Abstract
Biofuels are a promising long-term renewable energy source having potentials which could address both environmental impacts and security concerns posed by current dependence on petroleum-based fuels. Biodiesel fuel is a renewable energy fuel produced from biological materials or biomass. Development of biodiesel processors is still a novel technology in Nigeria where till date; much works has not been recorded on the development of biodiesel processors. A one-stage nine-flask continuous-batch biodiesel reactor is developed to withhold the heat generated during the reaction of hydrocarbons (vegetable oil), alcohols (methanol or ethanol), and the catalyst (sodium or potassium hydroxide) for biodiesel production. The stages include methoxide tank to mix the methanol; the reactor where the transesterification of the mixtures of vegetable oil, alcohol, and catalyst occur; the settling tower where freshly reacted batch of biodiesel is transferred to free up the reactor; first wash tank for wet washing; second wash tank for dry washing; dry tank to free up the wash tanks for the next batch and also dry the fuel much faster with much better results through heating; glycerin tank where the glycerin from the wash tank is drained; biodiesel tank in which the dry biodiesel is stored; and water tank used in washing the biodiesel.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Gasoline contributes to increase hazardous emissions (Abdulkareem et al. 2012), and according to Alamu (2007), diesel with higher carbon numbers contribute to emissions of high particulate matters such as high sulfur dioxide and high poly-aromatic hydrocarbons.
Also, the growing energy needs and irregular supply, unstable cost and increasing environmental concern, and protecting the environment and planning for long-term energy security become necessary (ECN 2008). This prompted investigation into alternative fuels with comparative properties to nonrenewable and environmentally unfriendly fossil fuel, and biodiesel-based fuels became a considerable choice because of their renewable, nontoxic, and safe to store characteristics. Biodiesel is one of such renewable alternative fuels that could be domestically produced from new or used vegetable oil and animal fat through a chemical reaction with methanol or ethanol. This reaction requires heat and a strong catalyst (alkalis, acids, or enzymes) to achieve complete conversion of the vegetable oil into the separated esters and glycerin. During the transesterification reaction, glycerin is obtained as a by-product which could be used in the pharmaceutical and cosmetic industries. Not only can biodiesel be used alone in neat form, but it can also be mixed with petroleum diesel fuel in any unmodified diesel engine (Nakpong and Wootthikanokkhan 2010).
It is a known fact that biodiesel is synthesized from a variety of feedstocks, such as soybean, rapeseed, palm, and others which are food grade raw materials. The competition in using these raw materials as both food and energy crops has become a great obstacle in biodiesel production on a commercial scale.
Also, waste cooking oil (WCO) is considered an economic and increasingly available resource for biodiesel production (Jain and Sharma 2010; Kee et al. 2010; Han et al. 2009).
The use of biodiesel as an alternative fuel has been globally accepted, and several research works have been done to first-generation biodiesel production where homogenous catalysts such as NAOH, KOH, etc. are used.
2 Aim and Objective
The broad aim of this research work is to develop a multi-tank biodiesel processor in Nigeria and carry out performance evaluation tests using waste vegetable oil (WVO). Many researchers had worked on one-, two-, and three-flask processor in the past in Nigeria, but this research work is basically on a one-stage nine-flask processor as listed below:
-
1.
Methoxide tank to mix the methanol and lye together before it is injected into the reactor
-
2.
The reactor where the transesterification of the mixtures (vegetable oil + alcohol + catalyst) occurs
-
3.
The settling tower where freshly reacted batch of biodiesel is transferred to free up the reactor for yet another batch
-
4.
First (1st) wash tank where the product is washed with water to decant fuel from soap and glycerin (i.e., wet washing)
-
5.
Second (2nd) wash tank to finally free the fuel from all impurities by passing it through wood shaving (i.e., dry washing)
-
6.
Dry tank to free up the wash tanks for the next batch and also dry the fuel much faster with much better results through heating
-
7.
Glycerin tank where the glycerin from the wash tank is drained
-
8.
Biodiesel tank where the dry biodiesel is stored
-
9.
Water tank used in washing the biodiesel
3 Previous Research Works on Biodiesel Processors
Many researchers have delved into the production of biodiesel and its processor worldwide. In developed nations, many manufacturing companies and home brewers have come up with various designs for biodiesel processor, and it is currently on sale in developed nations. Various types of biodiesel processors ranging from small-scale sizes to the large-scale plants have been built by different researchers in both developed and developing nations, but Nigeria as a nation does not have encouraging records in terms of development of processors and biodiesel production.
-
Mayvan et al. (2011) – designed and fabricated batch-type stirred tank reactor (STR) with a capacity of 70 l in which two methods of mechanical and hydraulic mixing were introduced and electrostatic coalescing method in separation of glycerin and dry wash by absorbing column and Magnesol powder as filter aid.
-
A Florida-based biodiesel processor manufacturing company partnered with a Nigerian company “Avandith Energy” in 2014 to get a B-60 biodiesel processor for their pilot transesterification facility. The B-60 plant will produce four batches of biodiesel in 24 h and will be used as hands-on educational tool to show students and government agencies how to make renewable energy (Fig. 1).
-
Also, Daniyan et al. (2013) designed a small-scale biodiesel processor capable of transesterifying 20 l of biodiesel per batch as represented in Fig. 2
-
Ramesh et al. (2013) presented a biodiesel plant of 250 l/day capacity at the Tamil Nadu Agricultural University, India (Fig. 3)
-
Tint and Mya (2009) also produced a 120 l biodiesel pilot plant from Jatropha oil (Jatropha curcas); warm water was used for washing and sand was used as filter
This has really shown that everyone knows the advantages of the eco-friendly properties of biodiesel and the need for a processor.
The research work on focus will see to the design and development of a processor with locally sourced materials to make available a 100-l Nigerian-made biodiesel processor that would be locally produced in Nigeria. It would save cost a great deal when compared with the foreign exchange between Nigeria and other nations on imported ones.
4 Materials and Methods
The design of this biodiesel processor was given utmost considerations on two subheads:
-
(i)
The process
-
(ii)
The processor
5 The Process
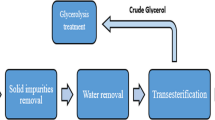
This comprises the reaction of methoxide, waste vegetable oil (WVO), and alkali such as sodium or potassium hydroxide under a specific temperature, but in this study, sodium hydroxide is considered, and the reaction would be aided by continuous stirring of the aforementioned in a mechanical reactor. At a given temperature, glycerol tap would be opened to give way for glycerin which goes into its own separate tank. Time is allowed for the reaction to complete, and then the biodiesel is transferred to a settling tank to give way for a new batch. Thereafter, from the settling tank, the product flows into a wet wash tank where it is washed with the introduction of hot water from the water tank. The mixture is stirred, biodiesel floats, and water goes down and drained out through the drain tap. This water is allowed to settle and treated with acid to neutralize its alkalinity before it could be disposed in order not to endanger plant and animal life. The biodiesel on the other hand is passed into a dry wash tower through wood shaving to further remove any left impurities. Thereafter, it goes into the final storage tank, ready for dispensing to the final consumer.
As illustrated in Fig. 4, the process comprises the use of dangerous chemicals such as NAOH, methanol, and acids, and as such metallic substances such as copper, zinc, lead, etc. that may catalyze or react with these chemicals were avoided in the design. Timers and thermostat on heating elements shall be installed in order to prevent overheating of oils. Generally, there are two available methods for washing biodiesel:
-
(i)
Wet washing
-
(ii)
Dry washing
Both methods shall be adopted to get a very clean biodiesel. Wet washing employs the use of water to separate glycerol from the final product which shall then be passed through wood shaving in the dry wash tank to remove every other impurity left in the product. The process is as illustrated in Figs. 4, 5, and 6.
6 Conclusions and Recommendations
Developing a biodiesel processor will contribute to reduction of greenhouse gases in the environment. So far, no research work on 100 l capacity in a nine-drum/flask plant using a combined wet and dry wash process has been recorded in Nigeria. Moreover, it is going to be a bridge between the first- and second-generation biodiesel productions, i.e., using nonedible feedstock/waste oil with homogeneous catalyst to produce a standard biodiesel fuel. Future consideration of designing a processor having two or more methoxide and settling tanks in order to prepare for two to three batches so as to save time and increase quantity produced (Fig. 7).
References
Abdulkareem AS, Jimoh A, Afolabi AS, Odigure JO, Odili UC (2012) Production and characterization of biofuel from refined groundnut oil. http://dx.doi.org/10.5772/52443
Alamu OJ (2007) Effect of ethanol-palm kernel oil ratio on Alkali-catalyzed biodiesel yield. Pac J Sci Technol 8(2):212–219
Daniyan IA, Adeodu AO, Dada OM, Aribidara AA (2013) Design of a small scale biodiesel processor. J Emerg Trends Eng Appl Sci (JETEAS) 4(4):576–580. © Scholarlink Research Institute Journals, 2013 (ISSN: 2141-7016) jeteas.scholarlinkresearch.org
Energy Commission of Nigeria (2008) National Energy Policy. http://www.energy.gov.ng
Han M, Yi W, Wu Q, Liu Y, Hong Y, Wang D (2009) Preparation of biodiesel from waste. Bioresour Technol 100:2308–2310
Jain S, Sharma MP (2010) Biodiesel production from Jatropha curcas oil. Renew Sustain Energy 14:3140–3157
Kee LM, Teong LK, Rahman MA (2010) Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol Adv 28:500–518
Mayvan AA, Ghobadian B, Najafi G, Omidkhah MR (2011) Design, fabrication and evaluation of a novel biodiesel processor system. SET2011. In: 10th international conference on Sustainable Energy Technologies, İstanbul, 4–7 September 2011
Nakpong P, Wootthikanokkhan S (2010) Roselle (hibiscus sabdriffa L.) oil as an alternative feedstock for biodiesel production in Thailand. Fuel 89:1806–1811
Ramesh D, Samapathrajan A, Venkatachalam P (2013) Production of biodiesel from Jatropha curcas oil by using Pilot biodiesel plant
Tint TK, Mya MO (2009) Production of biodiesel from Jatropha oil (Jatropha curcas) in pilot plant. World Acad Sci Eng Technol 50:477–483
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Openibo, A.O., Raji, N.A. (2018). Design of a Multi-tank Processor to Produce Biodiesel Using Waste Vegetable Oil in Nigeria. In: Ghosh, S. (eds) Utilization and Management of Bioresources. Springer, Singapore. https://doi.org/10.1007/978-981-10-5349-8_15
Download citation
DOI: https://doi.org/10.1007/978-981-10-5349-8_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5348-1
Online ISBN: 978-981-10-5349-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)