Abstract
Graphene oxide is single or few atomic layers of graphene attached to oxygen-containing groups. It is a flexible material and is prepared by the energetic oxidation of graphite. Graphene oxide has a lot of potential due to easy synthesis, cost-effectiveness and scope for mass scale production. It is one of the important materials for the futuristic memory devices. The band gap of graphene oxide can be easily tuned by varying the oxidation level. It is ideal as an electrical insulator as well as semiconductor, when it is fully oxidized and partially oxidized respectively. Graphene oxide is produced by the oxidation of graphite followed by exfoliation of oxidized graphite. Various methods for the synthesis of graphene oxide are discussed in this chapter. The reduction of graphene oxide to produce reduced graphene oxide is extremely important. The process used for reduction has a large impact on the quality of the reduced graphene oxide produced and therefore will determine how close reduced graphene oxide will come, in terms of structure and properties, to pristine graphene. For the industrial applications, there is a need to utilize large quantities of graphene, and reduced graphene oxide is the most obvious solution due to the relative ease in creating sufficient quantities of graphene to desired quality levels. Although, there are many reports on the reduction of graphene oxide available in the literature, the complete removal of oxygen-containing groups is still a challenge. This chapter presents a review of the research work reported on the synthesis and characterization of graphene oxide.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1.1 Introduction
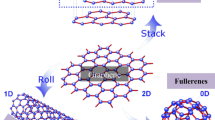
Carbon is one of the most vital elements found on earth. The word carbon is taken from the Latin word carbo, which means charcoal. Carbon is capable of forming many allotropes due to its valency. Well-known crystalline forms of carbon are diamond and graphite. On the other hand, amorphous forms of carbon include coal, soot and carbide-derived carbons. However, these materials are not amorphous in true crystallographic sense; rather, they are polycrystalline, consisting of combination of graphitic and diamond crystals. With the development of science and technology at small scale, known as nanotechnology, many more allotropes and forms of carbon have been discovered, which includes buckminsterfullerene, carbon nanotubes and graphene (Fig. 1.1).
Buckminsterfullerenes are cage-shaped molecules of carbon with molecular formula C60, which were discovered in 1985 by a group of scientists from Rice University and University of Sussex, and three of them got 1985 Chemistry Nobel Prize. Its shape resembles a football made up of 20 hexagons and 12 pentagons with a carbon atom at each vertex of each polygon and a bond along each polygon edge [1]. The name of buckminsterfullerene is in reference to Buckminster Fuller, a US architect, because the structure of C-60 resembled the geodesic dome invented by him. Buckminsterfullerene can withstand very high amounts of heat and pressure. After that, fullerenes having higher number of atoms, open-cage fullerenes, quasifullerenes were prepared, isolated and investigated [2]. Buckminsterfullerene, higher-order fullerenes and other derivatives of fullerene family found their applications in the field of medical and biological sciences [3] and photovoltaics [4] etc. The discovery of fullerenes triggered the scientists towards the preparation and isolation of other allotropes of carbon.
In 1991, a Japanese electron microscopist, Sumio Iijima, discovered a new form of carbon resembling a tube, called carbon nanotube. These are long hollow structures with their walls made up of single atomic layer of sp2-hybridized carbon [5]. According to the number of rolled atomic layers of carbon, carbon nanotubes can be categorized into multiwalled and single-walled carbon nanotubes. Single-walled carbon nanotube has only one rolled atomic layer of carbon, while multiwalled carbon nanotubes have two or more atomic layers of carbon rolled along single axis forming the concentric cylinders. According to the manner in which the carbon atomic layer/layers is/are rolled, carbon nanotubes can be categorized into armchair, zigzag and chiral. Normally, armchair nanotubes are metallic, while chiral and zigzag nanotubes are semiconducting in nature. Carbon nanotubes possess extraordinary physical properties [6, 7]. These are the stiffest and strongest material, very good conductor of heat and electricity with various other interesting properties. Since their discovery, carbon nanotubes have been employed into various applications such as electronic, optoelectronic, mechanical and medical applications due to their extraordinary properties [8].
Graphene is a single atomic layer of carbon which exists in the form of Van der walls stacked sheets in graphite [9] and in the rolled form in carbon nanotubes [10]. Theoretically, it was predicted long back that the stacked atomic layers of graphite can be isolated. It has likely been unknowingly produced in small quantities for centuries, through the use of pencils and other similar applications of graphite. It was actually observed in electron microscopes in 1962, but only studied while supported on metal surfaces [11]. The material was later rediscovered, isolated and characterized in 2004 by Andre Geim and Konstantin Novoselov at the University of Manchester [12, 13]. Since its discovery, graphene has proved to be one of the most promising materials due to its extraordinary properties. It is finest, strongest and stiffest material as well as a wonderful conductor of electricity and heat. With these excellent properties, graphene is being used as the building block for different applications in electronics and material science [13,14,15,16], electrochemistry [17], sensor technology [18, 19], biological engineering [20, 21], filtration [21, 22] and energy storage [23,24,25], etc.
Although graphene has a lot of interesting properties and applications, there are some drawbacks associated with it. The physical methods to synthesize graphene are so complex that its mass scale production is very difficult. On the other hand, graphene is a semimetal and is not suitable for semiconducting applications. Due to these limitations, researchers shifted their attention towards graphene oxide, which is an oxygenated derivative of graphene. These oxygenated graphene sheets have the same structure as graphene except oxygen-containing functional groups on their basal planes and edges (Fig. 1.2). Graphene oxide (GO) can be easily dispersed in water and is easy to process. It can be produced in large quantities easily. Most interestingly, it can be reduced (partly) to form reduced graphene oxide (rGO). The properties and behaviour of reduced graphene oxide depend on the oxygen content in it. The increasing degree of reduction decreases the oxygen content, which induces the properties and behaviour of rGO to shift towards graphene.
Structure of graphene oxide (Lerf-Klinowski model) (reprinted with permission from [11])
As-prepared and reduced graphene oxide can be extensively used in a variety of applications, especially for the mass scale production of graphene. Graphene oxide (GO) films can be deposited on any substrate and can be converted into a conductor (via reduction). This makes it suitable for the use in the production of transparent conductive films, flexible electronics, solar cells and chemical sensors [26,27,28,29]. Graphene oxide has a high surface area and can be suitable for the use as electrode material for batteries and capacitors [29,30,31]. GO can easily be mixed with different polymers and other materials to enhance their properties like tensile strength, elasticity and conductivity of composite materials [32]. In solid form, graphene oxide flakes are attached to one another to form thin and stable flat structures that can be folded, wrinkled and stretched. Such graphene oxide structures can be used for applications in hydrogen storage, ion conductors and nanofiltration membranes [14, 33]. Graphene oxide is a fluorescent material, which makes it appropriate for various medical applications. Biosensing and disease detection, drug carriers and antibacterial materials are just some of the possibilities GO holds for the biomedical applications [34, 35].
1.2 Synthesis of Graphene Oxide/Reduced Graphene Oxide
As mentioned earlier, graphite consists of several graphene layers stacked by weak van der Waals forces. Graphite oxide has more or less same structure of graphite with a little difference. Graphite can be oxidized using strong oxidizing agents to change hybridization of carbon atoms and attach oxygen. This transition not only increases the interlayer spacing of graphite, but it also enhances the hydrophilic nature of the system. Due to this property, GO can be dispersed more easily in aqueous or other polar solvents to get isolated graphene oxide layers. Various reduction strategies can be used to get reduced graphene oxide (rGO) sheets having oxygen content specifically required for desired applications (Fig. 1.3).
Schematic showing the chemical route for the synthesis of aqueous graphene dispersions. 1 Oxidation of graphite (black blocks) to graphite oxide (lighter coloured blocks) with greater interlayer distance. 2 Exfoliation of graphite oxide. 3 Reduction of graphene oxide [16]
1.2.1 Oxidation of Graphite
Oxidation of graphite to get graphite oxide is the first step towards the synthesis of graphene oxide. The oxidation methods utilize strong oxidation agents such as potassium chloride and potassium permanganate in the strong acidic medium. The main methods for the synthesis of graphite oxide include Broodie’s method, Hummer’s method and Tour’s method. Among these three methods, Hummer’s method is the most popular one, which is being widely used by the researchers worldwide with or without modifications.
1.2.1.1 Broodie’s Method
The very first report on the ability of graphite to get oxidized using oxidizing agents and strong acids was published by a British chemist B.C. Brodie in 1859 [36]. In this report, a mixture of KClO3 as the oxidizing agent in the presence of fuming nitric acid was used to prepare graphene oxide (GO). The investigation of chemical composition of resulting product suggested the presence of carbon, oxygen and hydrogen. The resulting product was washed to make it salt-free and dried at 100 °C. Further, the oxidized batch was kept in oxidizing environment using three sequential steps to obtain a blackish yellow material which did not change with additional oxidation. After elemental analysis, the chemical composition of the resulting material came out to be C11H4O5. Although an effort was made to analyse the chemical composition, structural properties and thermal decomposition process of oxidized graphite, these analyses were limited by the available characterization techniques at that time, leaving room for further improvement in the process. In 1898, Staudenmaier [37, 38] proposed an improvement over the method introduced by Broodie’s et al. [36] by adding H2SO4 to increase the acidic nature of the reaction medium. He further added multiple aliquots of potassium chlorate solution into the mixture several times during the reaction. These improvements enhanced the oxidation process of graphite in the single step and ruled out the need for repeated oxidation steps. In 1937, Hofmann et al. [39] reported further improvement in the process by using non-fuming nitric acid. This method provided an enhancement in the oxidation process of graphene oxide. However, these methods have several shortcomings such as the danger of explosion due to the evolution to chlorine dioxide [40].
1.2.1.2 Hummer’s Method
The method proposed by Hummer et al. [41] has been popularly used by the researchers worldwide with and without modifications. In their report, they used a mixture of sulphuric acid (H2SO4), sodium nitrate (NaNO3) and potassium permanganate (KMnO4) to oxidize graphite at a temperature of 20 °C [41]. KMO4 is a commonly used strong oxidizing agent. In Hummer’s method, dimanganese heptoxide (Mn2O7), formed by the reaction of KMnO4 with H2SO4, acts as the active species [42]. It is more reactive than KMO4 and acts as a strong oxidizing agent [43, 44]. The original Hummer’s process lasted for 2 h, and the resulting graphite oxide had a higher degree of oxidation than that of Staudenmaier’s method [45]. It was found that the Hummer’s method provided oxidation of the outer shell of graphite, leaving the graphite core unoxidized. Hummer’s method can be improved by the pre-treatment of graphite with a mixture of H2SO4, P2O8 and K2S2O8 [46] prior to the employment of Hummer’s method or by taking small-sized graphite flakes. Other commonly used modified Hummer’s methods include the use of increased amount of KMnO4 [46]. Hummer’s methods involve several purification and filtration steps, which make them quite lengthy and complicated. Despite this fact, the Hummer’s method and its modified forms remain to be the most popular method used for the oxidation of graphite.
1.2.1.3 Tour’s Method
The development of carbon nanotechnology inspired the researchers to discover new and improved methods to synthesize new carbon nanomaterials. Several methods for the synthesis of graphite oxide have also been proposed. Graphene oxide is considered as an intermediate material formed during the facile synthesis of graphene. Most of these methods may be regarded as the modified forms of Hummer’s methods. Some modifications in Broodie’s and Staudenmaier’s methods also came into the light. Among different methods reported in the first decade of twenty-first century, the method proposed by Tour et al. [47] made several modification and improvements over the earlier methods and it was established as the new method. In their method reported in 2010, Tour et al. [47] avoided the use of NaNO3 and introduced H3PO4 in the method originally proposed by Hummer’s et al. Their method provided graphite oxide having an improved degree of oxidation. Other features of their protocol are the absence of toxic gases such as NO2 and N2O4, more intact sp2 hybridized graphitic plane [47] and improved yield.
1.2.2 Exfoliation of Graphene Oxide
1.2.2.1 Chemical Exfoliation of Graphene Oxide
The oxidation of graphite increases the interlayer spacing between the atomic layers. The increase in the interplanner spacing decreases the degree of staking between the oxidized graphene sheets. Graphene oxide dispersions can be achieved by dissolving graphite oxide in aqueous and organic solvents using simple sonication [48–52]. Due to the hydrophilic nature of graphene oxide, stable dispersions with concentration up to 3 mg/ml can be achieved. The colour of these dispersions has been reported between yellowish brown to dark brown. Modification of graphene oxide sheets with organic molecules is also reported to be helpful to achieve stable graphene oxide suspension in polar and non-polar organic solvents [50]. In the repost published by Stankovich et al. [50], a dispersion of 1 mg/ml was achieved by treating GO with isocyanate followed by dissolution in organic aprotic solvent. The treatment of GO with organic isocyanates can lead to the derivatization of both the edge carboxyl and surface hydroxyl functional groups via formation of amides or carbamate esters, respectively (Fig. 1.4). These amide and carbamate functionalities can be used for covalent linking of variety of materials with GO [50]. Different dispersing agents used for preparing stable graphene oxide dispersions are presented in Table 1.1.
Formation of amide and carbamate functionalities on treating graphite oxide with isocyanates (reprinted with the permission from [50])
Although, ultrasonication in organic polar or aqueous medium has been proven to be the faster and more efficient way to exfoliate graphite oxide to graphene oxide sheets, it is also reported that ultrasonic method also induces structural damages and breaks the graphene oxide sheets [48]. As an alternative approach, graphite oxide can be exfoliated thermally via rapid heating of the graphite oxide powder.
1.2.2.2 Thermal Exfoliation of Graphene Oxide
When graphite oxide is put at high temperature, its exfoliation occurred due to the generation of high pressure between the layers of graphite oxide. This high pressure is the consequence of the conversion of carboxylic and other oxygen-containing groups into CO2 and CO [66, 67]. Akhawan et al. [67] put the alumina boat filled with graphite oxide in the pre-heated tube furnace at 1050 °C. They kept the boat till 30 s and then rapidly moved the boat out of the furnace to get graphene oxide sheets.
1.2.3 Reduction of Graphene Oxide
The most attractive property of graphene oxide is its partial reduction, which can convert it into reduced graphene oxide (rGO), having properties similar to that of pristine graphene. The shift in the properties of reduced graphene oxide towards graphene depends upon the degree of reduction. The reduction of graphene oxide is achieved by the removal of oxygen-containing groups. Various efforts have been made to reduce graphene oxide completely into graphene, but this ultimate goal is still a dream.
1.2.3.1 Thermal Annealing
Graphene oxide can be reduced by annealing at the temperatures greater than 1200 °C. In fact, thermal annealing was initially used to exfoliate graphite oxide [66, 67]. The exfoliation of graphene oxide occurs due to the generation of CO and CO2 by the thermal decomposition of oxygen-containing groups. In this way, thermal annealing not only exfoliates the graphite oxide into graphene oxide, but also removes the oxygen-containing groups from the graphite oxide and reduces it into reduced graphene oxide [66–68]. However, thermal annealing procedure creates lattice defects and breakage in the graphene sheets. This is due to the fact that the decomposition of carboxylic groups in the reduction process utilizes the carbon atoms of the graphene lattice. These lattice defects and breakages badly affect the conductivity of the resulting reduced graphene oxide sheets. The reported conductivity of the resulting reduced graphene oxide (rGO) is 10–23 S/cm, which is several orders lower than the pristine graphene [68, 69] (Fig. 1.5).
Pseudo-3D representation of a 600 nm × 600 nm AFM scan of an individual graphene sheet showing the wrinkled and rough structure of the surface, and an atomistic model of the graphite oxide to graphene transition (reprinted with permission from [68])
The most popular way to exfoliate graphite oxide into graphene oxide is sonication in variety of aqueous or organic solvents. In this way, large-area graphene oxide sheets can be deposited and subsequently reduced using thermal heating [70]. The reduction of pre-exfoliated graphene oxide depends upon the annealing temperature and the annealing atmosphere. At high temperatures, the degree of reduction increases, but increased chances of reaction of oxygen present in the atmosphere compensate the loss of oxygen achieved via thermal annealing. Therefore, vacuum or inert atmosphere is required for high degree of reduction. Sometimes a reducing gas such as hydrogen or ammonia is used to create reducing atmosphere to enhance the reducibility [68]. Youn et al. analysed the effect of hydrazine vapours to create the reducing atmosphere for efficient thermal reduction of graphene oxide [71].
1.2.3.2 Reduction Using High-Energy Radiations
High-energy radiations such as microwave [72, 73], gamma radiation [74], camera flash radiation [75, 76] and UV radiation [77, 78] may be used to reduce graphene oxide. Microwave radiation heats GO more rapidly than conventional heating. The time taken for the reduction of GO under microwave radiation is reported to be less than 1 min [72] (Fig. 1.6).
Optical photographs of GO before (a) and after (b) treatment in a microwave oven for 1 min. c Typical SEM image of as-prepared rGO by microwave irradiation with a high-magnification SEM image in the inset showing the crumpled rGO sheets. d Typical TEM image of the rGO and the corresponding electron diffraction pattern. e XPS C 1s spectra of GO and rGO (reprinted with permission from [72])
Cote et al. [75] reduced graphene oxide using camera flash. Camera flash induces rapid heating which reduces graphene oxide instantaneously. Matsumoto et al. [77] reported the reduction of graphene oxide using UV radiation in the mild atmosphere of H2 and N2. The UV radiation destroys the epoxy groups attached to the basic graphene lattice to convert graphene oxide into reduced graphene oxide. Laser radiation also reduces graphene oxide very efficiently. While the photo- and UV radiations need prolonged interaction with graphene oxide to convert it into reduced graphene oxide, laser radiation reduces graphene oxide ultimately fast [78]. Gamma radiation may also be used to reduce graphene oxide in alcohol/water [79]. Gamma radiation converts water or alcohol molecules into the highly reactive reductive species, which deoxidize the graphene oxide and convert it into reduced graphene oxide [79].
1.2.3.3 Chemical Reduction of Graphene Oxide
Chemical reduction is the most popular and one of the earliest reported methods to reduce graphene oxide. Hydrazine is one of the very first chemical reagents to be used to reduce graphene oxide in aqueous solution. Unlike other common strong reducing agents, hydrazine does not react with water; this property makes it most popular reducing agents for graphene oxide dispersed in aqueous solution [80]. Although the reaction mechanism of reduction of graphene oxide via hydrazine is not well supported, the proposed mechanism is presented in Scheme 1.1 [80].
rGO obtained after reduction is often aggregated due to increased hydrophobicity as a result of oxygen-containing groups [81]. Tung et al. [59] used pure hydrazine to reduce graphene oxide to get highly dispersed monolayers of graphene oxide.
Other nitrogen-containing compounds such as hydroxylamine [82], pyrrole [83], benzylamine [84], 5 p-phenylenediamine [85], ethylenediamine [86], urea [87], dimethyl ketoxime [88], hexamethylenetetramine [89], polyelectrolyte [90] and poly(amidoamine) [91] were used as the reducing agent to reduce graphene oxide.
Hydrohalic acids such as HI, HBr and HCl are used in electrophilic addition and nucleophilic substitution in synthetic chemistry. In addition to this, their capability of opening epoxides makes them good candidates for the reduction of graphene oxide [81]. Moon et al. [92] used the mixture of hydroiodic acid and acetic acid for the liquid-phase as well as solid-phase reduction of graphene oxide. They further tested the mixture of hydroiodic acid and trifluoroacetic acid and found that the latter reduces the graphene oxide more effectively due to strong acidic nature of trifluoroacetic acid. The more interesting point with the latter mixture is that it can reduce GO at sub-zero temperature [92, 93]. Pure hydroiodic acid [94] and hydrobromic [95] acid were also used to reduce graphene oxide. The conductivity of reduced graphene oxide obtained by the use of hydrobromic acid was low, which was attributed to the presence of residual bromides [95].
Metal hydrides such as sodium hydride, sodium borohydride and lithium aluminium hydride are considered as the strong reducing agents. Sodium borohydride (NaBH4) was used, which was proved as better reducing agent than hydrazine [96]. NaBH4 effectively reduces C=O species, but it shows less reducibility for epoxy and alcoholic groups [97]. An additional treatment with sulphuric acid, after the NaBH4 treatment, improves the reduction of graphene oxide [98].
Oxygen-containing compounds, such as common alcohols, l-ascorbic acid and hydroquinone, have also been used to reduce graphene oxide [96]. Su et al. demonstrated that the graphene oxide reduced by high-temperature alcohol vapours has excellent graphitic structure [99]. Keeping in view of good antioxidant property of l-ascorbic acid, commonly known as vitamin C, some groups used l-ascorbic acid as the reducing agent for graphene oxide and produced good quality rGO [100, 101].
Sulphur-containing compounds such as thiourea dioxide, ethanethiol–aluminium chloride, Lawessons’s reagent and thiourea [81] have also been used to reduce graphene oxide. Thiourea dioxide is a common reducing agent used in the paper and textile industry. It is used to reduce ketones, aromatic nitro-azo, azoxy and organosulphur compounds. It was used with NaOH [102] and with NH3 [103] by different groups for the reduction of GO.
Different metals in acid as well as alkaline environment have the potential to be used for the reduction of graphene oxide. Aluminium [104, 105], iron [106], zinc [107], tin [108] and magnesium [109] metals were used in HCl medium, while zinc was used with H2SO4 [110] to reduce graphene oxide. Some alkaline metal combinations such as Zn + NH3 [111], Zn + NaOH [105, 112], Al + NaOH [105] and Na + NH3 [113] were tried and successfully proven to act as the reducing agents for graphene oxide.
Most of the chemical reagents discussed so far for the reduction of GO are harmful and toxic in nature. Keeping in view of this, some researchers used natural and biocompatible materials for the reduction of GO. The use of these materials not only avoids the use of harmful and toxic materials at the processing stage, but also opens a way to the production of biocompatible rGO, which is free from metal and other toxic residuals. Amino acids are biologically important organic compounds. They contain amine and carboxyl functional groups along with a side chain. Chen et al. [114] used l-cysteine for the reduction of graphene oxide. rGO obtained in their study showed less conductivity. Further, they used glycin, l-lysine and l-glutathione to reduce graphene oxide [102,115,117]. The ongoing search of environment-friendly and green chemical reagents showed a new way to the researchers to utilize plant extract as the reducing agents. Suresh et al. used clove extract for the reduction of GO at 100 °C for 30 min [118]. Hatamie et al. [119] used curcumin extract to reduce chemically exfoliated graphene oxide sheets. Other plant extracts such as grape extract [120] and pomegranate juice were also used as the reducing agents for graphene oxide. Some micro-organisms, proteins and hormones have also been employed as the reducing agents for GO. Shewanella, a group of heterotrophic and facultative anaerobes, usually found in the environment, have the ability to utilize inorganic or organic compounds in their respiratory pathway as terminal electron acceptors. These organisms are also able to utilize solids as terminal electron acceptors. Salas et al. [121] highlighted the usage of Shewanella in a strictly anaerobic environment towards the reduction of graphene oxide. In other works, E. coli [122] and baker’s yeast [123] were also used to reduce graphene oxide. Bacteriorhodopsin is a protein, which captures the light energy and utilizes it for the movement of protons across the membrane. Akhawan [124] used this property and utilized it as the reducing agent for graphene oxide. Melatonin (N-acetyl-5-methoxytryptamine) is a hormone found in animals and other living organisms. It has an electron-donating property, which makes it an excellent antioxidant. Akhawan et al. [125] employed melatonin to reduce GO in the presence of NH3. They proposed a series of reaction mechanisms to understand the reduction to epoxide and hydroxyl present in GO by melatonin, as given in Scheme 1.2.
Proposed mechanisms for the reduction of epoxide and hydroxyl by melatonin (reproduced with permission from [81])
1.3 Characterizations of Graphene Oxide
Graphene oxide is single or few atomic thick layers of carbon atoms attached to different groups such as epoxy, hydroxyl, carbonyl and carboxyl. The exact structure of graphene oxide is still a matter of debate. The nature, quantity and distribution of different groups present in graphene oxide depend upon the method of synthesis, whereas the reduction depends on the method and the nature of reducing agent.
Solid-state nuclear magnetic resonance (NMR) is a good technique to analyse the structure of materials. 13C and 1H NMR spectra of graphene oxide exhibit three major peaks at 60, 70 and 130 pm [126]. In some studies, four peaks were found at 57.6, 69.2, 92.9 and 166.3 ppm [126]. In high resolution, 13C NMR spectra of graphene oxide also feature three minor peaks at 101, 167 and 191 ppm [52, 127]. Different peaks observed in 13C NMR spectra of graphene oxide and their respective assignments are summarized in Table 1.2. Figure 1.7 presents the typical 13C NMR spectra for GO.
13C NMR spectra of Go (reprinted with permission from [101])
X-ray photoelectron spectroscopy (XPS) reveals the information regarding the chemical environment of carbon atoms. Therefore, it is a good technique to investigate the structure of GO and understand the degree of oxidation and reduction of graphene oxide. These features can be investigated by comparing the XPS signals of pristine graphite and graphene oxide at different oxidation and reduction stages [132]. For fully oxidized graphene oxide, five different components are observed in C 1s spectra and their respective origins are given in Table 1.3.
Some groups reported only four chemically shifted peaks in the XPS spectrum of graphene oxide. They did not find the shifted peak at 287.5 eV, which is assigned to carbonyl group [133]. As the complementary to C 1s spectra, O 1s spectra gives the additional information of groups present in the graphene oxide. In O 1s spectra, three chemically shifted peaks are observed at 531.08, 532.03 and 533.42 eV and these peaks can be assigned to C=O [133, 138], C–O and phenolic groups [61, 139]. A typical XPS spectrum is provided in Fig. 1.8.
XPS spectra for graphene oxide films after 200, 500 and 1000 °C treatment in flowing argon: a comparison of C 1s peaks, b comparison of O 1s peaks (reprinted with permission from [131])
X-ray photoelectron spectroscopy can be effectively used to determine the degree of reduction of graphene oxide. This can be done by analysing the fraction of carbon sp2-bonded carbon atoms. The fraction of sp2 carbon is evaluated by dividing the area under sp2 carbon peak by the area under entire C 1s spectrum graphene oxide. The increase in this fraction depicts the increased degree of reduction [61, 133].
Fourier transform infrared (FTIR) spectroscopy can also be used to determine the functional groups present in graphene oxide. The FTIR spectrum of graphene oxide [138] may be composed of various peaks which can be assigned to different functional groups present in graphene oxide. The assignments of these peaks to the functional groups are presented in Table 1.4 (Fig. 1.9).
FTIR absorbance spectra of single-layer GO. After annealing at 423 K and referenced to the bare oxidized silicon substrate, the spectrum (1) shows hydroxyls (broad peak at 3050–3800 cm−1), carbonyls (1750–1850 cm−1), carboxyls (1650–1750 cm−1), C¼C (1500–1600 cm−1) and ethers and/or epoxides (1000–1280 cm−1). The differential spectrum after annealing to 448 K (reprinted with permission from [134])
At different levels of reduction, the peak positions associated with different groups diminish, some new peaks arise, and some peaks remain unaffected. This pattern depends upon the method of reduction. To understand the process of reduction, FTIR spectrum at different levels of reduction needs detailed analysis [134].
Raman spectroscopy is an excellent technique to study the carbonaceous materials. In case of graphene oxide, three different peaks are observed at 1350, 1580 and 2680 cm−1, which correspond to G band, D band and 2D band of carbonaceous materials. G band is the characteristic of graphitic (sp2) carbon, D band arises due to defects and the presence of different functional groups, and 2D band, which is an overtone of D band, is the measure of the number of graphene layers [140, 141]. The ratio of intensity of D peak and that of G peak (I D/I G) is a good measure of degree of oxidation or reduction. Increasing degree of oxidation increases the value of I D/I G, while increasing degree of reduction decreases the value of I D/I G. Reduction in the intensity of 2D band can be attributed to the breaking of stacking order associated with oxidation reaction [142–144] (Fig. 1.10).
Raman spectra of pristine and thermally reduced graphene oxide showing D, G and 2D (inset) bands (reprinted with permission from [134])
Transmission electron microscopy (TEM) is a good technique to study the microstructure of GO. It enables direct imaging of GO lattice and defects [145–147]. GO monolayer is highly transparent, and the samples with different oxidation levels show different transparencies [142] (Fig. 1.11). Increasing oxidation levels increase the transparency. High-resolution transmission electron microscopy (HRTEM) is capable of imaging honeycomb lattice of GO. In HRTEM image of GO, three different regions may be clearly visible. These regions may be termed as holes, graphitic and disordered regions. Holes are created by the release of CO and CO2 during the oxidation, while graphitic region is the region with less effect of oxidation. In the disordered region, there is a large quantity of oxygen functionalities attached to honeycomb lattice [146]. Selected-area electron diffraction (SAED) pattern shows clear diffraction spots which depict that the original graphene crystal lattice is preserved in GO [142] (Fig. 1.11).
TEM images of graphene oxide at increasing level of oxidation (S-1 to S-6) (reprinted with permission from [142])
Atomic force microscopy (AFM) is employed to investigate the thickness and lateral morphology of graphene oxide sheets. For AFM investigation, coating of graphene oxide on flat surface (usually silicon) is needed. The thickness of single or multiple sheets can be obtained either by taking a simple line scan going across the sheets and substrate or by obtaining the histogram of the area of interest in the image [148] (Fig. 1.12). Typically, the thickness of graphene oxide sheet is about 1–1.3 nm which is more than that of graphene. The reason behind the increased thickness of graphene oxide is the presence of different groups attached to the graphene lattice [148, 149].
a AFM image of graphene oxide. b Height profile of AFM image [40]
UV–visible spectroscopy is an analytical technique which is used in analytical chemistry for quantitative determination of different analytes [150]. In the case of graphene oxide, the UV–visible spectrum consists of a couple of peaks around 233 nm due to π–π* transition of C=C bond. A broad band between 290 and 300 nm is also evident in UV–visible spectrum of graphene oxide. This band can be attributed to π–π* transition of C=O band [151] (Fig. 1.13). Paredes et al. [51] recorded UV–visible spectra of graphene oxide dissolved in different organic solvents. They also observed a peak and a shoulder at 231 and 300 nm, respectively. On reduction, the characteristics peak of graphene oxide around 230 nm gets red-shifted. Li and Kaner [19] reduced graphene oxide using hydrazine and observed that UV–visible peak initially located around 230 nm was red-shifted on reduction. The shift in peak position increases with increase in reaction time. The intensity of peak located around 230 nm can be used to estimate the number of layers in graphene oxide. In their report, Lai et al. [152] showed that the intensity of peak located at 230 nm in UV–visible spectrum of graphene oxide decreases with the increase in number of layers.
UV–visible spectra of GO solution (reprinted with permission from [151])
X-ray diffraction spectrum of graphene oxide shows a broad peak around 2θ = 10° due to the interlayer distance of GO which is between 5.97 and 9.5 Å (Fig. 1.14). The XRD peak position and intensity are affected by oxidation, reduction, humidity and fractionalization [47, 153]. On reduction, the removal of oxygen-containing groups reduces the interlayer distance of graphene oxide, which, in turn, shifts the peak position towards higher 2θ values. Zhang et al. [154] reduced graphene oxide using l-ascorbic acid. They observed that the XRD peak position shifted from 2θ = 11.1° to 2θ = 24° which corresponded to interlayer spacing of 7.45 and 3.7 Å. The observed interlayer spacing of reduced graphene oxide, i.e. 3.7 Å, is very much close to the interlayer spacing of graphite (002) plane (~3.35 Å). Krishnamoorthy et al. [142] studied various graphene oxide samples having different levels of oxidation. They found that the XRD peak position shifts from higher values of 2θ to lower values of 2θ with decreasing degree of oxidation.
XRD spectrum of GO (reprinted with permission from [155])
1.4 Conclusion
Graphene oxide (GO), is very promising material due to its easy synthesis and extraordinary properties. Although the structure of graphene oxide depends upon the methods of preparation, it is an insulator in its unreduced form. On reduction, the oxygen-containing groups of graphene oxide are removed and it starts to behave as a semiconductor. Further reduction improves the conductivity of graphene oxide and it shifts towards graphene, which is a semimetal. Facile synthesis and wide range of reduction recipes make graphene oxide a cost effective replacement of pristine graphene. Although there are various reduction recipes used by the researchers worldwide, no one has claimed to produce pure graphene from graphene oxide. Use of different chemicals and reagents induces different types of impurities, which affect the properties of graphene oxide. Therefore, the complete reduction of GO is still an issue to be addressed. The mass scale production of graphene can be realized only if the complete reduction of GO becomes possible.
References
A. Hirsch, M. Brettreich, Fullerenes—Chemistry and Reactions (Wiley, New York, 2004). H. Yang et al., Angew. Chem. Int. Ed. 49, 886 (2010)
A. Hirsch, The era of carbon allotropes. Nat. Mater. 9(11), 868–871 (2010)
N. Tagmatarchis, H. Shinohara, Fullerenes in medicinal chemistry and their biological applications. Mini. Rev. Med. Chem. 1(4), 339–348 (2001)
E.A. Katz, Fullerene thin films as photovoltaic material, in Nanostructured Materials for Solar Energy Conversion, ed. by T. Sōga (Elsevier, Amsterdam, 2006), pp. 361–443
R.E. Smalley, Carbon Nanotubes: Synthesis, Structure, Properties, and Applications, vol. 80, ed. by M.S. Dresselhaus, G. Dresselhaus, P. Avouris (Springer Science & Business Media, 2003)
S.H. Jin, Single-walled carbon nanotubes (SWNTs); history and future prospects for electronic applications, in Active-Matrix Flatpanel Displays And Devices (AMFPD), 2016 The 23rd International Workshop on Active-Matrix Flatpanel Displays and Devices, FTFMD (2016), pp. 38–41
M. Husain, Z.H. Khan (eds.), Advances in Nanomaterials, vol. 79 (Springer, Berlin, 2016)
P.M. Ajayan, O.Z. Zhou, Applications of carbon nanotubes, in Carbon Nanotubes (Springer, Berlin, 2001), 391–425
H.P. Boehm, R. Setton, E. Stumpp, Nomenclature and terminology of graphite intercalation compounds (IUPAC Recommendations 1994). Pure Appl. Chem. 66(9), (1994), 1893–1901
E.T. Thostenson, Z. Ren, T.W. Chou, Advances in the science and technology of carbon nanotubes and their composites: a review. Compos. Sci. Technol. 61(13), 1899–1912 (2001)
H.P. Boehm, A. Clauss, G.O. Fischer, U. Hofmann, Das adsorptionsverhalten sehr dünner kohlenstoff‐folien. Zeitschrift für anorganische und allgemeine Chemie 316(3–4), 119–127 (1962)
K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, A.A. Firsov, Electric field effect in atomically thin carbon films. Science 306(5696), 666–669 (2004)
This Month in Physics History: October 22, 2004: Discovery of Graphene. APS News. Series II. 18(9), 2 (2009)
D. Chen, H. Feng, J. Li, Graphene oxide: preparation, functionalization, and electrochemical applications. Chem. Rev. 112(11), 6027–6053 (2012)
J.T. Robinson, F.K. Perkins, E.S. Snow, Z. Wei, P.E. Sheehan, Reduced graphene oxide molecular sensors. Nano Lett. 8(10), 3137–3140 (2008)
V. Dua, S.P. Surwade, S. Ammu, S.R. Agnihotra, S. Jain, K.E. Roberts, S. Park, R.S. Ruoff, S.K. Manohar, All-organic vapor sensor using inkjet-printed reduced graphene oxide. Angew. Chem. 122(12), 2200–2203 (2010)
S. Stankovich, D.A. Dikin, G.H.B. Dommett, K.M. Kohlhaas, E.J. Zimney, E.A. Stach, R.D. Piner, S.T. Nguyen, R.S. Ruoff, Graphene-based composite materials. Nature 442(7100), 282–286 (2006)
H. Zhang, X. Lv, Y. Li, Y. Wang, J. Li, P25-graphene composite as a high performance photocatalyst. ACS Nano 4(1), 380–386 (2009)
D. Li, R.B. Kaner, Graphene-based materials. Nat. Nanotechnol. 3, 101 (2008)
S. Goenka, V. Sant, S. Sant, Graphene-based nanomaterials for drug delivery and tissue engineering. J. Controll. Release 173, 75–88 (2014)
K. Shehzad, Y. Xu, C. Gao, X. Duan, Three-dimensional macro-structures of two-dimensional nanomaterials. Chem. Soc. Rev. 45(20), 5541–5588 (2016)
Y. Han, Z. Xu, C. Gao, Ultrathin graphene nanofiltration membrane for water purification. Adv. Func. Mater. 23(29), 3693–3700 (2013)
Martin Pumera, Graphene-based nanomaterials for energy storage. Energy Environ. Sci. 4(3), 668–674 (2011)
Z.S. Wu, G. Zhou, L.C. Yin, W. Ren, F. Li, H.M. Cheng, Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 1(1), 107–131 (2012)
M.F. El-Kady, R.B. Kaner, Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat. Commun. 4, 1475 (2013)
H. He, J. Klinowski, M. Forster, A. Lerf, Chem. Phys. Lett. 287, 53–56 (1998)
X. Wang, L. Zhi, K. Müllen, Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 8(1), 323–327 (2008)
G. Eda, G. Fanchini, M. Chhowalla, Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 3(5), 270–274 (2008)
X. Li, Y. Zhu, W. Cai, M. Borysiak, B. Han, D. Chen, R.D. Piner, L. Colombo, R.S. Ruoff, Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 9(12), 4359–4363 (2009)
G. Eda, Y.Y. Lin, S. Miller, C.W. Chen, Su WF, M. Chhowalla, Transparent and conducting electrodes for organic electronics from reduced graphene oxide. Appl. Phys. Lett. 92(23), 233305 (2008)
S. Chen, J. Zhu, X. Wu, Q. Han, X. Wang, Graphene oxide—MnO2 nanocomposites for supercapacitors. ACS Nano 4(5), 2822–2830 (2010)
X. Zhu, Y. Zhu, S. Murali, M.D. Stoller, R.S. Ruoff, Nanostructured reduced graphene oxide/Fe2O3 composite as a high-performance anode material for lithium ion batteries. ACS Nano 5(4), 3333–3338 (2011)
R.R. Nair, H.A. Wu, P.N. Jayaram, I.V. Grigorieva, A.K. Geim, Unimpeded permeation of water through helium-leak–tight graphene-based membranes. Science 335(6067), 442–444 (2012)
L. Wang, K. Lee, Y.Y. Sun, M. Lucking, Z. Chen, J.J. Zhao, S.B. Zhang, Graphene oxide as an ideal substrate for hydrogen storage. ACS Nano 3(10). 2995–3000 (2009)
C. Chung, Y.K. Kim, D. Shin, S.R. Ryoo, B.H. Hong, D.H. Min, Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 46(10), 2211–2224 (2013)
B.C. Brodie, On the atomic weight of graphite. Phil. Trans. R. Soc. London 149, 249–259 (1859)
L. Staudenmaier, Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 31, 1481–1487 (1898)
L. Staudenmaier, Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 32, 1394–1399 (1899)
U. Hofmann, R. Holst, Über die Säurenatur und die Methylierung von Graphitoxyd. Berichte der deutschen chemischen Gesellschaft (A and B Series), 72(4), 754–771 (1939)
W. Gao, Synthesis, structure and characterizations, in Graphene Oxide: Reduction Recipes, Spectroscopy, and Applications, ed. by W. Gao (Springer International Publishing, 2015), pp. 1–28
W.S. Hummers Jr, R.E. Offeman, Preparation of graphitic oxide. J. Am. Chem. Soc. 80(6), 1339–1339 (1958)
K.R. Koch, P.F. Krause, Oxidation by dimanganese heptoxide: an impressive demonstration. J. Chem. Ed 59, 973 (1982)
A. Simon, R. Dronskowski, B. Krebs, B. Hettich, The crystal structure of Mn2O7. Angew. Chem. Int. Ed. Engl. 26(2), 139–140 (1987)
S. Stankovich, R.D. Piner, X. Chen, N. Wu, S.T. Nguyen, R.S. Ruoff, Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly (sodium 4-styrenesulfonate). J. Mater. Chem. 16(2), 155–158 (2006)
N.I. Kovtyukhova, P.J. Ollivier, B.R. Martin, T.E. Mallouk, S.A. Chizhik, E.V. Buzaneva, A.D. Gorchinskiy, Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 11, 771–778 (1999)
J. Chen, B. Yao, C. Li, G. Shi, An improved hummers method for eco-friendly synthesis of graphene oxide. Carbon 64, 225–229 (2013)
D.C. Marcano, D.V. Kosynkin, J.M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L.B. Alemany, W. Lu, J.M. Tour, Improved synthesis of graphene oxide. ACS Nano 4, 4806–4814 (2010)
S. Park, R.S. Ruoff, Chemical methods for the production of graphenes. Nat. Nanotechnol. 4(4), 217–224 (2009)
S. Park et al., Graphene oxide papers modified by divalentions—Enhancing mechanical properties via chemical cross-linking. ACS Nano 2, 572–578 (2008)
S. Stankovich, R. Piner, S.T. Nguyen, R.S. Ruoff, Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 44, 3342–3347 (2006)
J.I. Paredes, S. Villar-Rodil, A. Martinez-Alonso, J.M.D. Tascón, Graphene oxide dispersions in organic solvents. Langmuir 24, 10560–10564 (2008)
S. Stankovich et al., Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007)
D. Li, M.B. Muller, S. Gilje, R.B. Kaner, G.G. Wallace, Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 3, 101–105 (2008)
Y. Xu, H. Bai, G. Lu, C. Li, G. Shi, Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 130, 5856–5857 (2008)
S. Park et al., Aqueous suspension and characterization of chemically modified graphene sheets. Chem. Mater. 20, 6592–6594 (2008)
Y. Si, E.T. Samulski, Synthesis of water soluble graphene. Nano Lett. 8, 1679–1682 (2008)
S. Niyogi et al., Solution properties of graphite and graphene. J. Am. Chem. Soc. 128, 7720–7721 (2006)
J.R. Lomeda, C.D. Doyle, D.V. Kosynkin, W.-F. Hwang, J.M. Tour, Diazonium functionalization of surfactant-wrapped chemically converted graphene sheets. J. Am. Chem. Soc. 130, 16201–16206 (2008)
V.C. Tung, M.J. Allen, Y. Yang, R.B. Kaner, High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 4, 25–29 (2008)
R. Muszynski, B. Seger, P.V. Kamat, Decorating graphene sheets with gold nanoparticles. J. Phys. Chem. C 112, 5263–5266 (2008)
H.C. Schniepp et al., Functionalized single graphene sheets derived form splitting graphite oxide. J. Phys. Chem. B 110, 8535–8539 (2006)
M.J. McAllister et al., Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 19, 4396–4404 (2007)
G. Williams, B. Serger, P.V. Kamat, TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2, 1487–1491 (2008)
P.V. Kamat, V. Bridewell, Electrocatalytic activity of graphene oxide: mediating electron transfer between two redox couples, in Meeting Abstracts, no. 6, The electrochemical society, (2016), pp. 551–551
Y. Wang, L. Li, C. Luo, X. Wang, H. Duan, Removal of Pb 2+ from water environment using a novel magnetic chitosan/graphene oxide imprinted Pb 2+. Int. J. Biol. Macromol. 86, 505–511 (2016)
Z.S. Wu, W. Ren, L. Gao, B. Liu, C. Jiang, H.M. Cheng, Synthesis of high-quality graphene with a pre-determined number of layers. Carbon 47(2), 493–499 (2009)
O. Akhavan, M. Choobtashani, E. Ghaderi, Protein degradation and RNA efflux of viruses photocatalyzed by graphene–tungsten oxide composite under visible light irradiation. J. Phys. Chem. C. 116(17), 9653–9659 (2012)
S. Pei, H.M. Cheng, The reduction of graphene oxide. Carbon 50(9), 3210–3228 (2012)
K.N. Kudin, B. Ozbas, H.C. Schniepp, R.K. Prud’homme, I.A. Aksay, R. Car. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 8(1), 36–41 (2007)
H.A. Becerril, J. Mao, Z. Liu, R.M. Stoltenberg, Z. Bao, Y. Chen, Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2(3), 463–470 (2008)
S.C. Youn, J. Geng, B.S. Son, S.B. Yang, D.W. Kim, H.M. Cho, H.T. Jung, Effect of the exposure time of hydrazine vapor on the reduction of graphene oxide films. J. Nanosci. Nanotechnol. 11(7), 5959–5964 (2011)
Y. Zhu, S. Murali, M.D. Stoller, A. Velamakanni, R.D. Piner, R.S. Ruoff, Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 48(7), 2118–2122 (2010)
H.M.A. Hassan, V. Abdelsayed, A.E.R.S. Khder, K.M. AbouZeid, J. Terner, M.S. El-Shall et al., Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 19(23), 3832–3837 (2009)
S. Sharin, I.A. Rahman, A.F. Ahmad, H.M.K. Mohd, F. Mohamed, S. Radiman, M.S. Yasir, S. Sarmani, M.T. Ayob, I.S.A. Bastamam, Reduction of graphene oxide to graphene by using gamma irradiation. Malays. J. Anal. Sci. 19(6), 1223–1228 (2015)
L.J. Cote, R. Cruz-Silva, J. Huang, Flash reduction and patterning of graphite oxide and its polymer composite. J. Am. Chem. Soc. 131(31), 11027–11032 (2009)
Y. Zhang, L. Guo, S. Wei, Y. He, H. Xia, Q. Chen et al., Direct imprinting of microcircuits on graphene oxides film by femtosecond laser reduction. Nanotoday 5(1), 15–20 (2010)
Y. Matsumoto, M. Koinuma, S.Y. Kim, Y. Watanabe, T. Taniguchi, K. Hatakeyama, H. Tateishi, S. Ida, Simple photoreduction of graphene oxide nanosheet under mild conditions. ACS Appl. Mater. Inter. 2(12), 3461–3466 (2010)
P. Kumar, K.S. Subrahmanyam, C.N.R. Rao, Graphene produced by radiation-induced reduction of graphene oxide. Int. J. Nanosci. 10(04n05), 559–566 (2011)
B. Zhang, L. Li, Z. Wang, S. Xie, Y. Zhang, Y. Shen, M. Yu et al., Radiation induced reduction: an effective and clean route to synthesize functionalized graphene. J. Mater. Chem. 22(16), 7775–7781 (2012)
D.R. Dreyer, S. Park, C.W. Bielawski, R.S. Ruoff, The chemistry of graphene oxide. Chem. Soc. Rev. 39(1), 228–240 (2010)
C.K. Chua, M. Pumera, Chemical reduction of graphene oxide: a synthetic chemistry viewpoint. Chem. Soc. Rev. 43(1), 291–312 (2014)
X. Zhou, J. Zhang, H. Wu, H. Yang, J. Zhang, S. Guo, J. Phys. Chem. C 115, 11957–11961 (2011)
T. Sakura, Y. Nagasaki, Preparation of gold colloid using pyrrole-2-carboxylic acid and characterization of its particle growth. Colloid Polym. Sci. 285(12), 1407–1410 (2007)
S. Liu, J. Tian, L. Wang, X. Sun, A method for the production of reduced graphene oxide using benzylamine as a reducing and stabilizing agent and its subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. Carbon 49(10), 3158–3164 (2011)
Y. Chen, X. Zhang, P. Yu, Y. Ma, Stable dispersions of graphene and highly conducting graphene films: a new approach to creating colloids of graphene monolayers. Chem. Commun. 30, 4527–4529 (2009)
J. Che, L. Shen, Y. Xiao, A new approach to fabricate graphene nanosheets in organic medium: combination of reduction and dispersion. J. Mater. Chem. 20(9), 1722–1727 (2010)
Z. Lei, L. Lu, X.S. Zhao, The electrocapacitive properties of graphene oxide reduced by urea. Energy Environ. Sci. 5(4), 6391–6399 (2012)
P. Su, H.L. Guo, L. Tian, S.K. Ning, An efficient method of producing stable graphene suspensions with less toxicity using dimethyl ketoxime. Carbon 50(15), 5351–5358 (2012)
X. Shen, L. Jiang, Z. Ji, J. Wu, H. Zhou, G. Zhu, Stable aqueous dispersions of graphene prepared with hexamethylenetetramine as a reductant. J. Colloid. Interf. Sci. 354(2), 493–497 (2011)
S. Zhang, Y. Shao, H. Liao, M.H. Engelhard, G. Yin, Y. Lin, Polyelectrolyte-induced reduction of exfoliated graphite oxide: a facile route to synthesis of soluble graphene nanosheets. ACS Nano 5(3), 1785–1791 (2011)
T. Wu, X. Wang, H. Qiu, J. Gao, W. Wang, Y. Liu, Graphene oxide reduced and modified by soft nanoparticles and its catalysis of the Knoevenagel condensation. J. Mater. Chem. 22(11), 4772–4779 (2012)
I.K. Moon, J. Lee, R.S. Ruoff, H. Lee. Reduced graphene oxide by chemical graphitization. Nat. Commun. 1(73), (2010)
P. Cui, J. Lee, E. Hwang, H. Lee, One-pot reduction of graphene oxide at subzero temperatures. Chem. Commun. 47(45), 12370–12372 (2011)
S.F. Pei, J.P. Zhao, J.H. Du, W.C. Ren, H.M. Cheng, Carbon 48, 4466–4474 (2010)
Y. Chen, X. Zhang, D. Zhang, P. Yu, Y. Ma, Carbon 49, 573–580 (2011)
H.J. Shin, K.K. Kim, A. Benayad, S.M. Yoon, H.K. Park, I.S. Jung et al., Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 19(12), 1987–1992 (2009)
M. Periasamy, M. Thirumalaikumar, Methods of enhancement of reactivity and selectivity of sodium borohydride for applications in organic synthesis. J. Organomet. Chem. 609(1–2), 137–151 (2000)
W. Gao, L.B. Alemany, L. Ci, P.M. Ajayan, New insights into the structure and reduction of graphite oxide. Nat. Chem. 1(5), 403–408 (2009)
C.Y. Su, X.U. Yanping, W. Zhang, J. Zhao, A. Liu, X. Tang, C.H. Tsai, Y. Huang, L.J. Li, Highly efficient restoration of graphitic structure in graphene oxide using alcohol vapors. ACS Nano 4(9), 5285–5292 (2010)
J. Gao, F. Liu, Y. Liu, N. Ma, Z. Wang, X. Zhang, Chem. Mater. 22, 2213–2218 (2010)
M.J. Fernández-Merino, L. Guardia, J.I. Paredes, S. Villar-Rodil, P. Solís-Fernández, A. Martínez-Alonso, J.M.D. Tascón, J. Phys. Chem. C. 114, 6426–6432 (2010)
Y. Wang, L. Sun, B. Fugetsu, Bull. Chem. Soc. Jpn. 85, 1339–1344 (2012)
Q. Ma, J. Song, C. Jin, Z. Li, J. Liu, S. Meng, J. Zhao, Y. Guo, Carbon 54, 36–41 (2013)
Z. Fan, K. Wang, T. Wei, J. Yan, L. Song, B. Shao, Carbon 48, 1686–1689 (2010)
V.H. Pham, H.D. Pham, T.T. Dang, S.H. Hur, E.J. Kim, B.S. Kong, S. Kim, J.S. Chung, J. Mater. Chem. 22, 10530–10536 (2012)
Z.-J. Fan, W. Kai, J. Yan, T. Wei, L.-J. Zhi, J. Feng, Y.-m. Ren, L.-P. Song, F. Wei, ACS Nano 5, 191–198 (2010)
X. Mei, J. Ouyang, Carbon 49, 5389–5397 (2011); P.B. Liu, Y. Huang, L. Wang, Mater. Lett. 91, 125–128 (2013)
N.A. Kumar, S. Gambarelli, F. Duclairoir, G. Bidan, L. Dubois, J. Mater. Chem. A 1, 2789–2794 (2013)
B.K. Barman, P. Mahanandia, K.K. Nanda, RSC Adv. 3, 12621–12624 (2013)
R.S. Dey, S. Hajra, R.K. Sahu, C.R. Raj, M.K. Panigrahi, Chem. Commun. 48, 1787–1789 (2012)
Y. Liu, Y. Li, M. Zhong, Y. Yang, W. Yuefang, M. Wang, J. Mater. Chem. 21, 15449–15455 (2011)
S. Yang, W. Yue, D. Huang, C. Chen, H. Lin, X. Yang, RSC Adv. 2, 8827–8832 (2012)
H. Feng, R. Cheng, X. Zhao, X. Duan, J. Li, Nat. Commun. 4, 1539 (2013)
D. Chen, L. Li, L. Guo, Nanotechnology 22, 325601 (2011)
S. Bose, T. Kuila, A.K. Mishra, N.H. Kim, J.H. Lee, J. Mater. Chem. 22, 9696–9703 (2012)
J.K. Ma, X.R. Wang, Y. Liu, T. Wu, Y. Liu, Y.Q. Guo, R.Q. Li, X.Y. Sun, F. Wu, C.B. Li, J.P. Gao, J. Mater. Chem. A 1, 2192–2201 (2013)
T.A. Pham, J. Kim, J.S. Kim, Y.T. Jeong, Colloids Surf. A 384, 543–548 (2011)
D. Suresh, H. Nagabhushana, S.C. Sharma, Clove extract mediated facile green reduction of graphene oxide, its dye elimination and antioxidant properties. Mater. Lett. 142, 4–6 (2015)
S. Hatamie, O. Akhavan, S.K. Sadrnezhaad, M.M. Ahadian, M.M. Shirolkar, H.Q. Wang, Curcumin-reduced graphene oxide sheets and their effects on human breast cancer cells, Mater. Sci. Eng. C 55, 482–489 (2015)
R.K. Upadhyay, N. Soin, G. Bhattacharya, S. Saha, A. Barman, S.S. Roy, Grape extract assisted green synthesis of reduced graphene oxide for water treatment application. Mater. Lett. 160, 355–358 (2015)
E.C. Salas, Z. Sun, A. Lüttge and JM Tour. ACS Nano 4, 4852–4856 (2010)
O. Akhavan, E. Ghaderi, Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon 50(5), 1853–1860 (2012)
P. Khanra, T. Kuila, N.H. Kim, S.H. Bae, D.S. Yu, J.H. Lee, Simultaneous bio-functionalization and reduction of graphene oxide by baker’s yeast. Chem. Eng. J. 183, 526–533 (2012)
O. Akhavan, Bacteriorhodopsin as a superior substitute for hydrazine in chemical reduction of single-layer graphene oxide sheets. Carbon 81, 158–166 (2015)
A. Esfandiar, O. Akhavan, A. Irajizad, Melatonin as a powerful bio-antioxidant for reduction of graphene oxide. J. Mater. Chem. 21(29), 10907–10914 (2011)
T. Szabó, O. Berkesi, P. Forgó, K. Josepovits, Y. Sanakis, D. Petridis, I. Dékány, Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 18(11), 2740–2749 (2006)
W. Cai, R.D. Piner, F.J. Stadermann, S. Park, M.A. Shaibat, Y. Ishii, D. Yang et al., Synthesis and solid-state NMR structural characterization of 13C-labeled graphite oxide. Science 321(5897), 1815–1817 (2008)
H. He, T. Riedl, A. Lerf, J. Klinowski, Solid-state NMR studies of the structure of graphite oxide. J. Phys. Chem. 100(51), 19954–19958 (1996)
A. Lerf, H. He, T. Riedl, M. Forster, J. Klinowski, 13C and 1H MAS NMR studies of graphite oxide and its chemically modified derivatives. Solid State Ionics. 101, 857–862 (1997)
A. Lerf, H. He, M. Forster, J. Klinowski, Structure of graphite oxide revisited. J. Phys. Chem. B 102(23), 4477–4482 (1998)
Q. Zhang, K. Scrafford, M. Li, Z. Cao, Z. Xia, P.M. Ajayan, B. Wei, Anomalous capacitive behaviors of graphene oxide based solid-state supercapacitors. Nano Lett. 14(4), 1938–1943 (2014)
J. Zhao, L. Liu, F. Li, Structural Characterizations in Graphene Oxide: Physics and Applications. (Springer, Berlin Heidelberg, 2015), pp. 15–29
C. Mattevi, G. Eda, S. Agnoli, S. Miller, K.A. Mkhoyan, O. Celik, D. Mastrogiovanni, G. Granozzi, E. Garfunkel, M. Chhowalla, Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv. Funct. Mater. 19(16), 2577–2583 (2009)
D. Yang, A. Velamakanni, G. Bozoklu, S. Park, M. Stoller, R.D. Piner, S. Stankovich et al., Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 47(1), 145–152 (2009)
O. Akhavan, The effect of heat treatment on formation of graphene thin films from graphene oxide nanosheets. Carbon 48(2), 509–519 (2010)
G. Abhijit, S. Sharma, P. Papakonstantinou, J. Hamilton, Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J. Phys. Chem. C 115(34), 17009–17019 (2011)
R.J.W.E. Lahaye, H.K. Jeong, C.Y. Park, Y.H. Lee. Density functional theory study of graphite oxide for different oxidation levels. Phys. Rev. B 79(12), 125435 (2009)
A. Bagri, C. Mattevi, M. Acik, Y.J. Chabal, M. Chhowalla, V.B. Shenoy, Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2(7), 581–587 (2010)
C. Hontoria-Lucas, A.J. Lopez-Peinado, J.D. de López-González, M.L. Rojas-Cervantes, R.M. Martin-Aranda, Study of oxygen-containing groups in a series of graphite oxides: physical and chemical characterization. Carbon 33(11), 1585–1592 (1995)
A.C. Ferrari, J.C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec et al., Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 97(18), 187401 (2006)
A. Gupta, G. Chen, P. Joshi, S. Tadigadapa, P.C. Eklund, Raman scattering from high-frequency phonons in supported n-graphene layer films. Nano Lett. 6(12), 2667–2673 (2006)
K. Krishnamoorthy, M. Veerapandian, K. Yun, S-J. Kim. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 53, 38–49 (2013)
G. Eda, M. Chhowalla, Chemically derived graphene oxide: towards large‐area thin‐film electronics and optoelectronics. Adv. Mater. 22(22), 2392–2415 (2010)
L.M. Malard, M.A. Pimenta, G. Dresselhaus, M.S. Dresselhaus, Raman spectroscopy in graphene. Phys. Rep. 473(5), 51–87 (2009)
C. Gómez-Navarro, J.C. Meyer, R.S. Sundaram, A. Chuvilin, S. Kurasch, M. Burghard, K. Kern, U. Kaiser, Atomic structure of reduced graphene oxide. Nano Lett. 10(4), 1144–1148 (2010)
K. Erickson, R. Erni, Z. Lee, N. Alem, W. Gannett, A. Zettl, Determination of the local chemical structure of graphene oxide and reduced graphene oxide, Adv. Mater. 22(40), 4467–4472 (2010)
J. Xie, F. Tu, Q. Su, G. Du, S. Zhang, T. Zhu, G. Cao, X. Zhao, In situ TEM characterization of single PbSe/reduced-graphene-oxide nanosheet and the correlation with its electrochemical lithium storage performance. Nano Eng. 5, 122–131 (2014)
K.A. Mkhoyan, A.W. Contryman, J. Silcox, D.A. Stewart, G. Eda, C. Mattevi, S. Miller, M. Chhowalla, Atomic and electronic structure of graphene-oxide. Nano Lett. 9(3), 1058–1063 (2009)
M.I. Katsnelson, K.S. Novoselov, A.K. Geim, Chiral tunnelling and the Klein paradox in graphene, Nat. Phys. 2(9), 620–625 (2006)
D.A. Skoog, F.J. Holler, S.R. Crouch, Principles of Instrumental Analysis, 6th edn. (Thomson Brooks/Cole, Belmont, CA, 2007), pp. 169–173
Z. Luo, Y. Lu, L.A. Somers, A.T.C. Johnson, High yield preparation of macroscopic graphene oxide membranes. J. Am. Chem. Soc. 131(3), 898–899 (2009)
Q. Lai, S. Zhu, X. Luo, M. Zou, S. Huang, Ultraviolet-visible spectroscopy of graphene oxides. AIP Adv. 2(3), 032146 (2012)
T. Szabó, O. Berkesi, I. Dékány, DRIFT study of deuterium-exchanged graphite oxide. Carbon 43(15), 3186–3189 (2005)
J. Zhang, H. Yang, G. Shen, P. Cheng, J. Zhang, S. Guo. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 46(7), 1112–1114 (2010)
Y. Wang, L. Tang, Z. Li, Y. Lin, J. Li, In situ simultaneous monitoring of ATP and GTP using a graphene oxide nanosheet–based sensing platform in living cells. Nat. Protoc. 9(8), 1944–1955 (2014)
K.R. Koch, Oxidation by Mn207: an impressive demonstration of the powerful oxidizing property of dimanganeseheptoxide. J. Chem. Educ. 59(11), 973 (1972)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Khan, M.B., Parvaz, M., Khan, Z.H. (2017). Graphene Oxide: Synthesis and Characterization. In: Khan, Z. (eds) Recent Trends in Nanomaterials. Advanced Structured Materials, vol 83. Springer, Singapore. https://doi.org/10.1007/978-981-10-3842-6_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-3842-6_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3841-9
Online ISBN: 978-981-10-3842-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)