Abstract
Biomass saccharification has assumed a significant importance in the context of the modern-day energy crisis and climate change scenarios. Waste biomass, which can constitute a significant portion of solid wastes, can be converted into value-added chemicals like ethanol by this process. The present investigation deals with the development of a solid acid catalyst for biomass saccharification using coconut shell, a cheap and abundant raw material, which has not been explored previously in this field. Coconut shell has been carbonised with zinc chloride at 723 K for 1 h to produce activated carbon which has been sulphonated with conc. H2SO4 (98%) at 403 K for 16 h to develop the solid acid catalyst. The catalyst has been characterised by scanning electron micrography, X-ray diffraction, FTIR spectroscopy and nitrogen adsorption. The X-ray diffraction studies have shown a graphene sheet content of 39% in the catalyst, while the FTIR spectra show the presence of SO3H, phenolic OH and COOH groups. The specific surface area measured by nitrogen adsorption was 10.162 m2/g. The catalyst has been used to hydrolyse pretreated sawdust from Acacia nilotica heartwood as well as microcrystalline cellulose under experimental conditions specified by central composite design. The yields of total reducing sugars in the hydrolysates have been analysed by UV spectrophotometry, and the produced sugars were identified by HPLC.Glucose constituted almost all of the produced sugars with negligible amounts of galactose being formed. The maximum sugar yield was 91% for pretreated sawdust and 93% for microcrystalline cellulose, indicating the excellent catalytic property of the catalyst. The results indicate the suitability of coconut shell as a source for developing biomass saccharification catalysts, as well as the efficacy of such a catalyst in the saccharification process.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Biomass constitutes a significant portion of solid wastes that are generated in human settlements, especially urban areas. The most common method of disposing of biomass is by landfilling or incineration. But these useful energy sources can also be disposed off in a novel way, i.e. converting them to value-added chemicals. As the main component of lignocellulosic biomass, cellulose is a biopolymer consisting of many glucose units connected through β-1,4-glycosidic bonds. Breakage of β-1,4-glycosidic bonds by acids leads to the hydrolysis of cellulose polymers, resulting in the formation of glucose or other oligosaccharides. These sugars can be further fermented to produce ethanol, which can be used as transportation fuel in blends with gasoline. Thus these novel methods of biomass waste disposal have the potential to reduce fossil fuel consumption and greenhouse gas emissions, which have a great implication in the modern industrial society. Mineral acids like HCL and H2SO4 have been used in the hydrolysis of cellulose. Since they suffer from problems of product separation, reactor corrosion and poor recyclability, emphasis is being placed on developing more efficient catalysts for these reactions. Research works are being carried out for the possibility of replacing mineral acids with solid acidic catalysts for these processes. Solid catalysts can be easily recycled for further use and pose no environmental hazards.
2 Literature Survey
Suganuma et al. (2008) developed a solid acid catalyst by sulphonation of activated carbon. The solid acid catalyst prepared by them had nanosized graphene sheets along with SO3H, phenolic OH and COOH groups. This catalyst reduced the activation energy of lignocellulose hydrolysis to 110 kJ mol−1.
A sulphonated porous catalyst was prepared from wood powder with high specific surface area (Kitano et al. 2009). Carbonisations at different temperatures were done, and 723 K was found as the optimum temperature for carbonisation with the carbon catalysts containing high densities of micro- and mesopores.
Huang et al. (Huang and Yao 2013) studied the hydrolysis of cellulose to glucose by solid acid catalysts. Advances in the hydrolysis of cellulose by different types of solid acids, such as sulphonated carbonaceous-based acids, polymer-based acids and magnetic solid acids, were summarised.
A sulphonated carbonaceous material was prepared from lignosulphonate, and its usefulness was studied as an esterification catalyst (Lee 2013).
Salmi et al. (2014) developed kinetic models for the hydrolysis of O-acetyl-galactoglucomannan (GGM), a hemicellulose appearing in coniferous trees. Mineral acid catalysts and heterogeneous solid catalysts were used to hydrolyse the hemicelluloses.
Various solid acid catalysts were prepared which were further used for hemicellulose hydrolysis (Cara et al. 2013). The reactions were conducted under neutral ph and relatively mild temperature and pressure conditions in water as the reaction medium.
Sulphonated silica/carbon nanocomposites were successfully developed as reusable, solid acid catalysts for the hydrolytic degradation of cellulose into high yields of glucose (Van de Vyver et al. 2010).
Onda et al. (2008) for the first time performed solid acid catalysis for the hydrolysis of cellulose. The sulphated and the sulphonated catalysts showed a remarkably high yield of glucose.
The objective of the present work is to develop a solid acid catalyst based on activated carbon prepared from coconut shell and study the effectiveness of the developed catalyst in the hydrolysis of pretreated sawdust from Acacia nilotica heartwood. Coconut shell, which is a highly abundant and cheap raw material, has not been explored so far for the development of solid acid catalysts for biomass hydrolysis. Literature on hydrolysis of actual lignocellulosic biomass by solid acid catalysts is also scant, with most researchers focussing on hydrolysis of pure cellulose and starch. Hence on this front, the present work is a novel research in this domain. The study of the recyclability of the developed catalyst has also been incorporated.
3 Materials and Methods
Chemicals used were:
-
ZnCl2 (Merck, >95%)
-
NaOH (Merck, 97%)
-
H2SO4 (Merck, 98%)
-
HCl (Merck, 35%)
-
NaCl (Merck, 99%)
Sawdust from Acacia nilotica heartwood was the substrate for hydrolysis. The composition of Acacia nilotica wood is detailed in Table 1. After pretreatment (Mallick et al. 2016), the initial lignin content was reduced by 60%.
3.1 Experimental Procedure
Coconut shell was used for the manufacture of activated carbon. Its composition on dry basis is given below:
Cellulose – 33.61% | Lignin – 36.51% |
Pentosans – 29.27% | Ash – 0.61% |
The high cellulose and pentosan content and low ash content make coconut shell an excellent substrate for preparing activated carbon. The high lignin content makes it suitable for preparation of the solid acid catalyst since lignin being an aromatic polymer, the produced activated carbon should contain a large proportion of aromatic rings, which would facilitate easy sulphonation.
3.1.1 Carbonisation
The coconut shells were crushed to obtain a definite particle size passing through 100 mesh. The shells were then impregnated with 10% ZnCl2 solution for 24 h. The shells were filtered and dried to room temperature. The coconut shells were then taken in silica crucibles and carbonised at 723 K in a muffle furnace for 1 h. Nitrogen purging was done to maintain an inert atmosphere. The carbonised mass so obtained was washed with deionised water to remove traces of chlorides. It was then air-dried for a day.
3.1.2 Sulphonation
In borosilicate glass beaker, 400 ml of sulphuric acid was taken. The carbonised mass was then slowly added to it and heated at 403 K for 16 h in a castor oil bath for digestion with continuous stirring by a magnetic stirrer. Following the digestion process, the mixture was cooled to room temperature and was diluted by adding 1000 ml of deionised water to the reaction mixture. A black precipitate was formed which was then filtered and washed repeatedly with deionised water to remove SO4 2− ions. The particle size of the sulphonated activated carbon was around 100 mesh.
3.1.3 Catalyst Characterisation
The developed catalyst was analysed for estimating the acidity in terms of SO3H group content and total acidity in terms of SO3H, phenolic OH and COOH group content (Lee 2013). The specific surface area of the catalyst was measured by nitrogen adsorption in a BET apparatus. Scanning electron micrography of the catalyst was carried out to determine its surface structure. X-ray diffraction studies of the activated carbon prior to sulphonation were conducted to determine the graphene sheet content. The functional groups in the catalyst were determined by FTIR spectroscopy.
3.1.4 Estimation of Catalyst Performance in Hydrolysis of Acacia nilotica Heartwood
The performance of the developed catalyst was estimated in the hydrolysis of pretreated Acacia nilotica heartwood. For design of the hydrolysis experiments, central composite design was followed. Accordingly the hydrolysis reactions were carried out between 372 and 413 K. The time of reaction was varied between 30 min and 2 h. The catalyst/biomass ratio was varied from 1:1 to 2:1. Appropriate amounts of biomass and catalyst were taken in a 500 ml conical flask. Two hundred millilitres of deionised water was added. The flask was placed in an autoclave and hydrolysis was carried out. The pH of the reaction mixture with the catalyst present was 1. A conical flask containing only the pretreated sawdust without the catalyst was also placed in the autoclave. After reaction, hydrolysates were collected and analysed for total reducing sugars (TRS) by UV spectrophotometry (Miller 1959), and identification of the reducing sugars was carried out by HPLC. For studying the reusability of the catalyst, the residue (catalyst + biomass) remaining in the conical flasks was filtered out and weighed. Then the residues were transferred in other conical flasks, and hydrolysis was again carried out under same experimental conditions. The hydrolysates from this second batch of hydrolysis were also analysed for total reducing sugars by UV spectrophotometry. The catalyst was also used for hydrolysis of microcrystalline cellulose to compare the catalytic performance.
4 Results and Discussions
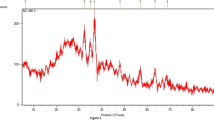
The SO3H content in the catalyst was found to be 1.27 mmol/g, and the total acidity was determined to be 10 mmol/g. The specific surface area of the developed catalyst was 10.162 m2/g. The pore volume was 0.36 cm3 g−1, and the average pore diameter was 1 μ. The XRD pattern of the activated carbon has been shown in Fig. 1.
The graphene sheet content in the prepared activated carbon was determined according to Liou and Huang (2013). It was found to be 39%. The FTIR spectrum of the sulphonated activated carbon catalyst is shown in Fig. 2.
The peaks at 1027 cm−1 and 889 cm−1 are due to the presence of SO3H group. The peaks corresponding to 1703 and 1383 cm−1 can be attributed to the presence of C=O stretching and carboxylic OH bending vibrations, respectively, indicating the presence of COOH group. The peak at 3530 cm−1 is due to phenolic OH stretching vibration, indicating the presence of phenolic OH. The peak at 812 cm−1 indicates aromatic C–H bending, and peaks at 1515 and 1643 cm−1 indicate aromatic C=C bending. This indicates the highly aromatic structure of the developed catalyst. Figure 3a, b shows the ultrastructure of the catalyst. As can be seen from the figures, the porosity of the catalyst is quite high, with average pore diameter ranging from 5 to 10 μ.
The principal hydrolysis product was glucose with negligible amounts of galactose. The glucose selectivity of the catalyst was more than 99%. The maximum yields of reducing sugars for hydrolysis of pretreated sawdust and microcrystalline cellulose have been detailed in Table 2.
The optimum time of reaction was found to be 1 h. For reaction times more than 1 h, the yield of reducing sugars decreased significantly at higher temperatures. This is due to the degradation of sugars to products like furfural, 5-hydroxymethylfurfural, acetic acid , etc. (Wei et al. 2008). Hence the studies on reusability of the catalyst were carried out for 1 h only. The yields of reducing sugars during recyclability studies are detailed in Table 3.
As evident from Table 2, the yield of reducing sugars is very high compared to earlier works in this field (Saganuma et al. 2008; Cara et al. 2013; Van de Vyver et al. 2010). The yield of sugars without the catalyst was negligible under the same experimental conditions, which indicate the excellent catalytic activity of the developed catalyst. The decrease in the sugar yield when the catalyst was reused is due to the leaching out of loosely bonded SO3H groups into the aqueous medium. The rate of this leaching increases at higher temperatures; hence, the percentage decrease in sugar yield is more at higher temperatures. The surface area of the developed catalyst is low in comparison with that developed by Kitano et al. (2009). It can be inferred from this that large hydrophobic molecules will not be adsorbed properly on the catalyst; hence, the efficacy of this catalyst in hydrophobic acid-catalysed reactions, e.g. esterification, will be low. But for a hydrophilic reaction like biomass saccharification, relatively smaller cellulose units are adsorbed and stabilised effectively onto the catalyst surface, which then makes them amenable to attack by H+ ions coming from adjacent SO3H groups on the catalyst surface.
5 Conclusions
The present investigation has been successful in developing a solid acid catalyst from coconut shell, a cheap and abundant raw material, for biomass saccharification. Heterogeneous catalytic hydrolysis of actual lignocellulosic biomass (viz. Acacia nilotica heartwood) has been explored, which has been, up to now, a relatively unexplored area. A high yield of reducing sugar and high glucose selectivity has been obtained at moderate temperatures (413 K) and relatively short reaction time (1 h). The catalyst can also be reused though with a moderate drop in sugar yields. All these indicate the applicability of the catalyst in industrial biomass saccharification units which will lead to increased glucose production and consequently increased production of bioethanol . This will also reduce the problem of accumulation of solid biomass wastes since every type of biomass waste can be hydrolysed to value-added chemicals using this catalyst.
6 Future Scope of Work
In the future, the kinetics of the heterogeneous catalytic hydrolysis needs to be explored which can lead to further improvement in the development of saccharification catalysts.
References
Cara PD, Pagliaro M, Bmekawy A, Brown DR, Verschuren P, Raveendran Shiju N, Rothenberg G (2013) Hemicellulose hydrolysis catalysed by solid acids. Cat Sci Technol 3:2057–2061
Huang Y-B, Yao F (2013) Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem 15:1095–1111
Kitano M, Arai K, Kodama A, Kousaka T, Nakajima K, Hayashi S, Hara M (2009) Preparation of a sulfonated porous carbon catalyst with high specific surface area. Catal Lett 131:242–249
Lee D (2013) Preparation of a sulfonated carbonaceous material from lignosulfonate and its usefulness as an esterification catalyst. Molecules 18:8168–8180
Liou Y-J, Huang W-J (2013) Quantitative analysis of graphene sheet content in wood char powders during catalytic pyrolysis. Mater Sci Technol 29(5):406–410
Mallick A, Ash SN, Mahapatra DK (2016) Pretreatment of Acacia nilotica sawdust by catalytic delignification and its fractal kinetic modeling. J Inst Eng India Ser E 97(1):39–45
Miller GI (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Onda A, Ochi T, Yanagisawa K (2008) Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem 10:1033–1037
Saganuma S, Nakajima K, Kitano M, Yamaguchi D, Kato H, Hayashi S, Hara M (2008) Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH and OH groups. J A Chem Soc 130:12787–12793
Salmi T, Murzin DYU, Maki-Arvela P, Kusema B, Holmbom B, Willfor S, Warna J (2014) Kinetic modeling of hemicellulose hydrolysis in the presence of homogeneous and heterogeneous catalysts’. AICHE J 60:1066–1077
Van de Vyver S, peng L, Geboers J, Schepers H, de Clippel F, Gommes CJ, Goderis B, Jacobs PA, Sels BF (2010) Sulfonated silica/carbon nanocomposites as novel catalysts for hydrolysis of cellulose to glucose. Green Chem 12:1560–1563
Wei Q, Su-ping Z, Qing-li X, Zheng-wei R, Yong-jie Y (2008) Degradation kinetics of xylose and glucose in hydrolysate containing dilute sulfuric acid. Chin J Process Eng 8(6):1132–1137
Acknowledgements
The authors wish to thank the laboratory staff members of the Department of Chemical Engineering, University of Calcutta, for their cooperation in the experimental work. The authors also acknowledge the SEM section of the Department of Metallurgical Engineering and Material Science, Jadavpur University, India, for their cooperation. Finally the authors would like to gratefully acknowledge the authorities of University of Calcutta, for providing the financial support for this research work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Mallick, A., Mukhopadhyay, M. (2018). Preparation and Characterisation of Solid Catalysts for Saccharification of Biomass. In: Ghosh, S. (eds) Utilization and Management of Bioresources. Springer, Singapore. https://doi.org/10.1007/978-981-10-5349-8_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-5349-8_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5348-1
Online ISBN: 978-981-10-5349-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)