Abstract
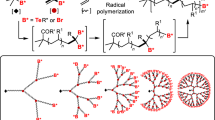
Following the extensive works on dendrimers which are structurally perfect but tedious to prepare, the need for the development of structurally imperfect hyperbranched (hb) polymers has gained momentum. A dendrimer is constituted of terminal units (at the globular surface) and dendritic units (inside the macromolecular framework). Whereas a hb polymer is constituted of terminal units (at the irregular surface), linear units and dendritic units (both of which are distributed randomly inside the macromolecular framework). These structural variations in dendrimers and hb polymers arise from the difference in synthesis strategies and mechanism of their formation
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Dendritic Units
- Macromolecular Framework
- Click Polymerization
- Enzyme-catalyzed Polymerization
- Strain-promoted Azide-alkyne Cycloaddition Reaction (SPAAC)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Introduction to Theoretical Approaches in Hyperbranched Polymerization
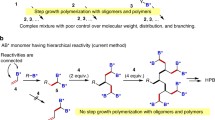
Following the extensive works on dendrimers which are structurally perfect but tedious to prepare, the need for the development of structurally imperfect hyperbranched (hb) polymers has gained momentum. A dendrimer is constituted of terminal units (at the globular surface) and dendritic units (inside the macromolecular framework). Whereas a hb polymer is constituted of terminal units (at the irregular surface), linear units and dendritic units (both of which are distributed randomly inside the macromolecular framework). These structural variations in dendrimers and hb polymers arise from the difference in synthesis strategies and mechanism of their formation. A lot of research has already been done and also ongoing to introduce new synthesis strategies for the development of hb polymers for commercialization. Hb polymers may be prepared via one of the three one pot, low-cost pathways- (1) bottom up approach (polymerization of monomers), (2) top down approach (breakdown of macromolecules) and (3) from polymer precursor molecules [1]. Among these, the bottom up approach is popular in the synthesis of hb polymers which is further categorized into four subdivisions-1) AB x polycondensation (where x > 2), (2) vinyl polymerization, (3) A2 + B3 polymerization; following Flory’s rule of equal reactivity and (4) polymerization of asymmetric monomer pairs; following the rule of non-equal reactivity. It is well established from our Nature that branching is an important phenomenon as it facilitates fast and efficient transfer and distribution of energy and mass. Hence, hb polymers would undoubtedly attract biomedical applications where transport phenomenon is one of the essential parts. In fact, there is a constant hunt for the new monomers to develop hb polymers with better tunable properties (say physiochemical properties, biodegradability, biocompatibility, self-assembling properties, peripheral functionality for target specific delivery applications, stimuli responsiveness, etc.) and controlled topologies than the existing ones, especially for the biomedical applications. This chapter is mainly focused on the synthesis of bio-medically important hb polymers (either established or with potential future prospects) through ABx polymerization and A2 + B3 polymerization which may follow either step-growth or chain-growth routes.
2.2 Hyperbranched Polymers from AB x -Type Monomers
Both in theory and practice, majority of the hb polymers with a multitude of functional end groups have been synthesized via one-pot step-growth reaction of AB x -type monomers (where x ≥ 2), following either single monomer methodology; SMM or double monomer methodology; DMM. Till today, AB2, AB3, AB4, and AB6 type monomers have successfully yielded hb polymers [2]. Polycondensation of AB x -type monomers produces random hb polymers unlike ideal generations of dendrimers, as in the former case the polymer chain formation occurs via random reaction sequences (via dimers, trimers, short/ long oligomers, etc.). The simplest and the most successful hb polymers are generally obtained from AB2-type monomers (trifunctional monomers), following Flory’s cascade theory (whereby crosslinking can be prevented as detailed below) [3]. In an AB2 polycondensation reaction, if both the B groups of one molecule react with the A group of two different molecules then only a branch unit (a three-arm structure) is generated (see Scheme 2.1). Otherwise, in the same case, if only one of the B groups of one molecule reacts with the A group of another molecule then a linear polycondensed polymer is generated. In an ideal hb polycondensed AB2 polymer, each molecule definitely contains at most, one unreacted A group (provided there is no intramolecular condensation reaction between A and B) and n + 1 unreacted B groups for n mer units.
However, often polycondensed AB2 polymers get gelled and hb polymers cannot be obtained owing to the uncontrolled growth in three dimensions. Gelation in an AB2 system occurs when a critical number of intermolecular linkages get exceeded which must be avoided in order to generate hb polymers [4]. Successful branching theory for the conventional hb polycondensed AB2 polymers was theoretically established by many groups among which those determined by Flory [5], Holter–Frey [6], Moller et al. [7], and Hult et al. [8] are well accepted. Traditional Flory’s cascade/ branching theory (in terms of the critical extent of reaction; conversion of B groups) predicted the critical branching coefficient (i.e., the probability for the attachment of a B group of a branch unit to another unit) for a polyfunctional condensation system employing A-R-Bf-1 monomer where f = number of functional groups per monomer of the same reactivity; f ≥ 3. Flory assumed,
where p B was the fraction of B groups that condensed (provided all the B groups were equally reactive). As A and B react stoichiometrically,
where p A was the fraction of A groups that reacted.
Thus, replacing p B , Flory obtained,
Now that as αmax = 1/ (f − 1) for any condensation reaction since p A (max) = 1, was actually considered as the critical condition for the network formation in a multi functional monomer system. Indirectly, it was assumed later that soluble hb polymers might be successfully generated if α × (f − 1) < 1. Such a prediction for the critical conditions for the network formation given by Flory was although a pathbreaking attempt for the generation of hb polymers, yet was certainly hypothetical as he considered three ideal situations- (1) A should react only with B in the reaction medium, (2) absence of cyclization and other side reactions, (3) the reactivity of B was totally independent of DP, and (4) both the B groups have equal reactivity. A typical hb macromolecule contains dendritic units (fully reacted B groups), terminal units (unreacted B groups), linear units (one reacted B group), and a focal point (A group); Scheme 2.1. Generally, an AB2-type monomer contains a focal point (say an imino group or an aromatic ring) which acts as the branching point. However, from Flory’s cascade theory any information about the extent of branching cannot be obtained as α which is basically the number of B groups that reacted, can either be part of a linear chain or a hb chain. In the year 1991, Frechet et al. established the expression for DB (DB F in terms of theoretical M.W.D which in turn is indirectly related to the number of different units present in a mass of polymer chains) for a hb polycondensed AB2 polymer [9]; see Scheme 2.1 for the location of different units which can be determined by NMR spectroscopy.
Again, in the year 1997, Frey and Holter provided another expression for DB (DB HF in terms of DP) for the AB2 system [6];
The group predicted that an AB2-type monomer with similar reactive both B groups yield hb polymers having maximum DB of 0.5 due to the statistical nature of an AB2 polymerization reaction. Finally, Moller’s group further modified Frechet and Frey–Holter branching parameters and established a corelation between DB and the conversion of A groups in an AB2 system [7]. Moller observed that the value of DBF decreased from 1.0 (for p A = 0) to 0.5 (for p A = 1) but never decreased below 0.5. Moller also observed that DB HF increased from 0 (for p A = 0) to 0.5 (for p A = 1). In this regard, Frey–Holter parameter provided a better understanding of the extent of branching in a hb AB2 polycondensate than Flory and Frechet parameter as DB HF increased with the conversion of A group which indirectly suggested the addition of more and more molecules to the branched structures at the branching points. However, Frechet parameter again suffers from the assumption of a generation of monodisperse hb polycondensed AB2 polymer (an ideal situation). In the present day, DB is considered as one of the important parameters for the determination of the structure of a hb polymer. There are numerous analytical techniques from which DB for an AB x polycondensate system may be determined (either directly or indirectly) [10] which are detailed in the subsequent Chap. 4 under the Sect. 4.2.
To generate AB2 hb structures, AB2 or latent AB2-type reactants may be used solely as the monomer, AB2-type monomers may be used as the additive seed in co-polycondensation reactions or AB2 polycondensation may be carried out in the presence of a third molecule core B f , where f ≥ 2 [11]. The first hb polycondensed AB2 polymer was commercialized under the trade name “Boltorn” by Berzelius (Perstorp Polyols Inc. USA) from the esterification reaction of 2,2 dimethylolpropionic acid. Following Berzelius’ work, numerous hb polycondensed AB2 polymers (from different classes of condensation polymers) have been prepared through controlled condensation reactions, of which some major works include polyarylenes-Suzuki coupling, polyaryleneacetylenes-Heck reaction, polyaryleneether/polyetherketones-aromatic substitution, polycarbosilanes/polycarbosiloxanes-hydrosilylation, etc. Even hb grades of polyesters, polyamides, polyethers, polyethersulfones, polycarbonates, polysiloxanes, polyphenyleneoxides, polyphenylenesulfides, and poly (bis-(alkylene) pyridinium)s.
2.2.1 Carbon–Carbon Coupling Reactions
2.2.1.1 Transition Metal Mediated C–C Coupling Reactions
One of the oldest techniques to synthesize new architectural macromolecules like hb polymers was relied on C–C bond formation, catalyzed by transition metals (mostly Pd and Ni). The first substitute to dendrimers, hb polyphenylenes, 6 was in fact developed by Kim and Webster through a one-step coupling reaction (a Suzuki type coupling reaction) between 3,5 dibromophenyl boronic acid (an unstable AB2-type monomer intermediate, 4), in the presence of catalytic amount of tetrakis(triphenylphosphine) Pd (II) in aqueous carbonate (Scheme 2.2) [12]. Alternately, successful hb polyphenylenes, 6 were also prepared from mono-Grignard compound (another unstable AB2-type monomer intermediate, 5), in the presence of catalytic amount of tetrakis(phenylphosphine) nickel chloride.
Scheme showing transition metal catalyzed C–C coupling reaction for the generation of hb polyphenylenes [12]
Polyphenylenes are the typical examples of transition metal catalyzed chemo-selective aryl–aryl coupling reactions. Unlike linear polyphenylenes, hb polyphenylenes are soluble in many organic solvents like THF, o-dichloro benzene, tetrachloroethane, etc., and thus processable owing to the presence of numerous voids spaces (where solvent molecules may be entrapped). Void spaces are generated from irregular microstructures of the hb polymers and also for reduction in π – π stacking interactions [13]. Hb polyphenylenes are basically nonconducting polymers because extended π conjugation is prevented in the microstructure due to the presence of packed and twisted phenylene units and hence they may be suitable as high-performance insulators. Diverse derivatives of functionalized hb polyphenylenes were also prepared by many researchers by electrophilic substitution reactions at positions bearing halogen groups either on polylithio-phenylenes or polyphenylenes, with electrophilies like –CO2, CH3OCH2Br, (CH3)2O, ROH, DMF, etc. Often with the introduction of hydrophilic electrophiles, hb polyphenylenes become water soluble and get suitable in many bio-based applications owing to the rendered biocompatibility and biodegradability. In an attempt to explore the world of aromatic hb polymers, Tanaka et al. synthesized hb poly (triphenylamine)s, 8 by Ni (II) catalyzed coupling of an AB2-type Grignard reagent, 7 (Scheme 2.3) [14]. However, the intermediate AB2-type monomer, 7 is highly moisture/air sensitive and hence restricted the production of hb polymer, 8 for commercial purposes.
Scheme showing Ni (II) catalyzed C–C coupling reaction for the generation of hb poly (triphenylamine)s [14]
So far, most of the successful formation of hb polymers through transition metal catalyzed C–C coupling reaction was carried out either by Suzuki coupling reaction or by Heck coupling reaction. Suzuki coupling reaction was employed in the synthesis of hb polyarylenes, poly (triphenylamine)s and poly (aryleneether)s. Sun et al. prepared hb poly (triphenylamine)s, 10 from 4-[bis(4-bromophenyl)amino]benzene boronic acid in its ester form, 9; a derivative of tris(4-bromophenyl)amine, by a Suzuki coupling polycondensation reaction in a one-pot system; Scheme 2.4 [15]. The same group also synthesized an alternating hb copolymer of poly (triphenylamine-fluorene), 12 of higher M.W and better solubility than the hb poly (triphenylamine)s-homo polymer, by a Suzuki coupling polycondensation reaction between 9 and a multifunctional core molecule named as 2,7-diiodo-9,9-dioctylfluorene, 11; Scheme 2.4 [15].
Scheme showing Suzuki coupling polycondensation reaction for the synthesis of hb poly (triphenylamine)s and hb poly (triphenylamine-fluorene)s [15]
Hb aryl/alkyl polymers with controlled architecture and high DB were prepared by Bo et al. from an AB2-type monomer, in the presence of an AC2-type monomer (where AC2 is more reactive than AB2 under mild temperature conditions) by a one-pot Suzuki coupling polycondensation reaction; Scheme 2.5 [16]. A two-step temperature variation was followed to ensure a controlled growth of the dendritic structures. AC2; an iodo-aromatic compound reacted faster than AB2; a bromo-aromatic compound with boronic esters, at a lower temperature and hence formed multi B functional AB n -type branched oligomers. In the further step, under refluxing condition, AB n -type oligomers polymerized to form highly branched structures. Often catalyst transfer reactions are favored to improve DB of the hb polymers. Huang et al. synthesized a hb polymer with a very high DB-100% [17]. There are reports on the improvement of DB of the hb polymers but they are less explored as there are only a few monomers which may allow such achievement [18]. The group of Huang used an AB2-type monomer containing one aromatic boronic pinacol ester and two aromatic bromo groups linked by an alkyl chain spacer for the catalyst transfer polymerization in the presence of P(t-Bu)3; the ligand and Pd2(dba)3CHCl3 as the source of Pd(0) catalyst, to develop hb polymers. Extensive research has found that selective functionalization of hb polyarylenes/poly (triphenylamine)s or their other derivatives through halogen exchange reaction with hydrophilic groups may produce biocompatible polymers for safe applications in diodes intended for making the components of electronic organ implants. Often hb polyphenylenes are used as multifunctional macroinitiators for the star polymers which may find usefulness in the design of various biomedical devices. Heck coupling polycondensation reaction is another important reaction scheme which is highly utilized in the synthesis of hb poly (arylenevinylene). Lim et al. in their study successfully synthesized hb poly (1,2,4-phenylene vinylene), 14 from 4-bromo-1,3-divinylbenzene, 13 (an AB2-type monomer) via Heck coupling reaction (employing Pd (II) catalyst); Scheme 2.6 [19, 20]. Nishide et al. used an asymmetrical AB2-type monomer, 15 to develop head to tail linked and 1,2,4,6 substituted-poly (phenylenevinylene), 16 through Pd (II) catalyzed Heck coupling reaction; Scheme 2.7 [21].
Schematic representation of a probable mechanism for the formation of a hb polymer through AB2 + AC2 type Suzuki coupling polycondensation reaction; there was a difference in the reactivity of the monomers under different reaction conditions [16]
Scheme showing Heck coupling reaction for the synthesis of hb poly (1,2,4-phenylenevinylene) [19]
Scheme showing Heck coupling polycondensation reaction for the synthesis of hb 1,2,4,6 substituted- poly (phenylenevinylene) [21]
Fukuzaki and Nishide developed a stable high spin and three-dimensional hb poly (1,2,4-phenylenevinyleneanisylaminium), 18 in the nanometer range from an asymmetric trifunctional monomer N-(3-bromo-4-vinylphenyl)-N-(4-methoxyphenyl)-N-(4-vinylphenyl)amine, 17 via a polycondensation route employing Pd (II) catalysts; Scheme 2.8 [22]. So far, there is no report on the applicability of such organic-derived magnetic polymers with high solubility in the realm of biomedical, yet additional functionalization of these polymers may make them as attractive constituents in the magnetic field operated medical devices, in the near future.
Scheme showing Heck coupling polycondensation reaction for the synthesis of hb poly (1,2,4-phenylenevinyleneanisylaminium) [22]
Other transition metals that are often used as the catalysts in the synthesis of AB2 hb polycondensed polymers include ruthenium and copper. Lu et al. reported successful generation of hb poly (4-acetylstyrene); an AB2-type monomer via Ru (II) catalyzed polycondensation reaction [23]. Acetophenone derivatives with a vinyl or an ethynyl group are mainly used as the AB2-type monomers in the dihydridocarbonyltris(triphenylphosphine)ruthenium; ([Ph3P]3RuH2CO) catalyzed polycondensation reactions for the generation of hb polymers. Utilization of Cu (I) catalyst in the synthesis of C–C coupled hb polymers was successfully done by In et al. who utilized Ullmann polycondensation reaction to generate hb poly (phenylene oxide). Mr. In prepared 3,5-dibromophenol, 19 from pentabromophenol and polymerized 19 to hb poly (phenyleneoxide), 20 via a two-step process, in the presence of Cu (I) catalyst at a very high-temperature range; Scheme 2.9 [24]. Although Ullmann reaction requires a much robust condition for the ether bond formation yet it is favored in many cases over the nucleophilic aromatic substitution reaction as the former reaction may be adapted to well-known monomers for the synthesis of hb polymers. In the same work, In’s group further converted bromine terminated hb poly (phenyleneoxide) to a lithium carboxylate derivative for which the structure 20 became water soluble.
Scheme showing Ullmann polycondensation reaction for the synthesis of hb poly (phenyleneoxide) [24]
Of the various cross-coupling reactions, recently Sonogashira reactions have gained impetus in the synthesis of hb polymers. Tolosa and his team produced hb polymer 22 from an AB2-type monomer, 21 via a polycondensation route employing Sonogashira reaction, in the presence of Pd (II) catalyst and Cu (I) cocatalyst; Scheme 2.10 [25]. Hb polymer 22 exhibited a M.W of 2.4 × 104 g mol−1 and a P.D.I of 2.0. However, owing to high iodine content, the fluorescence property of 22 was somewhat quenched by the heavy atom effect. Further functionalization of 22 with different types of terminal alkynes produced derivatives of 22 with higher quantum yields and thus could be considered as suitable candidates for sensory applications.
Scheme showing Sonogashira polycondensation reaction for the synthesis of a fluorescent hb polymer [25]
In another work, Li et al. developed an AB2-type hb nonlinear optical polymer 24 from monomer 23 via Sonogashira coupling reaction; Scheme 2.11 [26]. Hb polymer 24 was soluble in a range of solvents (CHCl3, THF, DMF, and DMSO), thermally stable, optically transparent and exhibited an M.W of 11,750 g mol−1 and second harmonic coefficient as high as 153.9 pm. V−1. Higher nonlinear optical effects of hb polymer 24 undoubtedly made them attractive for photonic applications which may find suitability in label-free bioimaging. In fact, nonlinear optical polymers are used in non linear optical microscopes for the imaging of drugs and dosages during the life cycle of the product, from manufacturing to their fate in the body (say distribution in tissues and live cells) [27]. The scope of nonlinear optics has gained much importance especially with the introduction of the concept of personalized therapy.
Scheme showing Sonogashira polycondensation reaction for the synthesis of a hb polymer with nonlinear optical properties [26]
From the different discussed works, it is observed that transition metal catalyzed C–C coupling polycondensation reactions of AB2-type monomers leave an indelible effect in the field of hb polymer synthesis. Through the correct choice of monomers and post polymer functionalization with biocompatible moieties, hb polycondensed AB2 polymers have already found applicability in the diverse biomedical arena. However, AB2-type hb polymers prepared through C–C coupling reactions in the absence of transition metals are gaining further importance to avoid toxicity arising from the presence of trace metal catalysts which otherwise may prove to be fatal to health (for in vivo applications) if not eradicated.
2.2.1.2 Polycycloaddition Type C–C Coupling Reactions- Metal Free and Metal Catalyzed Reaction Pathway
In the recent era, besides conventional polycondensation reactions, polycycloaddition reactions have gained much attention in the synthesis of hb polymers. Polycycloaddition reactions offer high selectivity and good yields in polymer synthesis. Age old cycloaddition reactions feature simultaneous breakage and formation of sigma bonds in a concerted manner via cyclic transition states. Cycloaddition reactions are possible only when phase matched interactions occur between the highest occupied molecular orbital (HOMO) of the unsaturated end and the lowest unoccupied molecular orbital (LUMO) of the other end, respectively, of the same substrate. Unlike condensation reactions, small molecules are not produced during cycloaddition reactions. Thus, species with high MWs may be obtained. Furthermore, unlike free radical reactions, as unwanted side reactions like reactions with oxygen and water do not happen during cycloaddition reactions, the latter is much favored in the polymer synthesis and modifications. Among the various types of polycycloaddition reactions, primarily [4 + 2] and 1,3-dipolar type cycloaddition step-growth reactions (where notions indicate the number of π electrons involved in the cycloaddition reaction) have been successfully utilized in the synthesis of hb polymers. Other reactions like [2 + 2] and [2 + 2 + 2] type polycycloaddition reactions have also received considerable attention in this regard. A typical example of [4 + 2] type polycycloaddition reaction which is often followed in the synthesis of hb polymers is Diels–Alder reaction (D.A). D.A reaction is pericyclic in nature which occurs between a conjugated diene (a 4π electron system) and a dieneophile (a 2π electron system) where frontier molecular orbitals combine in a suprafacial manner (i.e., addition to lobes occur on the same side of the π system). The resulting adduct is a highly regio-selective 6 membered ring structure. Unlike other polycycloaddition reactions, D.A reaction follows a thermally reversible mechanistic pathway during polymerization. Such thermo-reversibility often makes D.A cycloadducts attractive candidates in thermo-responsive drug/gene delivery devices. However, in practice, D.A reaction often fails to produce polymers with high M.Ws owing to the side reactions and retro [4 + 2] cycloadditions. By employing a strategy of synchronous aromatization and irreversible loss of a small molecule (say carbon monoxide), retro [4 + 2] cycloadditions may be prevented and polymerization is thus favored. After the pioneering work of Stille et al. where they synthesized linear polyphenylenes [28], Morgenroth and Mullen prepared hb polyphenylenes via repetitive inter molecular D.A-based C–C coupling technique, from two kinds of AB2-type monomers of tetraphenylcyclopentadienones; Scheme 2.12a [29]. In this notable work, they utilized AB2-type monomers comprising of one cyclopentadienone as a diene and two triple bonds as dienophiles. Typically, one equivalent of triisopropylsilyl protected diene, 25 was reacted with two equivalents of tetrabutylammonium fluoride at 180 °C in diphenylether to generate deprotected dienophiles in situ in the existing structure; thereby an AB2-type monomer was developed which finally got polymerized to hb polyphenylenes, 26 in a time period of 12 h. Polymeric cycloadduct 26 was soluble in most of the common organic solvents, exhibited an average M.W of ~17,000 g mol−1 and P.D.I of 6.85. The same group also synthesized hb polyphenylenes, 28 from a phenyl substituted in situ generated AB2-type monomer which in turn is obtained from a phenyl substituted diene, 27 in a similar fashion as the previous work; Scheme 2.12b. Polymeric cycloadduct 28 displayed higher average M.W (~1.07 × 105 g mol−1) but a lower P.D.I (4.3) than polymer 26, respectively. Unlike in Pd (II) catalyzed coupling of arylboronic acids with aryl halides where only structures with 1,3,5-linked triphenyl benzene units are produced, D.A reaction gives birth to architectures with densely packed benzene rings. Owing to the dense packing of benzene rings in the hb polyphenylenes prepared by D.A reaction, they undergo intramolecular dehydrogenation reactions which produce poly cyclic aromatic hydrocarbons. As often, poly cyclic aromatic hydrocarbons are used in gene therapy or drug delivery, the method of D.A is preferred in the synthesis of densely packed polyphenylenes over the other methods of preparation for the absence of harmful trace metals.
Scheme showing the synthesis of densely packed hb poly (phenylenes) via D.A type polycycloaddition [29]
Following the same methodology, Harrison and Feast prepared a hb polymer constituting of maleimide and cyclopentadienone (i.e., a polyimide) from an AB2-type monomer via D.A reaction [30]. The resulting hb polyimide was soluble in common organic solvents. Hb polyimides are very useful for encapsulation of biomacromolecules and insulation of active implants [31].
Nowadays, the combination of conventional D.A reaction and retro D.A cycloreversion reaction has gained significant importance in the synthesis of smart materials especially which display thermoresponsiveness with respect to physical properties (like color, viscosity, etc.) [33]. Such reactive polymers are highly useful for biomolecules immobilization, drug/gene delivery, and enzyme modifications. Gok and Sanyal prepared multi arm star polymers containing thiol reactive maleimide groups, 30 at the focal point; Scheme 2.13 [32]. The team deliberately masked double bonds of the reactive maleimide groups with furan via D.A reaction and generated a macroinitiator, which subsequently underwent a living polymerization with various methacrylate and acrylate monomers. Finally, they deprotected maleimide groups at the core of the polymers via retro D.A process (thermal treatment). Such macromolecular multi arm reactive polymeric scaffolds may be conjugated with drug molecules and such systems exhibit enhanced bioavailability and reduced clearance rate. In another work, Froimowicz and his team utilized anthracene functionalized hb polyglycerols in self-healing process via [4 + 4] reversible photo-cyclo-addition reactions [34]. Anthracenes undergo forward [4 + 4] dimerization process (or rather crosslinking in the case of anthracene containing polymers) when irradiated with 366 nm light and finally irradiation with 254 nm light induces backward decomposition of the dimmers (or rather de-crosslinking). In this regard, it is worthy to mention that self-healing polymers often find suitability in biological systems [34]. Hence, there is enough scope in exploring D.A/retro D.A reaction combination to prepare hb smart materials for biomedical applications. [2 + 2] cycloaddition dimerization reactions between bifunctional monomers often generate polymer with special architectures. Under thermal conditions, [2 + 2] cycloaddition reaction involves either a suprafacial or antarafacial molecular orbital interactions according to Woodward and Hoffmann selection rules. However, thermal [2 + 2] cycloaddition reactions involve highly strained transition states and thus are very difficult to follow. Thermal [2 + 2] cycloaddition reactions using ketenes moieties are very much favored as ketenes have linear structures which prevent steric repulsion in the antarafacial transition states and also ketenes cyclodimerize readily to produce 1,3-cyclobutanedione heterocycles. In this regard, interestingly isocyanates are isoelectronic with ketenes and can form cyclic dimers (1,3-diazetidine-2,4-diones) and trimers (tri substituted triazetidinediones). Both aliphatic and aromatic diisocyanates are precursors to an important class of polymers- polyurethane and polyurea; which hold high value in the future of biomedical applications. Itoya et al. produced hb poly (triazetidinediones) via cyclodimerization polymerization of aromatic diisocyanates at high-temperature (200 °C) and under high pressure (700 MPa) without the use of solvent or catalyst for 20 h [35]. Ta, Nb, or Cocatalysts catalyzed [2 + 2 + 2] cycloaddition polymerizations between two monoynes (one AB2-type monomer constituting of bifunctional alkynes and another is just a monofunctional alkyne) is just another approach to synthesize hb polymers. Tang et al. produced hb polyarylenes/poly (arylene ethynylene) utilizing [2 + 2 + 2] inter molecular cycloaddition reactions, i.e., via cyclotrimerization of alkynes. In one such work, Tang’s group prepared completely soluble hb polyphenylenes via polycyclotrimerization [36]. They observed that the nature of the catalysts played important roles in determining the molecular structures of hb polymers and thus affected their yields, solubility in various organic solvents and other physical properties. TaCl5 produced partially soluble hb polymers at low temperatures while NbCl5, Mo(CO)4(nbd), [Mo(CO)3(cp)]2, PdCl2-ClSiMe3, and Pd/C–ClSiMe3 gave birth to soluble hb polymers. In another work, Tang’s team further explored polycyclotrimerization reactions to prepare hb polyarylenes using Cocatalyst activated via UV irradiation [37].
Scheme showing the synthesis of multi arm star polymers containing reactive functional groups via D.A/retro D.A reaction strategy [32]
2.2.1.3 C–C Coupling Reactions via Nucleophilic Substitution Reactions
Polymerizations proceeded by nucleophilic substitution reactions are quite useful in the generation of hb polymers. Generally, AB2-type monomers constituting activated leaving groups are used for the purpose. One such example includes the use of activated methylene carbon as the branching origin. Jin et al. prepared controlled branched polymers from an AB2-type monomer containing a difunctional nucleophile, 32; 4-(4’-chloromethylbenzyloxy)phenylacetonitrile [38]. In this monomer 32, both the A (ClCH2) and 2B (CHCH2) sites were attached separately to the aromatic rings by a flexible ether bridge. The polymerization of activated methylene monomers was carried out in DMSO-NaOH (aq) medium in the presence of a phase transfer catalyst (tetrabutylammonium chloride; TBAC) without gelation; Scheme 2.14. Hb polymers 33 exhibited broad M.W.D and were soluble in organic solvents like DMSO, DMF, and THF. In et al. used aromatic nucleophilic substitution reactions on an AB2-type monomer 34 to generate hb poly (arylene ether amide)s with fluorine or hydroxyl end groups; Scheme 2.15 [39]. In this work, the two fluorine leaving groups (the B groups) of the AB2-type monomers were activated for the aromatic substitution reaction by the electron withdrawing carbonyl groups of the amide linkages. Hb polymers 35 showed high DB (0.43–0.53), high Tg (>200 °C), high thermal stability and were readily soluble in aprotic polar solvents.
Scheme showing nucleophilic substitution polymerization of activated methylene monomers to hb polymers [38]
Scheme showing the synthesis of hb poly (arylene ether amide) via nucleophilic aromatic substitution reaction [39]
Yang and Kong in their work, produced hb polymers with a high DB, via a Friedel–Crafts aromatic substitution reaction of an AB2-type monomer, 36; 5-(4-phenoxyphenoxy)isobenzofuran-1,3-dione [40]. Utilizing acid-catalyzed condensation reaction of isobenzofuran-1,3-dione with aromatic compounds which exclusively yields 3,3-diaryl compounds, the group produced hb poly (arylene isobenzofuran-1(3H)-one), 37; Scheme 2.16.
Scheme showing the synthesis of a hb polymer via Friedel–Crafts aromatic substitution reaction of an AB2-type monomer [40]
So far, we have not obtained any hb polymer synthesized via nucleophilic substitution reactions to be useful in biomedical applications. However, we fervently believe that in future, nucleophilic substitution-based polymerization reactions hold too much prospect in the generation of hb polymers for external medical devices.
2.2.1.4 C–C Coupling Reactions via Michael Addition Reactions
Michael addition reaction which is the nucleophilic addition of a carbanion or another nucleophile to an α, ß-unsaturated carbonyl compound, has also attracted the synthesis of hb polymers. Michael addition reaction proceeds rapidly at room temperatures and involves less toxic precursors which often suits biomedical applications. Endo et al. procured an AB2-type monomer, 38; 2-(acetoacetoxy)ethyl acrylate and carried out Michael addition reaction with 38 in the presence of a mid base 1,8-diazabicyclo-[5.4.0]undec-7-ene (DBU) catalyst, to generate hb poly (ß-ketoester); Scheme 2.17 [41]. Hb polymers 39 exhibited high DB around 0.43–0.829 and were highly soluble in DCM and CHCl3.
Scheme showing the synthesis of hb poly (ß-ketoester) from an AB2-type monomer via Michael addition reaction [41]
D.L Trumbo used Michael addition reaction to polymerize diacrylates (tripropylene glycol diacrylate) and bisacetoacetates to generate hb polymers in the presence of DBU catalysts [42]. Difunctionality of acetoacetate groups facilitated the formation of hb polymers. The hb polymers exhibited high M.W (~4.37 × 105 g mol−1) but broad M.W.D (P.D.I ~ 10). Concerning the field of biomedical applications, Gao et al. precisely developed highly water soluble, biodegradable hb polyesters with a large amount of terminal hydroxyl groups from an AB2-type monomer and found them to be suitable for drug delivery; Scheme 2.18 [43]. The group prepared the intermediate AB2/ADn type monomer (an ester diol, 40) from diethanolamine and methyl acrylate via Michael addition reaction in situ and continued the polymerization at a higher temperature. The hb polyester, 41 contained tertiary amino groups in the backbone/end hydroxyl groups with moderate M.W and DB was slightly greater than 0.5.
Scheme showing the synthesis of a hb polyester via Michael addition reaction [43]
Once again, Park and his team synthesized hb polymer-based gene transfection agents via Michael addition reaction between low M.W linear polyethylenimine, 42 and polyethylene glycol diacrylate, 43; Scheme 2.19 [44]. The resultant hb polymer 44 was highly branched due to the inherent branching in polyethylenimine and also for the reaction at multiple amine sites along the polymer backbone. The hb polymer 44 was water soluble, biodegradable, exhibited M.W around 103 g mol−1, had low cytotoxicity, formed complexes with plasmid DNA, and enabled gene transfection in HepG2/MG63 cell lines with high efficiency.
Scheme showing hb copolymer formation between polyethylenimine and polyethylene glycol diacrylate [44]
2.2.2 Carbon-Hetero Atom Coupling Reactions
2.2.2.1 C-N Coupling Reactions via Condensation of Amines and Acid Derivatives
Linear aromatic polyamides and polyimides are known as high-performance polymers owing to excellent thermal, mechanical, and chemical properties. However, these aromatic polymers are insoluble in common organic solvents at room temperature due to the presence of rigid repeating units. Often linear aromatic polymers are restricted in a number of applications because of their robust structures. Introducing dendritic units into the aromatic polymers not only improve solubility but also make them suitable in other sectors of applications including biomedical applications. In fact, hb aromatic polyamides are potentially used as supporting materials for protein immobilization [45]. Hb polyamides, hb polyimides, and hb polybenzoxazoles are generally prepared via condensation of amines and acid derivatives, i.e., via amidation of AB x -type monomers. It was for the first time Kim introduced liquid crystalline property in the hb aromatic polyamide materials [46]. He prepared hb polyamides (-COOH terminated-46 and –NH2 terminated-48) from AB2-type monomers, 45 and 47 in an amide solvent at low temperature; Scheme 2.20. Both hb polyamides 46 and 48 exhibited M.W of 2.4–4.6 × 103 g mol−1, P.D.I around 2.0–3.2. Hb polyamide 46 displayed nematic phase liquid crystalline properties and did not lose birefringence up to 150 °C. Often nematic liquid crystalline polymers are used in biosensors and ophthalmic lenses [47]. Hence, liquid crystalline hb polymers may be useful as potential biosensors.
Scheme showing the synthesis of hb polyamides via condensation of amine and acid derivatives [46]
Many a times, hb polyamides are synthesized via direct polycondensation reaction in the presence of condensing agents which activate –COOH groups of the AB x -type monomers in situ. The structure of AB x -type monomers very much affects DB of the hb polyamides during direct polycondensation reactions. Ishida and his team found that the use of AB x -type dendritic macromonomers enhances DB of the hb polymers [48]. AB4, AB6, and AB8 type dendrons of amino benzoic acids produced hb polyamides with DB as high as 0.7–0.8 unlike those obtained from AB2-type monomers (DB ~ 0.32). Direct/self-polycondensation reactions were further explored in the synthesis of hb polyimides in order to ensure high DB. Hb polyimides were prepared by a two-step process, via self-polycondensation of an intermediate polyamic acid methyl ester precursor; Scheme 2.21 [49]. Yamanaka et al. prepared an AB2-type monomer, 49 which was transformed into polyamic acid methyl ester precursor, 50 in situ using a condensing agent, (2,3-dihydro-2-thioxo-3-benoxazolyl)phosphonic acid diphenyl ester (DBOP) in NMP. Finally, hb poly imide, 51 was synthesized from 50 via chemical imidization process in the presence of acetic anhydride and pyridine in DMSO at 100 °C for 24 h. The resultant hb polyimide was soluble in DMF/DMSO/NMP, had a DB of 0.48, an M.W of 1.8 × 105 g mol−1 and a P.D.I of 3.0 with outstanding thermal stability.
Scheme showing the synthesis of hb polyimides via condensation of amine and acid derivatives [49]
2.2.2.2 Click Chemistry in C-N/C-S Coupling Reactions
[3 + 2] or 1,3-dipolar cycloaddition reactions are often considered as powerful tools in the development of dendritic structures via hetero atom coupling reactions. So far, Cu (I) catalyzed Huisgen 1,3-dipolar cycloaddition between an azide and terminal/internal alkyne derivatives (commonly known as azide–alkyne click reaction or CuAAC reaction), thiol–ene click reaction, and thiol–yne click reaction are extensively used in the synthesis of triazole functionalized hb polymers from various AB2-type monomers. The term “click chemistry” was first proposed by Sharpless who in his language gave the following criteria for the reaction strategy:
“The reaction must be modular, wide in scope, give very high yields, generate only inoffensive byproducts that can be removed by nonchromatographic methods, and be stereospecific (but not necessarily enantioselective). The required process characteristics include simple reaction conditions (ideally, the process should be insensitive to oxygen and water), readily available starting materials and reagents, the use of no solvent or a solvent that is benign (such as water) or easily removed, and simple product isolation. Purification-if required –must be by nonchromatographic methods, such as crystallization or distillation, and the product must be stable under physiological conditions.” [50] Sharpless [50] and Tornoe [51] claimed that the reactions to terminal alkynes exclusively yield anti-regioisomer of 1,4-disubstituted [1–3]—triazoles and thus is favored in the polymerization reactions. Click reaction is basically an A2 + B3 reaction strategy. However, with the advancement of research, many AB2-type monomers have been designed for the purpose. Depending upon the design of AB2-type monomers (having both two acetylene and one azide groups or vice versa), hb polytriazoles rich in acetylene or azide periphery could be obtained. Earlier, CuAAC polymerizations of AB2-type monomers yielded insoluble polymers owing to self-oligomerizations [52]. However, recent studies showed that self-oligomerization could be avoided to successfully generate soluble hb polymers. Li et al. designed AB2-bisazides, 52 & 54 and polymerized them via “click reaction” to azo chromophore containing soluble hb polymers 53 & 55, respectively, which exhibited nonlinear optical properties; Scheme. 2.22 [53]. The group added additional chromophores C1 & C2 (which are structurally similar to 52 & 54, respectively, but contains only alkyne groups) during the second stage of polymerization, in the respective reactions to avoid crosslinking through reactions with the unreacted azido groups.
Scheme showing the synthesis of hb polymer from AB2-bisazides via CuAAC click reaction [53]
In an attempt to prepare hb polymer from an AB2-bisalkyne, 56 (with terminal alkyne groups), Scheel et al. used both thermal click and CuAAC polymerization techniques [52]. Thermal click polymerization of 56 yielded soluble hb polymer 57 (containing 1,5-and 1,4-isomers in the ratio 36:64) whereas CuAAC polymerization of the former produced insoluble, rubbery hb polymer 58 (containing exclusively 1,4-isomer); Scheme 2.23.
Scheme showing the synthesis of hb polymer from an AB2-bisalkyne via thermal/CuAAC click reaction [52]
In some cases, when 1,5-regioisomer is preferred over 1,4-regioisomer and a faster controlled reaction than CuAAC polymerization is desired, Ru (II) catalyzed click polymerization (RuAAC) is employed [54]. Often click reaction is favored in the synthesis of polymers for biomedical applications. The 1,4-regioisomer of [1–3]—triazole bears a striking resemblance to peptide–amide bond in terms of geometry and thus is compatible with the functional groups present in the biological macromolecules like proteins, DNA, RNA, etc. In fact, [1–3]-triazole moiety has been utilized to mimic peptide–amide bond to generate dipeptide isotere in ß-strands and α-helical coiled structures [55]. However, there are few drawbacks associated with CuAAC polymerization or rather metal catalyst mediated click polymerization especially if the polymers synthesized are intended for biomedical applications. AB x -type monomers constituting of both alkyne and azide groups are highly reactive and are often difficult to store (even under ambient condition) or are rapidly consumed during the preparation owing to the uncontrolled self-oligomerization; even some materials are explosives. Again, metal catalysts are highly cytotoxic (especially neurotoxic) and the residues are difficult to eradicate from the reaction systems. The presence of trace metal catalysts also affects the physical properties (electronic, optical, etc.) of the synthesized polymers; say for example luminescence of the polymers gets quenched by the metal traps. Even, CuAAC polymerized products are often insoluble which defer the primary necessity of hb polymers. Ru (II) catalysts used in RuAAC polymerization are quite expensive and tedious to prepare. Cu (I) catalysts (either prepared in situ or purchased) are highly sensitive to air and require inert atmosphere for storage which in turn expedite cost. Hence, owing to these serious drawbacks, metal catalyst mediated click polymerization is often discouraged in biomedical applications. In this regard, metal free click polymerization of azides and alkynes, i.e., MFAAC polymerization has emerged as a potential alternative strategy for [1–3]–triazole-based hb polymers syntheses. To be precise, thermally carried out click reactions are not considered as MFAAC reactions because the former does not meet the general criteria for click reactions. In fact, thermally carried out click reactions (which is carried out at quite a high-temperature) are regio-random reactions and yield both 1,4-and 1,5-triazole isomers almost in equal ratios. Generally, alkynes or azides attached to electron withdrawing groups (carboxyl group, ether group, etc.), i.e., propiolates and aroylacetylenes facilitate MFAAC polymerization with high regio-selectivity [56]. The work of Li’s group included the synthesis of hb poly (aroxycarbonyltriazole)s via MFAAC polymerization, for the detection of explosives through aggregation induced emission [57]. Surprisingly, MFAAC polymerization technique is very less explored in the world of hb polymers for biomedical applications. Owing to the feasibility of a reaction and the absence of any cytotoxic elements, MFAAC reactions may attract further research. There is another variation in “click chemistry” like strain-promoted azide–alkyne cycloaddition reaction (SPAAC) which is gaining impetus in the synthesis of hb polymers with a high level of purity. SPAAC reaction belongs to the category of “bioorthogonal chemistry” which refers to the orthogonal reaction between a cyclooctyne and an azide without any significant interference from native biological processes, oxygen, and moisture. Hence, SPAAC reaction is highly favored in the fabrication of bioactive and cell-instructive materials. Ring strain of the cyclooctyne mainly drives the SPAAC reaction thermodynamically. In spite of the fact that SPAAC reaction is highly encouraging in labeling biomacromolecules and their use in living cells, owing to the limited commercial availability of the cyclooctyne reagents and tedious synthesis routes, SPAAC is not so much explored yet. With the progress in azide–alkyne click polymerization, recently thiol–ene/yne click polymerization has been much explored either to synthesize hb polymers or to functionalize them. Thiol–ene reaction which occurs between a thiol and an alkene to form an alkyl sulfide, is generally considered as the click reaction (as they are characterized by high thermodynamic driving force and occurs under extremely mild conditions). Thiol–ene reaction proceeds via an anti-Markovnikov addition of a thiol to an alkene and is quite favored in biomedical sciences. Much research has been carried out in the synthesis of hb polymers containing thioether and thioester groups, via free radical or base/nucleophile catalyzed Michael addition type thiol–ene click polymerization. Thiol–ene click polymerization is often followed in the synthesis of hb polycarbosilanes and polycarbosiloxanes and functionalization of other hb polymers. Polycarbosilanes and polycarbosiloxanes are potential antibacterial biocides and thus find too much prospect in biomedical applications including the drug delivery devices [58]. This phenomenon demands extensive research in the exploration of polycarbosilanes and polycarbosiloxanes. Xue et al. successfully synthesized hb organo silicon polymers 60 and 62 via thiol–ene click polymerization under UV light (which is basically a step-growth route involving hydrosilylation between an alkene and a thiol group), of an AB2-type monomer mercaptopropylmethyldiallylsilane, 59 and an AB3-type monomer mercaptopropyltriallyl silane, 61, respectively, Scheme 2.24 [59]. The hb polymers exhibited an M.W of 3279 g mol−1 for 60 and 2963 g mol−1for 62 whereas a DB of 0.6 for 60 and 0.22 for 62, respectively.
Scheme showing the synthesis of hb organosilicon polymer via step-growth thiol–ene click polymerization [59]
Many times, thiol–ene “click chemistry” is very much useful in the functionalization of polymers for effective biomedical applications. Moreno and coworkers at first developed hb aromatic polycarbosilane hydrophobic cores with allyl/vinyl terminal units, 64 from an AB2-type monomer, 63 via hydrosilylation polymerization [60]. The hb polymer 64 exhibited an M.W of 4500 g mol−1, P.D.I of 1.4, and DB of 0.43. Finally, the group carried out different thiol–ene functionalizations on 64 to generate hydrophilic or rather amphiphilic terminal units (anionic or cationic) in the respective hb polymers. The entire scheme for the synthesis of hb polymer is provided in Scheme 2.25.
Scheme showing the synthesis of amphiphilic hb polymers via a combination strategy of hydrosilylation polymerization and thiol–ene functionalization [60]
Similarly, Roy and Ramakrishnan designed an AB2-type monomer, 65 (bearing two allyl benzyl ether groups and an alcohol functionality), allowed self-condensation of 65 under acid-catalyzed melt transetherification to generate hb polymer 66 and then functionalized the peripheral allyl groups using variety of thiols via thiol–ene click reaction; Scheme 2.26 [61].
Scheme showing the functionalization of a hb polymer via thiol–ene click reaction [61]
Another variation, thiol–yne “click chemistry” also holds a strong position in the development of functionalized hb polymers intended for biomedical applications. Thiol–yne click reaction occurs between a thiol and an alkyne in an anti-Markovnikov fashion, to form an alkenyl sulfide. Thiol–yne click polymerization proceeds either via thermal or UV initiated route. Konkolewicz and coworkers synthesized a functional hb polymer 68 via photo-initiated step-growth thiol–yne click polymerization from an AB2-type monomer; prop-2-ynyl-3-mercaptopropanoate, 67, Scheme 2.27 [62]. The group used [2,2-dimethoxy-2-phenylacetophenone]; DMPA as the potential photo initiator. Thiol–yne click polymerization generally yields hb polymers with higher DB than the conventional AB2 polymerization (as the second B group reacts at a faster rate) and offers scope for further functionalization (owing to the presence of many Π bonds in their structures) [6, 63].
Scheme showing the synthesis of hb polymer via photo-initiated thiol–yne click reaction from an AB2-type monomer [62]
The major drawback of thiol–yne click polymerization is the synthesis of suitable AB2-type monomers containing both free SH and reactive ethynyl groups. These monomers are difficult to prepare and store as they react spontaneously even at low temperature. However, the use of double monomer strategy circumvents the problem of monomer handling for thiol–yne click polymerization. To overcome the problem of monomer handling, Han et al. reported for the first time, synthesis of a reactive AB2-type monomer (containing both thiol and alkyne groups) in situ and its subsequent polymerization to a highly functionalized hb polythioether-yne by following sequential “click chemistry” [64]. In the first step, the group generated an AB2-type monomer via thiol-Michael addition click reaction and in the subsequent step, they polymerized the monomer via thiol–yne click polymerization. The resultant hb polymer exhibited a high DB of 0.6–0.8, high M.W and a broad P.D.I. In spite of the fact that thiol-based chemistry is highly favored in the design of biological macromolecules, they are still avoided in many circumstances as thiol functionalized compounds are pungent, sensitive to oxidation, generate harmful reactive oxygen species, and are quite expensive.
2.2.2.3 C-O Coupling Reactions
C-O coupling reactions via nucleophilic substitution of alkoxides and phenoxides generally yield hb engineering plastics (e.g., poly (aryl ether)s, poly (ether ketone)s, poly (ether sulfone)s, etc.) which so far are hardly useful in the realm of biomedical applications. However, the esterification of carboxylic derivatives generates very useful hb polyesters and hb polycarbonates. For a long time, polyesters, both aliphatic and aromatic are highly recommended in the biomedical applications (especially in the design of drug/gene delivery devices) owing to certain useful properties like easy degradability of polyesters under physiological conditions and rapid metabolization of the degradation products in vivo. Often polyesters are functionalized with bioactive/bio-responsive constituents; for which they become sensitive to enzymes, to various redox conditions or to pH of the affected tissues (apart from the physiological conditions) [65]. However, drug-polymer conjugates demand high water solubility so that the vehicles can circulate the drug molecules easily in the blood stream. But in most of the cases, the drug molecules get detached from the vehicles uncontrollably once injected into the body. Hence, attaching the drug molecules covalently to polymeric scaffolds (say through ester linkages which can be readily broken only by esterase enzymes within the cells) often circumvents the problem of drug unloading in undesirable parts of the body. In this scenario, thus, hb polyesters have gained much impetus owing to high water solubility and superior encapsulating efficiency through covalent attachment with the desirable functional groups. “Boltorn” as introduced by Berzelius (Perstorp Polyols Inc. USA), obtained from the esterification reaction of 2,2 dimethylolpropionic acid was the first ever reported hb polymer which happened to be an aliphatic polyester with a high degree of hydroxyl functionality and is commercially highly successful. Malmstrom et al. synthesized hydroxyl rich hb aliphatic polyesters via co-condensation reaction of 2,2-bis(methylol)propionic acid; bis-MPA and a four functional polyol in a molten state [8]. They claimed that DB of the resultant hb polyester was around 0.8. Later, the same group rectified and suggested that the actual DB of the polyester was around 0.45. In the earlier version, such a high DB of the polyester was reported owing to the undue acetal formation during NMR analysis in acetone-d6 in the presence of trace amount of acid catalyst which could not be removed. Recently, as an alternative to petrochemical-based products, many times, aliphatic polyesters are developed from the renewable resources. Usage of biomass precursors for the bio-plastics reduces green house gas emissions and significantly prevents the depletion of scarce fossil resources. One of the important classes of commercially exploited bio-plastics is poly (lactic acid) which is actually an aliphatic polyester. Poly (lactic acid) is used in various biomedical devices (like as suture materials, in bone-fixation devices, implants for the repair of osseous and soft tissues, in controlled drug delivery, in medical packaging, etc.) due to high biocompatibility, biodegradability (can be degraded easily by the hydrolysis of ester linkages without the requirement of any enzymes which otherwise may have caused inflammatory reactions; the hydrolysis of ester linkages even provide spaces for the newly developing tissues) and bio-absorbable properties with low immunogenecity [66]. However, linear poly (lactic acid)s are often difficult to process (due to high crystallinity) and hence are prepared in conjunction with other comonomers [67]. Otherwise, the introduction of branching into the structure also ease the processing of poly (lactic acid) and encourage biomedical applications.
Tasaka et al. synthesized a hb copolymer, 71 of L-lactide (LA), 69 and a metabolically degradable bifunctional monomer; DL-mevalonolactone (ML), 70 via ring opening polymerization, ROP in the presence of Sn(Oct)2 catalyst; Scheme 2.28a [67]. Here, ML (containing a lactone ring and a pendant hydroxyl group) acted as a latent AB2-type comonomer (as the second hydroxyl group remained inactive until the lactone ring was attacked) and also as an initiator for ROP. In another instance, Pitet and his coworkers developed a hb copolymer, 73 of LA, 69 and glycidol, 72 via simultaneous ROP of epoxides and lactides in the presence of Sn(Oct)2 catalyst; Scheme 2.28b [68]. ROP leading to branched architectures is generally favored at a higher polymerization temperature (say around 110–130 °C) and continues for days before desirable products are obtained. To avoid the usage of metallic catalysts, Tsujimoto et al. prepared hb poly (lactic acid) using castor oil (bearing three secondary hydroxyl groups) as the initiator for the ring opening of lactide ring, which finally formed the core of the hb polymer [69]. Apart from poly (lactic acid), ester copolymers of glycerol precursors (obtained from renewable resources) are also considered as important bio-polyesters. Hb polyglycerol or polyglycidol (hb PG) consist of a polyether backbone and peripheral hydroxyl groups at every branch points. Hb PG is generally obtained via oxyanionic ROP of glycidol (a highly reactive hydroxyl epoxide) which acts as a latent cyclic AB2-type monomer (as it releases a second hydroxyl group upon ring opening) [70]. The hydrophilic nature and the presence of free hydroxyl groups in hb PGs suit the design of hydrogels for biomedical applications (drug delivery, tissue engineering, bioconjugation with peptides, protein immobilization, the suppression of protein adsorption to blood-contacting surfaces, etc.) [65, 71]. Moreover, PGs are highly biocompatible, exhibit low cytotoxicity against fibroblast and endothelial cells. For the first time, Sunder’s team synthesized hb PGs with controlled M.Ws, narrow P.D.I (1.13–1.47) and reasonable DB (0.53–0.59), via anionic ROP of glycidol and by making use of a fast proton exchange equilibrium (in the presence of a partially deprotonated triol as an alkoxide initiator) [72]. Robinson et al. developed hb aryl polyesters as viscosity improvers (VII) for lubricants via AB2 polycondensation of a monomer containing 12–16 methylene units in order to ensure good hydrophobicity for solubility in nonpolar medium [73]. Often in high operating temperature window (40–150 °C), lubricants suffer from thinning which adversely reduce application potentiality. Generally, polymers with high M.W (>100 kDa) are used as viscosity modifiers in commercial lubricants [74]. However, linear polymers are prone to degradation under high shear forces. Thus, hb polymers are favored as efficient viscosity modifiers as they are more resistant toward shear degradation [75]. Further, in the subsequent years, various hb copolymers of PGs have also been designed for desirable biomedical applications. Lee and his team made an approach to develop hb double hydrophilic block copolymer of poly (ethylene oxide)-hb-polyglycerol as an efficient drug delivery system with high loading capacity and controlled release properties [76]. The hb copolymer was capable of forming a self assembled micellar structure on conjugation with doxorubicin (a popular hydrophobic anticancer drug) when linked through pH sensitive hydrazone bonds. Following the protocol of Sunder and his coworkers, Garamus et al. prepared amphiphilic hb poly (glycerol ester)s with varying degrees of esterification, by partial esterification of a hb PG with palmitoyl chloride and studied their solution properties in different solvents, using SANS studies [72, 77]. Parzuchowski and his coworkers also synthesized highly functionalized hb polyesters from glycerol-based AB2-type monomers, ethyl{3-[2hydroxy-1-(hydroxymethyl)ethoxy]propyl}thioacetate via polycondensation in the presence of different catalysts [78]. Other biocompatible hb aliphatic polyesters include hb polycarbonates (hb PCs) and hb polyphosphates which have been explored in the last few years for various biomedical applications. Parzuchowski and his team successfully developed an AB2-type monomer, 5-{3-[(2-hydroxyethyl)thio)]propoxy}-1,3-dioxan-2-one from glycerol and subsequently polymerized the monomer to biocompatible and biodegradable hb PCs via ROP technique [79]. However, most of the hb PCs and hb polyphosphates are generated via A2 + B3 strategy and thus detailed under the Sect. 2.3. In a recent work, Testud and his team successfully developed hb polyesters via polycondensation of fatty acid-based ABx-type monomers [80]. The group designed four different types of AB2/AB3-type monomers constituting of a methyl ester group (A group) and two/three alcohol groups (B groups) via epoxidation of the internal bonds of vegetable oils and subsequent ring opening of the epoxide groups under acidic condition. Finally they carried out the polycondensation reaction on the multifunctional bio-based monomers in bulk to synthesize hb polyesters with tunable properties (M.W ~ 3000–10,000 gmol−1, P.D.I ~ 2–15 and DB ~ 0.07–0.45). It has been studied that the aliphatic monomers which are used for the synthesis of hb polyesters are often highly susceptible to thermal degradation reactions such as decarboxylation, cyclization, or dehydration [81]. Hence, the hunt for hb aromatic polyesters has been expedited. The most popular approach for the synthesis of hb aromatic polyesters is melt polycondensation of AB2-type monomers [82]. However, for the increment in the production of hb polyesters, more convenient method is necessary. So far, the AB2-type monomer which is extensively used in the synthesis of hb aromatic polyester is 3,5-dihydroxy benzoic acid; DBA [83, 84]. However, poor thermal stability of DBA restricts direct esterification and thus the hydroxy groups are often chemically modified by acetylation or trimethylsilylation prior to polycondensation reactions. In the earliest known work, Kricheldorf and his team used 3,5-bis(trimethylsiloxy)benzoyl chloride as an AB2-type monomer and condensed it with 3-(trimethylsiloxy)benzoyl chloride in bulk (at around 250–300 °C) to synthesize hb poly (3-hydroxybenzoate) which is an aromatic polyester [85]. They isolated the hydroxyl terminated hb polyesters from the synthesized hb polymers by adding methanol which hydrolyzed trimethylsiloxy groups. Similarly, Turner and his group used 3,5-diacetoxybenzoic acid as an AB2-type monomer and condensed it via acidolysis reaction at 250 °C in bulk to synthesize a hb aromatic polyester [86]. The resultant hb polyester was soluble and exhibited a M.W greater than 106 g mol−1. Generally, the polycondensation reaction of 3,5-diacetoxybenzoic acid requires higher polymerization temperature than that required for the polycondensation reaction of 3,5-bis(trimethylsiloxy)benzoyl chloride in order to obtain a polymer with high M.W. Fomine and his group reported the synthesis of a coumarin (which has medical approval in pharmaceutical chemistry) containing hb aromatic polyester via the esterification reaction of an AB2-type monomer [87]. Kricheldorf and his group reported the polycondensation reaction of an AB3-type monomer (triacetylated gallic acid) in bulk to generate a hb aromatic polyester [88]. Qie et al. reported the synthesis of carboxylic groups terminated aryl-alkyl hb polyester via melt polycondensation of an AB2-type monomer, 5-hydroxyethoxyisophthalic acid [89]. The resultant hb poly (5-hydroxyethoxyisophthalic acid) being a polycation, was used to generate self-assembly films with the assistance of a polyanion, poly (diallyldimethylammonium chloride) via layer by layer technique. Often, to replace the robust condition of melt/bulk polycondensation process, one-pot solution polycondensation of AB2-type monomer is favored in the synthesis of hb polyesters. Moreover, in the melt polycondensation process, if even a trace amount of impurities is present in the monomer then insoluble polymers are formed [90]. In this regard, Erber et al. showed the formation of hb aromatic polyesters with phenol terminal groups from an AB2-type monomer, 3,5-dihydroxybenzoic acid via solution polycondensation reaction, in the presence of 4-(dimethylamino) pyridinium 4-tosylate as the catalyst [91]. The resultant hb aromatic polyester exhibited a DB around 0.6 which was almost similar to those hb aromatic polyesters obtained via melt polycondensation of 3,5-bis(trimethylsiloxy) benzoyl chloride. Owing to the broad utility of the hb aromatic polyesters (in coatings, paints, adhesives, etc.), extensive researches are carried out to develop new interesting properties and even some of them have been successfully explored for commercialization. Generally, hb aromatic polyesters are used in vitro biomedical applications due to the question of biocompatibility of all grades.
2.2.2.4 Enzyme-Catalyzed Polymerization
These days, there is an urge for the development of nontoxic and environment friendly catalysts for the polymer syntheses. As an alternative to the existing metal catalysts used in polymerization, isolated enzymes have attracted much attention. Enzymes exhibit high catalytic activities, offer good enantio/regio/chemoselectivity, follow mild reaction conditions, have the ability to be used in bulk reaction media (without the use of organic solvents), are biodegradable, recyclable and maintain good biocompatibility [92]. Hence, enzyme-catalyzed polymerization is often highly favored in the synthesis of polymers specially intended for biomedical materials, drug/gene delivery vehicles and other pharmaceutical materials. So far, enzyme-catalyzed polymerization has successfully yielded many polymers like polyesters, polycarbonates, poly (amino acid)s, polyaromatics, etc. Popular commercially exploited enzymes, widely used in the polymerization have been listed by Uyama and Kobayashi which include oxidoreductases (for polyphenols, polyanilines), transferases (for polysaccharides, polyesters), hydrolases (for polysaccharides, polyesters, polycarbonates, etc.), lyases, ligases, etc. [93, 94]. Initially, enzyme-catalyzed polymerization of few selective monomers like ξ-caprolactone (CL), δ-valerolactone (VL), and γ-butyrolactone (BL) yielded polymers with low M.W and that too after many hours of reaction [95]. Hence, to circumvent the problems associated with the earlier version of enzyme-catalyzed polymerization, these days the polymerization is carried out using immobilized enzyme catalyst subtrates [92]. With the advancement in research, enzyme-catalyzed polymerization has also been explored in the synthesis of hb polymers which are more likely to suit biomedical applications. For the first time, Skaria et al. synthesized a series of hb copolyesters via a combination of ring opening AB polymerization (of ξ-caprolactone) and AB2 polycondensation (of 2,2-bis(hydroxymethyl)butyric acid, BHB), catalyzed by immobilized Lipase B (isolated from Candida antarctica) [95]. They were able to maintain 0 < DB < 0.33 for the different hb copolyesters just by controlling the comonomer ratio in the feed. Lopez-Luna and coworkers reported the synthesis of hb poly (VL-co-BHB) and poly (CL-co-BHB) via immobilized Lipase B catalyzed ring opening of the respective L-lactide in the presence of an AB2-type comonomer BHB core [96]. They carried out the enzyme-catalyzed ROP in 1,1,1,2-tetrafluroethane as a green, benign, polar, but hydrophobic solvent. However, the resultant hb copolyesters were semi crystalline and exhibited low DB (0.02–0.09) which might be due to the limited solubility of BHB in 1,1,1,2-tetrafluoroethane (~10 wt%). Later the same group improved DB of the resultant hb polylactones by carrying out the enzyme-catalyzed ROP of L-lactide (CL/VL) and an AB2-type core monomer BHB, in an ionic liquid (IL); 1-butyl-3-methylimidazolium hexafluorophosphate which is again considered as an environmentally benign solvent [97]. In a recent work, Xu et al. synthesized a series of hb poly (amine-ester)s with a value of DB (>0.8), via immobilized Lipase B catalyzed polycondensation of triethanolamine and diesters [98]. The resultant hb poly (amine-ester)s were biodegradable, exhibited low cytotoxicity/good biocompatibility/micellization ability and thus were suitable for loading/carrying drugs. There is immense scope in enzyme-catalyzed polymerization as many new enzymes from different sources are coming up commercially at acceptable prices. Hence, further research in the realm of enzyme-catalyzed polymerization would definitely develop new hb polymers with huge potentiality in biomedical applications.
2.3 Hyperbranched Polymers from A2 + B3 Monomer Pairs and Other Couple Monomer Methodologies
Apart from SMM (like ABx polymerization), the application of double monomer methodology; DMM in the synthesis of hb polymers is also highly acknowledged as an alternate strategy. DMM is further classified into A2 + B3 methodology and couple monomer methodology; CMM, depending upon the selection of monomer pairs and reaction pathways. Polymerization of the functionally symmetrical monomer pairs like A2/B4, A3/B3, A2/B3, etc., yields soluble hb polymers. Among these, A2 + B3 DMM (via condensation or addition mechanism) has received significant encouragement in the synthesis of hb polymers. A2 and B3 being separate entities are relatively easy to synthesize as compared to ABx monomers and thus facilitate commercialization. There is enough scope in expanding A2 + B3 strategy for biomedical applications owing to the existence/development of the vast choices of the monomer pairs. A2 + B3 strategy can be followed either in polycondensation or in self condensing vinyl polymerization. However, A2 + B3 polymerization often occur in an uncontrolled fashion and there is always a high risk of gelation and intra molecular cyclization reactions (especially when the molar feed ratio of A2: B3 > 0.9, at high monomer concentrations and at high conversions) [99]. In an A2 + B3 approach, AB2-type intermediates are formed at the initial stage of polymerization. In the subsequent reaction steps, AB2-type intermediates further reacts with unreacted A2 and B3 monomers and produces A x B y species (where x > 2 and y > 2). These A x B y being highly reactive species encourage intra molecular cyclization and crosslinking (after a certain conversion of the functional groups). In general, A2 + B3 strategy develops branched polymers with cyclic building blocks and/or a mixture of branched and cyclic polymers. In fact, earlier A2 + B3 strategy was solely used for the synthesis of crosslinked polymers. Aharoni et al. established a series of successful reactions between aromatic diamines (A2) and aromatic dicarboxylic acids (B3) but the resultant polymers were crosslinked networks [100, 101]. It was only after the pioneer work of Jikei and Kakimoto, it was established that when equimolar amounts of A2 and B3 monomers are used, soluble hb polymers could be synthesized [102]. The group prepared hb aromatic polyamides via condensation reactions between aromatic diamines (A2) and trimesic acid (B3) in the presence of condensing agents at 80 °C for 3 h. In this work, they maintained a low monomer concentration of monomers; 0.21 mol L−1 (3.3 wt%) to avoid gelation. They proposed that if the first condensation of A2 and B3 is faster than the following propagation, then an AB2-type intermediate is formed which subsequently undergoes polycondensation and thus prevents gelation. In fact, they compared the structural features of hb aromatic polyamides obtained from A2 + B3 polymerization and AB2 polymerization. To their surprise, they observed that the hb aromatic polyamides obtained from A2 + B3 strategy exhibited higher number of the dendritic units as compared to the terminal units whereas those obtained from AB2 polymerization exhibited equal number of the dendritic and the terminal units. Following this work, numerous patents and papers have been published which were mostly based on the polycondensation between glycerols and dicarboxylic acids/or cyclic anhydrides. So far, A2 + B3 strategy has been employed successfully to synthesize some important classes of hb polymers like hb polyamides, hb polyimides, hb polyesters, hb polyethers, hb polycarbonates, hb polyphosphates and hb polyurethanes; some of them are undoubtedly potential biomaterials. Fang et al. used A2 + B3 strategy to develop hb aromatic polyimides [103]. In this work, they used a series of dianhydrides (A2) and a triamine; tris(4-aminophenyl)-amine (B3) to prepare the hb polyamic acid precursors which were subsequently transformed to the respective hb aromatic polyimides via thermal or chemical imidization. Here, the order of monomer addition played a significant role in the development of hb aromatic polyimides either rich in terminal amines or anhydrides and it hardly affects DB. Often it is difficult to avoid gelation in an ideal A2 + B3 polymerization. Deviation from the ideal A2 + B3 strategy, i.e., by using a condensing agent, it is possible to prevent gelation significantly. Hao et al. followed a non ideal A2 + B3 strategy to prepare soluble hb polyimides from 1,4-phenylenediamine (A2) and tri (phthalic acid methyl ester) (B3) in the presence of diphenyl (2,3-dihydro-2-thioxo-3-benzoxazoyl) phosphonate condensing agent [104]. These hb polymers exhibited DB of 0.52–0.56 and inherent viscosities of 0.17–0.97. Unal and Long developed a hb poly (ether ester) via cyclization free melt condensation of A2 oligomers and B3 monomers [105]. In this novel work, they condensed oligomers of poly (propylene glycol) and trimethyl 1,3,5-benzenetricarboxylate in the presence of titanium tetraisopropoxide and stopped the reaction prior to gelation. Both hb PCs and hb polyphosphates are highly useful as functional materials and biomedicine, such as antibacterial/antifouling materials, in protein purification/detection/immobilization/delivery, in drug/gene delivery, in tissue engineering, in bioimaging, etc. Scheel et al. synthesized thermo-labile hb PCs via A2 + B3 route employing bis (carbonylimidazolide) and triethanolamine [106]. Such hb PCs may find usefulness in the preparation of nanoporous materials. In another work, Miyasaka and coworkers synthesized hb PCs via A2 + B3 polycondensation of di-tert-butyl tricarbonate (A2) and 1,1,1-tris(4-hydroxyphenyl)ethane (B3) [107]. The resultant hb PCs exhibited DB around 0.5–0.7. Apart from being biocompatible and biodegradable, polyphosphoesters are structurally similar to nucleic acids and teichoic acids. Under physiological conditions, polyphosphoesters easily degrade into harmless, low M.W materials either through hydrolysis or enzymatic degradation of the phosphate bonds. In an early work, Wang and Shi developed a reactive flame retardant hb polyphosphoester via A2 + B3 polycondensation of bisphenol-A (A2) and phosphoryl trichloride (B3) at 100 °C [108]. Following this work, many research has been focused on the synthesis of hb polyphosphates and functionalized hb polyphosphates which is elaborately detailed in the review of Lie et al. [109]. There are also significant works on the synthesis of soluble hb polymers via A2 + B3 CuAAC polymerization. Xie et al. prepared hb polytriazoles via A2 + B3 CuAAC polymerization of 4-N,N′-bis(2-azidoethyl)amino-4′-nitroazobenzene (A2) and 1,3,5-tris(alkynyloxy)benzene (B3) in a one pot at room temperature [110]. In another work, Qin et al. developed soluble, regio-regular hb poly (1,2,3-triazole)s via A2 + B3 CuAAC polymerization [111]. The resultant hb polytriazoles exhibited DB around 0.9 and quite high M.W. Chen et al. developed reduction cleavable disulfide bonds containing hb poly (ester triazole)s via A2 + B3 CuAAC polymerization of dipropargyl 3,3′-dithiobispropionate (A2) and tris(hydroxymethyl)ethane tri(4-azido butanoate) (B3) [112]. The hb poly (ester triazole)s exhibited M.W of 2.04 × 104 g mol−1 and P.D.I around 1.57–2.17. These hb polymers are highly recommended as stimuli responsive anticancer drug nanocarriers; except the fact that catalyst traces have to be eradicated. Often A2 + B3 strategy facilitate the synthesis of hb polymers with exceptional desirable properties, from uncommon monomer couple pairs. Kanai et al. designed hb poly (cyanurateamine) and hb poly (triacrylatetrimine) with good antimicrobial activities, from a pair of multifunctional monomers, via A2 + B3 Michael addition type polymerization [113]. In this work, they used diethylenetriamine and 2,4,6-triallylcyanurate/trimethylolpropane triacrylate as A2 and B3 monomer pairs, respectively. From the growing demand for the development of A2 + B3 strategy as an alternative to AB2 polymerization, it is obvious that A2 + B3 polymerization has a very good prospect in future. Hence, to encourage the establishment of A2 + B3 strategy for commercialization, gelation must to be prevented. Some ways of preventing gelation include the formation of reactive AB2-type intermediates (as gelation is easier to prevent in AB2 polymerization), partial functionalization of peripheral groups, stopping the polymerization through precipitation/end capping prior to the critical point of gelation, usage of suitable condensing agents, intense stirring, keeping low monomer concentration, and through other few methods as described later under the Sect. 2.4.
Although there are numerous methods to prevent gelation in A2 + B3 polymerization, yet most of them are not effective to the mark. Hence, as an alternative to A2 + B3 strategy, a new genre of synthesis strategy was developed. This new synthesis strategy is based on the in situ formation of AB x intermediates (during the initial stage of polymerization) from a specific pair of monomers with different reactivities of functional groups in each. These monomer pairs are basically functionally asymmetric in nature. This approach is analogous to nonideal A2 + B3 strategy and is popularly known as couple monomer methodology; CMM. Owing to the prevention of gelation, CMM is highly sought after for the scale up of hb polymers. Depending upon the reactivity of the monomer pairs, different categories of CMM have been reported [114]. For a monomer pair of AA′ and B′B2, in one of the instance when A′ is more reactive than A then CMM is named as AA′ + B3 strategy. In another instance, when B′ is more reactive than B then CMM is named as A2 + B′B2 strategy. Finally, when both A′ and B′ are differently reactive than A and B, respectively, then CMM is named as AC + DB2 strategy. In all the categories of CMM, AB2-type intermediates are formed in situ which eventually react to form hb polymers without any crosslinked structures. In CMM, M.W and the type of terminal functional groups on the hb polymers can be controlled by maintaining the molar feed ratio of the monomer pairs. When the molar feed ratio of AA′ to B′B2 is lower than 1:1 then the resultant hb polymers exhibit low M.W and are rich in B groups. Whereas when AA′:B′B2 molar feed ratio is greater than 2:1 then the resultant hb polymers have high M.W and are rich in A groups. It is only when AA′:B′B2 molar feed ratio is between 1:1 and 2:1, the hb polymers are equally rich in A and B groups with moderate M.W. So far, CMM has been successfully established in the generation of hb poly (sulfone amine)s, hb poly (ester amine)s, hb poly (urea urethane)s, hb poly (amide amine)s, hb polyesters, and hb poly (ester amide)s [114, 115].
2.4 Drawbacks of Hyperbranched Polymerization Techniques and Possible Remedies
From the vast studies of AB x polymerization or rather AB2 polymerization for the synthesis of hb polymers, it is clear that AB2 polymerization is a very important category of reaction. However, in an AB2 type batch polycondensation reaction, often there is hardly any control over the polymerization conditions owing to the complementary reactivity of A and two B functional groups which lowers M.W, broadens P.D.I, and DB is hardly beyond 0.5 for the resultant polymers [90]. Even yields of the polycondensed hb polymers in a step-growth reaction decrease owing to internal cyclization reactions, crosslinking reactions, and different reactivities of the similar B functional groups. Ideally, an AB2-type hb polymer should resemble the topology of a perfect dendrimer as two branches are expected to develop in a regular fashion, from each monomer. Although the two B groups of a terminal unit are equal in reactivity yet their ability to react depends strongly on the kinetic factors prevailing during the one-pot step-growth process. Once one of the B groups get reacted, a proximal steric hindrance is generated which generally prevents the other B group to react and thus the probability of development of branching units decrease (as observed in most of the reactions). Again, the regular dendritic growth of an AB2-type monomer gets impeded due to fast depletion of the monomer at an early stage of the reaction and thereby the polymerization continues through coupling of the sterically hindered oligomeric units. Thus, these limitations of AB2 polymerization hinder the development of hb polymers as advanced soft materials in the commercial world. Lot of efforts has been made to overcome the shortcomings of AB2 polymerization in order to add prosperity to the subject. In this regard, Shi et al. made a detailed and comprehensive study on the ongoing development of new reaction strategies for the synthesis of hb polymers with controlled topology and properties [116]. They claimed that there are three categories of reaction strategies which may be followed for any type of polymerization (for step growth, chain growth, and other reaction types) to improve the structure and properties (controlled M.W, narrow P.D.I and high DB) of the hb polymers (Scheme 2.29). These include-
Schematic representation of three different approaches to decrease P.D.I of the hb polymers. Reprinted (adapted) with permission from Shi et al. [116]. Copyright (2015) American chemical society
-
(I)
Slow addition of monomers to multifunctional core or chain terminator (a semi batch process). From the pioneer work of Malstorm et al. [8], Feast et al. [117] and Bharathi et al. [118], a concept of copolymerization of an AB2-type monomer in the presence of a multifunctional core monomer, B f was developed to control M.W and P.D.I of the hb copolymers significantly. During any polymerization reaction, probability of the addition of hb species to the other species (monomers, oligomers, etc.) is proportional to the number of functional groups and the number of functional groups is again proportional to DP. Thus, larger molecules grow faster than the smaller ones which in turn actually broadens M.W.D of the resultant hb copolymers [119]. Due to slow monomer addition, a very slow monomer concentration is maintained throughout, in the reaction mixture. Thus, the monomer almost exclusively reacts with the growing polyfunctional macromolecules (with complete exclusion of monomer–monomer reaction) which results in high M.W, narrow P.D.I, and high DB [120, 121]. Moreover, the B f molecule acts a chain terminator core which also restricts the undesirable side reactions significantly. In an ideal situation, the conversion of A groups should be 100% at all times of the reaction, i.e., the previously added monomer has to be consumed completely before a new monomer is being added, which is however not the case. But in reality, this approach is favorable when the rate of polymerization is reasonably fast. However, it is quite challenging to maintain the rate of monomer addition to be reasonably slow but constant during the polymerization reaction. The addition of a B f multifunctional core molecule to an AB2 system also makes the final hb copolymer to resemble the dendrimer like hierarchical architecture. In a random polymerization process, as most of the terminal units disperse at the periphery, the introduction of a core adds a focal point in the hb copolymer [122]. The molar feed ratio of the AB2-type monomer and the B f molecule is a highly critical parameter in determining M.W and P.D.I of the hb copolymers. Generally, a high molar feed ratio of AB2 and B f (say greater than 100) broadens M.W.D of the hb copolymers due to increased probability of the monomer–monomer reaction, i.e., self AB2 polymerization [118]. However, keeping the molar feed ratio of AB2 to B f less than 100 dramatically reduces M.W of the resultant hb copolymers which may limits their applications in many potential fields. The second comonomer multifunctional core molecule may be added to the AB2 system, either as a small molecule or as a polymer. When a multifunctional polymer or a macroinitiator is used as a core in an AB2 system, then it is a kind of hypergrafting strategy (grafting-from technique is generally followed) [123]. Hypergrafting strategy may involve grafting of a hb block copolymer either from the surface of a multifunctional linear macroinitiator (linear-graft-hyperbranched copolymer) [124] or from a multifunctional hb macroinitiator (hyperbranched-graft-hyperbranched copolymer) [125]. The multifunctional macroinitiator core molecule is generally composed of polyglycerols and poly (ethylene glycol)s. It has been found that hypergrafted hb copolymer products being core–shell type polymers are often highly useful in biomedical transport applications or for applications in bio-conjugations. In spite of using a multifunctional core molecule in the polymerization reaction mixture, often hb copolymers exhibit bimodal M.W.D; a narrow, high M.W peak, and a broad, long tail on the lower M.W end. Bharathi and Moore dramatically improved the GPC chromatograms of the hb copolymers prepared from an AB2-type monomer and a B f comonomer by carrying out the polymerization reaction on an insoluble solid (polymer, peptide, etc.) support [126]. They speculated that due to the limitations in the spaces that exists when a hb polymer attaches to a solid support, a high M.W could be maintained during the hb polymerizations. The solid support also inhibited any undesirable intramolecular cyclizations between the A and the B groups. In this novel work, they tethered a B f -type monomer core to an insoluble solid support suspension through a triazene linkage and then subsequently polymerized an AB2-type monomer in the presence of the core support suspension, Pd (II) catalyst and piperidine. The resultant hb copolymers (isolated from the solid support) exhibited highly controlled features like M.W of around 5–25 × 103 g mol−1 and P.D.I of around 1.1–1.5. The only problem associated with the technique of slow addition of the AB2-type monomer to a multifunctional core (either supported or unsupported) is the happening of self-polymerization of the AB2-type monomer which generally results in low yield of the desirable hb copolymers.
-
(II)
Maintaining different reactivities of the multifunctional core and the monomer in a real polymerization process, although a difference in reactivity between the AB2-type monomer and the B f molecule is always maintained but by maintaining a wide difference in their reactivities, further improvement in the GPC chromatogram of the resultant hb copolymers is possible [127]. This approach is analogous to slow monomer addition in the sense that randomness of the polymerization reaction is significantly reduced. Due to wide difference in the reactivity ratio between AB2 and B f (B f is preferably more reactive than the B groups of the AB2-type monomer due to higher degree of functionality), B f reacts with AB2 at a faster rate and delays undesirable homopolymerization of AB2. Bernal et al. prepared hb poly (arylene ether phosphine oxide)s in the presence of a series of B f molecules with varied reactivities and observed P.D.I as low as 1.25 [128]. Following this work, further improvement in the GPC chromatogram was procured by Roy et al. where they synthesized hb polyethers in the presence of highly active B3 core than the AB2-type monomer [129]. They observed on increasing the molar fraction of B3 to AB2, decrease in M.W (as low as 5400 g mol−1) and P.D.I (<1.5) occurred. Hence, in order to obtain hb copolymers with narrow P.D.I but high M.W and high DB in the true sense, more research has to be done to develop new pathbreaking strategies. These days, activation of the functional groups on the core polymer also significantly narrows M.W.D of the hb copolymers. On using the B f core molecule with higher reactivity than the AB2-type monomer, a faster consumption of the core molecules than the AB2-type monomer occurs in the initial stage. However, after complete reaction of the B with the A groups, the surface B groups on the produced 1st generation hb copolymer become equally reactive to that of the B groups on the monomer. This phenomenon significantly reduces the effect of faster reaction of the B f core than the monomer. Hence, a consecutive activation of the B groups on the 1st generation hb copolymer during the polymerization reaction is necessary. Suzuki et al. introduced the concept of multibranching polymerization (MBP) which generates a dendritic polymer with the initiator as the core [130]. MBP proceeds via multiplication of the propagating ends at every step of the propagation. To carry out a successful MBP reaction, a judicious choice of monomers and an adequate feed ratio between the monomers has to be maintained. In a later work, Suzuki et al. designed hb dendritic polyamine consisting of primary, secondary (a nonbranching point) and tertiary (a branching point) amino groups via Pd catalyzed decarboxylative ROP of a cyclic carbamate monomer in the presence of benzylamine initiator [131]. This type of MBP involves a monomer with a leaving group at the allylic position which is activated from time to time by the oxidative addition of Pd to form a Π-allylpalladium complex intermediate. The Π-allylpalladium intermediate is further nucleophilically attacked by an initiator fragment and a propagating end. Thus, here free amine initiator (benzylamine) or rather the growing polymer chains reacted with the monomer but the monomer could not undergo self-polymerization; thereby narrows M.W.D of the resultant hb copolymers. The hb copolymers exhibited DB around 0.6–0.8 and P.D.I < 1.5. In another attempt, Ohta and coworkers synthesized hb copolymers by exploiting the change of substituent reactivity effects between the monomer and the B f core as well as the growing polymer [132]. Generally, reactivity of the initially identical groups changes after one of them has reacted. In their study, they generated an AB2-type monomer anion with an amide anion (as A group) and two ethyl ester groups (as B groups) where the amide anion deactivated both the ester groups which subsequently prevented AB2 self-polymerization (through +I effect) and encouraged reaction of the AB2-type monomer anion exclusively with the initiator core molecule (constituting of two reactive ester groups). In the following step, the AB2-type monomer anion further reacts with the ester groups of the growing polymer chain which had been activated through the formation of amide linkage. The resultant hb copolymers exhibited P.D.I < 1.13 but DB was only 0.5 (due to equal reactivity of the terminal B groups on the polymer). Unfortunately, this approach is applicable only to certain classes of polymers (as the choice of monomer-core pair is critical) which restrict its exploration.
-
(III)
By carrying out the hb polymerizations in confined spaces- When a polymerization reaction (especially in FRP) is carried out in a homogeneous medium, variety of random reactions occur due to unconfined nature of the process (i.e., infinite space). Undoubtedly, such random reactions adversely affect the structure of the polymers with complex architectures where otherwise control in the reaction strategy is necessary. On the contrary, when a polymerization is carried out in a heterogeneous, dispersed medium (say in microemulsion), it is possible to synthesize polymers with controlled and complex architectures. A well dispersed medium or rather the presence of discreet micelles ensures compartmentalization which is basically segregation and/or confinement of the reactants within the discrete polymerizing particles. Such confinement of the reactant molecules dramatically reduce polymer–polymer inter particle reactions and thus narrows M.W.D of the resultant hb polymers. Each discrete polymerizing sites acts either as a microreactor or a nanoreactor with a well defined boundary. Each reactor is highly localized and independent and there is hardly any mass transfer between the adjoining reactors. This synthesis approach is mainly popular in the controlled radical polymerization technique. So far, the attempt to control the hierarchical structures of the hb copolymers with well defined properties has been mostly successful with this approach.
2.5 Conclusion
This chapter has covered some of the notable advancements in the synthesis of hb polymers via step-growth and chain-growth reactions since their birth. Typical approaches for the synthesis of hb polymers like AB x polycondensation, A2 + B3 polymerization and other categories which are quite widely followed worldwide is discussed in length. In each section, a brief detail has been provided how different methods can be used to produce different important classes of hb biomaterials. This chapter also focuses on the drawbacks associated with the different preparatory methods for the hb polymers and how various researches have come up with suitable remedies.
Abbreviations
- DB:
-
Degree of branching
- DP:
-
Degree of polymerization
- FRP:
-
Free radical polymerization
- Hb:
-
Hyperbranched
- M.W:
-
Molecular weight
- M.W.D:
-
Molecular Weight Distribution
- NMP:
-
N-methyl-2-pyrrolidinone solvent
- P.D.I:
-
Poly dispersity index
- PC:
-
Polycarbonate
- PG:
-
Poly glycerol
- ROP:
-
Ring opening polymerization
References
Gao C, Yan D, Frey H (2011) Promising dendritic materials: an introduction to hyperbranched polymers. In: Hyperbranched polymers, John Wiley & Sons, Inc.: pp 1–26
Voit B (2000) New developments in hyperbranched polymers. J Polym Sci A Polym Chem 38(14):2505–2525
Flory PJ (1953) Principles of polymer chemistry. 1st ed. Cornell University Press, Ithaca, United States
Flory PJ (1941) Molecular size distribution in three dimensional polymers. I. Gelation1. J Am Chem Soc 63(11):3083–3090
Flory PJ (1952) Molecular size distribution in three dimensional polymers. VI. Branched polymers containing A-R-Bf-1 type units. J Am Chem Soc 74(11):2718–2723
Hölter D, Burgath A, Frey H (1997) Degree of branching in hyperbranched polymers. Acta Polym 48(1–2):30–35
Beginn U, Drohmann C, Moller M (1997) Conversion dependence of the branching density for the polycondensation of AB n monomers. Macromolecules 30(14):4112–4116
Malmstrom E, Johansson M, Hult A (1995) Hyperbranched aliphatic polyesters. Macromolecules 28(5):1698–1703
Hawker CJ, Lee R, Frechet JMJ (1991) One-step synthesis of hyperbranched dendritic polyesters. J Am Chem Soc 113(12):4583–4588
Voit BI, Lederer A (2009) Hyperbranched and highly branched polymer Architectures synthetic strategies and major characterization aspects. Chem Rev 109(11):5924–5973
Korolev GV, Bubnova ML (2007) Synthesis, properties, and practical application of hyperbranched polymers. Polym Sci Ser 49(4):332–354
Kim YH, Webster OW (1992) Hyperbranched polyphenylenes. Macromolecules 25(21):5561–5572
Peng H, Dong Y, Jia D, Tang B (2004) Syntheses of readily processable, thermally stable, and light-emitting hyperbranched polyphenylenes. Chin Sci Bull 49(24):2637–2639
Tanaka S, Doke Y, Iso T (1997) Preparation of new branched poly(triphenylamine). Chem Commun 21:2063–2064
Sun M, Li J, Li B, Fu Y, Bo Z (2005) Toward high molecular weight triphenylamine-based hyperbranched polymers. Macromolecules 38(7):2651–2658
Bo Z, Schluter AD (2003) “AB2 + AC2” approach to hyperbranched polymers with a high degree of branching. Chem Commun 18:2354–2355
Huang W, Su L, Bo Z (2009) Hyperbranched polymers with a degree of branching of 100% prepared by catalyst transfer suzuki–miyaura polycondensation. J Am Chem Soc 131(30):10348–10349
Maier G, Zech C, Voit B, Komber H (1998) An approach to hyperbranched polymers with a degree of branching of 100%. Macromol Chem Phys 199(12):2655–2664
Lim S-J, Seok DY, An BK, Jung SD, Park SY (2006) A modified strategy for the synthesis of hyperbranched poly(p-phenylenevinylene): achieving extended π-conjugation with growing molecular weight. Macromolecules 39(1):9–11
Dieck HA, Heck RF (1974) Organophosphinepalladium complexes as catalysts for vinylic hydrogen substitution reactions. J Am Chem Soc 96(4):1133–1136
Nishide H, Nambo M, Miyasaka M (2002) Hyperbranched poly(phenylenevinylene) bearing pendant phenoxys for a high-spin alignment. J Mater Chem 12(12):3578–3584
Fukuzaki E, Nishide H (2006) Room-temperature high-spin organic single molecule: nanometer-sized and hyperbranched poly[1,2, (4)-phenylenevinyleneanisylaminium]. J Am Chem Soc 128(3):996–1001
Lu P, Paulasaari JK, Weber WP (1996) Hyperbranched poly(4-Acetylstyrene) by ruthenium-catalyzed step-growth polymerization of 4-acetylstyrene. Macromolecules 29(27):8583–8586
In I, Lee H, Kim SY (2003) Synthesis of hyperbranched poly(phenylene oxide) by ullmann polycondensation and subsequent utilization as unimolecular micelle. Macromol Chem Phys 204(13):1660–1664
Tolosa J, Kub C, Bunz UHF (2009) Hyperbranched: a universal conjugated polymer platform. Angew Chem Int Ed 48(25):4610–4612
Li ZA, Wu W, Ye C, Qin J, Li Z (2010) New second-order nonlinear optical polymers derived from AB2 and AB monomers via sonogashira coupling reaction. Macromol Chem Phys 211(8):916–923
Fussell AL, Isomaki A, Strachan CJ (2013) Non-linear optical imaging—introduction and pharmaceutical applications. Am Pharmaceut Rev 16(6):54–63
Stille JK (1972) Cycloaddition polymerization. Die Makromolekulare Chemie 154(1):49–61
Morgenroth F, Mullen K (1997) Dendritic and hyperbranched polyphenylenes via a simple Diels-Alder route. Tetrahedron 53(45):15349–15366
Harrison RM, Feast WJ (1997) ACS Polym Mater Sci Eng 77:162
Abadie MJM (2012) High performance polymers—polyimides based—from chemistry to applications. InTech:
Gok O, Durmaz H, Ozdes ES, Hizal G, Tunca U, Sanyal A (2010) Maleimide-based thiol reactive multiarm star polymers via Diels-Alder/retro Diels-Alder strategy. J Polym Sci A Polym Chem 48(12):2546–2556
Sanyal A (2010) Diels-alder cycloaddition-cycloreversion: a powerful combo in materials design. Macromol Chem Phys 211(13):1417–1425
Froimowicz P, Frey H, Landfester K (2011) Towards the generation of self-healing materials by means of a reversible photo-induced approach. Macromol Rapid Commun 32(5):468–473
Itoya K, Kakimoto M, Imai Y (1994) High-pressure synthesis of new aromatic poly(diazetidinediones) by cyclodimerization polymerization of aromatic diisocyanates. Macromolecules 27(25):7231–7235
Xu KT, Tang BZ (1999) Polycyclotrimerization of diynes, a new approach to hyperbranched polyphenylenes. Chin J Polym Sci 17(4):397–402
Peng H, Cheng L, Luo J, Xu K, Sun Q, Dong Y, Salhi F, Lee PPS, Chen J, Tang BZ (2002) Simple synthesis, outstanding thermal stability, and tunable light-emitting and optical-limiting properties of functional hyperbranched polyarylenes. Macromolecules 35(14):5349–5351
Jin RH, Motokucho S, Andou Y, Nishikubo T (1998) Controlled polymerization of an AB2 monomer using a chloromethylarene as comonomer: branched polymers from activated methylene compounds. Macromol Rapid Commun 19(1):41–46
In I, Kim SY (2005) Hyperbranched poly(arylene ether amide) via nucleophilic aromatic substitution reaction. Macromol Chem Phys 206(18):1862–1869
Yang D, Kong J (2016) 100% hyperbranched polymers via the acid-catalyzed friedel-crafts aromatic substitution reaction. Polym. Chem. 7(33):5226–5232
Kim Y-B, Kim HK, Nishida H, Endo T (2004) Synthesis and characterization of hyperbranched poly(β-ketoester) by the michael addition. Macromol Mater Eng 289(10):923–926
Trumbo DL (1991) Michael addition polymers from 1,4 and 1,3 benzenedimethanol diacetoacetates and tripropylene glycol diacrylate. Polym Bull 26(3):265–270
Gao C, Xu Y, Yan D, Chen W (2003) Water-soluble degradable hyperbranched polyesters: novel candidates for drug delivery? Biomacromol 4(3):704–712
Park MR, Han KO, Han IK, Cho MH, Nah JW, Choi YJ, Cho CS (2005) Degradable polyethylenimine-alt-poly(ethylene glycol) copolymers as novel gene carriers. J Control Release 105(3):367–380
Cosulich ME, Russo S, Pasquale S, Mariani A (2000) Performance evaluation of hyperbranched aramids as potential supports for protein immobilization. Polymer 41(13):4951–4956
Kim YH (1992) Lyotropic liquid crystalline hyperbranched aromatic polyamides. J Am Chem Soc 114(12):4947–4948
Lee CC (2015) The current trends of optics and photonics, vol 129. Springer Netherlands
Ishida Y, Sun ACF, Jikei M, Kakimoto M (2000) Synthesis of hyperbranched aromatic polyamides starting from dendrons as ABx monomers: effect of monomer multiplicity on the degree of branching. Macromolecules 33(8):2832–2838
Yamanaka K, Jikei M, Kakimoto MA (2000) Synthesis of hyperbranched aromatic polyimides via polyamic acid methyl ester precursor. Macromolecules 33(4):1111–1114
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40(11):2004–2021
Tornoe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67(9):3057–3064
Scheel AJ, Komber H, Voit BI (2004) Novel hyperbranched poly([1,2,3]-triazole)s derived from AB2 monomers by a 1,3-dipolar cycloaddition. Macromol Rapid Commun 25(12):1175–1180
Li ZA, Yu G, Hu P, Ye C, Liu Y, Qin J, Li Z (2009) New azo-chromophore-containing hyperbranched polytriazoles derived from ab2 monomers via click chemistry under copper(I) catalysis. Macromolecules 42(5):1589–1596
Zhang L, Chen X, Xue P, Sun HHY, Williams ID, Sharpless KB, Fokin VV, Jia G (2005) Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J Am Chem Soc 127(46):15998–15999
van Dijk M, Rijkers DTS, Liskamp RMJ, van Nostrum CF, Hennink WE (2009) Synthesis and applications of biomedical and pharmaceutical polymers via click chemistry methodologies. Bioconjugate Chem 20(11):2001–2016
Li H, Wang J, Sun JZ, Hu R, Qin A, Tang BZ (2012) Metal-free click polymerization of propiolates and azides: facile synthesis of functional poly(aroxycarbonyltriazole)s. Polym Chem 3(4):1075–1083
Li H, Wu H, Zhao E, Li J, Sun JZ, Qin A, Tang BZ (2013) Hyperbranched poly(aroxycarbonyltriazole)s: metal-free click polymerization, light refraction, aggregation-induced emission, explosive detection, and fluorescent patterning. Macromolecules 46(10):3907–3914
Ortega P, Cobaleda BM, Hernandez-Ros JM, Fuentes-Paniagua E, Sanchez-Nieves J, Tarazona MP, Copa-Patino JL, Soliveri J, de la Mata FJ, Gomez R (2011) Hyperbranched polymers versus dendrimers containing a carbosilane framework and terminal ammonium groups as antimicrobial agents. Org Biomol Chem 9(14):5238–5248
Xue L, Yang Z, Wang D, Wang Y, Zhang J, Feng S (2013) Synthesis and characterization of silicon-containing hyperbranched polymers via thiol-ene click reaction. J Organomet Chem 732:1–7
Moreno S, Lozano-Cruz T, Ortega P, Tarazona MP, de la Mata FJ, Gómez R (2014) Synthesis of new amphiphilic water-stable hyperbranched polycarbosilane polymers. Polym Int 63(7):1311–1323
Roy RK, Ramakrishnan S (2011) Thiol-ene clickable hyperscaffolds bearing peripheral allyl groups. J Polym Sci Part APolym Chem 49(8):1735–1744
Konkolewicz D, Gray-Weale A, Perrier SB (2009) Hyperbranched polymers by thiol-yne chemistry: from small molecules to functional polymers. J Am Chem Soc 131(50):18075–18077
Cook AB, Barbey R, Burns JA, Perrier S (2016) Hyperbranched polymers with high degrees of branching and low dispersity values: pushing the limits of thiol-yne chemistry. Macromolecules 49(4):1296–1304
Han J, Zhao B, Tang A, Gao Y, Gao C (2012) Fast and scalable production of hyperbranched polythioether-ynes by a combination of thiol-halogen click-like coupling and thiol-yne click polymerization. Polym Chem 3(7):1918–1925
Wang D, Zhao T, Zhu X, Yan D, Wang W (2015) Bioapplications of hyperbranched polymers. Chem Soc Rev 44(12):4023–4071
Lasprilla AJR, Martinez GAR, Hoss B (2011) Synthesis and characterization of poly (lactic acid) for use in biomedical field. Chem Eng 24:985–990
Tasaka F, Ohya Y, Ouchi T (2001) One-pot synthesis of novel branched polylactide through the copolymerization of lactide with mevalonolactone. Macromol Rapid Commun 22(11):820–824
Pitet LM, Hait SB, Lanyk TJ, Knauss DM (2007) Linear and branched architectures from the polymerization of lactide with glycidol. Macromolecules 40(7):2327–2334
Tsujimoto T, Haza Y, Yin Y, Uyama H (2011) Synthesis of branched poly(lactic acid) bearing a castor oil core and its plasticization effect on poly(lactic acid). Polym J 43(4):425–430
Frey H (2013) Hyperbranched polyglycerols (Synthesis and Applications). In: Encyclopedia of Polymeric Nanomaterials, Springer Berlin Heidelberg: Berlin, Heidelberg, pp 1–4
Wilms D, Stiriba S-E, Frey H (2010) Hyperbranched polyglycerols: from the controlled synthesis of biocompatible polyether polyols to multipurpose applications. Acc Chem Res 43(1):129–141
Sunder A, Hanselmann R, Frey H, Mulhaupt R (1999) Controlled synthesis of hyperbranched polyglycerols by ring-opening multibranching polymerization. Macromolecules 32(13):4240–4246
Robinson JW, Zhou Y, Bhattacharya P, Erck R, Qu J, Bays JT, Cosimbescu L (2016) Probing the molecular design of hyper-branched aryl polyesters towards lubricant applications. Sci Rep 6:18624
Khemchandani B, Verma HS (2012) High performance shear stable viscosity modifiers. In: Polymer processing and characterization, Apple Academic Press, pp 33–41
Stohr T, Eisenberg B, Muller M (2008) A new generation of high performance viscosity modifiers based on comb polymers. SAE Int J Fuels Lubr 1(1):1511–1516
Lee S, Saito K, Lee HR, Lee MJ, Shibasaki Y, Oishi Y, Kim BS (2012) Hyperbranched double hydrophilic block copolymer micelles of poly(ethylene oxide) and polyglycerol for pH-responsive drug delivery. Biomacromol 13(4):1190–1196
Garamus VM, Maksimova TV, Kautz H, Barriau E, Frey H, Schlotterbeck U, Mecking S, Richtering W (2004) Hyperbranched polymers: structure of hyperbranched polyglycerol and amphiphilic poly(glycerol ester)s in dilute aqueous and nonaqueous solution. Macromolecules 37(22):8394–8399
Parzuchowski PG, Grabowska M, Jaroch M, Kusznerczuk M (2009) Synthesis and characterization of hyperbranched polyesters from glycerol-based AB2 monomer. J Polym Sci A Polym Chem 47(15):3860–3868
Parzuchowski PG, Jaroch M, Tryznowski M, Rokicki G (2008) Synthesis of new glycerol-based hyperbranched polycarbonates. Macromolecules 41(11):3859–3865
Testud B, Pintori D, Grau E, Taton D, Cramail H (2017) Hyperbranched polyesters by polycondensation of fatty acid-based ABn-type monomers. Green Chem 19(1):259–269
Brenner AR, Voit BI, Massa DJ, Turner SR (1996) Hyperbranched polyesters: End group modification and properties. Macromol Symp 102(1):47–54
Ghosh A, Banerjee S, Voit B (2015) Aromatic hyperbranched polymers: synthesis and application. In: Long TE, Voit B, Okay O (eds) Porous carbons- hyperbranched polymers- polymer solvation. Springer International Publishing, Cham, pp 27–124
Hult A, Johansson M, Malmstrom E (1999) Hyperbranched Polymers. In: Roovers J (ed) Branched polymers II. Springer Berlin Heidelberg: Berlin, Heidelberg, pp 1–34
Zhang X (2010) Hyperbranched aromatic polyesters: From synthesis to applications. Prog Org Coat 69(4):295–309
Kricheldorf HR, Zang Q-Z, Schwarz G (1982) New polymer syntheses: 6. Linear and branched poly(3-hydroxy-benzoates). Polymer 23(12):1821–1829
Turner SR, Voit BI, Mourey TH (1993) All-aromatic hyperbranched polyesters with phenol and acetate end groups: synthesis and characterization. Macromolecules 26(17):4617–4623
Fomine S, Rivera E, Fomina L, Ortiz A, Ogawa T (1998) Polymers from coumarines: 4. Design and synthesis of novel hyperbranched and comb-like coumarin-containing polymers. Polymer 39(15):3551–3558
Kricheldorf HR, Stukenbrock T (1998) New polymer syntheses XCIII. Hyperbranched homo- and copolyesters derived from gallic acid and β-(4-hydroxyphenyl)-propionic acid. J Polym Sci APolym Chem 36(13):2347–2357
Qiu T, Tang L, Tuo X, Zhang X, Liu D (2001) Study on self-assembly properties of aryl-alkyl hyperbranched polyesters with carboxylic end groups. Polym Bull 47(3):337–342
Jikei M, Kakimoto MA (2001) Hyperbranched polymers: a promising new class of materials. Prog. Polym. Sci. 26(8):1233–1285
Erber M, Boye S, Hartmann T, Voit BI, Lederer A (2009) A convenient room temperature polycondensation toward hyperbranched AB2-type all-aromatic polyesters with phenol terminal groups. J Polym Sci Polym Chem 47(19):5158–5168
Gross RA, Kumar A, Kalra B (2001) Polymer synthesis by in vitro enzyme catalysis. Chem Rev 101(7):2097–2124
Uyama H, Kobayashi S (2002) Enzyme-catalyzed polymerization to functional polymers. J Mol Catal B Enzym 19–20:117–127
Reihmann M, Ritter H (2006) Synthesis of phenol polymers using peroxidases. In: Kobayashi S, Ritter H, Kaplan D (eds) Enzyme-catalyzed synthesis of polymers. Springer Berlin Heidelberg: Berlin, Heidelberg, pp 1–49
Skaria S, Smet M, Frey H (2002) Enzyme-catalyzed synthesis of hyperbranched aliphatic polyesters. Macromol Rapid Commun 23(4):292–296
Lopez-Luna A, Gallegos JL, Gimeno M, Vivaldo-Lima E, Barzana E (2010) Lipase-catalyzed syntheses of linear and hyperbranched polyesters using compressed fluids as solvent media. J Mol Catal B Enzym 67(1–2):143–149
Mena M, Lopez-Luna A, Shirai K, Tecante A, Gimeno M, Barzana E (2013) Lipase-catalyzed synthesis of hyperbranched poly-l-lactide in an ionic liquid. Bioproc Biosyst Eng 36(3):383–387
Xu F, Zhong J, Qian X, Li Y, Lin X, Wu Q (2013) Multifunctional poly(amine-ester)-type hyperbranched polymers: lipase-catalyzed green synthesis, characterization, biocompatibility, drug loading and anticancer activity. Polym Chem 4(12):3480–3490
Kricheldorf H (2013) Hyperbranched polymers by a2 + bn polycondensation. In: Polycondensation: history and new results. Springer Berlin Heidelberg: Berlin, Heidelberg, pp 147–159
Aharoni SM, Edwards SF (1989) Gels of rigid polyamide networks. Macromolecules 22(8):3361–3374
Aharoni SM (1991) Gels of two-step rigid polyamide networks. Macromolecules 24(15):4286–4294
Jikei M, Chon SH, Kakimoto MA, Kawauchi S, Imase T, Watanebe J (1999) Synthesis of hyperbranched aromatic polyamide from aromatic diamines and trimesic acid. Macromolecules 32(6):2061–2064
Fang J, Kita H, Okamoto KI (2000) Hyperbranched polyimides for gas separation applications. 1. Synthesis and characterization. Macromolecules 33(13):4639–4646
Hao J, Jikei M, Kakimoto MA (2002) Preparation of hyperbranched aromatic polyimides via A2 + B3 Approach. Macromolecules 35(14):5372–5381
Unal S, Long TE (2006) Highly Branched Poly(ether ester)s via cyclization-free melt condensation of A2 oligomers and B3 monomers. Macromolecules 39(8):2788–2793
Scheel A, Komber H, Voit B (2004) Hyperbranched thermolabile polycarbonates derived from a A2 + B3 monomer system. Macromol Symp 210(1):101–110
Miyasaka M, Takazoe T, Kudo H, Nishikubo T (2010) Synthesis of hyperbranched polycarbonate by novel polymerization of di-tert-butyl tricarbonate with 1,1,1-tris(4-hydroxyphenyl)ethane. Polym J 42(11):852–859
Wang Q, Shi W (2006) Synthesis and thermal decomposition of a novel hyperbranched polyphosphate ester used for flame retardant systems. Polym Degrad Stab 91(6):1289–1294
Liu J, Huang W, Pang Y, Yan D (2015) Hyperbranched polyphosphates: synthesis, functionalization and biomedical applications. Chem Soc Rev 44(12):3942–3953
Xie J, Hu L, Shi W, Deng X, Cao Z, Shen Q (2008) Synthesis and characterization of hyperbranched polytriazole via an ‘A2 + B3’ approach based on click chemistry. Polym Int 57(8):965–974
Qin A, Lam JWY, Jim CKW, Zhang L, Yan J, Haussler M, Liu J, Dong Y, Liang D, Chen E, Jia G, Tang BZ (2008) Hyperbranched Polytriazoles: click polymerization, regioisomeric structure, light emission, and fluorescent patterning. Macromolecules 41(11):3808–3822
Chen H, Jia J, Duan X, Yang Z, Kong J (2015) Reduction-cleavable hyperbranched polymers with limited intramolecular cyclization via click chemistry. J Polym Sci A Polym Chem 53(20):2374–2380
Tapan K, Thirumoolan D, Mohanram R, Vetrivel K, Basha KA (2015) Antimicrobial activity of hyperbranched polymers: synthesis, characterization, and activity assay study. J Bioact Compat Polym 30(2):145–156
Gao C, Yan D (2004) Hyperbranched polymers: from synthesis to applications. Prog Polym Sci 29(3):183–275
Gao C, Yan D (2011) Synthesis of Hyperbranched polymers via polymerization of asymmetric monomer pairs. In: Hyperbranched Polymers, John Wiley & Sons, Inc. pp 107–138
Shi Y, Graff RW, Gao H (2015) Recent progress on synthesis of hyperbranched polymers with controlled molecular weight distribution. In: Controlled Radical Polymerization: Materials, American Chemical Society, vol. 1188, pp 135–147
Feast WJ, Stainton NM (1995) Synthesis, structure and properties of some hyperbranched polyesters. J Mater Chem 5(3):405–411
Bharathi P, Moore JS (2000) Controlled synthesis of hyperbranched polymers by slow monomer addition to a core. Macromolecules 33(9):3212–3218
Yan D, Zhou Z (1999) Molecular weight distribution of hyperbranched polymers generated from polycondensation of AB2 type monomers in the presence of multifunctional core moieties. Macromolecules 32(3):819–824
Radke W, Litvinenko G, Muller AHE (1998) Effect of core-forming molecules on molecular weight distribution and degree of branching in the synthesis of hyperbranched polymers. Macromolecules 31(2):239–248
Satoh T (2012) Synthesis of hyperbranched polymer using slow monomer addition method. Int J Polym Sci p 8
Chen JY, Smet M, Zhang JC, Shao WK, Li X, Zhang K, Fu Y, Jiao YH, Sun T, Dehaen W, Liu FC, Han EH (2014) Fully branched hyperbranched polymers with a focal point: analogous to dendrimers. Polym Chem 5(7):2401–2410
Schull C, Frey H (2013) Grafting of hyperbranched polymers: from unusual complex polymer topologies to multivalent surface functionalization. Polymer 54(21):5443–5455
Schull C, Rabbel H, Schmid F, Frey H (2013) Polydispersity and molecular weight distribution of hyperbranched graft copolymers via “hypergrafting” of abm monomers from polydisperse macroinitiator cores: theory meets synthesis. Macromolecules 46(15):5823–5830
Popeney CS, Lukowiak MC, Bottcher C, Schade B, Welker P, Mangoldt D, Gunkel G, Guan Z, Haag R (2012) Tandem coordination, ring-opening, hyperbranched polymerization for the synthesis of water-soluble core-shell unimolecular transporters. ACS Macro Lett 1(5):564–567
Bharathi P, Moore JS (1997) Solid-Supported Hyperbranched Polymerization: Evidence for Self-Limited Growth. J Am Chem Soc 119(14):3391–3392
Zhou Z, Jia Z, Yan D (2012) Kinetic analysis of AB2 polycondensation in the presence of multifunctional cores with various reactivities. Polymer 53(15):3386–3391
Bernal DP, Bedrossian L, Collins K, Fossum E (2003) Effect of core reactivity on the molecular weight, polydispersity, and degree of branching of hyperbranched poly(arylene ether phosphine oxide)s. Macromolecules 36(2):333–338
Roy RK, Ramakrishnan S (2011) Control of molecular weight and polydispersity of hyperbranched polymers using a reactive B3 core: a single-step route to orthogonally functionalizable hyperbranched polymers. Macromolecules 44(21):8398–8406
Suzuki M, Ii A, Saegusa T (1992) Multibranching polymerization: palladium-catalyzed ring-opening polymerization of cyclic carbamate to produce hyperbranched dendritic polyamine. Macromolecules 25(25):7071–7072
Suzuki M, Yoshida S, Shiraga K, Saegusa T (1998) New ring-opening polymerization via a π-allylpalladium complex. 5. multibranching polymerization of cyclic carbamate to produce hyperbranched dendritic polyamine. Macromolecules 31(6):1716–1719
Ohta Y, Fujii S, Yokoyama A, Furuyama T, Uchiyama M, Yokozawa T (2009) Synthesis of well-defined hyperbranched polyamides by condensation polymerization of AB2 monomer through changed substituent effects. Angew Chem Int Ed 48(32):5942–5945
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Das, T., Sengupta, S., Bandyopadhyay, A. (2018). Part I—Synthesis of Hyperbranched Polymers: Step-Growth Methods. In: Hyperbranched Polymers for Biomedical Applications . Springer Series on Polymer and Composite Materials. Springer, Singapore. https://doi.org/10.1007/978-981-10-6514-9_2
Download citation
DOI: https://doi.org/10.1007/978-981-10-6514-9_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6513-2
Online ISBN: 978-981-10-6514-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)