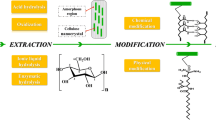
Abstract
Cellulose nanofibrils (CNFs) are one type of nanostructured cellulosic materials with a width below 100 nm and a length of several micrometers. CNFs have many desirable characteristics, such as a unique rheological behavior, high mechanical and barrier properties, and lightweight. They are produced from cotton, wood, grasses, and other lignocellulosic biomass. Thus, CNFs are abundantly available and can be a cheap alternative to petroleum-based polymers. Manufacturing of CNFs consists of pretreatment process and mechanical disintegration process. The pretreatment process makes cellulose fibers more responsive to be fibrillated, and pretreated fibers are mechanically disintegrated into nano-sized fibers in the next stage. Moreover, the type of raw materials can be a principal factor that affects CNFs production and properties. In this chapter, we reviewed the production, characterization, and the current applications of nanocellulose for food industries, such as food additives, food packaging, and coating.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Cellulose is the most abundant natural biopolymer derived from wood, cotton, and other non-wood fiber sources (agricultural residues and grasses). Paper and pulp industries are converting woody biomass into various formats of cellulose fibers for manufacturing papers, tissues, moisture absorbents, and several cellulose derivatives for chemical and pharmaceutical applications. For example, ethyl cellulose is commercially used in coating, binding, and controlled-release drug systems. Other ether derivatives, such as carboxymethyl cellulose and hydroxyethylcellulose, are also used as a viscosity modifier, a gelling agent, a foaming agent, and a binding agent (Dufresne 2012).
Cellulose nanofibrils (CNFs) are one type of nanostructured cellulose, which has a width of below 100 nm and a length of several micrometers. CNFs were first produced by Turbak et al. (1983a) and Herrick et al. (1983). Cellulose nanofibrils have many advantages over ordinary cellulose fibers since CNFs are much lighter but have more extensive network structures, resulting in higher mechanical and barrier properties. CNFs film has a tensile strength as high as 310 MPa and an oxygen permeability as low as 0.00006 cc.μm/m2.day.kPa, which are competitive with those of other commercial polymers (up to 70 MPa) (Stevens 1999; Saito et al. 2009; Nair et al. 2014). CNFs also have unique rheological properties. They have a high viscosity with shear-thinning behavior. The unique properties of CNFs have been studied in the broad range of applications such as food, cosmetic, electronic, and biomedical applications. Specifically, CNFs have been used in many different ways to food products, such as food additives, food packaging, and coating films.
CNFs are produced by disintegrating cellulose fibers using mechanical methods, such as high-pressure homogenization, micro-fluidization, and micro-grinding. The mechanical disintegration methods consume high energy and pose clogging issues to successfully produce CNFs. Cellulose fibers are pretreated using mechanical, chemical, and/or enzymatic methods to make the fibers more susceptible to disintegration. The selection of raw materials also affects the ease of CNFs production. The disintegration of hardwood cellulose fibers is more difficult than that of softwood cellulose fibers due to the rigidity and complexity of hardwood cell wall (Stelte and Sanadi et al. 2009). Compared with cellulose fibers from wood, cellulose fibers from non-woody plants might be more favorable to produce cellulose nanofibrils because non-woody plant fibers are present in thin primary cell walls.
This paper first aims to investigate the effects of raw materials on the production and properties of cellulose nanofibrils. Several review articles on cellulose nanofibrils produced from either cotton/wood sources or bacterial source have been published in the literature (Siqueira et al. 2010; Siro and Plackett 2010; Lavoine et al. 2012; Abdul Khalil et al. 2014; Jonoobi et al. 2015) with limited focus on the interaction effects of preparation methods and raw materials on CNFs production and their application to food. Also, since cellulose nanofibrils have a number of potential applications, reviews of nanocellulose applications in the fields of papermaking (Brodin et al. 2014; Osong et al. 2016), biomedical engineering (Lin and Dufresne 2014; Lu et al. 2014; Jorfi and Foster 2015; Guise and Fangueiro 2016), and electronics (Kim et al. 2015; Hoeng et al. 2016; Sabo et al. 2016) were found in the literature. The use of CNFs in food science has been recently summarized (Gómez et al. 2016). This chapter reviews a wide range of CNFs applications to food industries, including food additives, food packaging and edible film/coating applications.
1.2 Cellulose and Nanocellulose
1.2.1 Cellulose
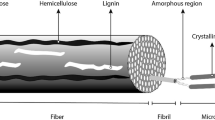
Cellulose is the most abundant natural polymer as it is the main component of plant cell walls. It is an almost inexhaustible source as the estimated annual biomass production is approximately 1.5 × 1012 tons in the US. Cellulose is a linear polymer of the repeating units of β-D-glucopyranose which are linked by linear β-1,4 glucosidic linkages (Fig. 1.1). Cellulose has a large number of hydrogen groups at C-2, C-3, and C-6 atoms. Due to its linear structure and multiple hydroxyl groups, cellulose can form extensive intra- and intermolecular hydrogen bonds, which enables cellulose to form a stable three-dimensional structure. Intramolecular hydrogen bonds are also responsible for cellulose stiffness and its insolubility in most solvents (Gavillon 2007; Wang 2008). Besides, cellulose has a hierarchical morphological structure: elementary fibrils (1.5–3.5 nm in widths), microfibrils (10–30 nm in widths), and microfibril bands (larger than 100 nm in widths) (Klemm et al. 2005). The elementary fibrils pack to the microfibrils, and the microfibrils form the core structural units of the plant cell wall. Each microfibril can be realized as a string of cellulose crystals, linked along the chain axis by disordered amorphous regions, merging into fibril bundles.
Cellulose is a skeletal component in plants that has ordered cell wall layers. Figure 1.2 schematically represents the cell wall of wood fibers. In most plant fibers, cellulose predominately exists in the central layer (S2) of a secondary cell wall, and it is surrounded by the amorphous matrix substances (hemicellulose and lignin, Fig. 1.3). The distribution of cellulose, hemicellulose, and lignin differs depending on the plant type. For instance, softwood typically has 42%, 27%, and 28% of cellulose, hemicellulose, and lignin content, respectively, while hardwood has 45%, 30%, and 20%, respectively (Smook 2002).
Schematic structure of wood fiber: ML middle lamella, P primary cell wall layer, R reversing point, S1 outer layer of secondary cell wall, S2 middle layer of secondary cell wall layer 2, S3 inner layer of secondary cell wall, W wart layer (Modified from Krässig 1993)
1.2.2 Nanocellulose
Nanocellulose refers to a cellulosic nanomaterial that has at least one dimension on a nanoscale. It can be classified into three types, cellulose nanofibrils (CNFs), cellulose nanocrystals (CNCs), and bacterial nanocellulose (BNC), adapted from Klemm et al. (2011). Each type of nanocellulose is obtained using different preparation methods. The production of CNFs and CNCs is the top-down process, where lignocellulosic materials are broken down into microscale cellulosic materials and further disintegrated into nanocellulose materials (Fig. 1.4). On the other hand, bacterial cellulose production is a bottom-up process where bacteria generate glucose and cellulose is synthesized by connecting glucose molecules. Each nanocellulose type has a different dimension that affects its functions. CNFs and CNCs can be produced from lignocellulosic materials as shown in Fig. 1.5.
First, cellulose nanofibrils, known as microfibrillated cellulose, nanofibrillated cellulose, and cellulose nanofibers, were first developed by Turbak et al. (1983a) and Herrick et al. (1983). They conducted mechanical refining and a high-pressure homogenization processes with wood cellulose fibers, and they obtained a gel-like material, naming this new material as microfibrillated cellulose. Since then, a number of studies have been conducted on cellulose nanofibrils. Cellulose nanofibrils typically have a diameter of below 100 nm and a length of several micrometers (Klemm et al. 2011). Due to high aspect ratio (length/width), they form rigid network structures that induce remarkable mechanical and barrier properties. It has been reported that the CNFs films have a tensile strength of up to 310 MPa, which is much higher than commercial polymers, such as polyethylene, polystyrene, and polycarbonate (up to 70 MPa) and high performance polymers, such as polyimide and polyetheretherketon (70–100 MPa) (Stevens 1999; Yano and Nakahara 2004; Abe and Yano 2009; Fukuzumi et al. 2009; Saito et al. 2009). CNFs have been incorporated as a reinforcing agent into various polymer matrices, such as polypropylene, poly(lactic acid), and starch. For instance, Fernandes et al. (2010) reported that chitosan/CNFs nanocomposite films had up to threefold higher Young’s modulus compared with pure chitosan at 60% CNFs loading. CNFs films also have excellent barrier performance. Aulin et al. (2010) described the oxygen permeability of CNFs films at 0% RH was 0.0006 cm3.μm/m2.day.kPa, which is much lower than that of polyethylene and polystyrene. Also, CNFs gels have not only high viscosity but also a high degree of shear thinning, which make them injectable as well as moldable for a number of biomedical and food applications (Pääkkö et al. 2007).
Mechanical disintegration process is required to obtain well-delaminated cellulose nanofibrils from cellulose fibers, which are held by hydrogen bonds. The equipment commonly used to produce CNFs includes a high-pressure homogenizer, a micro-fluidizer, and a micro-grinder. However, there are several technical problems in the mechanical disintegration process, such as high energy consumption and clogging in a homogenizer (Klemm et al. 2011). More specifically, the energy required by a high-pressure homogenizer reaches as high as 70,000 kWh/ton (Eriksen et al. 2008). By comparison, the average US household consumed 11,320 kWh of electricity in 2009 (EIA 2009). Despite this high energy demand, the research on CNFs has become a rapidly evolving research area because of the increasing demand for renewable materials and interest in nanotechnology since 2000. Researchers have developed mechanical, chemical, and/or enzymatic pretreatment methods that make large-scale CNFs production available since the pretreatment facilitates the fibrillation process, reducing energy consumption and clogging. Several pilot and commercial plants for cellulose nanofibrils have opened since 2011.
Cellulose nanocrystals, called nanocrystalline cellulose, cellulose whiskers, and cellulose nanowhiskers, consist of rodlike cellulose particles. CNCs have a width ranging from 5 to 60 nm, and length varies to several nanometers depending on the sources of cellulose. For instance, CNCs obtained from plants have a length of 100–250 nm, while CNCs from tunicate, algae, and bacterial celluloses have a length from 100 nm to several micrometers. CNCs are prepared by strong acid hydrolysis, often followed by sonication. During the acid hydrolysis, amorphous regions of cellulose fibers are removed as the hydronium ions attack weak points of cellulose (glucosidic bonds in amorphous regions); thus, cellulose nanocrystals are highly crystalline (Siqueira et al. 2010). Hydrochloric and sulfuric acids are the two most used acids for CNCs extraction. Sulfuric acid is more commonly used as it reacts with the hydroxyl groups of cellulose, rendering the negatively charged surface sulfate groups. The charged groups create the repulsive forces between cellulose, which allow CNCs homogeneously dispersed in water (Dong et al. 1998). CNCs can be used as a reinforcing agent in polymer composites, but they create a weak reinforcing effect compared with CNFs because of their lower aspect ratio (Xu et al. 2013).
Bacterial nanocellulose (BNC) is generated by Gluconacetobacter bacteria strains. BNC is produced by a biotechnological bottom-up method unlike CNFs and CNCs. It is synthesized from biochemically activated dextrose between the outer and plasma membranes of the bacterial cell by a cellulose-synthesizing complex. This complex is associated with pores at the surfaces of the bacterial cell, having a diameter of about 3 nm (Gatenholm and Klemm 2010). BNC has a width ranging from 20 to 100 nm (Klemm et al. 2011). It has extensive networks due to the random motion of bacteria. Moreover, BNC is pure cellulose that does not include functional groups, such as carbonyl and carboxyl groups, and other polymers, such as hemicellulose and lignin; therefore, it is the most used nanocellulose in the biomedical application (Gatenholm and Klemm 2010).
1.3 Production of Cellulose Nanofibrils
Cellulose nanofibrils are typically prepared by mechanical disintegration process using three common methods: high-pressure homogenization (Herrick et al. 1983; Turbak et al. 1983a, b; Dufresne et al. 2000; Iwamoto et al. 2005; Malainine et al. 2005; Besbes et al. 2011a, b; Li et al. 2012; Alila et al. 2013; Chaker et al. 2013), micro-fluidization (Pääkkö et al. 2007; Zimmermann et al. 2010; Spence et al. 2011; Zhu et al. 2011; Zhang et al. 2012), and micro-grinding (Iwamoto et al. 2005, 2007, 2008; Jonoobi et al. 2012; Josset et al. 2014; Sharma et al. 2015). Each method has its advantages and disadvantages.
High-pressure homogenization is the first method used to produce cellulose nanofibrils from woody cellulose fibers (Herrick et al. 1983; Turbak et al. 1983a). Cellulose aqueous suspension is pumped at high pressure through a spring-loaded valve assembly. The valve is opened and closed repetitively, so the fibers are subjected to a significant pressure drop with high shear and impact forces generated in the narrow slit of the valve and disintegrated into cellulose nanofibrils (Fig. 1.6). The process can be easily scaled up and operated continually. However, it consumes a substantial amount of energy over 25,000 kWh/ton and causes clogging in the homogenizer, particularly at the in-line valves, which must be dissembled and cleaned. A micro-fluidizer is an alternative to the high-pressure homogenizer. The cellulose/water slurry is passed through thin z-shaped chambers with different channel dimensions (commonly 100–400 μm) under high pressure (Fig. 1.7). Then, it is converted into a gel-like material by the application of shear and impact forces. This process does not use in-line moving parts, thus reduces the likelihood of clog. If the clogging occurs, the micro-fluidizer has to be cleaned by reverse flow through the chamber.
When processing with a micro-grinder, cellulose/water suspension is passed between a static and a rotating grindstone generally at 1500 rpm. Cell wall structures and hydrogen bonds are broken down by the shearing forces generated by the grinding stones, and nanofibers in a multilayer structure are liberated. This method is considered less energy demanding than the other two methods, and it does not have issues related to clogging. However, the main disadvantage of micro-grinding is disk maintenance and replacement due to frequent worn out.
Ultrasonication is an alternative method to produce cellulose nanofibrils (Chen et al. 2011a, b). Ultrasound energy is transferred to cellulose chains through a cavitation process where cavities in water are formed, grow, and are violently disrupted. The energy generated by cavitation is roughly 10–100 kJ per mol that is within the hydrogen bond energy scale. Therefore, the ultrasonic impact can break down hydrogen bonds and disintegrate cellulose fibers into cellulose nanofibrils. However, the multilayered cell wall structure and interfibrillar hydrogen bonds inhibit the complete fibrillation of cellulose fibers into individual microfibrils; thus CNFs are usually obtained as aggregates of microfibrils having a wide distribution in width (Chen et al. 2011a). For this reason, high output energy of ultrasonication is required to obtain well-fibrillated cellulose. When the output power of ultrasonication was 1000 W or higher, CNFs had a narrow distribution in width (5–20 nm) with a weblike network structure; on the other hand, 400 W ultrasonic treatment produced large aggregates of cellulose fibers with widths ranging from several nanometers to a hundred nanometers.
A high-speed blender has been recently introduced to prepare cellulose nanofibrils (Uetani and Yano 2011; Jiang and Hsieh 2013). Uetani and Yano (2011) produced cellulose nanofibers by agitating 0.7 wt.% pulp/water slurry using a blender at 37,000 rpm for 30 min. The resultant cellulose nanofibrils had a uniform width of 15–20 nm. They reported that high-speed blending process could yield the same extent of fibrillation of cellulose with less damage on CNFs compared with micro-grinding. Jiang and Hsieh (2013) also prepared CNFs using high-speed blending, and the CNFs exhibited superior crystallinity (81.5%) compared with original rice straw cellulose (72.2%), confirming high-speed blending did not cause severe damage on cellulose. Interestingly, these CNFs had a bimodal distribution of dimensions: an average width of 2.70 nm or 8.46 nm and a length of 100–200 nm or several micrometers.
1.4 Pretreatment Methods for CNFs production
1.4.1 Mechanical Pretreatments
Mechanical pretreatment methods aim to pre-fibrillate cellulose fibers and reduce fiber size to facilitate the fiber disintegration easily. They can damage the cellulose fibrillary structure, causing external fibrillation that exposes secondary cell wall, where individual cellulose fibrils are organized. They can also produce internal fibrillation that loosens the fiber wall (Hamad 1997; Nakagaito and Yano 2004). Alternatives to mechanical pretreatment include manual cutting, disk refiners, PFI mills, Valley beaters, and blenders (Herrick et al. 1983; Turbak et al. 1983a; Dinand et al. 1999; Dufresne et al. 2000; Chakraborty et al. 2005; Iwamoto et al. 2005; Malainine et al. 2005; Spence et al. 2011; Zhang et al. 2012). The PFI mill and the Valley beater are commonly used for refining pulps in the laboratory scale. Firstly, the PFI mill is a high-energy and low-intensity refiner (Gharehkhani et al. 2015). This device refines pulps between the inner roll and outer bedplate that rotate in the same direction but with different peripheral speeds; thus, the pulps are exposed to mechanical shearing action (TAPPI 2000). The Valley beater needs the larger amount of pulp samples and longer operating time (Gharehkhani et al. 2015). In the beating process, the pulps are looped around a well and forced between a rotor and loaded bedplate (TAPPI 2001). The energy consumption of PFI mill and Valley beater led to increased cost of manufacturing cellulose nanofibrils. It was reported that the effective beating energy in a Valley beater was about 482 and 578 kWh/ton for bleached eucalyptus and bleached pine, respectively, for a total beating time of 1 h. When no-load power was included, the total beating energy was approximately 3000 kWh/ton (Atic et al. 2005). It was also reported that the 3-h beating process consumed about 2000 kWh/ton while producing cellulose microfiber 0.24 μm in diameter from cellulose fibers 30 μm in diameter (Spence et al. 2011). Concerning the PFI mill, Welch and Kerekes (1994) estimated the refining energy for PFI milling was about 0.18 kWh/ton-rev. If the pulps are refined in a PFI mill for 20,000 revolutions prior to the mechanical disintegration process as described by Sharma et al. (2015), the PFI mill would consume approximately 3600 kWh/ton. Also, the refining energy required by a PFI mill was estimated as high as 21,700 kWh/ton to generate cellulose microfiber 1.3 μm in diameter from bleached softwood kraft pulp 13 μm in diameter (Chakraborty et al. 2007b). Therefore, the development of low-energy mechanical pretreatment technique can contribute to the reduction in the overall energy use for CNFs production. Moreover, the standard mechanical refining process is a wet processing. The mechanical pretreatment process using a PFI mill is performed at the initial pulp consistency of 10%. Specifically, 300 g of cellulose slurry containing 30 g of cellulose (dry basis) can be refined per run (TAPPI 2000). The process with a Valley beater is carried out at the pulp consistency of 1.57%, having 360 g of cellulose (dry basis) in 23 L (TAPPI 2001). This indicates that both mechanical pretreatment processes produce wet cellulose precursors, and their bulk volume would make it difficult to store and handle these cellulose precursors.
1.4.2 Chemical Pretreatments
Several chemical pretreatments have been developed to facilitate the mechanical disintegration of cellulose. 2,2,6,6,-tetramethylpiperidine-1-oxyl radial (TEMPO)-mediated oxidation has been widely studied as a chemical pretreatment for cellulose nanofibrils production (Alila et al. 2013; Besbes et al. 2011a, b; Chaker et al. 2013; Saito et al. 2006, 2007; Zhang et al. 2012). This process converts half of C6 primary hydroxyl groups on a cellulose surface into C6 carboxyl groups (Fig. 1.8). TEMPO oxidation helps cellulose fibers to be delaminated in several ways: (1) repulsive forces between cellulose fibers form the introduction of the carboxyl groups; (2) TEMPO oxidation favors the hydration and swelling of cellulose fibers, making the fibers more flexible and also increasing the accessibility of their crystalline regions; (3) the oxidation also makes the S2 layer more accessible and more prone to be fibrillated by loosening the primary S1 cell wall; and (4) the oxidation leads to chain scission the amorphous region, which creates weak points within the cell wall (Besbes et al. 2011a).
Carboxymethylation process is another common chemical pretreatment method. Cellulose fibers are first reacted with sodium hydroxide to become more accessible to chemicals and then with monochloroacetic acid to introduce carboxymethyl groups as shown in Fig. 1.9. The introduction of charged groups enhanced the delamination of cellulose fibers by giving repulsive electrostatic forces between fibers. Also, carboxymethylation leads to fiber swelling, and carboxymethyl groups should be in the form of their sodium salts in order to cause as much swelling of cellulose fibers as possible. Swollen fibers are more susceptible to be delaminated as they have lower cell-wall cohesion than less swollen fibers (Klemm et al. 2011). It was reported that with the carboxymethylation process, cellulose nanofibrils were successfully obtained only after one pass through a high-pressure fluidizer and had a diameter of 5–10 nm (Wågberg et al. 2008; Aulin et al. 2010).
There are several disadvantages of chemical pretreatment methods. First, these processes require various chemicals and organic solvents, which is far from environmentally benign technology. TEMPO oxidation uses sodium hypochlorite and/or sodium chlorite, sodium bromide, TEMPO, and ethanol. Carboxymethylation is based on the reaction in organic solvents, such as ethanol, methanol, and isopropanol. More specifically, a total of 30 kg of organic solvents are consumed during carboxymethylation to manufacture 1 kg of cellulose nanofibrils (Arvidsson et al. 2015). In addition, the introduction of functional groups, such as carboxyl and carboxymethyl groups, decreases thermal stability of cellulose due to decarbonation (Britto and Assis 2009). Fukuzumi et al. (2009) demonstrated that TEMPO-oxidized cellulose nanofibrils had an onset thermal degradation temperature (To) of approximately 200 °C, while original cellulose had a To of approximately 300 °C. Other studies also confirm that the thermal degradation of cellulose nanofibrils containing functional groups takes place at a lower temperature than pure cellulose nanofibrils (Eyholzer et al. 2010; Fukuzumi et al. 2010).
Ionic liquids have been used to promote mechanical disintegration process. Li et al. (2012) studied imidazolium-based ionic liquid treatment to obtain nanocellulose from sugarcane bagasse using a high-pressure homogenization. Before homogenization, cellulose fibers were pretreated with [Bmim]Cl ionic liquid (IL) which attacked and broke the hydrogen bonds between cellulose fibers. IL-treated fibers were passed through a high-pressure homogenizer, and then nanocellulose was generated with a width of 10–20 nm. However, IL treatment has some drawbacks. First, this treatment decreased the crystallinity of cellulose, thereby the thermal stability of cellulose. The crystallinity of original cellulose, IL-treated cellulose, and nanocellulose was 60%, 52%, and 36%, respectively, because the IL treatment and homogenization made cellulose more amorphous, resulting from the destruction of intermolecular hydrogen bonds. The decrease in an onset decomposition temperature is another evidence of the cellulose disruption. The onset decomposition temperature of untreated cellulose was 288 °C, while those of IL-treated cellulose and nanocellulose were 251 °C and 238 °C, respectively. Furthermore, imidazolium-based ionic liquid is expensive and non-environmentally friendly because the feedstock for the synthesis of the ionic liquid is of petroleum based (Hou et al. 2012).
1.4.3 Enzymatic Pretreatment
Enzymatic pretreatment has been used as an environmentally benign pretreatment for cellulose nanofibrils production, which is based on hydrolysis of cellulose, in particular, glucosidic bonds in amorphous regions (Zhu et al. 2011). Cellulases used for enzymatic pretreatment can be divided into two categories as follows (Dufresne 2012): (1) endoglucanases or β-1,4-endoglucanases, which randomly attack intramolecular β-1,4-glucosidic bonds, creating oligosaccharides of various lengths and hence new chain ends, and (2) exoglucanases or cellobiohydrolases, which act on the chain ends, generating cellobiose or glucose. The selection of cellulases used affects the type of nanocellulose obtained. Siqueira et al. (2010) pretreated sisal fibers with two types of cellulases, an endoglucanase and an exoglucanase. The enzymatic treatment was performed before (as pretreatment) or after (as posttreatment) mechanical disintegration process with a micro-fluidizer. It was reported that regardless of the order of the enzymatic treatment conducted (pre- or posttreatment), the use of endoglucanases produced a mixture of CNFs and rodlike nanoparticles, while CNFs with network structures was obtained with the exoglucanases used.
Pääkkö et al. (2007) attempted to obtain CNFs using a high-pressure homogenizer with or without mild enzymatic pretreatment using endoglucanases. They found that the use of untreated cellulose fibers caused the rapid blocking of the homogenizer and needed an enormous amount of energy to produce cellulose nanofibrils. A large portion of non-fibrillated cellulose was observed. In contrast, when enzymatically pretreated cellulose fibers were used, the homogenization was performed without clogging and with lower energy consumption of approximately 1100 kWh/ton. The resultant CNFs consisted of individual microfibrils with a width of 5 nm and microfibrils aggregates with a width of 10–20 nm. Also, a very small dosage of an enzyme (0.17 μl per gram of fiber) was sufficient to obtain cellulose nanofibrils; on the other hand, too high dosage (30 μl per gram of fiber) reduced the efficiency of fiber refining and homogenization. Henriksson et al. (2007) reported that cellulose nanofibrils were successfully prepared with low enzyme concentration (0.02%), while their molecular weight and fiber length were maintained.
1.5 Effects of Cellulose Sources on CNFs Production
The first raw material used for manufacturing cellulose nanofibrils was wood pulp (Turbak et al. 1983a; Herrick et al. 1983). Since then, wood pulp has been widely used as a source for CNFs production. More specifically, among 31 CNFs manufacturing plants at a pilot or commercial scale, 22 plants have used wood-based material (18 plants for wood and 4 plants for bleached wood pulp) as a starting material (TAPPI 2015, Fig. 1.10). Wood can be classified into two categories, softwood and hardwood, based on the differences in anatomical features. Softwood pulp is the most used raw material for producing CNFs since it is more responsive to be fibrillated even though it typically has higher lignin content compared with hardwood pulp. Hardwood fibers are less flexible than softwood fibers because the outer secondary wall of hardwood fibers is spirally layered, which reduces their flexibility and accessibility to the inner secondary wall (Stelte and Sanadi 2009). Stelte and Sanadi (2009) described that softwood cellulose fibers were fibrillated into aggregates of small fibers after 25 passes through a disk refiner, while hardwood cellulose fibers mostly remained intact after 75 passes. Moreover, the refined softwood fibers were well fibrillated into nanoscale fibers after ten passes through a high-pressure homogenizer, whereas the refined hardwood fibers required 100 passes to be nano-fibrillated.
In addition, softwood is more accessible to be chemically modified, which facilitates cellulose fiber disintegration, compared with hardwood. This is because the dominant component of the softwood hemicellulose is glucomannan which has C6 primary hydroxyl groups that can be converted into other functional groups, such as carboxyl groups. On the other hand, the hardwood hemicellulose is mainly xylan which does not have C6 primary hydroxyl groups (Sjöström 1993; Fukuzumi et al. 2009). Fukuzumi et al. (2009) reported that the light transmittance at 600 nm was 90% for the CNFs film made of TEMPO-oxidized softwood pulp, but it was about 78% for the CNFs film made of TEMPO-oxidized hardwood pulp, indicating that CNFs from softwood was largely nano-fibrillated than those from hardwood.
The demand for wood has been increased since wood is an important material not only for producing pulp but also for other products such as building products, furniture, and energy. For this reason, non-woody plants, such as agricultural crops and by-products, have been studied as a starting material to produce CNFs. In the literature, CNFs have been obtained from various non-woody plants, such as sugar beet pulp (Dinand et al. 1999; Leitner et al. 2007), wheat straw (Alemdar and Sain 2008; Zimmermann et al. 2010), rice straw (Hassan et al. 2012; Jiang and Hsieh 2013), swede root (Bruce et al. 2005), potato tuber (Dufresne et al. 2000), soy hulls (Alemdar and Sain 2008), cladodes (Malainine et al. 2005), sugarcane bagasse (Li et al. 2012), alfalfa (Besbes et al. 2011b; Chaker et al. 2013), sunflower (Chaker et al. 2013), flax, hemp, jute, abaca, and sisal (Alila et al. 2013). Six pilot or commercial CNFs manufacturing plants have used non-woody plants as a starting material for CNFs production.
Non-woody plants have several advantages over wood as a source for CNFs production. First of all, non-woody plants are annually harvested; in other words, the harvest cycle is much shorter compared with wood. Therefore, non-woody plants are considered as a cheaper and more renewable source than wood. These plants also contain a lower amount of lignin than wood (Alila et al. 2013); thus, the processing steps required to separate cellulose fibers from non-woody plants are expected to be fewer than those required with wood. More importantly, non-woody cellulose fibers are extracted from the primary wall, where microfibrils are organized in a loose network; on the other hand, wood cellulose fibers present in the secondary cell wall where microfibrils are tightly packed. Primary cell walls are more fragile with a thickness of 100 nm or less, compared with secondary cell walls with a thickness up to several tens of micrometers (Dinand et al. 1999). Therefore, the delamination of cellulose fibers from non-woody plants might be easier, requiring less energy than from wood.
Cellulose nanofibrils from non-woody plants have comparable characteristics to those of CNFs from wood. Table 1.1 summarizes morphological information of cellulose nanofibrils from different cellulose sources. The type of raw materials does not influence the morphology of the resultant CNFs. Regardless of cellulose sources, CNFs have a width of less than 100 nm and a length of several micrometers. Table 1.2 presents the light transmittance of cellulose nanofibrils suspensions or films. Since non-fibrillated particles scatter light due to the large size, the presence of non-fibrillated particles leads to the reduction in the transparency. In another word, as the degree of fibrillation increases, the light transmittance also increases. There is no significant difference between the light transmittance of CNFs from wood and from non-woody plants. In the literature, the lowest light transmittance value (at 600 nm) was 20% for CNFs films from softwood prepared solely by micro-grinding (Nogi et al. 2009). On the other hand, the highest value was 90% for CNFs film from softwood prepared by TEMPO oxidation and ultrasonication (Fukuzumi et al. 2009) and for CNFs suspension from abaca fibers prepared by TEMPO oxidation and high-pressure homogenization (Alila et al. 2013). In addition, the mechanical properties of CNFs films do not depend on the type of raw materials. The tensile strength of pure CNFs films from wood reaches as high as 310 MPa, while from non-woody plants, as high as 230 MPa. It should be noted that these values are much greater than that of commercial polymers, such as polyethylene, polystyrene, and polycarbonate (up to 70 MPa), and high-performance polymers, such as polyimide and polyetheretherketon (70–100 MPa) (Stevens 1999). Young’s modulus of CNFs films from non-woody plants (up to 11 GPa) is also comparable to that of CNFs film from wood (up to 18 GPa) (Table 1.3).
1.6 Cellulose Nanofibrils Applications to Food
Cellulose nanofibrils have numerous desirable properties for food applications (Fig. 1.11). Firstly, CNFs have unique rheological properties. They behave like a gel under normal conditions but flow when shaken or agitated. When the shearing forces are removed, CNFs return to their original state. This rheological behavior allows CNFs to be employed as food additives, such as a thickener, a suspension stabilizer, and a textile modifier. Also, CNFs have a great ability to form transparent films due to their nanoscale dimensions and hold outstanding mechanical and barrier properties due to their dense network structure. CNFs have a very high surface area with multiple hydroxyl groups, establishing a good bonding with polymer matrices. Therefore, CNFs films and CNFs-based composites are considered promising for food packaging and coating applications.
1.6.1 Food Additives
Cellulose nanofibrils in food applications were explored in the early 1980s. CNFs are a gel-like material having pseudoplastic or thixotropic viscosity properties. Therefore, CNFs can be used as various food additives. First, they can be used as an emulsion stabilizer. Turbak et al. (1983b) reported that adding a small amount of CNFs stabilized oil-in-water (o/w) emulsions; for instance, dressing mixtures containing CNFs yielded a stable suspension, showing the uniform distribution through the mix; thus, the mixtures did not require shaking before use. Other studies have also confirmed that CNFs are a useful stabilizer for o/w emulsions (Andresen and Stenius 2007; Xhanari et al. 2011; Ström et al. 2013; Winuprasith and Suphantharika 2013). Winuprasith and Suphantharika (2013) described that cellulose nanofibrils obtained at the higher number of homogenization passes (20 passes) could stabilize o/w emulsions more efficiently, improving the shelf life of the products and making them more aesthetically attractive, compared with CNFs obtained by zero, one, and five homogenization passes (Fig. 1.12). This is because the higher the number of passes, the denser network structures were formed, which are more effective to trap the emulsion droplets, thereby preventing the coalescence of these droplets. Cellulose nanofibrils can be also used as a moisture retention agent in food. Turbak et al. (1983b) reported that meat emulsion containing CNFs formed less fat globules. The hamburgers with CNFs lost less water during frying, which made the hamburgers juicier and tastier compared with those without CNFs. This is in agreement with Ström et al. (2013). They found that CNFs held more water without side effect as watery taste. Moreover, cellulose nanofibrils can be applied as a textile modifier. Kleinschmidt et al. (1988) described filling-containing, dough-based products containing cellulose fibrils and microfibrils. They described that cellulose fibrils and microfibrils formed a network, acting as a flow control agent that enabled the filling to be co-based, with the dough forming the crumb. After baking, the filling dispersed rapidly in the mouth, leading to a good eating quality and flavor release. Ström et al. (2013) also reported that bread containing CNFs was softer and had better appearance like higher volume and more even form (Fig. 1.13). Lastly, the addition of cellulose nanofibrils can thicken food products, such as gravies and soups. It was reported that the addition of 0.75% CNFs suspension was sufficient to produce creamed soups (Turbak et al. 1982). It should be noted that since cellulose is not digestible, the use of CNFs in food products does not increase or can reduce calories of the food products (Turbak et al. 1983b).
Oil-in-water emulsions (30% w/w oil) stabilized by CNFs prepared with different number of passes through a homogenizer (0, 1, 5, and 20 passes, from left to right, Winuprasith and Suphantharika 2013)
Buns baked from fresh dough with (a) no additives and (b) cellulose nanofibrils (Reprinted with permission from Innventia AB. Ström et al. 2013)
1.6.2 Food Packaging
CNFs-based materials have proved to be a promising material for food packaging because the addition of cellulose nanofibrils improves mechanical and barrier properties as well as biodegradability. CNFs-based composites have been fabricated with various polymer materials, including hydrophobic polymers, such as polyethylene (PE), polypropylene (PP), and polylactic acid (PLA), and hydrophilic polymers, such as starch, amylopectin, and polyvinyl alcohol (PVA). Moreover, CNFs/Ag composites have been recently developed and can be used as an antimicrobial food packaging film.
1.6.2.1 CNFs/PE and CNFs/PP Composites
It has been attempted to prepare CNFs-based composites with polyethylene (PE) and polypropylene (PP), which are the two most widely used polymers in food packaging industries (Cheng et al. 2007; Wang and Sain 2007a, b). It has been found that the addition of cellulose nanofibrils improves the mechanical and thermal properties of PE and PP composites with an increasing potential of biodegradability. Cheng et al. (2007) fabricated CNFs/polypropylene composites and reported that the tensile strength and Young’s modulus of CNFs/PP composites were higher than pure PP matrix. However, some holes in PP matrix and some gaps between the matrix and fibers were observed due to a lack of good adhesion between CNFs and PP matrix. Since cellulose nanofibrils have a hydrophilic surface, they have a weak interaction with hydrophobic matrices, such as PP and PE; thus, an additional treatment is needed to modify CNFs surface by coating with a dispersant or by chemical modification. Wang and Sain (2007a, b) coated cellulose nanofibrils with an ethylene-acrylic oligomer emulsion (as a dispersant) and successfully produced CNFs-reinforced composites with PE and PP. The mechanical properties of the composites were slightly improved compared to pure PE or PP matrix. Freire et al. (2008) modified the surface of cellulose fibers by acylation with a fatty acid to make them compatible with PE. This surface modification apparently improved interfacial adhesion between the cellulose fibers and the matrix and therefore increased mechanical properties and water resistance of the composite.
In addition, VTT recently developed the bio-based mineral oil barrier film by coating bio-high-density polyethylene (bio-HDPE) with TEMPO-oxidized CNF. This barrier film can be used as a “bag-in-box” liner for dry foods, such as breakfast cereals. It protected the foodstuffs from mineral oil migration. More specifically, the significant reduction (>90%) in mineral oil migration after 7 days of storage was achieved as compared to non-coated bio-HDPE film and other commercial cereal bag films (“VTT files patent” 2016).
1.6.2.2 CNFs/PLA Composites
Polylactic acid (PLA) has a great potential as a biodegradable food packaging material. PLA is safe for all food packaging applications according to the US Food and Drug Administration (FDA 2002). However, PLA is brittle with very little elongation of 7.2% (Kingsland 2010) and not thermally stable (Siro and Plackett 2010). One of the solutions to these problems is the addition of cellulose nanofibrils (Iwatake et al. 2008; Suryanegara et al. 2009; Nakagaito et al. 2009; Jonoobi et al. 2010). Nakagaito et al. (2009) fabricated CNFs/PLA composites using a papermaking-like process which enabled the uniform dispersion of CNFs in PLA. They concluded that the tensile strength, Young’s modulus, and strain at break increased linearly as the CNFs contents increased. In addition, the CNFs/PLA composites exhibited constant storage modulus above glass transition temperature of PLA (T g = 57 °C). In particular, at high CNFs content of 70 and 90 wt.%, the storage modulus did not drop up to 250 °C. This result indicated that cellulose nanofibrils network acted as a load-bearing framework, resisting the applied stress even after the PLA matrix was melt. The improvement of mechanical properties for CNFs/PLA composites was achieved even with a low CNFs content of 5 wt.% (Iwatake et al. 2008; Suryanegara et al. 2009; Jonoobi et al. 2010). Jonoobi et al. (2010) produced CNFs-reinforced PLA by twin screw extrusion, and they reported that the tensile strength and modulus increased from 58 MPa to 71 MPa and from 2.9 to 3.6 GPa, respectively, with 5 wt.% CNFs. Moreover, the addition of cellulose nanofibrils significantly improves barrier properties. Fukuzumi et al. (2009) described that 25 μm-thick PLA films had an oxygen transmission rate of about 746 ml/m2.day.Pa, and the value substantially decreased to 1 ml/m2.day.Pa by coating PLA film with 0.4 μm thick TEMPO-oxidized cellulose nanofibrils.
1.6.2.3 Other CNFs/Polymer Composites
Cellulose nanofibrils have been also used to reinforce other matrices, such as polyvinyl alcohol (PVA), starch, and chitosan, which have a great potential as food packaging films. First, PVA is a water-soluble and biodegradable polymer with excellent film forming property and chemical resistance. Due to these properties, it is used as a water-soluble film useful for packaging (Tripathi et al. 2009; Khan et al. 2014). Cellulose nanofibrils have been used as a reinforcement in PVA matrix, enhancing mechanical properties. Zimmermann et al. (2004) reported that CNFs/PVA composites presented up to three times higher Young’s modulus and five times higher tensile strength compared with pure PVA matrix. Such reinforcing effect of CNFs on PVA is also confirmed by others (Bhatnagar and Sain 2005; Wang and Sain 2007b; Lu et al. 2008).
Starch is the most common carbohydrate polymer used in human diets, produced from cereals and root vegetables (Singh et al. 2010). Starch has been extensively investigated as an attractive material for food packaging applications due to its environmental compatibility, wide availability, and low cost (Arora and Padua 2010). However, it has several drawbacks, such as poor mechanical properties and high water uptake, compared with synthetic polymers. In addition, its properties can be altered easily during processing (Siro and Plackett 2010). Reinforcement with cellulose nanofibrils is one of the approaches to overcome these problems. CNFs/starch composite presents improved thermomechanical behavior (Dufresne and Vignon 1998), bending strength (Yano and Nakahara 2004), tensile strength, and tensile modulus (Dufresne et al. 2000; Chakraborty et al. 2007a) compared with pure starch. Also, the barrier properties were significantly improved when CNFs were added in amylopectin, which is one of the two components of starch. Plackett et al. (2010) reported that CNF addition reduced an oxygen permeability. 15% CNFs/85% amylopectin composite had the oxygen permeability value of 0.034–0.037 ml.mm/m2.day.atm, and 50% CNFs/50% amylopectin composite presented the lower value of 0.02–0.013 ml.mm/m2.day.atm at 23 °C, 50% RH.
Chitosan is a biodegradable polymer, produced from hard outer skeleton of shellfish, such as crabs and shrimps. Chitosan films may be used for food packaging applications to develop edible films or coatings, extending the shelf life of food products (Bangyekan et al. 2006; Durango et al. 2006; Campaniello et al. 2008). However, chitosan films have usually poor mechanical and barrier properties compared with synthetic polymers. Their mechanical and barrier properties can be enhanced by adding cellulose nanofibrils. Fernandes et al. (2010) reported that chitosan/CNFs nanocomposite films had up to threefold higher Young’s modulus compared with pure chitosan at CNFs loading of 60%. Azeredo et al. (2010) also demonstrated that chitosan nanocomposites with 15% CNFs had comparable characteristics to some synthetic polymers, in terms of strength and stiffness. Interestingly, in another research, the addition of CNFs enhanced wet properties of the chitosan/CNFs composite by reducing the risk of creases and deformation, while the effect of CNFs on mechanical properties of dry chitosan films was small or absent (Nordqvist et al. 2007). Recently, antimicrobial chitosan/CNFs composite membranes were developed with a nitric oxide donor, S-nitroso-N-acetyl-D-penicillamine (SNAP), and tested for food packaging applications (Sundaram et al. 2016). They reported that the membranes mixed with SNAP showed excellent water barrier property, a low water vapor permeability, and apparent effects toward inhibition of Enterococcus faecalis, Staphylococcus aureus, and Listeria monocytogenes.
1.6.2.4 CNFs/Ag Composites
Cellulose nanofibrils can be used as an antimicrobial film for various applications. It has been reported that cellulose containing silver nanoparticles shows very strong antimicrobial activity because silver nanoparticles and non-reduced silver ions inhibit the growth of bacteria (Son et al. 2004, 2006; Fernández et al. 2010; Li et al. 2011; Díez et al. 2011; Martins et al. 2012). Son et al. (2004) found that the ultrafine cellulose acetate (CA) fibers electrospun with very small amount of the silver nanoparticles (AgNO3) exhibited antimicrobial properties. 99.9% of bacteria were reduced for CA fibers with 0.05 wt% AgNO3. Fernández et al. (2010) described the antimicrobial activity toward spoilage-related microorganisms of cellulose-silver nanoparticle absorbent pads during storage of fresh-cut melon. The presence of the silver-loaded pads in melon pieces reduced the growth of mesophilic and psychotropic microorganisms.
Cellulose nanofibrils/Ag nanocomposites with antimicrobial activity have been recently developed (Díez et al. 2011; Martins et al. 2012). Díez et al. (2011) prepared CNFs/Ag nanocomposite by dipping a CNFs film into silver solution protected by poly(methacrylic) acid. They found that the growth of bacteria was inhibited around the CNFs/Ag composites. More specifically, the ratio of the surface without bacterial growth to the surface of the CNFs/Ag composite film was approximately up to 5. In contrast, bacteria grew freely around a pure CNFs film. Martins et al. (2012) also found that CNFs/Ag composites treated with polyelectrolytes showed strong antibacterial activity at low-nutrient condition.
1.6.3 Food Coating
Cellulose nanofibril coating for protecting food products has been recently invented (Zhao et al. 2014). They developed an edible composition containing CNFs in an amount of up to 1 wt% and 0.1 wt% nano-calcium carbonate (NCC) to coat and protect a plant, fruit, and vegetable. The CNFs-coated plant, fruit, and vegetable exhibited reduced leaching of functional food substrates (e.g., anthocyanins), moisture loss, and gas exchange compared with the uncoated products. CNFs coating also protected foodstuff from UV damage. The CNFs-based films had the transmittance of 7.2–27.3% for UV light, and the transmittance was further reduced by the addition of NCC. The CNFs-coated apples showed a significant increase in color intensity (chroma) after UV exposure and storage. Moreover, CNFs coating reduced weight loss of foodstuff during thawing. For instance, the weight loss after thawing of CNFs-coated apple slices was around 17%, which was lower than that of uncoated apple slices, around 21%.
1.7 Safety Issues of Cellulose Nanofibrils in Food Applications
Cellulose nanofibrils have a broad range of potential applications in food industries. However, their toxicity to humans, in particular for food consumption, still needs to be validated. Various forms of cellulose are generally recognized as safe (GRAS) food substances, according to the US Food and Drug Administration. Small cellulose particles give several benefits to food products, such as smooth consistency and stickiness, while longer cellulose fibers provide structure and a firmer texture to baked goods (Li et al. 2015). However, because cellulose nanofibrils have unusual properties not found in the bulk cellulosic materials, it might cause unknown risks to humans and the environments.
In general, it has been reported that cellulose nanofibrils have no or low toxicity (Vartiainen et al. 2011; Norppa 2012; Pereira et al. 2013; Andrade et al. 2015). Vartiainen et al. (2011) performed the in vitro study on the health and environmental safety of cellulose nanofibrils. They concluded that no inflammatory effects or cytotoxicity in mouse and human macrophages was observed after short-term (6–24 h) exposure to cellulose nanofibrils. It was also reported that CNFs did not create cytotoxic effects and damage on DNA or chromosome (Norppa 2012). Pereira et al. (2013) assessed in vitro cytotoxicity and expression of genes in fibroblast cells. Interestingly, low concentrations of CNFs (0.02–100 μg.ml−1) did not have cytotoxicity, whereas high concentrations of CNFs (2000 and 5000 μg.ml−1) considerably decreased cell viability and affected the expression of stress- and apoptosis-associated molecular markers. Andrade et al. (2015) performed an in vivo study with mice with diets containing different amounts of CNFs (7 wt%, 14 wt%, and 21 wt%) to investigate the effects of CNFs addition in diets. They concluded that CNFs addition did not cause harmful effects in the animal metabolism, indicating CNFs could be used as dietary supplement.
Although earlier studies reported that cellulose nanofibrils did not cause toxic effects on humans, some toxicity and safety issues still need to be validated, including long-term in vivo toxicity of CNFs and the effects of large amounts of CNFs in animal diets. Further research would be necessary to achieve a better understanding of CNFs effects to food products before commercialization of CNFs use in food industries.
1.8 Conclusions
Cellulose nanofibrils (CNFs) are one type of nanostructured cellulose with a width of below 100 nm and a length of several micrometers. CNFs are an inexhaustible and renewable biopolymer derived from lignocellulosic materials, such as wood and agricultural crops. Interestingly, the selection of raw materials affects the ease of CNFs production. Non-woody plants may be more favorable for CNFs production than wood. Non-woody cellulose fibers exist in thin primary cell walls, while wood cellulose fibers exist in thick and tightly packed secondary cell walls, and CNFs prepared from non-woody has comparable characteristics to those of CNFs from wood.
Cellulose nanofibrils have an excellent ability to form network structures due to their nanoscale dimensions, making their remarkable mechanical and barrier properties. CNFs are a gel-like material with high viscosity, but they flow once shear forces are applied. These desirable properties make CNFs a promising material in a broad range of applications, especially to food industries. CNFs can be used to develop food products, such as food additives, food packaging, and food coating. Recently, the number of studies on CNFs-based materials for food applications has been increased, increasing the likelihood of their commercialization in food industries. Further research in this field is required for a better understanding of how the structure and properties of cellulose nanofibrils affect their function, suitability, and safety for food applications including encapsulation of fat globules in food matrix.
References
Abdul Khalil HPS, Davoudpour Y, Nazrul Islam MD, Mustapha A, Sudesh K, Dungani R, Jawaid M (2014) Production and modification of nanofibrillated cellulose using various mechanical processes: a review. Carbohydr Polym 99(2):649–665
Abe K, Yano H (2009) Comparison of the characteristics of cellulose microfibril aggregates of wood, rice straw and potato tuber. Cellulose 16:1017–1023
Abe K, Yano H (2010) Comparison of the characteristics of cellulose microfibril aggregates isolated from fiber and parenchyma cells of Moso bamboo (Phyllostachys pubescens). Cellulose 17(2):271–277
Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues-wheat straw and soy hulls. Bioresour Technol 99:1644–1671
Alila S, Besbes I, Vilar MR, Mutje P, Boufi S (2013) Non-woody plants as raw materials for production of microfibrillated cellulose (MFC): a comparative study. Ind Crops Prod 41:250–259
Andrade DRM, Mendonca MH, Helm CV, Magalhães WLE, Muniz GIB, Kestur SG (2015) Assessment of nano cellulose from peach palm residue as potential food additive: part II: preliminary studies. J Food Sci Technol 52(9):5641–5650
Andresen M, Stenius P (2007) Water-in-oil emulsions stabilized by hydrophobized microfibrillated cellulose. J Dispers Sci Technol 28:839–844
Arora A, Padua GW (2010) Review: nanocomposites in food packaging. J Food Sci 75(1):R43–R49
Arvidsson R, Nguyen D, Svanstrom M (2015) Life cycle assessment of cellulose nanofibrils production by mechanical treatment and two different pretreatment processes. Environ Sci Technol 49:6681–6890
Atic C, Immamoglu S, Valchev I (2005) Determination of specific beating energy-applied on certain pulps in a valley beater. J Univ Chem Technol Metall 40(3):199–204
Aulin C, Gällstedt M, Lindström T (2010) Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 17:559–574
Azeredo HMC, Mattoso LHC, Avena-Bustillos RJ, Filho GC, Munford ML, Wood D, McHugh TH (2010) Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J Food Sci 75(1):N1–N7
Bangyekan C, Aht-Ong D, Srikulkit K (2006) Preparation and properties evaluation of chitosan-coated cassava starch films. Carbohydr Polym 63(1):61–71
Besbes I, Alila S, Boufi S (2011a) Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibers: effects of the carboxyl content. Carbohydr Polym 84:975–983
Besbes I, Vilar MR, Boufi S (2011b) Nanofibrillated cellulose from Alfa, Eucalyptus and Pine fibres: preparation, characteristics and reinforcing potential. Carbohydr Polym 86:1198–1206
Bhatnagar A, Sain M (2005) Processing of cellulose nanofiber reinforced composites. J Reinf Plast Compos 24:1259–1268
Britto D, Assis OBG (2009) Thermal degradation of carboxymethylcellulose in different salty forms. Thermochim Acta 494:115–122
Brodin R, Gregersen ØW, Syverud K (2014) Cellulose nanofibrils: challenges and possibilities as a paper additive or coating material – a review. Nordic Pulp Paper Res J 29(1):156–166
Bruce DM, Hobson RN, Farrent JW, Hepworth DG (2005) High-performance composites from low-cost plant primary cell walls. Compos Part A Appl Sci Manuf 36(11):1486–1493
Campaniello D, Bevilacqua A, Sinigaglia M, Corbo MR (2008) Chitosan: antimicrobial activity and potential applications for preserving minimally processed strawberries. Food Microbiol 25(8):992–1000
Chaker A, Alila S, Mutje P, Vilar MR, Boufi S (2013) Key role of the hemicellulose content and the cell morphology on the nanofibrillation effectiveness of cellulose pulps. Cellulose 20:2860–2875
Chakraborty A, Sain M, Kortschot M (2005) Cellulose microfibrils: a novel method of preparation using high shear refining and cryocrushing. Holzforschung 59:102–107
Chakraborty A, Sain M, Kortschot M, Cutler S (2007a) Dispersion of wood microfibers in a matrix of thermoplastic starch and starch-polylactic acid blend. J Biobaased Mater Bioenergy 1:71–77
Chakraborty A, Sain MM, Kortschot MT, Ghosh SB (2007b) Modeling energy consumption for the generation of microfibres from bleached kraft pulp fibres in a PFI mill. Bioresources 2(2):210–222
Chen W, Yu H, Liu Y, Chen P, Zhang M, Hai Y (2011a) Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr Polym 83:1804–1811
Chen W, Yu H, Liu Y, Hai Y, Zhang M, Chen P (2011b) Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 18:433–442
Cheng Q, Wang S, Rials TG, Lee S (2007) Physical and mechanical properties of polyvinyl alcohol and polypropylene composite materials reinforced with fibril aggregates isolated from regenerated cellulose fibers. Cellulose 14:593–602
Díez I, Eronen P, Österberg M (2011) Functionalization of nanofibrillated cellulose with silver nanoclusters: fluorescence and antibacterial activity. Macromol Biosci 11:1185–1191
Dinand E, Chanzy H, Vignon RM (1999) Suspensions of cellulose microfibrils from sugar beet pulp. Food Hydrocoll 13:275–283
Dong XM, Revol J, Gray DG (1998) Effect of microcrystalline preparation conditions on the formation of colloid crystals of cellulose. Cellulose 5:19–32
Dufresne A (2012) Nanocellulose: from nature to high performance tailored materials. Walter de Gruyer, Berlin/New York
Dufresne A, Vignon MR (1998) Improvement of starch film performances using cellulose microfibrils. Macromolecules 31:2693–2696
Dufresne A, Dupeyre D, Vignon MR (2000) Cellulose microfibrils from potato tuber cells: processing and characterization of starch–cellulose microfibril composites. J Appl Polym Sci 76:2080–2092
Durango AM, Soares NFF, Andrade NJ (2006) Microbiological evaluation of an edible antimicrobial coating on minimally processed carrots. Food Control 17:336–341
EIA (2009) Residential energy consumption survey. U.S. Energy Information Administration. http://www.eia.gov/consumption/residential/reports/2009/state_briefs. Accessed 2 July 2016
Eriksen Ø, Syverud K, Gregersen Ø (2008) The use of microfibrillated cellulose produced from kraft pulp as strength enhancer in TMP paper. Nordic Pulp Paper Res J 23(3):299–304
Eyholzer C, Bordeanu N, Lopez-Suevos F, Rentsch D, Zimmermann T, Oksman K (2010) Preparation and characterization of water-redispersible nanofibrillated cellulose in powder form. Cellulose 17:19–30
Fernandes SCM, Freire CSR, Silvestre AJD, Neto CP, Gandini A, Berglund LA, Salmén L (2010) Transparent chitosan films reinforced with a high content of nanofibrillated cellulose. Carbohydr Polym 81(2):394–401
Fernández A, Picouet P, Lloret E (2010) Cellulose-silver nanoparticle hybrid materials to control spoilage-related microflora in absorbent pads located in trays of fresh-cut melon. Int J Food Microbiol 140:222–228
Freire CSR, Silvestre AJD, Pascoal Neto C, Gandini A, Martin L, Mondragon I (2008) Composites based on acylated cellulose fibers and low density polyethylene: effect of the fiber content, degree of substitution and fatty acid chain length on final properties. Compos Sci Technol 68:3358–3364
Fukuzumi H, Saito T, Iwata T, Kumamoto Y, Isogai A (2009) Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 10:162–165
Fukuzumi H, Saito T, Okita Y, Isogai A (2010) Thermal stabilization of TEMPO-oxidized cellulose. Polym Degrad Stab 95:1502–1508
Gatenholm P, Klemm D (2010) Bacterial nanocellulose as a renewable material for biomedical applications. MRS Bull 35:208–213
Gavillon R (2007) Preparation and characterization of ultra porous cellulosic materials. Thesis, Mechanics. Ecole Nationale Superieure des Mines de Paris
Gharehkhani S, Sadeghinezhad E, Kazi SN, Yarmand H, Badarudin A, Safaei MR, Zubir MN (2015) Basic effects of pulp refining on fiber properties – a review. Carbohydr Polym 115:785–803
Gómez HC, Serpa A, Velásquez-Cock J, Gañán P, Castro C, Vélez L, Zuluaga R (2016) Vegetable nanocellulose in food science: a review. Food Hydrocoll 57:178–186
Guise C, Fangueiro R (2016) Biomedical applications of nanocellulose. In: Rangueiro R, Rana S (eds) Natural fibres: advances in sciences and technology towards industrial applications. Springer, Dordrecht, pp 155–169
Hamad WY (1997) Some microrheological aspects of wood-pulp fibres subjected to fatigue loading. Cellulose 4:51–56
Hassan ML, Mathew AP, Hassan EA, El-Wakil NA, Oksman K (2012) Nanofibers from bagasse and rice straw: process optimization and properties. Wood Sci Technol 46(1):193–205
Henriksson M, Berglund LA (2007) Structure and properties of cellulose nanocomposite films containing melamine formaldehyde. J Appl Polym Sci 106(4):2817–2824
Henriksson M, Henriksson G, Berglund LA, Lindström T (2007) An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (CNFS) nanofibers. Eur Polym J 43:3434–3441
Henriksson M, Berglund LA, Isaksson P, Lindström T, Nishino T (2008) Cellulose nanopaper structure of high toughness. Biomacromolecules 9:1579–1585
Herrick FW, Casebier RL, Hamilton JK, Sandberg KR (1983) Microfibrillated cellulose: morphology and accessibility. J Appl Polym Sci Appl Polym Symp 37:797–813
Hoeng F, Deneulin A, Bras J (2016) Use of nanocellulose in printed electronics: a review. Nanoscale 8:13131–13154
Hou XD, Smith TJ, Li N, Zong MH (2012) Novel renewable ionic liquids as highly effective solvents for pretreatment of rice straw biomass by selective removal of lignin. Biotechnol Bioeng 109(10):2484–2493
Iwamoto S, Nakagaito AN, Yano H, Nogi M (2005) Optically transparent composites reinforced with plant fiber-based nanofibers. Appl Phys A Mater Sci Process 81:1109–1112
Iwamoto S, Nakagaito AN, Yano H (2007) Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl Phys A Mater Sci Process 89:461–466
Iwamoto S, Abe K, Yano H (2008) The effect of hemicellulose on wood pulp nanofibrillation and nanofiber network characteristics. Biomacromolecules 9:1022–1026
Iwatake A, Nogi M, Yano H (2008) Cellulose nanofiber-reinforced polylactic acid. Compos Sci Technol 68:2103–2106
Jiang F, Hsieh Y (2013) Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr Polym 95:32–40
Jonoobi M, Harun J, Mathew AP, Oksman K (2010) Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos Sci Technol 70(12):1742–1747
Jonoobi M, Khazaeian A, Tahir PM, Azry SS, Oksman K (2011) Characteristics of cellulose nanofibers isolated from rubberwood and empty fruit bunches of oil palm using chemo-mechanical process. Cellulose 18(4):1085–1095
Jonoobi M, Mathew AP, Oksman K (2012) Producing low-cost cellulose nanofiber from sludge as new source of raw materials. Ind Crops Prod 40:232–238
Jonoobi M, Oladi R, Davoudpour Y, Oksman K, Dufresne A, Hamzeh Y, Davoodi R (2015) Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: a review. Cellulose 22(2):935–969
Jorfi M, Foster EJ (2015) Recent advances in nanocellulose for biomedical applications. J Appl Polym Sci 132(14):41719. doi:10.1002/app.41719
Josset S, Orsolini P, Siqueira G, Tejado A, Tingaut P, Zimmermann T (2014) Energy consumption of the nanofibrillation of bleached pulp, wheat straw and recycled newspaper through a grinding process. Nordic Pulp Paper Res J 29(1):167–175
Khan A, Huq T, Khan RA, Riedl B, Lacroix M (2014) Nanocellulose-based composites and bioactive agents for food packaging. Crit Rev Food Sci Nutr 54:163–174
Kim J, Shim BS, Kim HS, Lee Y, Min S, Jang D, Abas Z, Kim J (2015) Review of nanocellulose for sustainable future materials. Int J Precis Eng Manuf-Green Technol 2(2):197–213
Kingsland C (2010) PLA: a critical analysis. Mohawk College of Applied Arts and Technology. http://www.iopp.org/files/public/KingslandCaseyMohawk.pdf. Assessed 15 Sept 2015
Kleinschmidt DC, Roberts BA, Fuqua DL, Melchion JR (1988) Filling-containing, dough-based products containing cellulosic fibrils and microfibrils. US Patent 4,774,095A, Sept 1988
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393
Klemm D, Kramer F, Mortiz S, Lindström T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50(24):5438–5466
Krässig HA (1993) Cellulose-structure, accessibility, and reactivity. Gordon and Breach, Amsterdam
Lavoine N, Desloges I, Dufresne A, Bras J (2012) Microfibrillated cellulose – its barrier properties and applications in cellulosic materials: a review. Carbohydr Polym 90(2):735–764
Leitner J, Hinterstoisser B, Wastyn M, Keckes J, Gindl W (2007) Sugar beet cellulose nanofibril-reinforced composites. Cellulose 14:419–425
Li S, Jia N, Ma M, Zhang Z, Liu Q, Sun R (2011) Cellulose–silver nanocomposites: microwave-assisted synthesis, characterization, their thermal stability, and antimicrobial property. Carbohydr Polym 86:441–447
Li J, Wei X, Wanga Q, Chena J, Changa G, Kongc L, Sud J, Liue Y (2012) Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr Polym 90:1609–1613
Li F, Mascheroni E, Piergiovanni L (2015) The potential of nanocellulose in the packaging field: a review. Packag Technol Sci 28:475–508
Lin N, Dufresne A (2014) Nanocellulose in biomedicine: current status and future prospect. Eur Polym J 59:302–325
Lu J, Wang T, Drzal LT (2008) Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos Part A Appl Sci Manuf 39:738–746
Lu Y, Tekinalp HL, Peter WH, Eberle C, Naskar AK, Ozcan S (2014) Nanocellulose in polymer composites and biomedical: research and applications. TAPPI J 13(6):47–54
Malainine ME, Mahrouz M, Dufresne A (2005) Thermoplastic nanocomposites based on cellulose microfibrils from opuntia ficus-indica parenchyma cell. Compos Sci Technol 65:1520–1526
Martins NCT, Freire CSR, Pinto RJB, Fernandes SCM, Neto CP, Silvestre AJD, Causio J, Baldi G, Sadocco P, Trindade T (2012) Electrostatic assembly of Ag nanoparticles onto nanofibrillated cellulose for antibacterial paper products. Cellulose 19(4):1425–1236
Nair SS, Zhu LY, Deng Y, Ragauskas AJ (2014) High performance green barriers based on nanocellulose. Sustain Chem Processes 2:23. doi:10.1186/s40508-014-0023-0
Nakagaito AN, Yano H (2004) The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl Phys A Mater Sci Process 78:547–552
Nakagaito AN, Fujimura A, Sakai T, Hama Y, Yano H (2009) Production of microfibrillated cellulose (MFC)-reinforced polylactic acid (PLA) nanocomposites from sheets obtained by a papermaking-like process. Compos Sci Technol 69:1293–1297
Nogi M, Iwamoto S, Nakagaito AN, Yano H (2009) Optically transparent nanofiber paper. Adv Mater 21:1595–1598
Nordqvist D, Idermark J, Hedenqvist MS (2007) Enhancement of the wet properties of transparent chitosan-acetic-acid-salt films using microfibrillated cellulose. Biomacromolecules 8:2398–2403
Norppa H (2012) Nanofibrillated cellulose: results of in vitro and in vivo toxicological assays. Paper presented at Sunpap conference, 19–20 June 2012
Okahisa Y, Abe K, Nogi M, Nakagaito AN, Nakatani T, Yano H (2011) Effects of delignification in the production of plant-based cellulose nanofibers for optically transparent nanocomposites. Compos Sci Technol 71(10):1342–1347
Osong SH, Norgren S, Engstrand P (2016) Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: a review. Cellulose 23:93–123
Pääkkö M, Ankerfors M, Kosonen H, Nykänen A, Ahola S, Österberg M, Ruokolainen J, Laine J, Larsson PT, Ikkala O, Lindström T (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 8:1934–1941
Pereira MM, Raposo NRB, Brayner R, Teixeira EM, Oliveira V, Quintão CCR (2013) Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology 24:075103. doi:10.1088/0957-4484/24/7/075103
Plackett D, Auturi H, Hedenqvist M, Ankerfors M, Gällstedt M, Lindström T, Siro I (2010) Physical properties and morphology of films prepared from microfibrillated cellulose and microfibrillated cellulose in combination with amylopectin. J Appl Polym Sci 117(6):3601–3609
Rodionova G, Saito T, Lenes M, Eriksen Ø, Gregersen Ø, Fukuzumi H, Isogai A (2012) Mechanical and oxygen barrier properties of films prepared from fibrillated dispersions of TEMPO-oxidized Norway spruce and Eucalyptus pulps. Cellulose 19:705–711
Sabo R, Yermakov A, Law CT, Elhajjar R (2016) Nanocellulose-enabled electronics, energy harvesting device, smart materials and sensors: a review. J Renew Mater. http://dx.doi.org.proxy-remote.galib.uga.edu/10.7569/JRM.2016.634114
Saito T, Nishiyama Y, Putaux JL, Vignon M, Isogai A (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 7:1687–1691
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8:2485–2491
Saito T, Hirota M, Tamura N, Kimura S, Fukuzumi H, Heuz L, Isogai A (2009) Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation suing TEMPO catalyst under neutral conditions. Biomacromolecules 7:1687–1691
Sehaqui H, Zhou Q, Ikkala O, Berglund LA (2011) Strong and tough cellulose nanopaper with high specific surface area and porosity. Biomacromolecules 12(10):3638–3644
Sharma S, Nair SS, Zhang Z, Ragauskas AJ, Deng Y (2015) Characterization of micro fibrillation process of cellulose and mercerized cellulose pulp. RSC Adv 5:63111–63122
Singh J, Dartois A, Kaur L (2010) Starch digestibility in food matrix: a review. Trends Food Sci Technol 21(4):168–180
Siqueira G, Tapin-Lingua S, Bras J, da Silva PD, Dufresne A (2010) Morphological investigation of nanoparticles obtained from enzymatic and acid hydrolysis of sisal fibers. Cellulose 17:1147–1158
Siro I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17:459–494
Sjöström E (1993) Wood chemistry: fundamentals and applications, 2nd edn. Gulf Professional Publishing, Houston
Smook GA (2002) Handbook for pulp & paper technologists, 3rd edn. Angus Wilde Publications Inc., Vancouver
Son W, Youk J, Lee T, Park W (2004) Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol Rapid Commun 25(18):1632–1637
Son W, Youk J, Lee T, Park W (2006) Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr Polym 65(4):430–434
Spence KL, Venditti RA, Habibi Y, Rojas OJ, Pawlak JJ (2010a) The effect of chemical composition on microfibrillar cellulose films from wood pulps: mechanical processing and physical properties. Bioresour Technol 101:5961–5968
Spence KL, Venditti RA, Habibi Y, Rojas OJ, Pawlak JJ (2010b) The effect of chemical composition on microfibrillar cellulose films from wood pulps: water interactions and physical properties for packaging applications. Cellulose 17:835–848
Spence KL, Benditti RA, Rojas OJ, Habibi Y, Pawlak JJ (2011) A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 18:1097–1111
Stelte W, Sanadi AR (2009) Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind Eng Chem Res 48:11211–11219
Stevens MP (1999) Polymer chemistry: an introduction, 3rd edn. Oxford University Press, New York
Ström G, Ohgren C, Ankerfors M (2013) Nanocellulose as an additive in foodstuff. Innventia Report B, 403. http://www.innventia.com/Documents/Rapporter/Innventia%20report403.pdf. Accessed 29 July 2016
Sundaram J, Pant J, Goudie MJ, Mani S, Handa H (2016) Antimicrobial and physicochemical characterization of biodegradable, nitric oxide-releasing nanocellulose−chitosan packaging membranes. J Agric Food Chem 64:5260–5266
Suryanegara L, Nakagaito AN, Yano H (2009) The effect of crystallization of PLA on the thermal and mechanical properties of microfibrillated cellulose-reinforced PLA composites. Compos Sci Technol 69:1187–1192
Syverud K, Stenius P (2009) Strength and barrier properties of MFC films. Cellulose 16:75–85
Taniguchi T, Okamura K (1998) New films produced from microfibrillated natural fibres. Polym Int 47:291–294
TAPPI (2000) T248 sp-00, Laboratory beating of pulp (PFI mill method). Technical Association of the Pulp and Paper Industry, Norcross
TAPPI (2001) T200 sp-01, Laboratory beating of pulp (Valley beater method). Technical Association of the Pulp and Paper Industry, Norcross
TAPPI (2015) Summary of international activities on cellulosic nanomaterials. Technical Association of the Pulp and Paper Industry, Norcross. http://www.tappinano.org/media/1096/tc6-world-cnm-activities-summary-july-29-2015.pdf. Accessed 15 Nov 2015
Tripathi S, Mehrotra GK, Dutta PK (2009) Physicochemical and bioactivity of cross-linked chitosan–PVA film for food packaging applications. Int J Biol Macromol 45:372–376
Turbak AF, Snyder FW, Sandberg KR (1982) Food products containing microfibrillated cellulose. US Patent 43,418,071,982, July 1982
Turbak AF, Snyder FW, Sandberg KR (1983a) Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. J Appl Polym Sci Appl Polym Symp 37:815–827
Turbak AF, Snyder FW, Sandberg KR (1983b) Suspensions containing microfibrillated cellulose, US Patent 4,378,381 A, Mar 1983
Uetani K, Yano H (2011) Nanofibrillation of wood pulp using a high-speed blender. Biomacromolecules 12:348–353
Vartiainen J, Pöhler T, Sirola K, Pylkkänen L, Alenius H, Hokkinen J (2011) Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 18:775–786
VTT files patent for new bio-based mineral oil barrier technology for food packaging (2016) Bioplastics Magazine. http://www.bioplasticsmagazine.com/en/news/meldungen/2016-02-09-VTT-files-patent-mineral-oil-barrier-technology.php. Accessed 1 Aug 2016
Wågberg L, Norgren GDM, Lindström T, Ankerfors M, Axnas K (2008) The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 24:784–795
Wang Y (2008) Cellulose fiber dissolution in sodium hydroxide solution at low temperature: dissolution kinetics and solubility improvement. Dissertation, Georgia Institute of Technology
Wang B, Sain M (2007a) Dispersion of soybean stock-based nanofiber in a plastic matrix. Polym Int 56(4):538–546
Wang B, Sain M (2007b) Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos Sci Technol 67:2521–2527
Welch LV, Kerekes RJ (1994) Characterization of the PFI mill by the C-factor. APPITA 47(5):387–390
Winuprasith T, Suphantharika M (2013) Microfibrillated cellulose from mangosteen (Garcinia mangostana L.) rind: preparation, characterization, and evaluation as an emulsion stabilizer. Food Hydrocoll 32(2):383–394
Xhanari K, Syverud K, Stenius P (2011) Emulsions stabilized by microfibrillated cellulose: the effect of hydrophobization, concentration and O/W ratio. J Dispers Sci Technol 32:447–452
Xu X, Liu F, Jiang L, Zhu JY, Haagenson D, Wiesenborn DP (2013) Cellulose nanocrystals vs. cellulose nanofibrils: a comparative study on their microstructures and effects as polymer reinforcing agents. Appl Mater Interfaces 5:2999–3009
Yano H, Nakahara S (2004) Bio-composites produced from plant microfiber bundles with a nanometer unit web-like network. J Mater Sci 39:1635–1638
Zhang J, Song H, Lin L, Zhuang J, Pang C (2012) Microfibrillated cellulose from bamboo pulp and its properties. Biomass Bioenergy 39:78–83
Zhao Y, Simonsen J, Cavender G, Jung J, Fuchigami LH (2014) Nano-cellulose coatings to prevent damage in foodstuffs. US Patent 20,140,272,013 A1, Sept 2014
Zhu JY, Sabo R, Luo X (2011) Integrated production of nano-fibrillated cellulose and cellulosic biofuel (ethanol) by enzymatic fractionation of wood fibers. Green Chem 13:1339–1344
Zimmermann T, Pohler E, Geiger T (2004) Cellulose fibrils for polymer reinforcement. Adv Eng Mater 6(9):754–761
Zimmermann T, Bordeanu N, Strub E (2010) Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohydr Polym 79:1086–1093
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lee, H., Sundaram, J., Mani, S. (2017). Production of Cellulose Nanofibrils and Their Application to Food: A Review. In: Prasad, R., Kumar, V., Kumar, M. (eds) Nanotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-10-4678-0_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-4678-0_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4677-3
Online ISBN: 978-981-10-4678-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)