Abstract
Fluorescence analysis at low-level light intensity is important and inevitable for flow cytometry and cell biology. The photomultiplier (PMT) has been used as a photon-detection device for many years because of its high sensitivity; it can amplify a single photoelectron to millions of electrons by a cascade of dynodes in a vacuum. In addition, the photocathode in the PMT has the advantage of a wide detection area and wide dynamic range through analog photocurrent detection. Recently, microelectromechanical system (MEMS)-based PMTs and many solid-state sensors such as Si photodiodes (PDs), avalanche photodiodes (APDs), and Si photomultipliers (SiPMs) have been developed and improved in UV to near-IR wavelengths. Advancements in photosensors especially for photon detection and potential applications are described in this chapter.
I therefore take the liberty of proposing for this hypothetical new atom, which is not light but plays an essential part of every process of radiation, the name Photon—Lewis [1]
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Single-Photon Detection and Photocurrent Detection
Since Max Planck suggested that “radiation is quantized as quanta” in 1900 and Albert Einstein proposed the quantum theory of light in Annus Mirabilis (Miracle Year) 1905, the properties of the light particle known as the photon have become well known and commonly understood in this 21st century [2]. From its beginnings in the 1950s, flow cytometry has developed into an increasingly powerful tool for cell-population analysis through detection of fluorescence photons from conjugated cell surfaces. On the other hand, for the past half-century the photon signal has been detected by an analog photocurrent signal from vacuum PMTs. Based on new understanding of detection systems, it is likely that the feasibility of single-photon detection by the latest photon sensors and electronics will contribute significantly to next-generation cellular analysis. That is the motivation and objective for this chapter.
Flow cytometry uses “molecules of equivalent soluble fluorochrome” (MESF), a concept formalized by 2004 [3], for sensitivity evaluation. MESF refers to a standard fluorochrome solution and is useful to compare various data obtained under similar detection configurations. But in order to study physical characteristics of photons, it is necessary to go back to the système international d’unités (SI units) to investigate as physical energy-packet characteristics.
Planck and Einstein defined the photon energy of an electric magnetic wave as proportional to frequency, with Planck’s constant as the proportionality constant:
where h is Planck’s constant (6.626 × 10−34 Js) and ν is the radiation frequency (1/s). The wave frequency is calculated as follows:
where c is the speed of light (3.0 × 108 m/s) and λ is the wavelength in meters. Combining (1) and (2),
where E is the photon energy in Joules (J). Expressing wavelength λ in nm and energy in eV, Eq. (3) is simplified as
where 1 eV = 1.60 × 10−19 J.
From Eq. (4), photon energy versus wavelength is calculated in Fig. 1. Values for typical laser wavelengths in flow cytometry are:
Owing to the very small energy per photon, the number of photons per picoW (pW = 10−12W) is a surprisingly large number, expressed as megacounts per second (Mcps):
In general, one pW is the lowest detection limit of a PMT photocurrent signal.
The photon pulse detected by a sensor is not equal to the incident photon number. The conversion coefficient from photon to detected photoelectron (PE) is called the quantum efficiency (QE). Depending on sensor efficiency and wavelength, the maximum QE lies in the range of 0.2 to ~0.9. Conversely, detection of one photon per second (if possible) is the ultimate in photo detection efficiency. One-photon energy is calculated as follows;
where aW is an attoWatt, equal to 10−18 W.
The calculated photon number as a function of optical energy is shown in Fig. 2.
In general, a photon sensor has thermal noise, known as dark count, in the range of 1 cps to ~1 Mcps. Dark count is sensitive to temperature and determines the detection limit. In addition, dark-count standard deviation per second (σ) and coefficient of variation CV(%) are considered as the resolution limit of light intensity. Temperature control of the sensor can improve dark count and its standard deviation.
Another aspect of photon detection is the internal gain of the sensor. A simple model to obtain a 1-mV pulse height across 50 Ω in 1 ns requires a gain >104. This means that a photodiode with a gain of 1 and an avalanche photodiode with a gain of ~100 have difficulty in obtaining sufficient signal amplitude as a photon detector. Both photodiodes have excellent linearity and dynamic range as photocurrent sensors.
2 Photon Counting, Spectrum Analysis, and Time Domain Analysis
Photon counting is the digital measurement of light intensity with extremely high sensitivity and linearity. If a detected photon pulse is an ideal impulse with pulse width “zero” and dark count “zero,” the photo detection system is ideal. Unfortunately, real photon pulses have finite pulse width and waveform. The upper count rate is determined by pulse width and the lower limit by dark-count rate [3]. Owing to pulse overlapping, the true count value N and measured value M are described as follows;
where t is the pulse pair resolution. The calculated M/N ratio in the case of t = 1 ns is described in Fig. 3. This graph shows a small error up to 10 Mcps and a gradually larger error for higher count rates. If necessary, it is possible to correct the measured value up to ~1 Gcps by this model. 1 Gcps is equal to 0.5/QE nW at 405 nm and 0.25/QE nW at 780 nm. The figure suggests that it might be possible to achieve over six decades of magnitude and linearity if low dark count were less than 1 kcps and pulse pair resolution 1 ns. But this is quite challenging because conventional photon pulse pair resolution is longer than 10 ns. In addition, the above model is based on a continuous pulse train. The actual measured signals have fluctuations that produce counting threshold deviation. This is an additional source of error. Nevertheless, the latest sensor improvement and ultrahigh-speed electronics have potential to achieve the target performance.
Dark count rate (DCR) is another key factor to determine sensitivity limit. In general, dark count is proportional to sensor area. A smaller photocathode or sensor active area usually reduces dark counts. A tenth of sensor area may reduce a tenth of dark count. This is attractive, but there are trade-off with optics design. Conventional flow cytometer optics for fluorescence detection have relatively large aberrations as well as a large spot image due to broad wavelength and high-NA collection lens without compensation. Reflective optics or in combination with optical fiber coupling may resolve this trade-off.
Sensor structure and material can also contribute significantly to dark counts. Dark-count origins include thermal noise in the sensor or photocathode. Materials with higher sensitivity in the IR region have higher dark-count characteristics. For example, comparing bialkali and multi-alkali photocathode materials for detection at extended longer wavelengths, multi-alkali shows a higher dark count.
Dark count and signal deviations can be reduced by temperature control. Peltier cooling is an effective solution to reduce dark count. For cooling purpose, a smaller sensor is easier to implement. If our target is to achieve a six-orders-of-magnitude dynamic range with a maximum 1 Gcps, the dark count must be less than 1 kcps.
Light intensity with theoretical linearity is measured by photon counting in the digital domain. The next question is how should we analyze the photon spectrum or photon energy. Hyperspectral analysis is a very important opportunity for cellular analysis. Photon spectroscopy can be implemented in two ways. One is in combination with a motorized monochromator and photon detector using a long capture time (>1 s). A recent motorized monochromator has a wavelength scanning speed of 500 nm/s. Because photon measurement for flow particles is in the ms to μs time domain, it is necessary to build a parallel photon-detection system. Technically, this is feasible, but cost and implementation as a system may be a hurdle.
Time-domain analysis by a sub-ns photon pulse is an interesting topic and could become an important frontier. Several applications are reported, such as TOF-PET for cancer diagnostics, single-molecule detection, various types of fluorescence resonance-energy transfer (FRET), DNA analysis, fluorescence correlation spectroscopy (FCS), photon imaging, and others.
These analyses are highly dependent upon photon-detection performance, optics, electronics, and software. “What kind of contribution single-photon detection might make to cytometry” is the key question for next-generation cellular analysis.
3 Advancement of Photon Detectors for Cytometry
Biological fluorescence analysis requires high sensitivity and wide dynamic range with linearity in visible wavelengths. Photon detection has ultimate sensitivity if dark count is low. The upper dynamic range of photon detection is mainly determined by maximum count capability per second. The challenges is to obtain the shortest photon pulse width and reduce dead time. A conventional photon sensor is limited by a maximum count rate in the range of 1 to 10 Mcps. Thus a photon pulse width with minimum dead time is the key sensor characteristics for cytometry application.
3.1 The Photo Multiplier Tube (PMT)
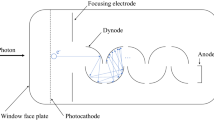
The discovery of the photon by Planck and Einstein in 1905 was based on the photoelectric effect. Elster and Geiger invented the photoelectric tube in 1913. Twenty years later, RCA laboratories commercialized the first PMT in 1936 [4]. Inevitably, the PMT became the choice of flow cytometry for fluorescence detection from the very beginning and has continued in use to this day. A new small PMT combined with Si-MEMS structure, the micro PMT, has been developed by Hamamatsu. Unlike a conventional PMT with metal dynode structure, the micro PMT dynode is made by a Si MEMS process, which accurately produces a small and thin structure. When a photon pulse from a micro PMT is amplified by a high-speed preamp, it is possible to obtain a shorter photon pulse of 4–5 ns (Fig. 4). The pulse waveform shows lower noise and distortion from smaller input capacitance owing to short distance and small area. In addition, a small photocathode area (1 × 3 mm) achieved very low dark-count rates at room temperature. According to the velocity distribution of cascade electrons, the photon pulse for the PMT shows a continuous PE level. The combination of low dark count and narrower pulse width can provide a photon detection system with a wider dynamic range. The drawbacks are the small photocathode area and the cost. In order to collect fluorescence light and couple to the sensor, the utilization of fiber- or aberration-free optics is advisable. PMT output is flexible to control gain and dynamic range for both photocurrent and photon modes. It is necessary to calibrate the detected value to the absolute power level for each measurement condition.
3.2 The Si Photomultiplier (SiPM)
Early development of solid-state single-photon detectors took place in the 1960s and early 70s at RCA and in Russia and Japan. The first single-photon avalanche diode (SPAD) was produced by Perkin-Elmer, and a solid-state Geiger-mode photomultiplier was developed by Rockwell in 1987 [4]. Since then, many developments and improvement have been made for a variety of applications, mainly in nuclear physics, astronomy, elementary particle physics, medical imaging, and the like.
APDs have been proposed for flow cytometry for some time [4, 5]. Recently an avalanche photo diode (APD) has been installed in a commercial compact flow cytometer instead of a PMT [6].
A solid-state photon detector has the advantage of small size, high quantum efficiency, lower bias voltage, light durability, insensitivity to magnetic fields, lower cost, and more, compared to a conventional PMT. In a PMT, an incident photon produces an electron-hole pair in the photocathode, which is an electrical insulator; vacuum and high voltage are mandatory for capture of the induced electron. On the other hand, the electron-hole pair of a solid-state sensor is produced in the p-n junction, which is semi-conductive material. The produced electron moves rapidly and the acceleration depends on the reverse bias electric field. There are three phases of operation: P-I-N mode with gain = 1, linear avalanche mode with gain ~100, and Geiger mode over break-down voltage with gain ~106 (see Fig. 5).
Geiger mode is highly sensitive for incident photons owing to a high QE ~0.8 and gain >106. Once a photon has hit, a quenching register is required to recharge electrons. Quenching time, typically 50 ns, is called dead time because the detector will not fire even if struck by a photon. In order to expand dynamic range, a Geiger-mode sensor has a structure arrayed as pixels (Fig. 6). SiPM photon detection efficiency PDE is defined as QE × εgeo × εtrig, where εgeo is the geometrical fill-factor and εtrig is the avalanche triggering probability. When a pixel is “fired,” a SiPM has secondary fire phenomena called “cross-talk,” at adjacent pixels, and “afterpulse,” a delayed signal in the fired pixel. Another issue is dark-count rate caused by thermal excitation and field-assisted excitation [5]. These drawbacks of SiPMs have been amended and SiPMs serve in several applications with very high time resolution on the order of picoseconds for TOF-PET, nuclear physics, and the like.
A typical photon pulse waveform and PE characteristics are shown in Fig. 7. Owing to the micron-length distance in the p-n junction, avalanche electrons are accelerated in a short path and the SiPM pulse-height distribution shows steps designated 0.5 PE and 1.5 PE. The dynamic range of a SiPM, typically three orders of magnitude, is limited by the number of pixels and the quench time. In order to be useful in biological applications, a wider dynamic range is clearly required.
The SiPM output waveform has a very fast avalanche process measured in picoseconds and a slow recharge process measured in nanoseconds. It is suggested that a highpass filter and signal differentiation may detect only the avalanche process. Experiments using an ultrahigh-speed differentiating circuit with GHz-bandwidth amp or equivalent differentiation in pixels show the feasibility of multiple photon detection even during quenching dead time. This is a significant breakthrough for wide-dynamic-range photon detection. This method combined with signal processing we have termed “differential Geiger mode.” Multiple photon detection during quenching is shown in Fig. 8.
4 Performance of Differential Geiger Mode and Preliminary Evaluation
Differential Geiger mode with signal processing is a new approach for photon detection by SiPMs. It is possible to expand the dynamic range of detection by multiple photon detection during quenching time. The current status of detection performance and a preliminary evaluation of basic material for flow cytometry are described.
4.1 Linearity Between Incident Light Intensity and Counting Rate
Sensing linearity is evaluated by a 10-mW 405-nm laser and optics as shown in Fig. 9. Laser power is adjusted using a rotational polarization beam splitter (PBS) and neutral density filters (ND2 & ND3), and coupled to a 0.6 mm—core optical fiber through a lens. The Q8230 (Advantest) optical power meter is calibrated to 405 nm with 0.01 nW resolution. The SiPM and power meter are connected to the optical fiber by an SMA connector to eliminate ambient light. The incident 405-nm light is adjusted by the power meter and the photon count measured. Measurement results show excellent linearity to 400 pW, after which the line curves as the system gradually becomes saturated. Photon counting sensitivity is described as count per pW. The 405-nm and 1-pW light includes 2.04 Mcps photons. The PDE of the tested sensor is 0.24 and the estimated photoelectron number is 489 kcps/pW. The measured rate, 358 kcps/pW, is reasonable considering the coupling efficiency (~75%) from the fiber to the 1 × 1-mm sensor area. In addition, the measured rate is confirmed to be constant within the operational range of the bias voltage. As shown in the figure, the maximum count rate is over 250 Mcps and the measured time pair resolution is 1.20 ns. In order to achieve a target of 1 Gcps and 1 ns, it is necessary but feasible to improve ultrahigh-speed signal processing by applying the latest and highest-bandwidth ICs.
4.2 Dark Count Rate by Cooling and Dynamic Range
Dark-count rate (DCR) is the lower limit of sensitivity and determines the dynamic range of photon counting. The main cause of high dark counts is thermal noise in p-n junctions. Thermal electrons are amplified in a similar manner to incident photons. Unlike photocurrent noise (thermal noise, shot noise, amp noise, and so on), the dark count can easily be subtracted from the evaluated count rate. Peltier cooling for SiPMs is very effective to reduce dark count. Figure 10 shows DCR temperature dependence of a 1-mm2 sensor. At temperatures higher than 25 °C, the DCR is over 100 kcps, but is reduced to 2 kcps at −10 °C. The approximate curve suggests that a target of 1 kcps would be possible at temperatures lower than −20 °C. Our experimental evidence showed that a DCR of 100 cps is confirmed by dry-ice cooling at −50 °C.
A counting range from 1 kcps to 1 Gcps means a six-orders-of-magnitude dynamic range with theoretical linearity in the digital environment. Further development is required, but current performance is very close to achieving the targeted function. DCR standard deviation and CV % are considered as the resolution of light intensity difference by sensor and statics. Dark count is sensitive to temperature change, so temperature control is certainly required for biological measurements for data accuracy, stability, and reproducibility. The measured value of DCR controlled at 4 °C is 5 kcps, the standard deviation per second, σ, is 10 to 50 cps, and the coefficient of variation is 0.2–1.0%, respectively.
4.3 Excited Fluorescence Evaluation by Photon Counting
Photon counting is the ultimate high-sensitivity approach for photon measurement. In the case of 10 kcps photons at 405 nm, the intensity is approximately 5 femtoW (fW). This is roughly 1000 times higher sensitivity than is obtained with a conventional photocurrent approach. It is very difficult to analyze the causes in photocurrent noise; photon counting can distinguish each cause and opens a new frontier in cellular analysis. In order to evaluate basic material in flow cytometry and biology, we have configured autofluorescence (AFL) evaluation optics as shown in Fig. 11. An excitation wavelength of 405 nm is the shortest wavelength in the visible region with excitation energy that provides a full visible spectrum longer than the laser wavelength. A laser spot 100 μm in diameter illuminates the sample behind a bandpass filter to remove stimulated spontaneous emission in the laser beam. Excited fluorescence photons are collected by an NA-0.125 lens and coupled to an NA-0.22 optical fiber through a 405-nm notch filter and a longpass filter to remove excitation photons. Detection occurs at 420–900 nm and count is given as total number of photoelectrons (PEs) without detection-efficiency correction. Assuming that excited fluorescence is emitting uniformly to any solid angle, the total number of emitted photons is estimated as 1000 times the measured PE number because of lens collection efficiency (1/250) and sensor PDE (1/4).
Excited autofluorescence intensity is basically proportional to illuminating power and sample thickness under fixed optics. In order to compare autofluorescence, an excitation coefficient k is defined as the detected PE number per μW illumination for a 1-mm sample thickness (PEcps/μWmm). Interestingly, quartz, glass, and many materials show AFL and photobleaching (Fig. 12). It is necessary to check the excitation coefficient before and after photobleaching. Photobleaching is difficult to observe with the noncoherent light source in a conventional fluorescence spectrometer. Lasers can provide very high illumination intensity, over 106 J/m2, which is not proportional to total illumination energy (J). Measurement is first dark count, system AFL, and dry vial for liquid, and finally the sample to calculate a count only for that sample.
As an example, an excitation coefficient of 1000 cps/μWmm means that the total number of emitted photons is estimated as 1 Mcps (1 k × 1 kcps) under measurement conditions. 1 Mcps photons is about 1 pW. Illumination at 405 nm/1 μW contains 2.04 Gigaphotons. This equals 1 Mcps/2 Gcps, ~1/2000; it takes 2000 illuminating photons to produce a single emitted photon. Using a measured excitation coefficient, it is possible to estimate the AFL from the illumination level. A material with k = 1000 cps/μW mm emits 1 k × 1 kcps = 1 Mcps, ~1 pW AFL under 1 mW illumination.
Several material-evaluation results are shown in Table 1. We have to recognize that every material for flow cytometry exhibits autofluorescence, even distilled water, sheath, or clean beads. Yellow-Green dye diluted in water for flow check in a cytometer has a count of 1.2 Mcps/μWmm, meaning roughly one emitted photon for every two excitation photons in the illuminated volume. In order to reduce the influence of AFL from the tube, a trapping method involving illumination along the coaxial direction has been developed for liquid-sample evaluation.
Many researchers may recognize the influence of autofluorescence at the time of measurement, but it is difficult to understand quantitatively. Photon counting can provide a method for order estimation. The influence of excited AFL depends on optics configuration. In-line detection to laser incident direction may be highly affected, but perpendicular fluorescence detection as in a conventional flow cytometer may be minimal. In the case of confocal fluorescence imaging or flow cytometry with longer gate period for small particles, optics AFL must be reduced for higher contrast and accurate detection. AFL reduction for optical glass and coatings is quite challenging, but it may be possible to improve after establishing an evaluation approach.
5 Conclusion and Discussion
We have developed a wide-dynamic-range Si photon-detection system, differential Geiger mode, and confirmed the potential performance for flow cytometry and cellular analysis. Photon counting with sensitivity over three orders higher than that of conventional photocurrent detection has made clear that optical and biological materials are the limit of background noise such as autofluorescence. The next step is single-photon spectrum analysis for identifying the origins of autofluorescence and for the highest sensitivity analysis on biological assays. Photon counting is simply intensity analysis with time deviation. In addition, single-photon detection with picosecond time resolution could open new frontiers for live single-cell and population analysis in flow cytometry and cellar analysis.
References
Lewis GN (1926) Letters to the editor; conservation of photons. Nature 118:874
Okun LB (2006) Formulae E=MC2 in the year of physics. Acta Physica Polonica B 37:1127–1132
Schwartz A, Gaigalas AK, Wang L, Marti GE, Vogt RF, Fernandez-Repollet E (2004) Formalization of the MESF unit of fluorescence intensity. Cytometry B Clin Cytom 57:1–6
Shutao Z, Xiaodong W, Yongqin C, Ce W, Tang Y (2011) High gain avalanche photodiode (APD) arrays in flow cytometer opitical system. In: 2011 international conference on multimedia technology
Lawrence WG, Varadi G, Entine G, Podniesinski E, Wallace PK (2008) Enhanced red and near infrared detection in flow cytometry using avalanche photodiodes. Cytom Part A: J Intl Soc Anal Cytol 73:767–776
Tung J, Condello D, Donnenberg AD, Duggan E, Lemus JA, Nolan J, Ragheb K, Sturgis J, Robinson JP, Jing Z, Fenoglio D, Huang Y Scibelli P(2015) CytoFLEX system performance evaluation. In: CYTO2015. Wiley, Liss, Glasgow
Acknowledgements
I would like to acknowledge the substantial contributions of Keegan Hernandez on electronics and software development, Kathy Ragheb, Jennifer Sturgis, and Eva Biela on biological samples, Gretchen Lawler on chapter editing, and J. Paul Robinson on the opportunity and support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yamamoto, M. (2017). Photon Detection: Current Status. In: Robinson, J., Cossarizza, A. (eds) Single Cell Analysis. Series in BioEngineering. Springer, Singapore. https://doi.org/10.1007/978-981-10-4499-1_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-4499-1_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4498-4
Online ISBN: 978-981-10-4499-1
eBook Packages: EngineeringEngineering (R0)