Abstract
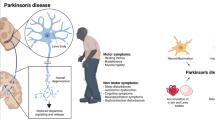
Parkinson’s disease (PD) is late-onset, progressive neurodegenerative, and hypokinetic movement disorder characterized by relatively selective degeneration of nigrostriatal dopaminergic neurons and presence of fibrillar cytoplasmic inclusions containing α-synuclein and ubiquitin. Major pathological features of PD include degeneration of dopaminergic neurons coupled with fibrillar intracytoplasmic inclusions known as Lewy bodies, and these Lewy bodies are also found in the hypothalamus, cranial nerve motor nuclei, locus coeruleus, nucleus basalis, cerebral cortex, and central and peripheral components of the ANS. PD is presented with four primary motor manifestations: tremor at rest, rigidity, bradykinesia (or slowing of movement), and postural instability. Initially, not all patients present with all of the classic signs of PD, there may be only one or two and non-motor symptoms includes neuropsychiatric disturbances (i.e., depression, anxiety, and dementia), cognitive impairment, sleep disturbances or hallucinations, autonomic dysfunctions, fatigue, apathy, and orthostatic hypotension. It is estimated that approximately 5–10% of cases are occur or happened due to inheritable genetic mutation. The remaining 90% of newly diagnosed PD cases are of idiopathic origin. PD is a second most common age related neurodegenerative disease after Alzheimer’s disease. PD is thought to affect more than 1 million people in the USA alone, 1 of every 100 individuals, beyond the age of 55 suffered with disease. The prevalence of PD is also increases with age, affecting about 1–2% adults above the age of 60 years and 4% of above the age of 80 years.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Fibrillar Cytoplasmic Inclusions
- Cranial Nerve Motor Nuclei
- Inheritable Genetic Mutation
- Lewy Bodies
- Substantia Nigra Pars Compacta (SNPc)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Parkinson’s disease (PD) is late-onset, progressive neurodegenerative, and hypokinetic movement disorder characterized by relatively selective degeneration of nigrostriatal dopaminergic neurons and presence of fibrillar cytoplasmic inclusions containing α-synuclein and ubiquitin. Major pathological features of PD include degeneration of dopaminergic neurons coupled with fibrillar intracytoplasmic inclusions known as Lewy bodies, and these Lewy bodies are also found in the hypothalamus, cranial nerve motor nuclei, locus coeruleus, nucleus basalis, cerebral cortex, and central and peripheral components of the ANS. PD is presented with four primary motor manifestations: tremor at rest, rigidity, bradykinesia (or slowing of movement), and postural instability. Initially, not all patients present with all of the classic signs of PD, there may be only one or two and non-motor symptoms includes neuropsychiatric disturbances (i.e., depression, anxiety, and dementia), cognitive impairment, sleep disturbances or hallucinations, autonomic dysfunctions, fatigue, apathy, and orthostatic hypotension. It is estimated that approximately 5–10% of cases are occur or happened due to inheritable genetic mutation. The remaining 90% of newly diagnosed PD cases are of idiopathic origin. PD is a second most common age related neurodegenerative disease after Alzheimer’s disease. PD is thought to affect more than 1 million people in the USA alone, 1 of every 100 individuals, beyond the age of 55 suffered with disease. The prevalence of PD is also increases with age, affecting about 1–2% adults above the age of 60 years and 4% of above the age of 80 years.
Pathophysiology
Pathophysiology of PD includes 70–80% dopaminergic neurons cell’s death in the substantia nigra pars compacta (SNPc) and specifically the ventral part of the pars compacta. Without treatment, PD progresses over 5–10 years to rigid, akinetic state in which patients are incapable of caring for themselves. Loss of dopaminergic neurons of SNPc leads to functional changes in complex circuit of basal ganglia which further resulting into enormous motor inhibition, and there is development of Parkinsonism like symptoms. A reduced activity of the mitochondrial complexes I has been observed in patients with PD in the SNPc. Mitochondrial complex I toxic derangement promotes alpha-synuclein aggregation and formation of Lewy body-like inclusions. There is possibility that protein aggregation may play a role in Parkinsonism had long been suggested by the presence of Lewy bodies in brain. Increasing evidences indicate that the ubiquitin–proteasome pathway dysfunction occurs in PD. A dysfunction in proteasome might contribute to the accumulation and aggregation of alpha-synuclein and other neurotoxic proteins because the finding suggested that Lewy bodies are ubiquitin-positive aggregates. The most important metal involved in the pathogenesis of PD is iron because of its ability to generate free radicals. It accumulates in different brain regions in high concentration and forms selectively complexes with neuromelanin that may induce oxidative stress. During the metabolism of dopamine, reactive oxygen species (ROS) are formed in very high concentrations. Then, there is formation of hydrogen peroxide, which further converted to either hydroxyl radical via a Fenton reaction or to superoxide anion and peroxynitrite. The reactive oxygen species (ROS) further activates mitogen-activated protein kinase and c-Jun N-terminal kinase, which further leads to the liberation of cytochrome C from mitochondria and sequential activation of caspase 9 and caspase 3, which results in activation of apoptotic factors. Elevated levels of glutamate in the synaptic cleft also contribute to the neurodegeneration of dopaminergic neurons in PD.
After more than 40 years of clinical use, levodopa (LD) remains the gold standard treatment for PD. On comparison with other available dopaminergic therapies, dopamine substitution with levodopa (LD) is associated with significantly reducing disability with the greatest improvement in motor function and mortality and increasing patient quality of life. Long-term conventional levodopa therapy is associated with wearing-off and dyskinesia which can result in motor dysfunction.
Need of animal model
The animal models are extremely useful to understand the origin or pathophysiology of the disease and discovery of novel treatments. Models based on specific pathogenic mechanisms may subsequently lead to the development of neuroprotective agents for PD. So there is a need for development of new animal models by activating pathogenic mechanism involved in PD so that we can develop target specific drugs.
2 Classification of Animal Models of PD (Fig. 1)
2.1 Toxin-Induced Models
2.1.1 6-Hydroxy Dopamine (6-OHDA)-Induced Parkinsonism
6-OHDA is a classic animal model for PD. 6-OHDA was first isolated in 1960 and used by Ungerstedt to produce animal model of akinesia. 6-OHDA is toxic both at a peripheral and central level; however, 6-OHDA is unable to cross the blood–brain barrier (BBB), toxicity in the CNS is accomplish only when 6-OHDA directly injected into the brain (SNpc or the striatum) by means of stereotaxic surgery. Neurotoxic effects of 6-OHDA occur by accumulation of the toxin into catecholaminergic neurons. It uses DAT and NAT transporters to gain access to the cytosol where it can auto-oxidize, hence generating an intracellular hydrogen peroxide, superoxide, and hydroxyl radicals. These free radical cause neuronal cell death by oxidative stress. The intracellular storage of 6-OHDA is mediated by the dopamine or noradrenaline membrane transporters (DAT and NAT respectively), which recognize and uptake 6-OHDA due to its structural similarity with endogenous catecholamines (Fig. 1). The primary endpoint for the unilateral 6-OHDA model is rotation, as assessed in a rotameter. Rotation results from an imbalance in functionality of dopamine systems on the left and right sides of the brain following the unilateral lesion. The chances to rotate and the direction of rotation (i.e., ipsilateral or contralateral to the lesion) is affected by pharmacological agents that either simulate or block dopamine pathways. One week following injection, d-amphetamine can be administered in a dose of (2.5 mg/kg i.p) to subjects and the net rotations (i.e., rotational asymmetry) over a 90-min trial recorded. Alternatively, 3 weeks postlesioning, apomorphine in a dose of (0.25 mg/kg) can be administered, and monitored rotational asymmetry over 40 min. D-amphetamine acts by releasing dopamine stored in the remaining nerve terminals, whereas apomorphine is an agonist for both D1/D2 receptors.
Different doses & routes of administration of 6-OHDA
S. no. | Dose | Route | Species | References |
|---|---|---|---|---|
1. | Single-dose administration of 6-OHDA (2 μg/1 μl or 8 μg/μl) | Nucleus caudatus putamen (NCP) | Sprague–Dawley rats | Urban Ungerstedt (1968) |
2. | Single-dose administration of 6-OHDA (8 μg/4 μl or 8 μg/2 μl) | Right unilateral SNPc | Male Wistar rats | Hritcu (2008) |
3. | 6-OHDA (6 μg/2 µl) for 14 days | Intracerebroventricular (I.C.V) | Male Wistar rats | Kahale et al. (2014) |
Procedure:
-
Brain surgery is performed using a stereotaxic apparatus.
-
The rats are anaesthetized using ketamine (80 mg/kg, i.p) and xylazine (5 mg/kg, i.p), then their heads are mounted in a stereotaxic apparatus frame at flat skull position.
-
The cannulas are implanted unilaterally above dorsal striatum and 6-OHDA is administered using following coordinates: anteroposterior (AP) −0 mm from the bregma, mediolateral (ML) ±3.6 mm from the midline, and dorsoventral (DV) −4.5 mm from the skull surface.
-
After surgery, immediately the rats are injected with gentamycin (5 mg/kg i.p) to avoid infection and are left in observational boxes until they had recovered from anesthesia.
-
Then, they are housed individually in polypropylene cages (30 cm × 19 cm × 12 cm) for a week.
Advantages
6-OHDA is the first animal model of PD associated with SNPc dopaminergic neuronal death was introduced more than 30 years ago.
Limitation
The time course and severity of the PD model depend on the number, amount, and location (striatum, substantia nigra, or medial forebrain bundle) of the 6-OHDA injections
Clinical relevance
6-OHDA is mediated its action by DAT and NAT transporters and generating the free radicals. These free radical cause neuronal cell death by oxidative stress, and oxidative stress is the contributing factor in the pathogenesis of PD.
2.1.2 Mitochondrial Complex I (NADPH Dehydrogenase) Inhibitors
2.1.2.1 MPTP-Induced Neurotoxicity
MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is a neurotoxin precursor to MPP+, which causes Parkinson’s disease by destroying dopaminergic neurons in the substantia nigra pars compacta (SNPc) of the brain. MPTP as a model of PD was developed after an accidental event that happened in 1980s when several drug addicts from California developed Parkinsonian like syndrome after intravenous use of synthetic meperidine that was contaminated with MPTP.
MPTP is highly lipophilic and after systemic administration crosses the blood–brain barrier and is converted by monoamine oxidase B (MAO-B) to MPP+ within astrocytes. From the astrocytes with the help of cation transporter 3 (OCT-3), MPP+ is released into extracellular spaces. From extracellular spaces, MPP+ is taken up by the dopamine (DA) transporter. Since midbrain neurons contain the highest concentration of dopamine transporters/cell once in the cell, MPP+ can move through several cellular compartments: it can enter into mitochondria where it interferes with complex I of the electron transport chain and inhibits it. Blockade of this complex I leads to a reduction in cellular ATP. Inhibition of mitochondrial complex I not only interferes with adenosine triphosphate (ATP) synthesis, but also results in enhanced production of superoxide anion radical (Fig. 2).
Different doses & routes of administration of MPTP
MPTP can be given by a number of different routes, including subcutaneous, intravenous, intraperitoneal, and stereotaxic injection into the brain.
S. No | Dose | Route | Species | References |
|---|---|---|---|---|
1. | MPTP (30 mg/kg per day) for 10 consecutive days | i.p. | Male C57BL/6 mice | Nicholas (2007) |
2. | Chronic MPTP/Probenecid model-10 doses of MPTP (25 mg/kg s.c) and probenecid 250 mg/kg for over 5 weeks at 3.5 days interval | s.c. | Male C57Bl/6 mice | Meredith et al. (2008) |
3. | Single-dose administration of MPTP (1 µm/2 µl) | Bilaterally SNPc | Male Wistar rats | Wang et al. (2010) |
4. | MPTP (40 mg/kg) four injections of 10 mg/kg i.p. in divided doses at an interval of 1 h | i.p. | Male Laca mice | Gupta et al. (2011) |
5. | MPTP (100 µg/µl in 3 doses 1st, 7th and 14th day) | Intranigral | Male Wistar rats | Sharma et al. (2015) |
Procedure:
-
Brain surgery is performed by using a stereotaxic apparatus.
-
The rats are anaesthetized using ketamine (80 mg/kg, i.p) and xylazine (5 mg/kg, i.p) and the cannulas are implanted bilaterally 2 mm above the SNpc using following coordinate’s anteroposterior (AP) −5.0 mm from the bregma, mediolateral (ML) ±2.1 mm from the midline, and dorsoventral (DV) −7.7 mm from the skull and AP: −5.0 mm, ML: ±2.1 mm, DV: 8.0 mm from the bregma, midline, and skull surface.
-
MPTP (100 µg/µl) is administered bilaterally on 1st, 4th, and 7th day.
-
Immediately after surgery, the rats are injected with gentamycin (5 mg/kg i.p) to avoid infection and are left in observational boxes until they had recovered from anesthesia.
-
Then they are housed individually in polypropylene cages (30 cm × 19 cm × 12 cm) for a week.
Species
The most commonly used regimens of MPTP in monkeys are the multiple intraperitoneal or intramuscular injections and the intracarotid infusion. It is easy to perform and produce a bilateral Parkinsonian syndrome. Mice are much less sensitive to MPTP than monkeys; thus, to produce significant dopaminergic damage in this animal species much higher amount of doses are required presenting a much more difficult situation. Second, in contradiction to the monkeys, mice treated with MPTP do not develop PD. The rats are relatively insensitive to MPTP, but despite of drawback, the MPTP is continuously used in rats.
Advantages
-
1.
The MPTP model exhibits many features of PD, including dopaminergic neurodegeneration, motor deficits, alpha-synuclein inclusion formation, and neuroinflammation. This model is thus an appealing choice for developing biomarkers for early detection of PD.
-
2.
MPTP has been used as a toxin in large range of species.
-
3.
MPTP has been administered by all common routes to the rodents.
Drawbacks
-
1.
The effect of MPTP on the mouse DA system could be obtained depending upon the dose, dosing interval, animal strain etc.
Clinical relevance
Metabolite of MPTP is inhibitor of mitochondrial complex I. Dysfunction of complex increases the levels of free radicals. A reduced activity of the mitochondrial complexes I and IV have been observed in patients with Parkinson’s disease in the SNPc. In vitro experiments on mitochondria isolated from whole brain demonstrate that complex I activity must be inhibited by approximately 70% to significantly impair ATP production. But PD postmortem tissues demonstrate only 40% inhibition of mitochondrial complex I activity.
2.1.2.2 Rotenone-Induced Parkinsonism
Rotenone is widely used as a pesticide and is derived from the roots of tropical plants in the Pisum sativum family that are native of Malaysia, South America, and East Africa. In 1895, A French botanist Emmanuel Geoffroy isolated the active chemical constituent from these plants and named it “nicouline.” Later on, a Japanese chemist, Nagai isolated a pure crystalline compound which he called rotenone from Derris elliptica, after the Japanese name of the plant “roten.”
Rotenone exposure replicates all the hallmarks of PD including complex inhibition, behavioral alterations, inflammation, alpha-synuclein aggregation, Lewy body formation, and oxidative stress. In 1985, Rotenone was first used as animal model for PD when its direct injection (5 mm) into the left median forebrain bundle (MFB) causes degeneration of dopaminergic nigrostriatal neurons. Rotenone is highly lipophilic in nature, and it easily crosses the blood–brain barrier, unlike many other toxic agents bypasses the dopamine transporter (DAT) for cellular entry. After entry into brain, it accumulates in subcellular organelles including the mitochondria, and specifically inhibit complex I, thereby disrupting mitochondrial respiration, increases “reactive oxygen species” (ROS) production and oxidative stress. Due to these actions, rotenone is used as classic mitochondrial poison in various in vitro and in vivo models. Rotenone acts systemically as a potent inhibitor of mitochondrial complex I (NADH ubiquinone oxido-reductase), whose activity levels are usually reduced in SNPc, frontal cortex, blood platelets, and skeletal muscle of Parkinson’s patients. Recently, it has been found that systemic long-term administration of rotenone produces selective degeneration of dopaminergic neurons and induces PD like locomotor symptoms in rats. In the case of DA neurons, the cargo is dopamine; oxidation of dopamine produces large quantities of ROS and may provoke cell death (Fig. 2).
Different doses & routes of administration of Rotenone
Rotenone has been administered by various routes in animals. Oral administration of rotenone appears to cause less neurotoxicity.
S. no. | Dose | Route | Species | References |
|---|---|---|---|---|
1. | Single-dose administration of Rotenone dissolved in absolute alcohol at (0.4 µg/µl) Coordinates A: 4.9, L: 1.6, D: −2.3) | MFB (left median forebrain bundle) | Female Sprague–Dawley rats | Heikkila et al. (1985) |
2. | Rotenone (3.0 mg/kg/day) rotenone for up to 5 weeks. | s.c. | Male Lewis rats | Sherer et al. (2003) |
Rotenone (2.5 mg/kg) once daily for 60 days. | i.p. | Male Wistar rats | ||
3. | Rotenone (2 mg/kg) daily for 5 weeks | s.c. | Male Sprague–Dawley | Sharma et al. (2013) |
4. | Rotenone (2–3 mg/kg) for 28–36 days | i.v. | Lewis rats | Betarbet al. (2000) |
5. | Rotenone (1.5 and 2.5 mg/kg) daily for 60 days | i.p. | Male Sprague–Dawley | Alam and Schmidt (2002) |
6. | Rotenone (1.5 mg/kg) three times per week for 2 weeks | s.c. | Male Sprague–Dawley rats | Meabdel-Salam et al. (2014) |
7. | Rotenone (12 mg/kg) for a period of 12 days | Per oral (p.o) | Male Wistar rats | Ittiyavirah and Ruby (2014) |
8. | Rotenone (3, 6, 12 μg) for 7 days Coordinates for SN are AP: 5.3; L: 2.0; DV: 7.8 | Intranigral | Sprague–Dawley rats | Swarnkar et al. (2013) |
PD is induced by rotenone by different doses as given in above table. It produces a loss of striatal DA terminals results from the progressive degeneration of dopaminergic neurons of SNpc neurons. Notably, dying DA neurons contain cytoplasmic inclusions, which like Lewy bodies, are immune positive for alpha-synuclein and ubiquitin.
Advantages of Rotenone model of PD
-
1.
Rotenone represents an easy model to induce PD because it can be administered peripherally and locally in the rodents. The neurodegeneration is slow which makes the rotenone model appropriate to study neuroprotective agents for PD.
-
2.
Rotenone-induced PD is the only model in which formation of α-synuclein occurs.
-
3.
Rotenone and other pesticides are powerful inhibitors of mitochondrial respiration. The model mimics general complex I deficiency which is considered to play a role in PD.
Drawbacks
-
1.
In comparison to MPTP or 6-OHDA, the lesions produced by rotenone are more variable and the extent of the lesion varies considerably.
-
2.
Rotenone does not induce Parkinsonism in all animals.
-
3.
Individual rodents develop the symptoms of PD varies considerably in this model.
Clinical relevance
Rotenone is the specific inhibitor of mitochondrial complex I. A reduced activity of the mitochondrial complexes I and IV has been observed in patients with Parkinson’s disease in the SNPc. In early 1924, researchers found that autopsy results showed that the regions with the greatest iron deposition were the substantia nigra (SN) and the globus pallidus (Xiong et al. 2012) Iron also accumulates in the SN in the rotenone-induced rat model of PD.
2.1.2.3 Paraquat-Induced Parkinsonism
The herbicide (weed killer) paraquat (N, N’-dimethyl-4-4’-bipyridinium) also induces a toxic model of PD. Paraquat shows structural similarity to MPP+ and is present in the environment. Exposure to paraquat cause an increased risk for PD toxicity appears to be mediated by the formation of superoxide radicals. The levels of alpha-synuclein are significantly elevated in both SNPc and frontal cortex.
Paraquat is administered systemically in (10 mg/kg i.p.) once a week for three weeks. Paraquat leads to degeneration of SNPc dopaminergic neuron following by α-synuclein containing inclusions, as well as there is advancement in α-synuclein immunostaining in frontal cortex.
Clinical relevance
Paraquat causes mitochondrial dysfunction or oxidative stress is associated with PD and produced clinical features of PD in humans.
2.1.2.4 Maneb-Induced PD
Maneb is a manganese containing fungicide and is extensively used in agriculture. Parkinson’s like symptoms have been reported to develop after chronic exposure to manganese containing fungicides like maneb. Surprisingly, in combination, the neurotoxic effects of maneb or paraquat on the nigrostriatal DA system in mice are synergistically potentiated. The specific toxicity of these environmental neurotoxins on nigral dopaminergic neurons remains unexplored. The marked use of paraquat and maneb in agricultural applications means that people living in these areas are more prone to nigral neurodegeneration.
Different doses & routes of administration of Maneb
S. no. | Dose | Route | Species | References |
|---|---|---|---|---|
1. | Single-dose administration of maneb (100 mg/2 ml/kg of body weight) | i.p. | Female Sprague–Dawley rats | Konno et al. (2001) |
2. | Paraquat (5 mg/kg) or Paraquat (5 mg/kg) + maneb (15 mg/kg) twice a week for 9 weeks (a total of 18 injections) | i.p. | Non-transgenic mice | Thiruchelvam et al. (2005) |
Clinical relevance
Maneb increases the levels of ROS and produces the symptoms of PD. Oxidative stress has been involved in the pathogenesis of PD.
2.1.3 Metal-Induced Parkinsonism
2.1.3.1 Manganese
The manganese (Mn) was firstly recognized as an element in 1774 by Carl Wilhelm Scheele the Swedish chemist, and it is isolated by Johan Gottlieb Gahn Coevally. During the last three decades, a high-risk occupational factor for the development of neurological defects associated with exposure to Mn gradually come into focus in workers engaged in welding and workers engaged in the ferromanganese-alloy industry and the manufacturing of dry-cell batteries.
Oxidative stress has been involved as a contributing factor by which manganese may be cytotoxic. The oxidation of DA by Mn is a potential mechanism by which Mn-induced oxidative stress can be induced after the prolonged exposure to rodents and primates, Mn can accumulate in DA-rich brain regions of rodents and primates (for example, basal ganglia). Another possible mechanism is that, through dysfunction in mitochondria, interferes with proper respiration, thereby causing the excessive production of ROS. Mn efflux has been suggested to account for the net accumulation of Mn in mitochondria. Mn alone inhibits the antioxidant system and disturbs mitochondrial respiratory chain.
Different doses & routes of administration of Manganese
S. no. | Dose | Route | Species | References |
|---|---|---|---|---|
1. | MnCl2 (6 mg/kg) once daily for 4 weeks | i.p. | Male Sprague–Dawley rats | Zheng et al. (1999) |
2. | MnCl2 (0.04 M) and Mn(Oac)3 (0.02 M) twice a week for 5 months | Male CD-1 mice | Ordonez-Librado et al. (2011) | |
MnCl2 (0.04 M) and Mn(Oac)3 (0.02 M) three times a week for 6 months | Inhalation | |||
3. | Mn (0, 25, or 50 mg Mn/kg/d) from PND 1-21 | Orally | Male Long–Evans rats | Beaudin et al. (2013) |
Vanadium-Induced Neurotoxicity
1. | V2O5 (182 μg/50 μL) for 3 times a week for 1 month. | Intranasally | Male C57BL/6 mice | Ngwa et al. (2014) |
Subchronic Diesel Exhaust Exposure
1. | Diesel exhaust (991.8, 311.2, 100.3, 34.9, and 0 μg PM/m3) daily for 6 months | Subchronic Diesel exhaust exposure | Male Fischer 344 rats | Levesque et al. (2011) |
Advantage
-
1.
Mn-induced PD is more reliable model of Parkinsonism because it is progressive and bilateral.
Drawbacks
-
1.
Despite the similarities in extra pyramidal symptoms between Mn neurotoxicity and PD,
Mn-induced lesions are approximately different from those lesions observed in PD.
Clinical relevance
The oxidation of DA by Mn is a potential mechanism by which Mn-induced oxidative stress can be induced in rodents. Oxidative stress has been involved as a contributing factor for PD. The first report of neurological effects associated with exposure to manganese (Mn) in the scientific literature in 1837 by John Couper when he described motor symptoms salivation, weakness in muscles, limb tremor, whispering speech, and posture abnormalities in five men working in a Mn ore crushing plant in France. He called this compilation of symptoms “manganese crusher’s disease.”
2.1.4 Neuro-inflammatory Models
2.1.4.1 LPS-Induced Parkinsonism
LPS is large molecule consisting of lipids and sugars joined by chemical bonds. LPS are the major outer surface membrane components present in almost all Gram-negative bacteria and act as exceptionally strong stimulators of immune system almost in all the organisms. In 1998, Bing and Castano reported that intranigral LPS injection induces inflammation on the nigrostriatal system and produces symptoms of PD.
LPS is well known to produce neurodegeneration, and this is closely linked to neuroinflammation. Numerous evidences indicate inflammation plays a major role in the pathogenesis of PD. Intranigral administration of LPS results in neuroinflammation in the nigrostriatal pathway, long-lasting elevations in tumor necrosis factor alpha (TNF-α), increased microglia, increased levels of pro-oxidants, and reduced amounts of antioxidant glutathione (GSH). Increased activity of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and inducible nitric oxide synthase (iNOS) has been found in cerebrospinal fluid of patients with PD. Systemic LPS injection also activates apoptotic cell death in SNpc.
Different doses & routes of administration of LPS
S. no. | Dose | Route | Species | References |
|---|---|---|---|---|
1. | Single-dose administration of LPS (10 mg/kg) or LPS (5 mg/kg) repeatedly for 5 days | i.p. | Male Wistar–Han rats | Noworyta-Sokolowska et al. (2013) |
2. | Single-dose administration of LPS (5 and 10 μg/4 μl) | Intrastriatal SNPc | Wistar–Han rats | Noworyta-Sokolowska et al. (2013) |
3. | LPS (10 μl) into nasal cavity for 5 months | Unilateral Intranasal | Female C57BL/6 mice | He et al. (2013) |
4. | Single-dose administration of LPS (2.5 μg/μl or 5 μg/μl) | Right or left striatum | Male Sprague–Dawley rats | Choi et al. (2009) |
5. | Single-dose administration of LPS (10 μg/4 µl, bilaterally) | Intrapallidal | Fisher F344 rats | Zhang et al. (2005) |
Drawbacks
-
1.
In LPS-induced dopaminergic neurodegeneration, gender differences seem to be an important factor in the sensitivity to the LPS-induced PD. In systemic LPS administration, C57BL/6 female mice are more resistant than male mice.
Clinical relevance
The first evidence for a role of inflammation in PD came from McGeer and colleagues who observed activated microglia and T cells in the postmortem SNpc of a PD patient. LPS causes neuroinflammation in the nigrostriatal pathway, including lifelong elevations in TNF-α, increased levels of oxidants, and reduced amounts of antioxidant enzymes. Inflammation plays a major role in the pathogenesis of PD.
2.2 Drug-Induced Parkinsonism
Drug-induced Parkinsonism (DIP) is the second most common cause of Parkinsonism after idiopathic PD and has been assume the most prevalent form of secondary Parkinsonism in the Western world. Common clinically used drug-induced PD is (Fig. 3):
2.2.1 Antipsychotic Drugs-Induced Parkinsonism
2.2.1.1 Haloperidol
In one of the largest series, an overall incidence of 15% for neuroleptics-induced Parkinson’s syndrome (NIPS) in over 3700 patients has been reported. The symptoms of muscle rigidity and catalepsy induced within 60 min of haloperidol (0.5–5 mg/kg, i.p.) injection. Haloperidol catalepsy is produced by blockade of postsynaptic striated DA receptors, leading to the functional lack of DA at postsynaptic receptor sites. Neurotoxic effect is caused by an alteration in the iron transport to the CNS and subsequent iron deposition in the basal ganglia. It was also suggested that the favorable side effect profile of the atypical antipsychotics might be related to the dual blockade of D2 and 5-HT2A.
Different doses & routes of administration of Haloperidol
S. no. | Dose | Route | Species | Reference |
|---|---|---|---|---|
1. | Administration of Haloperidol (1 mg/kg, for 1 week or 21 days) | i.p. | Male Wistar albino rats Male Swiss albino rats– Laca mice of either sex | Naidu et al. (2000) |
2.2.1.2 Flufenazine
Fluphenazine is a typical antipsychotic drug used for the treatment of chronic psychoses such as schizophrenia. Flufenazine is a phenothiazine derivative with D1 and D2 receptor blockade activity.
Different doses & routes of administration of Flufenazine
S. no. | Dose | Route | Species | References |
|---|---|---|---|---|
1. | Single-dose administration of Flufenazine (0.3–3.0 µg/µl) Stereotaxic coordinates −2.4 mm from bregma; ±2.3 mm from midline; −2.0 mm from dura; Incisor bar +0.5 mm | Bilaterally Intrastriatal (i.s) | Male random-bred hooded rats | Elliott et al. (1990) |
2. | Single-dose administration of Flufenazine (2 mg/kg) | s.c. | Male random-bred hooded rats | Elliott et al. (1990) |
Clinical relevance
Haloperidol and fluphenazine causes blockade of postsynaptic striated DA receptors, leading to the functional lack of DA at postsynaptic receptor sites and level of dopamine is already decreasing in PD.
2.2.2 Indole Alkaloids-Induced Parkinsonism
2.2.2.1 Reserpine
Reserpine is a dopamine depletor that blocks vesicular storage of monoamines. In 1950s, Carlsson first demonstrated that reserpine can be used to develop animal model for PD using rabbits. Reserpine is first recognized pharmacological model of PD. He showed that treatment of rats with reserpine results in deficiency of dopamine and other catecholamines in brain, thereby resulting in akinesia, which could be successfully reversed by L-dopa. The akinetic state arising due to dopamine depletion in the caudate and putamen and helped Carlsson to speculate that PD results from striatal dopamine depletion. Reserpine acts by inhibiting the vesicular monoamine transporter, VMAT 2. This leads to loss of storage capacity and subsequent depletion of brain (and peripheral) monoamines including noradrenaline, dopamine, and 5-HT.
Different doses & routes of administration of Reserpine
S. no. | Dose | Route | Species | Reference |
|---|---|---|---|---|
1. | Administration of Reserpine (2, 2.5 & 5 mg/kg) | i.p. | Mice or rats | Kumar and Kulkarni (2006) |
2.2.2.2 Harmaline
Harmaline is a fluorescent psychoactive indole alkaloid from the group of harmala alkaloids and beta-carbolines.
Different doses & routes of administration of Harmaline
S. no. | Dose | Route | Species | Reference |
|---|---|---|---|---|
1. | Single-dose administration of harmaline (20 mg/kg) | i.p. or s.c | Male Wistar rats or mice | Iseri et al. (2011) |
2.2.3 CNS Stimulants
2.2.3.1 Methamphetamine
Methamphetamine is one of a family of drugs called amphetamines, which acts as CNS stimulants. Methamphetamine increases the auto-oxidation of dopamine, because methamphetamine displaces the dopamine in the vesicles; large amounts of dopamine are released in synaptic cleft and cytosol where it then undergoes auto-oxidation. Hydrogen peroxide is produced as a by-product in the synthesis and metabolism of dopamine, leading to increased signaling of oxidative stress.
Different doses & routes of administration of Methamphetamine
Clinical relevance
Methamphetamine increases the auto-oxidation of dopamine. Hydrogen peroxide is produced as a by-product in the synthesis and metabolism of dopamine, leading to increased signaling of oxidative stress. Oxidative stress is the contributing factor in the development of PD.
2.2.4 Anti-emetics-Induced Parkinsonism
2.2.4.1 Metoclopramide
Anti-emetics are usually overlooked as a causative agent of DIP. Metoclopramide, the prototypical antiemetic, accounts for nearly a third of all drug-induced movement disorders. It is commonly prescribed in the elderly. Metoclopramide-induced Parkinsonism is typically encountered within the first 3 months of Metoclopramide therapy; the parkinsonian findings resolve in most patients within 2 months after drug therapy is discontinued.
2.3 Others Models of PD
2.3.1 Drosophila as a Model of PD
Seventy-seven percentage of human disease genes are conserved in the complete sequence of the Drosophila genome. The clusters of dopaminergic neurons are present in adult brain of Drosophila and these neurons degenerate when rotenone (a complex I inhibitor) are fed to flies that also triggers dopaminergic neuronal degeneration in mammals. Among the genes that mediate familial PD, only a-synuclein does not have a homolog in Drosophila.
Advantages
-
1.
Drosophila melanogaster has emerged as an especially effective tool to study the genes of PD. Drosophila has been used extensively for investigating biological processes, such as cell death as well as cell proliferation, growth, and migration.
Clinical relevance
These features make flies an excellent model system in which to study the function of disease genes including those involved in neurodegenerative diseases.
2.3.2 Zebrafish as a New Model for PD
Zebrafish are among few newly emerged animal models for neurodegenerative diseases. They come under the vertebrates and therefore more closely relate to humans than other animal models such as Drosophila or C. elegans. Their dopaminergic nervous system is well studied and characterized. Zebrafish embryos are transparent, and this transparency allows visualization and study of various macroscopic and microscopic changes that occurs during embryogenesis and organogenesis. In addition, transgenic zebrafish allows gene expression studies during early development and create easy access to new animal models.
Clinical relevance
The zebrafish brain does not contain a mesencephalic region comparable to the substantia nigra, treatment with MPTP (a PD-inducing drug in humans, and to a lesser extent mice) showed a direct effect on diencephalic dopaminergic neurons, illustrating that zebrafish can develop a phenotype comparable to PD.
2.3.3 Encephalitis Virus-Induced PD
A number of viruses like Japanese Encephalitis Virus (JEV) have ability to penetrate BBB and selectively infect the substantia nigra and induce PD. JEV infection is one of the most common cause of arthropod-borne human encephalitis worldwide. JEV infection is associated with postencephalitis Parkinsonism syndrome in man. In vitro studies have suggested that JEV inhibit the proliferation of T-lymphocytes and evades host immune system. It remains unclear why the neurons of the substantia nigra remain susceptible to JEV infection as opposed to other regions of the brain.
Procedure:
-
Thirteen days after birth, Fischer rats were infected with Japanese encephalitis virus (JEV) and sacrificed 12 weeks later.
-
Brain histological analysis revealed similar changes as found in PD. TH-positive neurons was significantly decreased in the substantia nigra. Specifically, there was neuronal loss mainly to the zona compacta of the substantia nigra.
-
Specifically when animals infected at 1–3 days of age developed widespread infection throughout the brain, whereas in animals infected at age 14-day old, JEV antigen was essentially restricted to the basal ganglia and substantia nigra.
Clinical relevance
JEV antigen was essentially restricted to the basal ganglia and substantia nigra. The limitation of infection to these areas suggested that infection of rats between days 12 and 14 after birth may result in pathological and clinical processes which might resemble PD.
3 Conclusion
Rodents and non-human primates are important tools for better understanding of PD and also helpful in understanding of the pathophysiology of PD. These newer animal models for PD have been less commonly used for drug development and also represent an easy model to induce Parkinsonism. In our review, we have also explored some toxins and drugs that can be used as targets for future research to replace classical toxic PD models. No model is perfect, but in various models rodents can demonstrate many pathophysiological features of PD.
Ethical Statement
All institutional guidelines, national guidelines, and state and local laws and regulations with professional standards for the care and use of laboratory animals should be followed. Studies involving animals must state that the institutional animal ethical committee has approved the protocol. For authors using experimental animals, a statement should be made that the animals’ care is in accordance with institutional guidelines, and animals used have been treated humanely and with regard for the alleviation of suffering. Researchers should treat animals as sentient and must consider their proper care and use and the avoidance or minimization of discomfort, distress, or pain as imperatives. Animal experiments should be designed only after due consideration of animal health. It should be ensured that all researchers who are using animals have received instruction in research methods and in the care, maintenance, and handling of the species being used. All the surgical procedures should be performed under appropriate anesthesia and follow only those procedures which avoid infection and minimize pain during and after surgery.
References
Abdel-Salam OM, Omara EA, Youness ER, Khadrawy YA, Mohammed NA, Sleem AA (2014) Rotenone-induced nigrostriatal toxicity is reduced by methylene blue. J Neurorestoratol 2:65–80
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136(1):317–324
Beaudin SA, Nisam S, Smith DR (2013) Early life versus lifelong oral manganese exposure differently impairs skilled forelimb performance in adult rats. Neurotoxicol Teratol 38:36–45
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12):1301–1306
Choi DY, Liu M, Hunter RL, Cass WA, Pandya JD, Sullivan PG, Shin EJ, Kim HC, Gash DM, Bing G (2009) Striatal neuroinflammation promotes Parkinsonism in rats. PLoS One 4(5):e5482
Elliott PJ, Close SP, Walsh DM, Hayes AG, Marriott AS (1990) Neuroleptic-induced catalepsy as a model of Parkinson’s disease II. Effect of glutamate antagonists. J Neural Transm Parkinson’s Dis Dement Sect 2(2):91–100
Gupta A, Kumar A, Kulkarni SK (2011) Targeting oxidative stress, mitochondrial dysfunction and neuroinflammatory signaling by selective cyclooxygenase (COX)-2 inhibitors mitigates MPTP-induced neurotoxicity in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 35(4):974–981
He Q, Yu W, Wu J, Chen C, Lou Z, Zhang Q, Zhao J, Wang J, Xiao B (2013) Intranasal LPS-mediated Parkinson’s model challenges the pathogenesis of nasal cavity and environmental toxins. PLoS One 8(11):e78418
Heikkila RE, Nicklas WJ, Vyas I, Duvoisin RC (1985) Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: implication for the mechanism of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine toxicity. Neurosci Lett 62(3):389–394
Hritcu L (2008) Hematological disorders in 6-hydroxydopamine-induced rat model of Parkinson’s disease. Turk J Hematol 25:140–144
Iseri PK, Karson A, Gullu KM, Akman O, Kokturk S, Yardýmoglu M, Erturk S, Ates N (2011) The effect of memantine in harmaline-induced tremor and neurodegeneration. Neuropharmacology 61(4):715–723
Ittiyavirah SP, Ruby R (2014) Effect of hydro-alcoholic root extract of Plumbago zeylanica l alone and its combination with aqueous leaf extract of Camellia sinensis on haloperidol induced parkinsonism in wistar rats. Ann Neurosci 21(2):47
Kahale V, Mhaiskar A, Shelat P, Pooja RU, Gaikwad NJ, Mundhada DR (2014) To determine the Effect of Berberine on 6-OHDA induced memory impairment in Parkinson’s disease in rodents. Pharma Innov J 3(7):101–108
Konno N, Tsunoda M, Nakano K (2001) The effect ofin vitro andin vivo ethylenbis dithiocarbamate fungicides on NMDA receptors in rat brain membranes. Environ Health Prev Med 6(1):54–59
Kumar A, Kulkarni SK (2006) Effect of BR-16A (Mentat), a polyherbal formulation on drug-induced catalepsy in mice. Indian J Exp Biol 44(1):45–48
Levesque S, Surace MJ, McDonald J, Block ML (2011) Air pollution & the brain: subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation 8(1):1
Meredith GE, Sonsalla PK, Chesselet MF (2008) Animal models of Parkinson’s disease progression. Acta Neuropathol 115(4):385–398
Moszczynska A, Yamamoto BK (2011) Methamphetamine oxidatively damages parkin and decreases the activity of 26S proteasome in vivo. J Neurochem 116(6):1005–1017
Naidu PS, Singh A, Kulkarni SK (2003) Quercetin, a bioflavonoid, attenuates haloperidol-induced orofacial dyskinesia. Neuropharmacology 44:1100–1106
Ngwa HA, Kanthasamy A, Jin H, Anantharam V, Kanthasamy AG (2014) Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity. Neurotoxicology 43:73–81
Nicholas AP (2007) Levodopa-induced hyperactivity in mice treated with 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Mov Disord 22(1):99–104
Noworyta-Sokolowska K, Gorska A, Golembiowska K (2013) LPS-induced oxidative stress and inflammatory reaction in the rat striatum pharmacological reports, vol 65, pp 863–869
Ordoñez-Librado JL, Anaya-Martínez V, Gutierrez-Valdez AL, Colín-Barenque L, Montiel-Flores E, Avila-Costa MR (2010) Manganese inhalation as a Parkinson disease model. Parkinson’s Disease, 2011
Robinson TE, Yew J, Paulson PE, Camp DM (1990) The long-term effects of neurotoxic doses of methamphetamine on the extracellular concentration of dopamine measured with microdialysis in striatum. Neurosci Lett 110(1):193–198
Sharma S, Deshmukh R (2015) Vinpocetine attenuates MPTP-induced motor deficit and biochemical abnormalities in Wistar rats. Neuroscience 286:393–403
Sharma N, Nehru B (2013) Beneficial effect of vitamin E in rotenone induced model of PD: behavioural, neurochemical and biochemical study. Exp Neurobiol 22(3):214–223
Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT (2003) Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 23(34):10756–10764
Swarnkar S, Goswami P, Kamat PK, Patro IK, Singh S, Nath C (2013) Rotenone-induced neurotoxicity in rat brain areas: a study on neuronal and neuronal supportive cells. Neuroscience 230:172–183
Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Richfield EK, Buckley B, Mirochnitchenko O (2005) Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat+ manebinduced Parkinson disease phenotype. J Biol Chem 280:22530–22539
Ungerstedt U (1968) 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol 5(1):107–110
Wang AL, Liou YM, Pawlak CR, Ho YJ (2010) Involvement of NMDA receptors in both MPTP-induced neuroinflammation and deficits in episodic-like memory in Wistar rats. Behav Brain Res 208(1):38–46
Xiong P, Chen X, Guo C, Zhang N, Ma B (2012) Baicalin and deferoxamine alleviate iron accumulation in different brain regions of Parkinson’s disease rats. Neural Regeneration Res 7(27):2092
Zhang J, Stanton DM, Nguyen XV, Liu M, Zhang Z, Gash D, Bing G (2005) Intrapallidal lipopolysaccharide injection increases iron and ferritin levels in glia of the rat substantia nigra and induces locomotor deficits. Neuroscience 135(3):829–838
Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH (1999) Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res 833(1):125–132
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sharma, N., Jamwal, S., Singh, S., Gill, H.K., Bansal, P.K. (2017). Animal Models of Parkinson’s Disease. In: Bansal, P., Deshmukh, R. (eds) Animal Models of Neurological Disorders. Springer, Singapore. https://doi.org/10.1007/978-981-10-5981-0_3
Download citation
DOI: https://doi.org/10.1007/978-981-10-5981-0_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5980-3
Online ISBN: 978-981-10-5981-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)