly, isothermal assembly, golden gate assembly, etc.
6.1.3 Alteration of Host Metabolism—Metabolic Flux Control
The engineering of host metabolism to redirect the metabolic flux in favour of the newly introduced biosynthetic pathway is extremely important. The newly introduced pathway normally depends on the cellular machinery for the generation of initial building blocks as well as the necessary cofactors like ATP, NADH and NADPH (Chubukov et al. 2014). Cells normally sense the increased demand for all these factors and normalize their internal metabolism. Sometimes this imposes extra burden on cell and resulted in stressed condition and leads to decreased expression of introduced biosynthetic gene cluster (Wu et al. 2015). To resolve all these problems, several stoichiometric—metabolic models are applied for considering the whole metabolic network and for studying the effect of host cell metabolism during the introduction of new biosynthetic gene cluster (King et al. 2015).
Table 2 summarizes various synthetic biology tools at different modification levels that have recently been used for heterologous protein production by yeast. Synthetic biology tools for the assembly of genes of multigene pathways include Gibson assembly, gateway recombination, DNA assembler and other gene assembly techniques like BioBrick™ and ePathBrick (Gibson et al. 2009; Endy and Knight 2008). Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), yeast oligo-mediated genome engineering, CRISPR-associated (Cas) systems, Transcription Activator-Like Effector Nucleases (TALENs) and Zinc Finger Nucleases (ZFNs) are the important genome editing and engineering tools. Recent technique, CRISPR–Cas9 systems revolutionized the field of genome editing which efficiently and quickly modifies several endogenous genes in different biomedically important cell types and in other organisms in which the genetic manipulation is challenging. This technology has greatly helped for the genome engineering of model yeasts (S. cerevisiae and Schizosaccharomyces pombe) and resulted in the increased homologous recombination efficiency and further genetic manipulation.
6.1.4 CRISPR–Cas
Yeast synthetic biology witnessed a huge transformation with the advent of CRISPR–Cas (Clustered Regularly Interspaced Short Palindromic Repeat) (Cong et al. 2013). CRISPR-mediated RNA-guided control of gene using nuclease-deficient Cas9 (dCas9) was experimentally proved to regulate the expression of genes inside the yeast, with the help of synthetic single stranded-guide RNAs (sgRNAs) which specifically target the genes to silence. This technique helped to introduce a series of simultaneous targeted genome integrations and double-stranded breaks in S. cerevisiae genome easily and efficiently (Bao et al. 2015). The dCas9 was fused to Mxi1, another protein that involved in the formation of histone deacetylase Sin3p homolog, a component of gene repressor complex in yeast. Further, this technique has been used to repress the TEF promoter of S. cerevisiae about 10-fold (Gilbert et al. 2013). This technique has also been applied to improve industrially important non-conventional K. lactis strain, where muconic acid production was improved by incorporating six genes at three targeted loci (Horwitz et al. 2015).
7 Conclusions
Modern innovations in yeast have revolutionized the field of heterologous protein production. Yeast systems are established end-to-end platforms for the discovery, development, and manufacture of biopharmaceuticals. Notable developments include glycoengineering systems, humanized strains, established synthetic biology tools, display technologies and libraries, and high throughput and scalable fermentation and downstream processing platforms to aid the use of yeast in all aspects of research and development. These new technologies have a high potential to boost the production of biopharmaceuticals.
References
Ahmad M, Hirz M, Pichler H, Schwab H (2014) Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Environ Microbiol 98:5301–5317
Amano K, Chiba Y, Kasahara Y, Kato Y, Kaneko MK, Kuno A, Ito H, Kobayashi K, Hirabayashi J, Jigami Y, Narimatsu H (2008) Engineering of mucin-type human glycoproteins in yeast cells. Proc Nat Acad Sci USA 105(9):3232–3237
Anelli T, Sitia R (2008) Protein quality control in the early secretory pathway. EMBO J 27:315–327
Baeshen NA, Baeshe MN, Sheikh A, Bora RS, Ahmed M, Ramadan HI, Saini K, Redwan EM (2014) Cell factories for insulin production. Microb Cell Fact 13:141
Bao Z, Xiao H, Liang J, Zhang L, Xiong X, Sun N (2015) Homologyintegrated CRISPR-Cas (HI-CRISPR) system for one-step multigene disruption in Saccharomyces cerevisiae. ACS Synth Biol 4:585–594
Bause E (1983) Structural requirements of N-glycosylation of proteins. Stud Proline Peptides Conformational Probes Biochem J 209:331–336
Becker SA, Feist AM, Mo ML, Hannum G, Palsson B, Herrgard MJ (2007) Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox. Nat Protoc 2:727–738
Bretthauer RK (2003) Genetic engineering of Pichia pastoris to humanize N-glycosylation of proteins. Trends Biotechnol 21:459–462
Brophy JAN, Voigt CA (2014) Principles of genetic circuit design. Nat Methods 11:508–520
Burda P, Aebi M (1998) The ALG10 locus of Saccharomyces cerevisiae encodes the alpha-1,2 glucosyltransferase of the endoplasmic reticulum: the terminal glucose of the lipid linked oligosaccharide is required for efficient N-linked glycosylation. Glycobiology 8:455–462
Camirand A, Heysen A, Grondin B, Herscovics A (1991) Glycoprotein biosynthesis in Saccharomyces cerevisiae. Isolation and characterization of the gene encoding a specific processing alpha-mannosidase. J Biol Chem 266:15120–15127
Carrera J, Jaramillo A (2013) Automated design of bacterial genome sequences. BMC Syst Biol 7:108
Caspeta L, Castillo T, Nielsen J (2015) Modifying yeast tolerance to inhibitory conditions of ethanol production processes. Front Bioeng Biotechnol 3:184
Chan LY, Kosuri S, Endy D (2005) Refactoring bacteriophage T7. Mol Syst Biol 1:0018
Cho EY, Cheon SA, Kim H, Choo J, Lee DJ, Ryu HM, Rhee SK, Chung BH, Kim JY, Kang HA (2010) Multiple-yapsin-deficient mutant strains for high-level production of intact recombinant proteins in Saccharomyces cerevisiae Biotechnol. 149(1–2):1–7
Chou C, Chang W, Chiu C, Huang C, Huang H (2009) FMM: a web server for metabolic pathway reconstruction and comparative analysis. Nucleic Acids Res 37:W129–W134
Chubukov V, Gerosa L, Kochanowski K, Sauer U (2014) Coordination of microbial metabolism. Nat Rev Microbiol 12:327–340
Chung BH, Park KS (1998) Simple approach to reducing proteolysis during the secretory production of human parathyroid hormone in Saccharomyces cerevisiae. Biotechnol Bioeng 57:245–249
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N (2013) Multiplex genome engineering using CRISPR/Cas systems Science 339:819–823
Conibear E, Stevens TH (1998) Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim Biophys Acta 1404:211–230
Cvijovic M, Olivares-Hernández R, Agren R, Dahr N, Vongsangnak W, Nookaew I, Patil KR, Nielsen J (2010) BioMet toolbox: genome-wide analysis of metabolism. Nucleic Acids Res 38:W144–W149
De Pourcq K, Vervecken W, Dewerte I, Valevska A, Van Hecke A, Callewaert N (2012a) Engineering the yeast Yarrowia lipolytica for the production of therapeutic proteins homogeneously glycosylated with Man8GlcNAc2 and Man5GlcNAc2. Microb Cell Fact 11:53
De Pourcq K, Tiels P, Van Hecke A, Geysens S, Vervecken W, Callewaert N (2012b) Engineering Yarrowia lipolytica to produce glycoproteins homogeneously modified with the universal Man3GlcNAc2 N-glycan core. PLoS ONE 7(6):e39976
De Pourcq K, Vervecken W, Dewerte I et al (2012c) Engineering the yeast Yarrowia lipolytica for the production of therapeutic proteins homogeneously glycosylated with Man8GlcNAc2 and Man5GlcNAc2. Microb Cell Fact 11:53
De Schutter K, Lin YC, Tiels P, Van Hecke A, Glinka S, Weber- Lehmann J, Rouze P, Van de Peer Y, Callewaert N (2009) Genome sequence of the recombinant protein production host Pichia pastoris. Nat Biotechnol 27:561–566
Dujon B, Sherman D, Fischer G et al (2004) Genome evolution in yeasts Nature 430:35–44
Durante Rodríguez G, Mancheño JM, Díaz E, Carmona M (2016) Refactoring the λ phage lytic/lysogenic decision with a synthetic regulator. Microbiol Open 5:575–581
Ecker M, Mrsa V, Hagen I, Deutzmann R, Strahl S, Tanner W (2003) O-mannosylation precedes and potentially controls the N-glycosylation of a yeast cell wall glycoprotein. EMBO Rep 4:628–632
Eiden-Plach A, Zagorc T, Heintel T, Carius Y, Breinig F, Schmitt MJ (2004) Viral preprotoxin signal sequence allows efficient secretion of green fluorescent protein by Candida glabrata, Pichia pastoris, Saccharomyces cerevisiae, and Schizosaccharomyces pombe. Appl Environ Microbiol 70:961–966
Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4:181–191
Endy D, KnightJr TF (2008) Engineering BioBrick vectors from BioBrick parts. J Biol Eng 2:5
Feng Y, De Franceschi G, Kahraman A, Soste M, Melnik A, Boersema PJ, de Laureto PP, Nikolaev Y, Oliveira AP, Picotti P (2014) Global analysis of protein structural changes in complex proteomes. Nat Biotechnol 32:1036–1044
Fidan O, Zhan J (2015) Recent advances in engineering yeast for pharmaceutical protein production. RSC Adv 5:86665
Finnis CJA, Payne T, Hay J et al (2010) High-level production of animal free recombinant transferrin from Saccharomyces cerevisiae. Microb Cell Fact 9:87
Fuller RS, Brake AJ, Thorner J (1989) Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science 246:482–486
Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Meth 6:343–345
Giga-Hama Y (1997) Fission yeast Schizosaccharomyces pombe: an attractive host for heterologous protein production. In: Giga- Hama Y, Kumagai H (eds) Foreign gene expression in fission yeast Schizosaccharomyces pombe. Springer, Berlin, pp 3–28
Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE et al (2013) CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154:442–451
Goffeau A, Barrell BG, Bussey H et al (1996) Life with 6000 genes. Science 274:547–563
Gonzalez-Lopez CI, Szabo R, Blanchin-Roland S, Gaillardin C (2002) Genetic control of extracellular protease synthesis in the yeast Yarrowia lipolytica. Genetics 160:417–427
Goodman M (2009) Market watch: sales of biologics to show robust growth through to 2013. Nat Rev Drug Discovery 8:837
Guerfal M, Ryckaert S, Jacobs PP, Ameloot P, van Craenenbroeck K, Derycke R, Callewaert N (2010) The HAC1 gene from Pichia pastoris: characterization and effect of its overexpression on the production of secreted, surface displayed and membrane proteins. Microb Cell Fact 9:49–60
Hamilton SR, Gerngross TU (2007) Curr Opin Biotechnol 18:387–392
Hamilton SR, Davidson RC, Sethuraman N, Nett JH, Jiang Y, Rios S, Bobrowicz P, Stadheim TA, Li H, Choi BK, Hopkins D, Wischnewski H, Roser J, Mitchell T, Strawbridge RR, Hoopes J, Wildt S, Gerngross TU (2006) Humanization of yeast to produce complex terminally sialylated glycoproteins. Science 313(5792):1441–1443
Hamilton SR, Cook WJ, Gomathinayagam S, Burnina I, Bukowski J, Hopkins D, Schwartz S, Du M, Sharkey NJ, Bobrowicz P, Wildt S, Li H, Stadheim TA, Nett JH (2013) Production of sialylated O-linked glycans in Pichia pastoris. Glycobiology 23(10):1192–1203
Harmsen M, Bruyne M, Raué H, Maat J (1996) Overexpression of binding protein and disruption of the PMR1 gene synergistically stimulate secretion of bovine prochymosin but not plant thaumatin in yeast. Appl Microbiol Biotechnol 46:365–370
Horwitz AA, Walter JM, Schubert MG, Kung SH, Hawkins K, Platt DM et al (2015) Efficient multiplexed integration of synergistic alleles and metabolic pathways in yeasts via CRISPR-Cas. Cell Systems 1:1–9
Hou J, Tyo KE, Liu Z, Petranovic D, Nielsen J (2012) Metabolic engineering of recombinant protein secretion by Saccharomyces cerevisiae. FEMS Yeast Researh. 12:491–510
Idiris A, Tohda H, Sasaki M, Okada K, Kumagai H, Giga-Hama Y, Takegawa K (2010a) Enhanced protein secretion from multiproteasedeficient fission yeast by modification of its vacuolar protein sorting pathway. Appl Microbiol Biotechnol 85:667–677
Idiris A, Tohda H, Kumagai H, Takegawa K (2010b) Engineering of protein secretion in yeast: strategies and impact on protein production. Appl Microbiol Biotechnol 86:403–417
Iwaki T, Hosomi A, Tokudomi S, Kusunoki Y, Fujita Y, Giga-Hama Y, Tanaka N, Takegawa K (2006) Vacuolar protein sorting receptor in Schizosaccharomyces pombe. Microbiology 152:1523–1532
Jiang M, Stephanopoulos G, Pfeifer BA (2012) Downstream reactions and engineering in the microbially reconstituted pathway for Taxol. Appl Microbiol Biotechnol 94:841–849
Jønson L, Rehfeld JF, Johnsen AH (2004) Enhanced peptide secretion by gene disruption of CYM1, a novel protease in Saccharomyces cerevisiae. Eur J Biochem 271:4788–4797
Jung YK, Kim TY, Park SJ, Lee SY (2010) Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol Bioeng 105:161–171
Kakule TB, Lin Z, Schmidt EW (2014) Combinatorialization of fungal polyketide synthase—peptide synthetase hybrid proteins. J Am Soc 136:17882–17890
Kalir S, McClure J, Pabbaraju K, Southward C, Ronen M, Leibler S, Surette MG, Alon U (2001) Ordering genes in a flagella pathway by analysis of expression kinetics from living bacteria. Science 292:2080–2083
Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopaedia of genes and genomes. Nucleic Acids Res 28:27–30
Kang HA, Choi ES, Hong WK, Kim JY, Ko SM, Sohn JH, Rhee SK (2000) Proteolytic stability of recombinant human serum albumin secreted in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 53:575–582
Kauffman K, Pridgen E, Fr D, Dhurjati P, Robinson A (2002) Decreased protein expression and intermittent recoveries in BiP levels result from cellular stress during heterologous protein expression in Saccharomyces cerevisiae. Biotechnol Prog 18:942–950
Kida Y, Morimoto F, Sakaguchi M (2007) Two translocating hydrophilic segments of a nascent chain span the ER membrane during multispanning protein topogenesis. J Cell Biol 179:1441–1452
Kida Y, Morimoto F, Sakaguchi M (2009) Signal anchor sequence provides motive force for polypeptide chain translocation through the endoplasmic reticulum membrane. J Biol Chem 284:2861–2866
Kim H, Yoo SJ, Kang HA (2014) FEMS Yeast Res 15:1–16
Kim H, Yoo SJ, Kang HA (2015) Yeast synthetic biology for the production of recombinant therapeutic proteins. FEMS Yeast Res 15:1–16
King ZA, Lloyd CJ, Feist AM, Palsson BO (2015) Next-generation genome-scale models for metabolic engineering. Curr Opin Biotechnol 35:23–29
Komeda T, Sakai Y, Kato N, Kondo K (2002) Construction of protease-deficient Candida boidinii strains useful for recombinant protein production: cloning and disruption of proteinase A gene (PEP4) and proteinase B gene (PRBI). Biosci Biotechnol Biochem 66:628–663
Kosuri S, Church GM (2014) Large-scale de novo DNA synthesis: technologies and applications. Nat Meth 11:499–507
Lee JW, Kim TY, Jang Y, Choi S, Lee SY (2011) Systems metabolic engineering for chemicals and materials. Trends Biotechnol 29:370–378
Lepenies B, Seeberger PH (2014) Simply better glycoproteins. Nat Biotechnol 32:443–445
Liu L, Redden H, Alper HS (2013) Frontiers of yeast metabolic engineering: diversifying beyond ethanol and Saccharomyces. Curr Opin Biotechnol 24(6):1023–30
Madhavan A, Sukumaran RK (2016) Secreted expression of an active human interferon—beta (HuIFNβ) in Kluyveromyces lactis. Eng Life Sci 16:379–385
Martinez JL, Liu L, Petranovic D, Nielsen J (2012) Pharmaceutical protein production by yeast: towards production of human blood proteins by microbial fermentation. Curr Opin Biotechnol 23:965–971
Mattanovich D, Branduardi P, Dato L, Gasser B, Sauer M, Porro D (2012) Recombinant protein production in yeasts. Methods Mol Biol 824:329–358
Mavromatis K, Chu K, Ivanova N, Hooper SD, Markowitz VM, Kyrpides NC (2009) Gene context analysis in the Integrated Microbial Genomes (IMG) data management system. PLoS ONE 4:e7979
Medema MH et al (2011) Anti SMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res 39:W339–W346
Medema MH, Raaphorst R, Takano E Breitling R. (2012) Computational tools for the synthetic design of biochemical pathways. Nat Rev Microbiol 10:191–202
Medema MH, Cimermancic P, Sali A, Takano E, Fischbach MA (2014) A systematic computational analysis of biosynthetic gene cluster evolution: lessons for engineering biosynthesis. PLoS Comput Biol 10:e1004016
Müller U, van Assema F, Gunsior M, Orf S, Kremer S, Schipper D, Wagemans A, Townsend CA, Sonke T, Bovenberg R, Wubbolts M (2006) Metabolic engineering of the E. coli l-phenylalanine pathway for the production of d-phenylglycine (d-Phg). Metab Eng 8:196–208
Na D, Lee D (2010) RBSDesigner: software for designing synthetic ribosome binding sites that yields a desired level of protein expression. Bioinformatics 26:2633–2634
Nevoigt E (2008) Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol Mol Biol Rev 72:379–412
Nielsen J (2013) Production of biopharmaceutical proteins by yeast: advances through metabolic engineering. Bioengineered 4(4):207–211
Osterlund T, Bordel S, Nielsen J (2015) Controllability analysis of transcriptional regulatory networks reveals circular control patterns among transcription factors. Integr Biol 7:560–568
Otero JM, Cimini D, Patil KR, Poulsen SG, Olsson L, Nielsen J (2013) Industrial systems biology of Saccharomyces cerevisiae enables novel succinic acid Cell factory. PLoS ONE 8:1–10
Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D (2013) High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496:528–532
Partow S, Siewers V, Bjørn S, Nielsen J, Maury J (2010) Characterization of different promoters for designing a new expression vector in Saccharomyces cerevisiae. Yeast 27:955–964
Payne T, Finnis C, Evans LR, Mead DJ, Avery SV, Archer DB, Sleep D (2008) Appl Environ Microbiol 74:7759–7766
Pharkya P, Burgard AP Maranas CD (2004) OptStrain: a computational framework for redesign of microbial production systems. Genome Res 14: 2367–2376
Picotti P, Clement-Ziza M, Lam H, Campbell DS, Schmidt A, Deutsch EW, Rost H, Sun Z, Rinner O, Reiter L, Shen Q, Michaelson JJ, Frei A, Alberti S, Kusebauch U, Wollscheid B, Moritz RL, Beyer A, Aebersold R (2013) Complete mass spectrometric map of the yeast proteome applied to quantitative trait analysis. Nature 494:266–270
Piirainen MA, de Ruijter JC, Koskela EV, Frey AD (2014) Glycoengineering of yeasts from the perspective of glycosylation efficiency. New Biotechnol 31(6):532–537
Prather KLJ, Martin CH (2008) De novo biosynthetic pathways: rational design of microbial chemical factories. Curr Opin Biotechnol 19:468–474
Rabinovich GA, van Kooyk Y, Cobb BA (2012) Glycobiology of immune responses. Ann N Y Acad Sci 1253:1–15
Rapoport TA (2007) Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 450:663–669
Ravin NV, EldarovMA Kadnikov VV et al (2013) Genome sequence and analysis of methylotrophic yeast Hansenula polymorpha DL1. BMC Genom 14:837
Robinson A, Hines V, Wittrup K (1994) Protein disulfide isomerase overexpression increases secretion of foreign proteins in Saccharomyces cerevisiae. Biotechnol (NY) 12:381–384
Robson GD (2007) Exploitation of fungi. Cambridge University Press, Cambridge, UK
Rodrigo G, Carrera J, Prather KJ, Jaramillo A (2008) DESHARKY: automatic design of metabolic pathways for optimal cell growth. Bioinformatics 24:2554–2556
Sanchez BJ, Nielsen J (2015) Genome scale models of yeast: towards standardized evaluation and consistent omic integration. Integr Biol 7(8):846–858
Sánchez C, Zhu L, Braña AF, Salas AP, Rohr J, Méndez C, Salas JA (2005) Combinatorial biosynthesis of antitumor indolocarbazole compounds. Proc Natl Acad Sci 102:461–466
Schroder M (2007) The cellular response to protein unfolding stress. In: Robson GD, van West P, Gadd GM (eds) Exploitation of fungi. British mycological society symposium series, vol 26. Cambridge University Press, Cambridge, pp 117–139
Schröder M (2008) Engineering eukaryotic protein factories. Biotechnol Letters 30(2): 187–196
Shao Z, Rao G, Li C, Abil Z, Luo Y, Zhao H (2013) Refactoring the silent spectinabilin gene cluster using a plug-and-play scaffold. ACS Synth Biol 2:662–669
Singh V (2014) Recent advancements in synthetic biology: current status and challenges. Gene 535:1–11
Song Y, Choi MH, Park JN, Kim MW, Kim EJ, Kang HA, Kim JY (2007) Engineering of the yeast Yarrowia lipolytica for the production of glycoproteins lacking the outer-chain mannose residues of N-glycans. Appl Environ Microbiol 73:4446–4454
Stephanopoulos G (2012) Synthetic biology and metabolic engineering. ACS Synth Biol 1:514–525
Strahl-Bolsinger S, Gentzsch M, Tanner W (1999) Protein O-mannosylation. Biochim Biophys Acta 1426:297–307
Swartz JR (2001) Advances in Escherichia coli production of therapeutic proteins. Curr Opin Biotechnol 12:195–201
Temme K, Zhao D, Voigt CA (2012) Refactoring the nitrogen fixation gene cluster from Klebsiella oxytoca. Proc Natl Acad Sci 109:7085–7090
Tyo KE, Liu Z, Magnusson Y, Petranovic D, Nielsen J (2014) Impact of protein uptake and degradation on recombinant protein secretion in yeast Applied Microbiology and Biotechnology 98(16):7149
Valkonen M, Penttilä M, Saloheimo M (2003) Effects of inactivation and constitutive expression of the unfolded-protein response pathway on protein production in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol 69:2065–2072
Van Ooyen AJ, Dekker P, Huang M, Olsthoorn MM, Jacobs DI, Colussi PA, Taron CH (2006) Heterologous protein production in the yeast Kluyveromyces lactis. FEMS Yeast Res 6:381–392
Vogl T, Glieder A (2013) Regulation of Pichia pastoris promoters and its consequences for protein production. New Biotechnol 30:385–404
Walsh G (2010) Biopharmaceutical benchmarks 2010. Nat Biotechnol 28:917–924
Wriessnegger T, Pichler H (2013) Yeast metabolic engineering—targeting sterol metabolism and terpenoid formation. Prog Lipid Res 52:277–293
Wu SG, He L, Wang Q, Tang YJ (2015) An ancient Chinese wisdom for metabolic engineering. Microb Cell Fact 14:39
Yoshida H (2007) ER stress and diseases. FEBS J 274:630–658
Zhang W, Zhao HL, Xue C, Xiong XH, Yao XQ, Li XY, Chen HP, Liu ZM (2006) Enhanced secretion of heterologous proteins in Pichia pastoris following overexpression of Saccharomyces cerevisiae chaperone proteins. Biotechnol Prog 22:1090–1095
Zhu F, Zhong X, Hu M, Lu L, Deng Z, Liu T (2014) In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene over production in Escherichia coli. Biotechnol Bioeng 111:1396–1405
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Madhavan, A., Sindhu, R., Arun, K.B., Pandey, A., Binod, P. (2018). Advances and Tools in Engineering Yeast for Pharmaceutical Production. In: Varjani, S., Parameswaran, B., Kumar, S., Khare, S. (eds) Biosynthetic Technology and Environmental Challenges. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-10-7434-9_3
Download citation
DOI: https://doi.org/10.1007/978-981-10-7434-9_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7433-2
Online ISBN: 978-981-10-7434-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)