Abstract
The engineering of skin substitutes and their applications on the regeneration of damaged skin have advanced dramatically in the past decades. However, scientists are still struggling with the generation of full-thickness skin with native structure and completed functions. In this chapter, classified by sources, recent developments of biomaterials for skin regeneration have been summarized. Then the most common formats of the engineering skin substitutes are introduced. The strategies of the biological functionalization in the design of skin substitutes are further summarized. Some important challenges in the field of skin substitutes such as angiogenesis, scarring, and appendages loss are particularly focused on. Finally, a brief conclusion and some perspectives are given in terms of the future trend of biomaterials for skin regeneration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The skin, the largest organ of the human body, provides a protective barrier against physical, chemical, and biological pathogens to support and maintain human health. In addition, the skin also has the function of temperature regulation, external insult protection, and detoxing. Typically, the skin has hierarchical structures including the upper epidermal layer, interlayer dermis, and subcutaneous tissue. The epidermis whose thickness is 0.1–0.2 mm consists mainly of keratinocytes derived from the capillary network. The dermis layer composes of fibroblasts and extracellular matrix (ECM) including collagen, glycosaminoglycans (GAGs), and elastin. Skin appendages such as hair follicles, sweat glands, and sebaceous glands are from the subcutaneous tissue and play a great role in the sensation, temperature regulation, and detoxing (Fig. 10.1) [1].
The structure of the human skin (Reprinted from Ref. [1] with permission. Copyright 2007, Rights Managed by Nature Publishing Group)
Burn, trauma, or chronic diseases frequently cause the loss of the skin, leading to descent of nonspecific immunity and bacterial infection, which is one of the most severe problems affecting human life quality. Thus, skin regeneration has become a major aim in the field of wound healing. In the past several decades, surgical therapies including skin transplantation have been applied to treat the loss of the skin and have achieved great success in skin regeneration. Autologous skin graft is the “gold standard” for clinical treatment of skin defect, and allograft plays a big role in the early period of skin repair as a temporary cover until a permanent skin graft is available. However, skin autograft and allograft are limited by the timely availability and donor sites. In addition, current skin grafts often suffer from a range of problems including incomplete biological functions, scar formation, and bacterial or virus infection during surgical therapies [2]. Thus, it has been becoming more and more urgent to find effective therapy strategies for the treatment of skin loss facing with the increasing clinical need and a vast patient resource.
Recently, skin substitute based on tissue engineering is being rapidly developed to bypass the limitations of conventional tissue transplantation and provide new therapeutic strategy to restore skin function [3]. Tissue engineering combines scaffold, cells, and biofactors to remodel the target tissue or organ in vitro, followed by in vivo transplantation according to the principles of materials, medicine, and biology. Skin tissue substitutes based on tissue engineering have been fabricating over the past several decades to provide more suitable therapeutic schemes for skin loss, and some commercial products are available in clinical application. For example, Dermagrafts®, a dermal skin substitute consists of poly(glycolic acid) (PGA), poly(lactic acid) (PLA), and fibroblasts, has been used to treat diabetic ulcers [4]. However, the fully functional skin regeneration is still a big challenge for skin tissue engineering. Better tissue engineering strategy in the aspect of exploitation of new biomaterials and novel design of biomaterial scaffolds should be developed to fulfill the increasing demand of skin regeneration.
This chapter focuses on the application of tissue engineering and regenerative medicine approach for the fabrication of bioengineered constructs for skin regeneration. First of all, the materials and the design of material scaffold for skin regeneration are summarized. Then the bio-functionalization of biomaterial scaffold is reviewed by using proteins, genes, and cytokines. Finally, some important challenges for skin regeneration including angiogenesis, scarring, appendages regeneration, and in situ tissue regeneration are discussed.
2 Materials for Skin Regeneration
2.1 Natural Materials
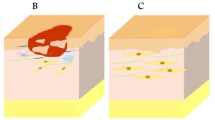
Collagen, one of the most important components of ECM and composed of a triple helix, is widely used in skin regeneration due to its good biocompatibility, biodegradability, flexibility, and structural and functional similarity to ECM [5]. Besides the collagen derived from animal, plant-derived human collagen has been shown to be a promising biomaterial for skin tissue engineering because of its low risk of an allergic response or disease transmission [6]. However, the poor mechanical properties of collagen limit its application in skin substitute. A variety of methods including cross-linking and blending with other substances have been established to improve the mechanical properties of collagen-based scaffold [7]. For example, synthetic human elastin/collagen composite scaffolds were fabricated by electrospinning for tissue engineering dermis [8]. The scaffold supported fibroblast infiltration, de novo collagen deposition, and new capillary formation. Recently, a full-thickness skin equivalent consists of collagen and silk was prepared to study skin biology [9]. In our lab, a collagen/chitosan scaffold cross-linked with glutaraldehyde has been fabricated to promote the growth of fibroblasts and dermis regeneration. The cross-linking of glutaraldehyde and introduction of chitosan can enhance the biostability of the scaffold [10, 11]. Wang et al. used PLGA-knitted mesh to integrate with collagen/chitosan scaffold to improve the mechanical strength of the scaffolds [12]. Some commercial products based on collagen have been applied in clinical practice. For example, Integra® fabricated by cross-linked bovine collagen and chondroitin-6-sulfate was employed for dermal regeneration. Apligraf®, a collagen-based hydrogel seeded with dermal fibroblasts and epidermal cells, has been widely applied to treat burns and several kinds of ulcers in the clinic (Fig. 10.2) [13, 14]. But it remains a challenge to regenerate the skin with complete appendages.
Histology of Apligraf compared with normal human skin (Reprinted from Ref. [13] with permission. Copyright 2010 John Wiley & Sons, Inc)
Chitosan, the deacetylated derivative of chitin, is a linear polysaccharide consisting of glucosamine and N-acetyl glucosamine [15]. Chitosan can be tailored with various molecular weights (50–2000 kDa) as well as degrees of deacetylation (30–95 %), allowing wide adjustment of mechanical and biological properties [16]. Cross-linking is usually made to control the degradation rate and enhance the mechanical properties of chitosan matrix for skin tissue engineering as well [17]. Chitosan can be applied to deliver bioactive molecules. For example, the human epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) were encapsulated in chitosan scaffold to promote wound healing [18, 19]. In addition, membranes based on chitosan are widely used as wound dressings because of the antibacterial capacity of chitosan [20]. However, the most extensive application of chitosan for skin regeneration is serving as a three-dimensional matrix. Tchemtchoua et al. prepared chitosan nanofibrillar scaffold for skin repair. Compared to chitosan sponge, the chitosan nanofibrillar scaffold induced a faster regeneration of both the epidermis and dermis (Fig. 10.3) [21]. Kiyozumi et al. fabricated a photo-cross-linkable chitosan hydrogel containing DMEM/F12 medium (medium-Az-CH-LA) for skin regeneration [22]. The hydrogel promoted re-epithelialization, vascularization, and wound repair. Moreover, compared to collagen sponge, thicker granulation tissue and earlier neovascularization were found in medium-Az-CH-LA [23].
Compared with chitosan films and sponges, the nanofibrillar structure strongly improved cell adhesion and proliferation in vitro. When used as a dressing covering full-thickness skin wounds in mice, chitosan nanofibrils induced a faster regeneration of both the epidermis and dermis compartments (Reprinted from [21] with permission. Copyright 2011 American Chemical Society)
Other natural biomaterials for skin regeneration including gelatin, hyaluronan, and fibrin are widely used as well. Shevchenko et al. designed a gelatin scaffold with attached silicone pseudo-epidermal layer for wound repair using a cryogelation technique [24]. The mechanical properties of the scaffold were comparable to the clinical product Integra®. In vivo test showed that the gelatin scaffold supports wound healing by allowing host cellular infiltration, bio-integration, and remodeling. Monteiro et al. utilized a spray-assisted layer-by-layer assembly technique to fabricate a multilayer film composed of poly-L-lysine (the epidermal component) and porous hyaluronic acid scaffold (the dermal component) in a rapid and controlled manner for skin tissue engineering [25]. The multilayer film enhances cell adhesion and regeneration of the epidermal barrier functions. Losi et al. prepared a fibrin-based scaffold with incorporated VEGF- and bFGF-loaded nanoparticles to stimulate wound healing [26]. The scaffold induces re-epithelialization and enhances granulation tissue formation/maturity and collagen deposition in genetically diabetic mice. Acellular dermal matrix (ADM) is used to obtain scaffolds with similar components and structure of ECM of the natural skin. For instance, AlloDerm® made of ADM by LifeCell® Corporation is extensively employed for full-thickness skin regeneration.
2.2 Synthetic Polymers
The mechanical property is the biggest drawback of natural materials for skin regeneration: thus, natural materials usually need to be cross-linked or combined with other materials. On the contrary, synthetic polymers with predictable and flexible physical and chemical properties including mechanical properties, functional groups, and degradation rate can be obtained under controlled conditions with mature techniques. Besides, synthetic polymers are biodegradable, are less expensive, and have lower immunological response than natural materials [27]. Furthermore, synthetic polymers such as PGA, PLA, poly(lactide-co-glycolide) (PLGA), and polycaprolactone (PCL) have been approved by the Food and Drug Administration (FDA) of the USA.
Synthetic polymers are important materials for skin regeneration. TransCyte® developed by the Advanced Tissue Science Company consisting of PLA scaffold and fibroblasts has been approved by the FDA for the healing of degree III burns [28]. PLGA matrices with fiber diameters varying from 150 to 6000 nm were fabricated via electrospinning [29]. Human skin fibroblasts acquire a well-spreading morphology and show significant growth on fiber matrices in the 350–1100 nm diameter range. Fibrous scaffolds composed of PLA and poly(ethylene glycol) (PEG) were prepared by electrospinning for skin tissue engineering [30]. The scaffold containing 30 % PEG exhibited most beneficial properties including wettability, and adaptable bulk biodegradation, and promoted the penetration and growth of human dermal fibroblasts. However, synthetic polymers are usually hydrophobic, and their lack of functional groups leads to limited capacity to combine with biomolecules. To enforce the bioactivity of synthetic polymers, natural materials are widely applied with synthetic polymers to design hybrid scaffolds. Chen et al. fabricate a hybrid scaffold composed of knitted PLGA and weblike collagen micro-sponges to facilitate cell seeding and distribution and rapid formation of the dermal tissue [31]. A porous polycaprolactone (PCL)/collagen membrane was designed by Venugopal et al. via electrospinning. The well-defined nanostructure can well promote the growth and adhesion of cells [32]. The post-modification of synthetic polymers is another important method for the enhancement of bioactivity. Yang et al. used anhydrous ammonia plasma treatment to modify surface properties to improve the cell affinity of a PLA/PLGA scaffold [33]. The modified scaffold facilitated the growth of fibroblasts. Nanofibrous PCL/gelatin scaffolds were modified by collagen type I grafting [34]. The diameter of the fiber and porosity decreased with the increase of grafted collagen, and the collagen-modified nanofibrous PCL/gelatin scaffolds can maintain characteristic shape and promote proliferation of fibroblasts.
3 Scaffold Design for Skin Regeneration
The scaffold plays a vital role for skin regeneration by serving as a three-dimensional matrix for maintaining cell activities and promoting extracellular matrix formation, delivering biofactors, preserving tissue volume, and providing temporary mechanical function. As the skin is complicated with a multilayer structure, how to design a scaffold mimicking the hierarchical structure and ultrastructure of ECM of the skin is an issue of great importance. So far different formats of scaffolds have been designed to treat different kinds of damaged skins. For example, chitosan/poly(vinyl alcohol) (PVA) nanofibrous membrane has been prepared by electrospinning as a wound dressing [35]. However, for dermal or even full-thickness skin regeneration, the most common scaffold formats are porous scaffolds and hydrogels.
3.1 Porous Scaffolds
The porous scaffold is the most common format for skin regeneration. Typically a porous scaffold possesses unique microstructures similar to native ECM, showing high surface area which facilitates cell attachment and growth. Both natural and synthetic polymers can be fabricated into the porous scaffolds with controlled three-dimensional structures by methods such as gas foaming, freeze-drying, and electrospinning.
A novel porous scaffold composed of collagen, hyaluronic acid, and gelatin was fabricated by freeze-drying for skin repair [36]. The average pore diameter of the scaffold was 132.5 ± 8.4 μm, which is beneficial for cell attachment and infiltration. The in vivo histological results revealed that the scaffold promoted wound healing compared to the group without treatments. Lu et al. fabricated a funnel-like porous PLLA-collagen and PLLA-gelatin hybrid scaffolds by forming collagen or gelatin sponge on a woven PLLA mesh for skin tissue engineering [37]. PLLA-collagen and PLLA-gelatin porous scaffolds promoted the regeneration of the dermal tissue and reduced contraction during the formation of new tissues. In our lab, Ma et al. have developed a collagen/chitosan hybrid porous scaffold which was cross-linked by glutaraldehyde (GA) to improve their biostability for skin regeneration [11]. Collagen and chitosan are evenly distributed in the scaffolds with high porosity and good interconnectivity. In vitro culture suggested that the porous scaffold could maintain the original good cytocompatibility of collagen and effectively accelerate infiltration and proliferation of human dermal fibroblasts. In vivo test revealed that the scaffold could induce the fibroblast infiltration from the surrounding tissues. Besides, collagen/chitosan-silicone membrane bilayer dermal equivalent (BDE) was designed, in which the silicone membrane covers the hybrid scaffold to prevent water evaporation and infection (Fig. 10.4) [38]. The porous BDE can be functionalized by plasmid DNA to form a gene-activated scaffold for more complicated reconstruction of the damaged skin. For example, the porous BED combined with N,N,N-trimethyl chitosan chloride (TMC)/pDNA-VEGF complexes can significantly enhance the expression of VEGF, which in turn facilitates the regeneration of full-thickness incisional wounds [39]. To inhibit the scar formation during wound healing, TMC/siRNA-TGF-β1complexes were incorporated into the BDE to interfere in transforming growth factor-β1 (TGF-β1) signal pathway and suppress the expression of TGF-β1 [40]. The functionalized porous BDE can inhibit scar formation compared to the normal BDE. These results indicate that the porous BDE holds great promise for skin regeneration in clinical application.
(a) A view of the scaffold after being combined with a silicone membrane with a thickness of about 0.14 mm. (b) The microstructures of the scaffold observed under a scanning electron microscope (Reprinted from Ref. [38] with permission. Copyright 2006 John Wiley & Sons, Ltd)
3.2 Hydrogel
Hydrogels are three-dimensional cross-linked polymer networks that are capable of absorbing large amount of water, which is important for the absorption of the excess of wound exudates [41]. In addition, hydrogels can protect the wound site from infection and promote the healing process by providing a moisturized environment. Moreover, hydrogels can preserve the bioactivity of growth factors, antibiotics, cytokines, and cells, making them ideal carriers for the delivery of biomolecules to realize complete skin regeneration. Many studies have been focused on the applications of hydrogel for skin regeneration [42–45].
Chitosan hydrogel is well known as a wound dressing, showing good biocompatibility, anti-infective activity, and the ability to accelerate wound healing. Thermo-responsive hydrogel was developed by using chitosan and agarose for skin regeneration [46]. The hydrogel prevented water loss and wound dehydration and was in favor of cell internalization and proliferation. A bilayer physical hydrogel of chitosan without any external cross-linking agent was used to induce inflammatory cell migration and angiogenesis [47]. A hydrogel sheet composed of alginate, chitin/chitosan, and fucoidan (ACF-HS) has been developed for wound dressing (Fig. 10.5) [48]. The hydrogel can provide a moist environment for rapid wound healing. Significantly advanced granulation tissue was observed in the healing-impaired wounds being treated with the hydrogel. Wong et al. fabricated a pullulan-collagen composite hydrogel by using a salt-induced phase inversion technique, which can recapitulate the reticular structure of human dermal ECM [49]. The hydrogel promoted wound closure due to the increased recruitment of stromal cells as well as the formation of the granulation tissue. A versatile, nontoxic, in situ cross-linkable biodegradable dextran hydrogel loaded with chitosan microparticles containing VEGF and EGF was designed for skin regeneration. In vivo results showed that the hydrogel improved the physical, chemical, and biological protection of the damaged skin [50]. Besides, the spatiotemporally controlled release of VEGF- and EGF-enhanced angiogenesis and re-epithelialization are crucial for the reconstruction of the native skin. More excitingly, a recent study shows that dextran-based hydrogels supported the infiltration of inflammatory cells, resulting in its rapid degradation and promoted infiltration of angiogenic cells and endothelial cells into the healing wounds [51]. In addition, the remarkable neovascularization and regeneration with hair follicles and sebaceous glands were observed after 21 days, and new hair was observed 5 weeks later. These results indicate that dextran-based hydrogel alone without bioactive factors can promote complete skin regeneration with appendages.
(a) Preparative procedures for ACF-HS. (b) Histological examination of wounds covered with ACF-HS or Kaltostat® and controls (uncovered). In the left panel, black arrows show formed granulation tissues. In the right panel, squares show the sites for microphotographs, and black arrows show blood vessels containing erythrocytes (Reprinted from Ref. [48] with permission. Copyright 2009 Published by Elsevier Ltd)
4 Bio-functionalization of Skin Regeneration Scaffolds
Skin repair is the result of synergistic effect of different kinds of cells whose proliferation, migration, differentiation, and ECM secretion are well regulated by bioactive factors such as growth factors, genes, and cytokines. Combining bioactive factors with scaffolds is a promising way to promote the efficiency and quality of the regenerated skin.
4.1 Growth Factors
Growth factors are capable of stimulating cellular growth, cellular proliferation, and cellular differentiation. Usually they are proteins or steroid hormones regulating a variety of cellular processes. Growth factors typically act as signaling molecules between cells to promote cell differentiation or maturation and have been widely used in tissue engineering skin constructs. Some growth factors are promising mediators of wound healing, such as the epidermal growth factor (EGF), fibroblast growth factor (FGF), vascular endothelial growth factors (VEGF), platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1), transforming growth factors α and β (TGF-α and TGF-β), and hepatocyte growth factor (HGF). Site-specific delivery of growth factors in micro-devices could provide an efficient means of stimulating localized recruitment to the cell transplants and would ensure cell survival and functions. By combining growth factors with micro-vehicles, bioactive skin scaffolds can be constructed. Richardson et al. incorporated VEGF and PDGF into a porous PLGA scaffold to realize a controlled dose and rate of delivery, pioneering the research of a vehicle delivering multiple angiogenic factors with distinct kinetics [52]. Perets et al. incorporated bFGF-loaded microspheres with three-dimensional porous alginate scaffolds, achieving enhanced vascularization in vivo [53]. Tabata et al. combined FGF, HGF, and VEGF with collagen gels to promote the regeneration of hair follicles after implantation [54]. Mao et al. combined FGF on a substrate via a layer-by-layer technique to fabricate bioactive films, on which fibroblasts proliferated better and secreted more ECM [55]. Regarding to hair follicle regeneration, HGF, a mitogen, motogen, and morphogen for lots of different organs, is expressed by the cells in human hair follicles and involved in the cycle of hair growth. Uijtdewilligen et al. incorporated insulin-like growth factor-2 (IGF-2) and sonic hedgehog (SHH) into a collagen type I heparin scaffold to form an embryonic-like scaffold which could help to repair the skin without contraction or scar formation [56].
4.2 Genes
As summarized above, with the ability to modulate and direct cells efficiently, the growth factors have been extensively used as bioactive factors to combine with tissue engineering scaffolds. However the most challenging limitation is their short half-lives. The emerging gene technique provides an optional method to make cells produce growth factors constantly. Hence, functional genes can be incorporated into scaffolds as bioactive factors and are locally expressed to encode specific growth factors at wound site. Specific examples of application of gene therapy in skin tissue engineering will be introduced below.
Shea et al. incorporated plasmid DNA encoding platelet-derived growth factor (PDGFB) into a three-dimensional PLGA sponge, implantation of which in a rat dermis showed enhancement of granulation tissue and vascularization [57]. Other types of matrices such as collagen and PVA sponges loaded with genes are also developed and used to treat cutaneous wounds, resulting in improvement of flap survival, granulation tissue formation, angiogenesis, and re-epithelialization [39, 58].
Alternatively, the nucleic acids (e.g., plasmid, siRNA) are complexed with cationic polymers or lipids, with the design of these transfection reagents depending upon the nucleic acid properties, such as size [59]. Complexation with polymers or lipids protects against degradation, creates a less negative particle relative to naked plasmid, and facilitates internalization and intracellular trafficking [60].
4.3 Cytokines
Cytokines, a family of small molecules of approximately 8–10 kDa in size, are key regulators of cell migration, immune responses, and wound healing [61]. Pro-inflammatory cytokines, particularly IL-1 and IL-6 and TNF-α, are upregulated during the inflammatory phase of wound healing.
IL-1 is produced by monocytes, neutrophils, macrophages, and keratinocytes and is immediately released by keratinocytes during wound healing. IL-1 activates fibroblasts and promotes the secretion of FGF [62]. IL-6 is secreted by neutrophils and monocytes and has been shown to be involved in healing response. Evidence shows that IL-6 is closely related with wound healing by regulating leukocyte infiltration, angiogenesis, and collagen accumulation [63]. TNF-α can promote the expression of FGF-7, indicating that it can favor the process of re-epithelialization [64].
Stromal cell-derived factor-1α (SDF-1α, CXCL12) chemokine is a member of the CXC family and works via the CXCR4 receptor. It plays a role in the inflammatory response by recruiting lymphocytes to the wound and promoting angiogenesis. Endothelial cells, myofibroblasts, and keratinocytes express SDF-1. A number of researches have proved that SDF-1α plays a pivotal role in the recruitment of stem cells. For example, PLGA scaffolds incorporated with SDF-1α can recruit more stem cells, which favors angiogenesis and decreases fibrotic and inflammatory responses. More interestingly, mechanical stretch can upregulate SDF-1α in the skin tissue and promote migration of circulating bone marrow-derived mesenchymal stem cells (BMSCs) [65]. The application of SDF-1α provides an avenue for the recruitment of stem cells, which is crucial for the in situ skin regeneration. For example, Nakamura et al. used mesenchymal stem cells (MSCs) genetically engineered with (SDF-1α) to heal skin wounds [66]. SDF-1α-engineered MSCs (SDF-MSCs) expressed more SDF-1α and enhanced the migration of MSCs and dermal fibroblasts and promoted skin wound closure.
5 Important Challenges and Strategies
Although many significant milestones of bioengineered skin have been reached for clinical therapies of full-thickness defects, challenges still remain to fulfill the criteria of “regenerated skin” with complete structural, esthetic, and functional properties as the nature of the skin. How to achieve rapid angiogenesis, inhibited scarring, and regeneration of appendages is a key issue related to the quality of the regenerated skin. In addition, in situ regeneration, which should be particularly concerned, would be less costly and complex than those approaches that require ex vivo cell manipulation.
5.1 Angiogenesis
One of the most critical issues for most bioengineered tissues is the rapid and appropriate angiogenesis of the constructs, since a tissue beyond a certain size generally cannot survive without the supply of nutrients and oxygen, and removal of waste products of cells [67]. For materials with a thickness larger than 0.4 mm, new blood vessels are not able to penetrate rapidly [68]. The delayed or poor angiogenesis of the reconstructed skin will hinder the nourishment of the overlaying epidermal layer and result in the failure of the graft. Moreover, if the transplantation of split-thickness skin grafts is delayed, the timely healing of the damaged skin will be hampered and the risk of death will increase [69]. It is clear that the blood supply is essential to realize the long-term integration of the reconstructed skin with the host tissue. Therefore, acceleration of the angiogenesis rate to achieve rapid formation of new blood capillaries is urgently required, whereas still remains a research focus for improvement of existing skin substitutes.
Suitable pore size of tissue-engineered scaffold plays an important role in improving permeability, facilitating cell migration, and enhancing angiogenesis. Yannas et al. found that a pore size ranging from 90 to 150 μm and porosity larger than 90 % promote vessel formation [70]. Pruitt et al. reported that only when pore size of scaffold is larger than 80 μm it can be conducive to the ingrowth of connective tissue and neovascularization [71]. Another possible approach to enhance the angiogenesis of the tissue-engineered skin is to combine the prefabricated vessels with some special kinds of cells to achieve better initial onset of revascularization for early anastomoses between graft and bed vessels. As one example, human dermal microvascular endothelial cells were incorporated into collagen or fibrin hydrogels. Three-dimensional capillaries were formed after transplantation of the pre-vascularized substitutes [72]. The engineered capillaries were further stabilized by pericytes and smooth muscle cells and ultimately connected to the microvessels of the wound ground. It is also found some glycosaminoglycans have angiogenetic effect. Pieper et al. reported that the incorporation of glycosaminoglycans could increase angiogenesis degree in vivo [73]. However, a sufficient vasculature still takes more than 4 weeks to develop.
The most powerful approach to induce angiogenesis in the engineered tissues is to use angiogenetic growth factors such as bFGF, VEGF, and PDGF. So far many efforts have been made to enhance angiogenesis by incorporation of angiogenic growth factors into tissue engineering scaffolds. With the loading of bFGF, the fibrin and collagen scaffolds show enhanced angiogenesis when applied to the rabbit ear ulcers, therefore greatly improving the healing of full-thickness skin defects [74]. Wissink et al. used heparin to realize a controlled release of bFGF through specific binding, which is effective in promoting the growth of endothelial cells within the collagen scaffold in vitro [75]. Perets et al. encapsulated bFGF into PLGA microspheres and then loaded the microspheres into a porous sodium alginate scaffold [53]. The formation of large mature vessels was greatly promoted by the bFGF-loaded scaffold in a rat peritoneal model.
However, delivery of the growth factors faces some challenges due to their sensitivity and instability, and their half-lives are only on the order of minutes in serum. In addition, the high cost of growth factors also limits their trial in practice. The gene technique has been considered as an alternative way in order to overcome the drawbacks of growth factors. By loading of more stable and functional genes into the scaffolds, the gene-activated matrix (GAMs) can be generated to locally transfect cells and constantly produce targeted growth factors at wound site. Endowed with the advantages of localized treatment, maintenance of effective amount of bioactive DNAs, and protection of DNAs against immune responses and nuclease degradation, the GAMs have shown great promise for the enhanced angiogenesis of the engineered skin. Mao et al. used TMC as a cationic gene delivery vector to carry plasmid DNA encoding VEGF (pDNA-VEGF) and constructed a gene-activated collagen scaffold for skin repair [76]. The in vivo application to Sprague-Dawley mice demonstrated that the TMC/pDNA-VEGF complexes remarkably promoted the in vivo expression of VEGF and thus enhanced the angiogenesis of the scaffolds. Recently, a gene-activated collagen-chitosan/silicone membrane bilayer dermal equivalent (BDE) has been prepared and evaluated for treatment of the full-thickness incisional wounds in terms of histology, immunohistochemistry, immunofluorescence, real-time quantitative PCR, and Western blotting analysis in a porcine model [39]. The TMC/pDNA-VEGF group shows highest level of VEGF expression at both mRNA and protein levels, resulting in the highest densities of newly formed and mature vessels. After 112 days of ultrathin skin graft transplantation, the healing skin has a similar structure and ~80 % tensile strength of the normal skin. Exploitation of the gene-activated BDE for the healing of full-thickness burns was also performed, showing very positive angiogenesis and repair results similar to those for incisional wounds (Fig. 10.6) [77].
(a) Cumulative release of DNA from the scaffolds as a function of time. (b) TMC/pDNA-VEGF group had a significantly higher number of newly formed and mature blood vessels (Reprinted from Ref. [77] with permission. Copyright 2010 Elsevier Ltd)
5.2 Scarring
Although skin substitutes based on principle of regeneration have achieved important progresses, scarring still remains a problem which results in issues such as disfiguration, itching, and local ulceration [78]. Prevention of scarring is therefore a major challenge to be addressed for the repair and regeneration of skin defects, and anti-scarring technologies should be incorporated for the new generation of the skin constructs.
Scars are the outcome of postnatal healing process of normal acute mammalian tissue repair including integration of bioengineered skins [79]. Scarring is a rapid tissue repairing process driven by an evolutionarily devised mechanism, allowing rapid recover of tissue integrity to fill tissue voids. Scarring involves a series of cellular events related to tissue repair including inflammation, migration/proliferation and ECM deposition, and also the inputs of numerous cell types, matrix components, and signaling molecules. Essentially, the excessive and disordered accumulation of ECM such as collagen, as well as the imbalance of new deposition and destruction of collagen, directly leads to the scar formation. By contrast, the scar-free regeneration should have features including complete restoration of skin structure, normal collagen deposition, and regular distribution of hair follicles, capillaries, and glands, which reflects a focus of interest for the emerging fields of regenerative medicine. Central to a material-based approach for skin regeneration is to build a suitable environment where cells are exposed to a complex pattern of bioactive molecules, which direct desired cell behaviors and right tissue regeneration.
Fetal wound repair is essentially a scar-free regenerative process. It has been extensively studied and confirmed that embryonic scarless wound repair exhibits reduced fibrin clots and platelet degranulation, and suppressed inflammatory response, which has provided therapeutic strategies for scar-free repair. Most importantly, the growth factor profile is also quite different for embryonic wound and adult wound. The large family of TGF-β protein, which can be secreted by multiple cell types such as platelets, macrophages, and fibroblasts, is one of the most important biosignal molecules during the wound healing process. It acts as a chemokine for fibroblasts, induces differentiation of myofibroblasts, regulates the collagen synthesis, and modulates the matrix turnover. Recent investigations have confirmed that the level of TGF-β1 and TGF-β2 in fetal skin injuries is lower than that in adult skin, while the level of TGF-β3 is elevated [80]. Inspired by these findings, successful reduction of scarring of adult skin wounds has been reported by interrupting TGF-β1 and TGF-β2 signaling pathway through neutralization with antibodies, inactivation by proteoglycan-like decorin, and blockage of function by exogenous receptors, as well as exogenous addition of the TGF-β3 isoforms. Samuels et al. [81] found that hypertrophic scar was induced in a rabbit embryos subcutaneous model by injecting the TGF-β1 and TGF-β2, while the addition of their polyclonal neutralizing antibody could inhibit scarring and generate a normal tensile strength and more physical dermal architecture. A recent research proves that after infected by adenovirus encoding a truncated TGF-β receptor II, normal dermal fibroblasts could result in wounds with an average of 49 % reduction of the scar area and less inflammatory reaction in the full-thickness incisional wounds in rats [82].
The recently emerging biomolecular cues of RNA interference (RNAi) offer a fascinating and prospective alternative to specifically silence targeted genes and downregulate targeted protein levels [83]. Delivery of exogenous small interfering RNA (siRNA) mediated by three-dimensional scaffolding materials is right now at the frontier of current research. Compared with other strategies, the RNAi not only shows higher efficiency and specificity during a long duration time but also avoids risks of immunogenicity and inactivation in the antibody method. In a recent research in our lab, TMC/siRNA complexes targeting TGF-β1 were incorporated into the collagen-chitosan/silicone membrane bilayer dermal equivalent (BDE) to fabricate an RNAi functionalized bioengineered skin (RNAi-BDE), aiming to interfere TGF-β1 signal pathway, directing cell behaviors, and ultimately inhibiting scarring (Fig. 10.7). The RNAi-BDE functioned as a reservoir for the incorporated TMC/siRNA complexes, enabling a prolonged siRNA release. Application of the RNAi-BDE on the full-thickness skin defects of pig backs confirmed the in vivo inhibition of TGF-β1 expression by immunohistochemistry, real-time quantitative PCR, and Western blotting during 30 days post-surgery. The levels of other scar-related factors such as collagen type I, collagen type III, and α-smooth muscle actin (α-SMA) were also downregulated. In combination with the ultrathin skin graft transplantation for 73 days, the regenerated skin by RNAi-BDE had an extremely similar structure to that of the normal one with significant scar inhibition.
(a) The siRNA could silence special gene expression. (b) Combination of polycations TMC with siRNA to form complexes which could be transfected into cells. Wound formation is mainly caused by massive and disordered deposition of Col I and Col III. (c) TMC/siRNA can suppress the expression of both Col I and Col III significantly (Reprinted from Ref. [40] with permission. Copyright 2012 Elsevier Ltd)
5.3 Appendages
Skin appendages such as hair follicle, sweat gland, sebaceous gland make skin functions well in touch, temperature sensation, excretion, perspiration, and thermoregulation. Regeneration of skin appendages is an important symbol of skin recovery and functionalization. Although some commercial artificial skin substitutions can achieve a structural repair in the epidermis and the dermis, it remains a challenge to regenerate the skin with complete appendages [84]. Hence, regenerated skin cannot fully replace normal skin in function.
Hair follicle is a mini-organ which produces hair. It is composed of hair papilla, matrix, root sheath, hair fiber, bulge, and so on. There are three stages in hair growth: the growth phase (anagen), the regressing phase (catagen), and the quiescent phase (telogen). Growth cycles are controlled by chemical signals like epidermal growth factor. Efforts of reconstructing hair follicles have been made decades ago. Lin et al. combined epidermal stem cells in collagen/gelatin scaffold with fibroblasts; hair follicle-like structure was formed after implantation. It is also reported that the interaction between epidermal cells and mesenchymal cells contributes to the formation of hair follicles. More recently, MSCs are induced into hair papilla-like cells [85]. Moreover, polysaccharide, as one main component of dermal ECM, is reported to induce the regeneration of hair follicles on a mice model [51]. Recently, it was reported that hair growth was promoted by adipose-derived stem cell (ASC) transplantation in animal experiments, and a conditioned medium of ASCs (ASC-CM) induced the proliferation of hair-compositing cells in vitro. Jin et al. introduced some ASC stimulators in preconditioning to enhance hair regeneration [86]. They also highlighted the functional role of ASCs in hair cycle progression and concluded the advantages and disadvantages of their application in hair regeneration.
Sweat glands are small tubular structures of the skin that can produce sweat. There are two kinds of sweat glands. Eccrine sweat glands are distributed all over the body (except for the lips, tip of the penis, and the clitoris), although their density varies a lot from region to region, while apocrine sweat glands are larger, having different mechanisms of secretion, and are limited to axilla (armpits) and perianal areas. Sweat glands play a key role in thermoregulation and inner balance. Therefore, it is vital to reconstruct sweat glands especially for large-area burns. Fu et al. cultured sweat gland cells (SGCs) on gelatin microspheres containing EGF and delivered the SGCs-microspheres complex into an engineered skin construct mainly composed of a fibroblast-embedded collagen-based matrix [87]. This engineered skin construct was then transplanted onto full-thickness cutaneous wound in an athymic murine model. Remarkably, sweat gland-like structure can be achieved in vitro within the hybrid matrix. Huang Sha et al. designed a functional in vitro cell-laden three-dimensional extracellular matrix mimics (3D-ECM) with composite hydrogels based on gelatin and sodium alginate. It provides the spatial inductive cues for enhancing specific differentiation of epidermal lineages to regenerate sweat glands [88].
Sebaceous glands are kind of microscopic glands in the skin that secrete an oily/waxy matter, called sebum, to lubricate and waterproof the skin and hair of mammals. In human beings, they are found in greatest abundance on the face and scalp, though distributed throughout all skin sites except the palms and soles. Compared with hair follicles and sweat glands, sebaceous glands are later to be studied. But exciting progress has been made related to reconstruction of sebaceous glands. Hair bulge cells have been reported to possess the potential of differentiating into sebaceous glands. Horsley et al. firstly found a kind of progenitor cells that can secret factor Blimp1, which can stimulate the regeneration of sebaceous glands when they get hurt [89].
Appendages do have a firm interaction rather than separate growth, although most researches are still confined to one certain appendage. With further study of biology of appendages, and clarifying the interaction of materials and related cells (specifically stem cells) and appendages themselves, it is quite possible to functionalize skin constructs by reconstructing different appendages together in the future.
5.4 In Situ Skin Regeneration
Along with the advancement of science and technology in biology, medicine, and material science, the insight mechanism of wound healing is better understood. Diverse methods for skin repair and regeneration have also been developed. Stem cell-based therapy, which is a promising cure for a multitude of diseases and disorders, has been one of the best documented approaches in regenerative medicine. However, the ex vivo expansion of stem cells and their in vivo delivery are restricted by the low survival rate and the limited availability of stem cell sources.
It has been demonstrated that endogenous stem cells can be actively attracted to sites of injury [3]. Thus, recruiting sufficient endogenous stem cells to the wound area and inducing them to repair the structure and functions of skin become a key challenge. This technique, known as in situ regeneration, has the potential to provide new therapeutic options for all kinds of tissues and organs. The in situ tissue regeneration method relies on endogenous stem cell homing, proliferation, differentiation, and rebuilding functional skins. Such options would be less costly and complex than the traditional approaches which require substantial ex vivo cell manipulation.
Microenvironment, which could be changed by cytokines, surface topology, and so on, could influence stem cell recruiting. Some cytokines can enhance tissue regeneration by facilitating cell homing. Chen et al. fabricated a radially oriented scaffold which could effectively promote BMSCs migration, whose effect was further enhanced by addition of stromal cell-derived factor-1 (SDF-1) (Fig. 10.8) [90]. It is reported that the migration abilities of PDMSCs exposed to hypoxic conditions are significantly increased. Interestingly, decreased integrin alpha4 in PDMSCs under hypoxia increases PDMSC migration ability [91]. In addition, bone morphogenetic protein-7 (BMP-7) is another cell homing factor [3]. Shao et al. found that a peptide sequence (E7, EPLQLKM) with seven amino acids has a high specific affinity to bone marrow-derived MSCs [92]. In the subsequent work, E7 peptide was immobilized to a collagen scaffold via a collagen-binding domain (CBD) to construct a functional collagen scaffold, which could enhance the speed of healing process [93]. It is reported that E7-modified scaffolds incorporated with rhTGF-β1 could maintain a sustained release and bioactivity. A series of analyses indicate that the E7 peptide promotes BMSC initial adhesion and that the scaffolds containing both E7 and rhTGF-β1 are the most favorable for BMSC survival (Fig. 10.9) [94].
(a) Fabrication of the radially oriented (RO) and random collagen scaffolds. (b) The radially oriented scaffolds had significantly better mechanical property compared with the random scaffolds. (c) The cell number was quantified at low magnification and the radially oriented scaffolds accelerated cell infiltration (Reprinted from Ref. [90] with permission. Copyright 2014 Elsevier Ltd)
The preparation process for coaxial electrospun fiber scaffolds. (a) The process of coaxial electrospinning: the spinneret is composed of two concentric needles; the outer needle is used to deliver the shell solution (blue, PCL), while the inner needle is used to eject the core solution (red, rhTGF-β 1). (b) Scaffold composed of electrospun coaxial fibers, with core (red) and shell (blue) structure. (c) Scaffold conjugated with the BMSC-specific affinity peptide (E7) (green). (d) E7-modified scaffold facilitates adhesion of BMSCs onto the surface. (e) The E7-modified surface and sustained release of rhTGF-β 1 in the core of the coaxial fibers promote adhesion and differentiation of BMSCs [94] (Reprinted from Ref. [94] with permission. Copyright 2014 Elsevier Ltd)
6 Conclusions and Future Perspectives
Skin regeneration is one of the most serious problems in clinical medicine. The use of skin grafts is still an important therapy for damaged skin. So far decades of efforts have focused on the development of the tissue-engineered skin based on material technologies, chemistry, biology, and medicine. Bioengineered skins for epidermal, dermal, and full-thickness defects have been fabricated, and some of them are currently commercially available. However, most of them are not sufficient to regenerate new skin similar with native skin.
To realize the regeneration of the skin with a complicated structure and complete functions, it is becoming increasingly popular to design “smart biomaterial system” to provide instructive signals to stimulate target cell responses in the processes of skin regeneration. “Smart biomaterials” can be obtained by adjusting the properties of biomaterials including physical properties, chemical compositions, and bio-functions. Meanwhile, the application of electronic devices into skin tissue engineering is able to regulate and control the environment such as temperature and wettability of the wound and thereby to provide more suitable conditions for skin regeneration. Moreover, by the combination of stem cells and in situ skin regeneration, the induced differentiation of endogenous stem cells for the in situ skin regeneration with appendages has attracted more and more interest. We believe that with the development of biomaterial science and regenerative medicine, the skin with a complicated structure and multifunction similar to those of the native skin can be regenerated sooner or later.
References
MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874–80.
Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface. 2009;6(35, Suppl 3):S311–24.
Chen FM, Wu LA, Zhang M, et al. Homing of endogenous stem/progenitor cells for in situ, tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011;32(12):3189–209.
Marston WA. Dermagraft, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Rev Med Devices. 2004;1(1):21–31.
Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863–87.
Willard JJ, Drexler JW, Das A, et al. Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Eng Part A. 2013;19(13–14):1507–18.
Cao H, Chen MM, Liu Y, et al. Fish collagen-based scaffold containing PLGA microspheres for controlled growth factor delivery in skin tissue engineering. Colloids Surf B Biointerfaces. 2015;136:1098–106.
Rnjak-Kovacina J, Wise SG, Zhe L, et al. Electrospun synthetic human elastin: collagen composite scaffolds for dermal tissue engineering. Acta Biomater. 2012;8(10):3714–22.
Bellas E, Seiberg M, Garlick J, et al. In vitro 3D full-thickness skin-equivalent tissue model using silk and collagen biomaterials. Macromol Biosci. 2012;12(12):1627–36.
Ma L, Gao C, Mao Z, et al. Thermal dehydration treatment and glutaraldehyde cross-linking to increase the biostability of collagen-chitosan porous scaffolds used as dermal equivalent. J Biomater Sci Polym Ed. 2003;14(8):861–74.
Ma L, Gao C, Mao Z, et al. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 2003;24(26):4833–41.
Wang X, You C, Hu X, et al. The roles of knitted mesh-reinforced collagen-chitosan hybrid scaffold in the one-step repair of full-thickness skin defects in rats. Acta Biomater. 2013;9(8):7822–32.
Zhong SP, Zhang YZ, Lim CT. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:510–25.
Edmonds M. Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Lower Extrem Wounds. 2009;8(1):11–8.
Montembault A, Viton C, Domard A. Physico-chemical studies of the gelation of chitosan in a hydroalcoholic medium. Biomaterials. 2005;26(8):933–43.
Ribeiro MP, Ana E, Daniela S, et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009;17(6):817–24.
Adekogbe I, Ghanem A. Fabrication and characterization of DTBP-crosslinked chitosan scaffolds for skin tissue engineering. Biomaterials. 2005;26(35):7241–50.
Hong JP, Kim YW, Lee SK, et al. The effect of continuous release of recombinant human epidermal growth factor (rh-EGF) in chitosan film on full thickness excisional porcine wounds. Ann Plast Surg. 2008;61(4):457–62.
Mizuno K, Yamamura K, Yano K, et al. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res A. 2003;64(1):177–81.
Abdelgawad AM, Hudson SM, Rojas OJ. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr Polym. 2014;100(100):166–78.
Tchemtchoua VT, Atanasova G, Aqil A, et al. Development of a chitosan nanofibrillar scaffold for skin repair and regeneration. Biomacromolecules. 2011;12(9):3194–204.
Kiyozumi T, Kanatani Y, Ishihara M, Saitoh D, Shimizu J, Yura H, et al. Medium (DMEM/F12)-containing chitosan hydrogel as adhesive and dressing in autologous skin grafts and accelerator in the healing process. J Biomed Mater Res B Appl Biomater. 2006;79:129–36.
Kiyozumi T, Kanatani Y, Ishihara M, et al. The effect of chitosan hydrogel containing DMEM/F12 medium on full-thickness skin defects after deep dermal burn. Burns. 2007;33(5):642–8.
Shevchenko RV, Eeman M, Rowshanravan B, et al. The in vitro characterization of a gelatin scaffold, prepared by cryogelation and assessed in vivo as a dermal replacement in wound repair. Acta Biomater. 2014;10(7):3156–66.
Monteiro IP, Shukla A, Marques AP, et al. Spray-assisted layer-by-layer assembly on hyaluronic acid scaffolds for skin tissue engineering. J Biomed Mater Res A. 2015;103(1):330–40.
Losi P, Briganti E, Errico C, et al. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013;9(8):7814–21.
Rezwan K, Chen QZ, Blaker JJ, et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–31.
Amani H, Dougherty WR, Blome S. Use of Transcyte® and dermabrasion to treat burns reduces length of stay in burns of all size and etiology. Burns J Int Soc Burn Injuries. 2006;32(7):828–32.
Kumbar SG, Nukavarapu SP, James R, et al. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. 2008;29(30):4100–7.
Cui W, Zhu X, Yang Y, et al. Evaluation of electrospun fibrous scaffolds of poly(dl -lactide) and poly(ethylene glycol) for skin tissue engineering. Mater Sci Eng C. 2009;29(6):1869–76.
Chen G, Sato T, Ohgushi H, et al. Culturing of skin fibroblasts in a thin PLGA-collagen hybrid mesh. Biomaterials. 2005;26(15):2559–66.
Venugopal JR, Zhang Y, Ramakrishna S. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif Organs. 2006;30(6):440–6.
Yang J, Shi G, Bei J, Wang S, Cao Y, Shang Q, et al. Fabrication and surface modification of macroporous poly (L-lactic acid) and poly (L-lactic-co-glycolic acid)(70/30) cell scaffolds for human skin fibroblast cell culture. J Biomed Mater Res. 2002;62:438–46.
Gautam S, Chou CF, et al. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater Sci Eng C Mater Biol Appl. 2014;34C(1):402–9.
Zhou Y, Yang D, Chen X, et al. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules. 2008;9(1):349–54.
Wang HM, Chou YT, Wen ZH, et al. Novel biodegradable porous scaffold applied to skin regeneration. Plos One. 2013;8(6):e56330.
Lu H, Oh HH, Kawazoe N, et al. PLLA-collagen and PLLA-gelatin hybrid scaffolds with funnel-like porous structure for skin tissue engineering. Sci Technol Adv Mater. 2012;13(6):64210–8.
Shi Y, Ma L, Zhou J, et al. Collagen/chitosan-silicone membrane bilayer scaffold as a dermal equivalent. Polym Adv Technol. 2005;16(11–12):789–94.
Rui G, Xu S, Ma L, et al. Enhanced angiogenesis of gene-activated dermal equivalent for treatment of full thickness incisional wounds in a porcine model. Biomaterials. 2010;31(28):7308–20.
Liu X, Liang J, Zhang B, et al. RNAi functionalized collagen-chitosan/silicone membrane bilayer dermal equivalent for full-thickness skin regeneration with inhibited scarring. Biomaterials. 2013;34(8):2038–48.
Vlierberghe SV, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12(5):1387–408.
Yanchun Liu MD, Cai S, Xiao ZS, et al. Release of basic fibroblast growth factor from a crosslinked glycosaminoglycan hydrogel promotes wound healing. Wound Repair Regen. 2007;15(2):245–51.
Shepherd J, Sarker P, Rimmer S, et al. Hyperbranched poly(NIPAM) polymers modified with antibiotics for the reduction of bacterial burden in infected human tissue engineered skin. Biomaterials. 2011;32(32):258–67.
Peattie RA, Nayate AP, Firpo MA, et al. Stimulation of in vivo angiogenesis by cytokine-loaded hyaluronic acid hydrogel implants. Biomaterials. 2004;25(14):2789–98.
Lee PY, Cobain E, Huard J, et al. Thermosensitive hydrogel PEG-PLGA-PEG enhances engraftment of muscle-derived stem cells and promotes healing in diabetic wound. Mol Ther J Am Soc Gene Ther. 2007;15(6):1189–94.
Miguel SP, Ribeiro MP, Brancal H, et al. Thermoresponsive chitosan-agarose hydrogel for skin regeneration. Carbohydr Polym. 2014;111(20):366–73.
Boucard N, Viton C, Agay D, et al. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials. 2007;28(24):3478–88.
Murakami K, Aoki H, Nakamura S, et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials. 2010;31(1):83–90.
Wong VW, Rustad KC, Galvez MG, et al. Engineered pullulan-collagen composite dermal hydrogels improve early cutaneous wound healing. Tissue Eng Part A. 2011;17(5–6):631–44.
Ribeiro MP, Morgado PI, Miguel SP, et al. Dextran-based hydrogel containing chitosan microparticles loaded with growth factors to be used in wound healing. Mater Sci Eng C. 2013;33(5):2958–66.
Sun G, Zhang X, Shen YI, et al. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Acad Sci U S A. 2011;108(52):20976–81.
Richardson TP, Peters MC, Ennett AB, et al. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–34.
Perets A, Baruch Y, Weisbuch F, et al. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J Biomed Mater Res A. 2003;65a(65):489–97.
Ozeki M, Tabata Y. In vivo promoted growth of mice hair follicles by the controlled release of growth factors. Biomaterials. 2003;24(13):2387–94.
Mao Z, Ma L, Zhou J, et al. Bioactive thin film of acidic fibroblast growth factor fabricated by layer-by-layer assembly. Bioconjug Chem. 2005;16(5):1316–22.
Uijtdewilligen PJE, Versteeg EMM, Gilissen C, et al. Towards embryonic-like scaffolds for skin tissue engineering: identification of effector molecules and construction of scaffolds. J Tissue Eng Regen Med. 2013;10(1):E34–44.
Shea LD, Smiley E, Bonadio J, et al. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17(6):551–4.
Hijjawi J, Mogford JE, Chandler LA, et al. Platelet-derived growth factor B, but not fibroblast growth factor 2, plasmid DNA improves survival of ischemic myocutaneous flaps. Arch Surg. 2004;139(2):142–7.
Putnam D, Doody A. RNA-interference effectors and their delivery. Crit Rev Ther Drug Carrier Syst. 2006;23(2):137–64.
Laporte LD, Rea JC, Shea LD. Design of modular non-viral gene therapy vectors. Biomaterials. 2006;27(7):947–54.
Vanden BergFoels WS. In situ tissue regeneration: chemoattractants for endogenous stem cell recruitment. Tissue Eng Part B Rev. 2014;20(1):28–39.
Tang A, Gilchrest BA. Regulation of keratinocyte growth factor gene expression in human skin fibroblasts. J Dermatol Sci. 1996;11(1):41–50.
Lin ZQ, Kondo T, Ishida Y, et al. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73(6):713–21.
Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):333.
Zhou SB, Wang J, Chiang CA, et al. Mechanical stretch upregulates SDF-1α in skin tissue and induces migration of circulating bone marrow-derived stem cells into the expanded skin. Stem Cells. 2013;31(12):2703–13.
Nakamura Y, Ishikawa H, Kawai K, et al. Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials. 2013;34(37):9393–400.
Zhang B, Liu X, Wang C, et al. Chapter 52 – bioengineering skin constructs. In: Stem cell biology and tissue engineering in dental sciences. London: Academic; 2015. p. 703–19.
Black AF, Berthod F, L’Heureux N, et al. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. Faseb J. 1998;12(13):1331–40.
O’Ceallaigh S, Herrick SE, Bluff JE, et al. Quantification of total and perfused blood vessels in murine skin autografts using a fluorescent double-labeling technique. Plast Reconstruct Surg. 2006;117(1):140–51.
O’Brien FJ, Harley BA, Yannas IV, et al. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26(4):433–41.
Pruitt Jr B, Levine NS. Characteristics and uses of biologic dressings and skin substitutes. Arch Surg. 1984;119(3):312–22.
Böttcher-Haberzeth S, Biedermann T, Klar AS, et al. Tissue engineering of skin: human tonsil-derived mesenchymal cells can function as dermal fibroblasts. Pediatr Surg Int. 2014;30(2):213–22.
Pieper JS, Wachem PBV, Luyn MJAV, et al. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials. 2000;21(16):1689–99.
Pandit AS, Feldman DS, Caulfield J. In vivo wound healing response to a modified degradable fibrin scaffold. J Biomater Appl. 1998;12(3):222–36.
Wissink MJB, Beernink R, Poot AA, et al. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J Control Release. 2000;64(1–3):103–14.
Mao Z, Shi H, Rui G, et al. Enhanced angiogenesis of porous collagen scaffolds by incorporation of TMC/DNA complexes encoding vascular endothelial growth factor. Acta Biomater. 2009;5(8):2983–94.
Guo R, Xu S, Ma L, et al. The healing of full-thickness burns treated by using plasmid DNA encoding VEGF-165 activated collagen-chitosan dermal equivalents. Biomaterials. 2011;32(4):1019–31.
Costa AMA, Desmoulire A. Mechanisms and factors involved in development of hypertrophic scars. Eur J Plast Surg. 1998;21(1):19–23.
Lappert PW. Scarless fetal skin repair: “unborn patients” and “fetal material”. Plast Reconstr Surg. 1996;98(6):1125.
Chalmers RL. The evidence for the role of transforming growth factor-beta in the formation of abnormal scarring. Int Wound J. 2011;8(3):218–23.
Samuels P, Tan AK. Fetal scarless wound healing. J Otolaryngol. 1999;28(5):296–302.
Liu W, Chua C, Wu X, et al. Inhibiting scar formation in rat wounds by adenovirus-mediated overexpression of truncated TGF-beta receptor II. Plast Reconstr Surg. 2005;115(3):860–70.
Monaghan M, Pandit A. RNA interference therapy via functionalized scaffolds. Adv Drug Deliv Rev. 2011;63:197–208.
Zhong SP, Zhang YZ, Lim CT. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(5):510–25.
Yoo BY. Application of mesenchymal stem cells derived from bone marrow and umbilical cord in human hair multiplication. J Dermatol Sci. 2010;60(2):74–83.
Jin SE, Sung JH. Hair regeneration using adipose-derived stem cells. Histol Histopathol. 2015;31:249–56.
Huang S, Xu Y, Wu C, et al. In vitro, constitution and in vivo, implantation of engineered skin constructs with sweat glands. Biomaterials. 2010;31(21):5520–5.
Huang S, Yao B, Xie J, et al. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater. 2015;32:170–7.
Horsley V, O’Carroll D, Tooze R, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126(3):597–609.
Chen P, Tao J, Zhu S, et al. Radially oriented collagen scaffold with SDF-1 promotes osteochondral repair by facilitating cell homing. Biomaterials. 2015;39:114–23.
Ho CJ, Mook LS, In YY, et al. Microenvironmental interaction between hypoxia and endothelial cells controls the migration ability of placenta-derived mesenchymal stem cells via alpha4 integrin and rho signaling. J Cell Biochem. 2015;117(5):1145–57.
Shao Z, Zhang X, Pi Y, et al. Polycaprolactone electrospun mesh conjugated with an MSC affinity peptide for MSC homing in vivo. Biomaterials. 2012;33(12):3375–87.
Wang H, Yan X, Shen L, et al. Acceleration of wound healing in acute full-thickness skin wounds using a collagen-binding peptide with an affinity for MSCs. Burns Trauma. 2014;2(4):181–6.
Man Z, Yin L, Shao Z, et al. The effects of co-delivery of BMSC-affinity peptide and rhTGF-β1 from coaxial electrospun scaffolds on chondrogenic differentiation. Biomaterials. 2014;35:5250–60.
Acknowledgement
We acknowledge financial support by the Key Science Technology Innovation Team of Zhejiang Province (2013TD02), the Natural Science Foundation of China (51322302, 20934003) and the National Key Research Program of China (2016YFC1101001).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Zheng, X., Li, Q., Ma, L., Gao, C. (2016). Skin Regeneration. In: Gao, C. (eds) Polymeric Biomaterials for Tissue Regeneration. Springer, Singapore. https://doi.org/10.1007/978-981-10-2293-7_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-2293-7_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2292-0
Online ISBN: 978-981-10-2293-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)