Abstract
Latin for “sacred bone” and so named for its recurrent role in ancient Greek and Egyptian mythology [1], the os sacrum remains the subject of much discourse and scholarship in modern-day musculoskeletal oncology. The treatment of sacral tumors demands attention to the complex interplay of anatomic, biomechanical, and oncologic considerations. However, with meticulous preoperative planning and input from a specialized multidisciplinary team, good functional and oncologic results can be obtained in the surgical management of these tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Latin for “sacred bone” and so named for its recurrent role in ancient Greek and Egyptian mythology [1], the os sacrum remains the subject of much discourse and scholarship in modern-day musculoskeletal oncology. The treatment of sacral tumors demands attention to the complex interplay of anatomic, biomechanical, and oncologic considerations. However, with meticulous preoperative planning and input from a specialized multidisciplinary team, good functional and oncologic results can be obtained in the surgical management of these tumors.
2 Anatomy
The surgical management of sacral tumors demands a detailed understanding of the bony, ligamentous, vascular, and nervous anatomy of the pelvis [2].
Bony: A single bone formed by the fusion of five vertebrae, the sacrum articulates laterally with the ileum via paired L-shaped facets. Inferior articulation (or fusion) with the coccyx involves the two horn-like coccygeal cornua and their sacral counterparts. Four pairs of anterior and four pairs of posterior foramina carry the anterior and posterior rami, respectively, of the S1-S4 nerve roots, as they emerge from the sacral canal. The termination of the sacral canal, which itself is the caudal continuation of the vertebral canal, is the sacral hiatus.
Joints and Ligamentous: The articulation between L5 and S1 involves the two zygapophyseal joints and the intervertebral disc. As a result of lordosis at this junction, the L5/S1 disc is wedge-shaped, thicker anteriorly than posteriorly. Stout iliolumbar and lumbosacral ligaments, extending from the transverse processes of L5, reinforce the lumbosacral junction. The synovial sacroiliac joints, prone to fibrosis and fusion with aging, are likewise stabilized by thick anterior and posterior ligaments. Finally, the sacrospinous and sacrotuberous ligaments serve to stabilize the bony pelvis, reinforce the lateral pelvic walls, and define the greater and lesser sciatic foramina.
Muscular: Relevant muscular anatomy about the sacrum includes the paired piriformis and coccygeus muscles, as well as the anococcygeal ligament. The piriformis originates on the anterior surface of the sacrum and exits the pelvis through the greater sciatic foramen, en route to its tendinous insertion on the greater trochanter. The piriformis serves as an important landmark within the greater sciatic foramen; contents of the suprapiriform foramen include the superior gluteal nerve and vessels, while structures exiting inferiorly include the inferior gluteal vessels, sciatic nerve, pudendal nerve, internal pudendal vessels, posterior femoral cutaneous nerve, and nerves to the obturator internus and quadratus femoris.
Contents of the lesser sciatic foramen, separated from its superior counterpart by the sacrospinous ligament, include the tendon of the obturator internus as it exits the pelvis and the pudendal nerve and internal pudendal vessels as they re-enter the pelvis. The coccygeus, which originates on the inner surface of the sacrospinous ligament, inserts on the lateral borders of the sacrum and coccyx. The anococcygeal ligament is at the midline raphe of the left and right levator ani musculature, which form the pelvic floor and help to maintain anal and vaginal closure; the anococcygeal ligament inserts posteriorly on the coccyx.
Peritoneum and Viscera: The sacrum is a retroperitoneal structure. It should be noted that the rectum is retroperitoneal as well; the upper two-thirds of this structure is draped anteriorly by peritoneum, while the lower one-third is completely uncovered by peritoneum.
Vascular: The internal iliac vascular system is relevant to surgery of the sacrum and pelvis. The paired internal iliac arteries typically branch from the common iliac arteries at the level of L5/S1, anteromedial to the SI joint. At the superior border of the greater sciatic foramen, the internal iliac artery divides into anterior and posterior trunks, which each subsequently gives rise to multiple named vessels. The posterior trunk of the internal iliac supplies the posterior pelvic wall and gluteal region; branches include the iliolumbar artery, which ascends superiorly out of the posterior pelvis and gives off a spinal branch that passes through the L5/S1 intervertebral foramen; the lateral sacral artery, which gives multiple branches that pass into each anterior sacral foramina; and the superior gluteal artery, which exits the pelvis through the suprapiriform greater sciatic foramen and supplies the abductor musculature.
Relevant branches of the anterior trunk of the internal iliac include the internal pudendal artery, which runs through Alcock’s canal with the pudendal nerve and supplies the external genitalia; the obturator artery, which exits the pelvis through the obturator canal into the adductor compartment of the thigh; the inferior gluteal artery, which exits the pelvis through the infrapiriform greater sciatic foramen and supplies the gluteus maximus and piriformis; and multiple branches to the pelvic viscera. The median sacral artery, an unpaired, midline vessel, branches off the abdominal aorta just above its bifurcation and travels down the anterior surface of the sacrum and coccyx.
Nervous: The sacral and coccygeal plexuses, formed by the anterior rami of S1-Co with contributions from L4-L5, carry primarily somatosensory fibers, with sympathetic and parasympathetic components as well. As noted above, four paired anterior and four paired posterior foramina transmit the anterior and posterior rami of the S1-S4 nerve roots. Each anterior ramus, except at the S4 level, in turn divides into ventral and dorsal divisions.
The sacral plexus gives rise to multiple somatic nerves, including the sciatic (L4-S2), superior and inferior gluteal, and pudendal nerves, as well as smaller motor branches to the quadratus femoris, gemelli, obturator internus, levator ani, and coccygeus muscles, and two sensory nerves (the posterior femoral cutaneous and perforating cutaneous). The pudendal nerve is of particular importance to surgery of the sacrum and pelvis. Arising from the ventral divisions of the anterior rami of S2-S4, the pudendal nerve exits the pelvis through the infrapiriform greater sciatic foramen, passes dorsal to the sacrospinous ligament, and re-enters the pelvis through the lesser sciatic foramen. As it does so, it courses along the lateral wall of the ischioanal fossa within Alcock’s canal (pudendal canal), inferior to the pelvic floor and accompanied by the internal pudendal vessels. The pudendal nerve innervates the skeletal muscle of the external anal and urethral sphincters as well as the levator ani and provides sensory innervation to much of the perineum and penis or clitoris. Compression or injury to the pudendal nerve or its sacral nerve roots can result in bowel, bladder, and sexual dysfunction, which is of great concern in the management of sacral tumors as discussed further below.
In addition to its somatic nervous functions, the sacral plexus provides parasympathetic visceral innervation through its contributions to the inferior hypogastric plexus of the pelvis. The S2-S4 nerve roots contribute to parasympathetic pelvic innervation, including vasodilation of the erectile tissue of the penis or clitoris, stimulation of bladder contraction during micturition, and modulation of activity of the descending colon and rectum. This parasympathetic outflow is carried by the pelvic splanchnic nerves, which originate from the S2-S4 roots. The pelvic splanchnic nerves join fibers from the superior hypogastric plexus, which descends along the posterior abdominal wall and carries both sympathetic and parasympathetic fibers, to form the inferior hypogastric plexus.
Sympathetic functions of the inferior hypogastric plexus include innervation of smooth muscle within the pelvic vasculature, internal anal and urethral sphincters, and reproductive tract (i.e., critical for the processes of ejaculation). It should be noted that sympathetic fibers within the inferior hypogastric plexus are supplied by the roots of T10-L2, but not by sacral nerve roots.
Finally, the somatic coccygeal plexus, with contributions from S4, S5, and Co, gives rise to the anococcygeal nerve, which contributes to motor and sensory innervation of the perineum.
3 Surgical Anatomy
The bony, ligamentous, and nervous anatomy of the pelvis, as related to pelvic biomechanics and function, must be considered in the course of preoperative planning. Partial transverse sacrectomies have been classified with respect to nerve root anatomy, wherein preservation of S3, S2, and S1 correspond to low, middle, and high sacrectomies, respectively [3]. The level of resection is of critical importance not only with respect to margin status but also with respect to preservation of mechanical stability and bowel, bladder, and sexual function. It should also be noted, however, that the level of bony resection does not necessarily correlate with extent of sacral root sacrifice: intraoperative oncologic considerations might require sacrifice of more cephalad nerve roots in order to achieve negative margins, or conversely, tumor location might allow for the sparing of nerve roots contralateral or caudal to the bone cut [4].
3.1 Biomechanical Considerations
Spinopelvic fixation is typically performed after total sacrectomy; in the absence of reconstruction, as elaborated by one author, the resultant “flail axial skeleton precludes the ability to ambulate” [5]. On the other hand, low partial sacrectomy does not warrant reconstruction. However, the indications for reconstruction after high or middle partial sacrectomy are less clear. Early biomechanical work performed by Gutenberg [6] found that resection through (or just cephalad to) the S1 foramina weakened the pelvic ring by 50%, while resection between the S1 and S2 foramina resulted in only 30% weakening. Regardless, the former was thought to preserve sufficient bony stability to allow for standing, and therefore it was concluded that reconstruction is not required for resections that preserve any amount of S1 body.
In contrast, a more recent cadaveric study, which purported to model physiologic loading more accurately, found that pelvises with sacral resections just caudal to the S1 foramina could withstand forces associated with postoperative mobilization, while those with resections just cephalad to the S1 foramina could not [7]. These authors highlighted the importance of at least partial preservation of the sacroiliac joint to pelvic stability and noted that bone cuts just cephalad vs. just caudal to the S1 foramina are associated with preservation of 75% vs. 84%, respectively, of the sacroiliac joint—perhaps signifying a biomechanically significant cutoff point within that range. Clinical outcomes data have largely confirmed these findings. One review found that three of nine sacrectomies involving the S1 body failed via fracture, ultimately requiring reconstruction [8]; another series reported a 76% rate of postoperative sacral insufficiency following high sacrectomy (and adjuvant radiation), as compared with 0% after low sacrectomy [9].
It should be noted, however, that not all authors advocate for reconstruction after total or high sacrectomy. Ruggieri et al. [10] have suggested that muscle and scar tissue may form a “biologic sling” between the unreconstructed pelvis and lower lumbar spine, which may migrate inferiorly toward the ilia; the use of a lumbosacral corset may increase stability and ambulatory ability in these cases [11].
3.2 Neurologic Considerations
The S3 nerve roots are thought to play a critical role in supplying normal bowel, bladder, and, to a lesser extent, sexual function. Sacrectomy with nerve root sacrifice cephalad to S3 may result in loss of normal bowel and bladder function in many [3, 8, 12] or all [6, 13] patients. Specifically, S2 may allow for weak internal and external anal sphincter activity but neither for discrimination between different rectal contents or sensation of rectal distention nor for maintenance of the micturition reflex. A review of sacrectomies performed at the Mayo Clinic found that preservation of bilateral S3 nerve roots ensured maintenance of normal bowel and bladder function in 100% and 69%, respectively, of patients; unilateral S3 preservation ensured maintenance of function in approximately two-thirds of patients. All patients with sacrifice of bilateral S2 roots had abnormal bowel and bladder function, and a minority of patients enjoyed normal bowel and bladder function with bilateral S3 sacrifice [8]. However, a more recent case series [13] found slightly improved outcomes with S3-sacrificing sacrectomies: normal bowel and bladder function in 63% and 71%, respectively.
Preservation of the S2 nerve roots—and perhaps even S1 nerve roots alone—might be sufficient for maintenance of sexual function [6, 14, 15]. Unilateral S1-S5 resection, with preservation of contralateral nerve roots, has minimal impact on bowel, bladder, or sexual function [3, 6, 8].
Investigations of patient-reported outcomes at our center, utilizing PROMIS questionnaires, have confirmed the negative impact on pain and quality of life resulting from more proximal resections. A study of 74 patients undergoing total or partial transverse sacrectomy identified significantly lower physical and mental health scores in patients for whom S3 was the most cephalad nerve root spared, as compared with those in whom S3 was preserved. Additionally, sparing of S2 resulted in higher orgasm scores. Interestingly, no difference in quality of life metrics was observed in patients with and without colostomy creation [4].
Evaluation of PROMIS questionnaire data for patients with low sacral resections (bony resection through S4 or S5) demonstrated particularly good functional outcomes. These patients, unsurprisingly, reported better physical health, mobility, and ability than patients undergoing total sacrectomies. However, quality of life and functional scores following low sacrectomy were in fact equivalent or superior to normative, general population PROMIS data [16]. High partial and total sacrectomies were also consistently associated in chronic pain in the reviews of PROMIS data [4, 16]; this finding was echoed in another review of 21 patients treated with sacrectomy for chordoma, which found that neuropathic pain persisted at final follow-up in 8 of 11 patients who presented with this complaint preoperatively [17]. As with bowel, bladder, and sexual function, therefore, chronic pain must be addressed as a known risk of high sacrectomy in preoperative patient discussions.
Of course, sacral nerve root status is only one of a number of factors that may contribute to post-sacrectomy function. High partial sacrectomies may necessitate lumbo-pelvic fixation, as noted above, which can be associated with sciatic nerve dysfunction or pain-generating hardware failure. Patients undergoing more proximal resections are also typically those with larger tumors or more significant preoperative sacral root involvement. Indeed, it has been demonstrated that the greatest predictor of postoperative bowel and bladder outcome is preoperative function [13]. Disruption of the hypogastric plexus, as may occur during a direct approach to the spine or direct injury to the pudendal nerves, can also result in bowel, bladder, or sexual dysfunction, even if the sacral nerve roots proper are preserved.
4 Clinical Management of Sacral Tumors
Sacral tumors may present with pain, perineal sensory changes, and sexual, bowel, and bladder dysfunction, the latter of which may be the result of either nervous or direct visceral compression. Additionally, given their location, sacral tumors may be quite large before symptoms arise. As with evaluation of any bone tumor, initial work-up should consist of history, physical examination, and imaging studies, including plain radiographs, CT and MRI of the sacrum and pelvis, and bone scan and CT of the chest for staging. Complete imaging of the mobile spine should be performed as well; additional lesions may be present in 17% of patients with sacral chordomas [18].
Tissue is necessary for histologic analysis and should preferably be obtained through image-guided needle (aspirate or core) biopsy. Open incisional biopsy of chordomas, especially when performed outside of the ultimate treating center, is associated with a higher risk of local recurrence, metastasis, and tumor-related death [3, 19, 20]. Analysis of sacral mass biopsy tissue should include immunohistochemical staining for cytokeratins, EMA, and vimentin, which stain strongly in chordomas, as well as for Ki-67, which is associated with a poor prognosis when present with a high degree of proliferative activity [19].
Tumor characteristics dictate surgical management, including extent of resection and use of adjuvants. Chordomas are the most common primary sacral malignancy; sacral chondrosarcomas, osteosarcomas, and Ewing’s sarcomas are seen as well. Benign primary tumors include giant cells tumors, aneurysmal bone cysts, and osteoid osteomas/osteoblastomas. Metastatic disease, multiple myeloma, and lymphomas are commonly encountered. Teratomas are the most common sacral tumors in children [21].
4.1 Management of Chordomas
Deriving from notochordal tissue, chordomas represent 1–4% of all primary malignant bone tumors and occur more commonly in the sacrum (50%) than in the skull base (35%) or the mobile spine (15%) [22]. Intralesional resection (or resection with inadequate margins) is associated with a higher rate of local recurrence (up to 83%) and, in turn, a lower survival [10, 12, 19, 22,23,24]. In one series, local recurrence was associated with a 23-fold increased risk for metastases and a 21-fold increased risk for tumor-related death [19]. Another large series found a more modest 2.5-fold increase in metastatic risk among patients with local recurrence, and survival among those with metastatic chordoma ranges from 20 months for cervical disease to 130 months for sacral disease [25]. Wide, en bloc resection is therefore recommended in the management of chordomas.
It has been suggested that tumor invasion into the piriformis or gluteus maximus muscles, or involvement of the sacroiliac joints, is an independent predictor of local recurrence, regardless of margin status at the time of resection [23]. Indeed, local recurrences may tend to occur in the posterior musculature, and wider margins may be required posteriorly as compared with anteriorly, where the presacral fascia may pose a barrier to tumor spread [23, 26].
High-dose (~70 Gy) proton/photon beam radiation therapy is now standard in the management of chordomas and other spinal malignancies at our institution. For sacral tumors, preoperative radiation of ~20 Gy is administered, with the remainder administered as a postoperative boost. A phase II clinical trial performed at the Massachusetts General Hospital, evaluating patients undergoing surgical resection of spinal chordomas and sarcomas (predominantly sacral), demonstrated that high-dose radiotherapy in addition to surgical resection was associated with a 74% rate of local control at 8-year follow-up [27, 28]. Notably, local control for primary tumors (94% at 5 years and 85% at 8 years) was far superior to that for locally recurrent tumors (~50% re-recurrence rate).
Chordomas, in particular, appear to benefit from high-dose radiation. A retrospective review of spinal chordomas treated at our institution demonstrated 5-year overall survival and local control of 81% and 62%, respectively [24]. This study found further improvement in local control when surgical resection of primary chordoma was accompanied by neoadjuvant and adjuvant radiation, as opposed to adjuvant radiation alone: 85% vs. 56% at 5 years. Most strikingly, among the 28 patients in this series who underwent en bloc resection and received both neoadjuvant and adjuvant radiation, no cases of recurrence were observed. Even in cases of margin-positive primary resection, good results may be salvaged: the use of adjuvant high-dose radiation achieved local control at 8.8 years in 10 of 11 patients with primary sacral chordomas (but in 0 of 5 patients with recurrent disease) treated at out institution [29]. Taken together, these results high lighted not only the excellent results achieved with neoadjuvant and adjuvant radiation and wide resection, but also the critical importance of initiation of aggressive treatment at first presentation.
Additionally, in patients for whom surgical resection is not feasible, due to risk of intraoperative neural injury (i.e., high sacral tumors) or medical comorbidities, definitive management with high-dose radiation is reasonable. In a review of 24 spinal chordomas, of which 19 were located in the sacrum, treated at our institution with proton or photon radiotherapy alone, local control rates were 90.4% and 79.8% at 3 and 5 years, respectively. All surviving patients maintained ambulatory status [30]. A follow-up study noted an ongoing tumor volumetric reduction up to 5 years after definitive radiation treatment [31]. However, high-dose radiation therapy to the sacrum can be associated with significant adverse effects. Specifically, delivery of greater than 77 Gy, which is required in cases of definitive treatment with radiation alone, has been associated with higher rates of neuropathy and erectile dysfunction, with lower rates of neural injury in patients receiving ~70 Gy or less [24, 27, 28, 30]. Sacral insufficiency fractures have been noted to occur in roughly half of all patients with sacral chordomas treated with definitive high-dose radiation [30, 31] and in over three-quarters of patients undergoing high sacrectomy [9]. For this reason, we avoid radiation doses greater than 70 Gy for patients undergoing surgical resection.
4.2 Management of Other Sacral Malignancies
Chondrosarcoma of the sacrum represents approximately 20% of spinal chondrosarcomas and 5% of all chondrosarcomas [32] and is the second most commonly resected primary sacral tumor after chordoma [3, 28, 33]. As is the case with other sacral malignancies, en bloc resection with negative margins is likely associated with decreased rates of local recurrence and improved disease-free survival [32, 34, 35]. Chondrosarcomas, like chordomas, are treated with high-dose neoadjuvant and adjuvant radiation, in addition to wide surgical resection, at our institution [28]. Spinal osteosarcoma is rare, but sacral involvement has been reported in 31–68% of cases [36, 37]. At our institution, treatment includes en bloc resection, when feasible, as well as high-dose radiation therapy and neoadjuvant and adjuvant chemotherapy [37], though outcome is poor and prognosis is worse for osteosarcoma of the sacrum as compared with that of the mobile spine [36].
Locally recurrent rectal cancer may be treated with aggressive re-resection, to include partial sacrectomy in cases of cortical invasion or tumor adherence to bone. Overall mean survival of 22–40 months has been reported following re-resection with sacrectomy, with improved outcomes in cases of negative margins [38,39,40]. The use of pedicled omental or rectus abdominis flaps has been described as a means of decreasing wound complications [41].
4.3 Management of Giant Cell Tumors
Traditionally, giant cell tumors (GCTs) of the sacrum have been treated with intralesional curettage, but high rates of recurrence—up to one-third to one-half of patients—have been reported [42, 43]. The authors of a review of a pooled cohort of 166 patients with sacral GCTs, therefore, recommended wide surgical resection for lower sacral lesions and for recurrent proximal sacral lesions. Notably, this study reported a 23% disease-related mortality, of which approximately one-third was related to treatment complications, at 8-year follow-up. Radiation may be utilized in cases of large or challenging sacral GCTs but is associated with high recurrence rates when used alone or as an adjuvant following curettage [42]. Radiation-related malignant transformation is also a concern [42,43,44]. Arterial embolization may represent a more successful nonoperative modality, and good results with respect to symptomatic improvement and low recurrence have been reported with the use of serial arterial embolizations (typically every 4–6 weeks) as monotherapy for sacral GCTs [45,46,47]. Additionally, embolization may be performed concurrently with local intra-arterial injection of cisplatin [47] or may be employed as a preoperative adjunct to limit surgical bleeding [48].
5 Total and Partial Sacrectomy: Surgical Techniques
Meticulous preoperative planning is a necessity for successful surgical management of sacral tumors. Preoperative considerations include options for preservation of fertility, plastic surgery consultation, and vascular embolization, while intraoperative considerations include choice of approach (combined anterior-posterior vs. posterior-only), use of computer-assisted navigation, and method of spinopelvic fixation, when needed.
5.1 Preoperative Preparation
We routinely refer reproductive-aged female patients for gynecologic evaluation prior to initiation of oncologic treatment. If desired, laparoscopic oophoropexy may be performed prior to initiation of radiation therapy, relocating the ovaries within the abdominal cavity such that they are outside of the planned radiation field, thereby preserving future fertility options.
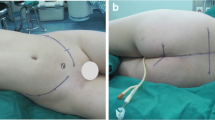
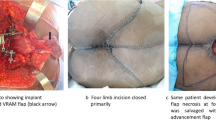
Preoperative consultation with a plastic surgeon should also be pursued prior to sacral resection; wound closure often proves difficult, given location, extent of surgery, prior radiation, and poor patient nutrition. Options for soft tissue wound closure include mobilization of pedicled omental flap; transpelvic vertical rectus abdominis myocutaneous (VRAM) flap, fed by the inferior epigastric vessels; and local tissue advancement techniques. A reconstructive algorithm put forth by Miles et al. [49] recommends that bilateral gluteus advancement flaps are associated with the lowest complication rate among reconstructive options and therefore should be the first choice for post-sacrectomy closure if the gluteal vessels are intact; transpelvic VRAM reconstruction should be performed in the setting of preoperative radiation, though is contraindicated for patients with prior laparotomy; and free flap coverage may be performed if other options are unavailable. In their review of outcomes following surgical management of sacral chordomas, Schwab et al. [12] found a significant decrease in wound complications in patients in whom rectus flap coverage was performed, as compared with patients for whom no flap coverage was performed. Reconstruction of the pelvic floor can also prove problematic; sacroperineal hernia is a rare complication but a risk in cases of large pelvic floor defects. Good results have been reported with reconstruction utilizing acellular dermal collage grafts [50] or mesh [51].
Intraoperative blood loss may be minimized by bilateral internal iliac ligation, which is the standard practice at our institution; however, preoperative embolization and intraoperative aortic balloon pump occlusion are options as well. Embolization of the internal iliac, median sacral, and other tumor-feeding arteries, typically within 24 h of surgery, may be performed with Gelfoam, thereby potentially minimizing longer-term devascularization of healthy tissues [52]. Additionally, the use of occlusive aortic balloon pump has been reported as a technique to reduce intraoperative blood loss in sacral tumor resections [53, 54]. In their review of 215 sacral resections, Tang et al. [53] found that balloon occlusion, utilized in 120 patients, was associated with significantly lower blood loss (2.2 vs. 3.9 L).
5.2 Staged Anterior and Posterior Approaches for Total and Partial Sacrectomy
5.2.1 Anterior Approach
Following induction of general anesthesia, administration of antibiotics for coverage of both bowel and skin flora, and establishment of central venous access, the patient is positioned supine on a flexible Jackson table, broken in the middle to improve intraoperative sacral exposure. With the assistance of a general surgeon, a low midline incision is made from the pubis to the umbilicus, and the peritoneal cavity is entered. An Omni retractor is utilized. The rectosigmoid colon and left ureter are mobilized away from the tumor, pelvic side wall, and sacrum; this process is repeated on the right side. Great care must be taken not to inadvertently enter the tumor in the course of mobilizing the overlying tissues. For chordomas in particular, violation of the tumor pseudocapsule confers a poor chance for curative resection. Subsequently, following identification and mobilization of the common, internal, and external iliac arteries, the trunks of the left and right internal iliac arteries and veins are ligated just distal to their respective bifurcations; large branches, such as the gluteal and iliolumbar vessels, may be identified and individually ligated as well. Exposure of the anterior sacrum will typically also require ligation of the median sacral artery. The L5 nerve root, which is closely associated with the anterior surface of the sacral ala, is identified and protected. The L5 root may be traced distally to facilitate further exploration of the lumbosacral plexus and identification of the sciatic nerve within the greater sciatic foramen.
En bloc partial sacrectomy: If a partial sacrectomy is to be performed, a high-speed burr is used to create a transverse osteotomy through the anterior sacral cortex, cephalad to the tumor. An intraoperative radiograph is made to assess the level of the osteotomy. The osteotomy is started in the midline, advanced laterally toward the left and right SI joints (or toward the greater sciatic foramina, in cases of more distal resections), and continued posteriorly until the posterior sacral cortex is reached. As the osteotomy is extended laterally from the midline, exiting nerve roots cephalad to the level of the cut are protected and gently retracted laterally; the more proximal L5 nerve root will need to be retracted as well. Additionally, osteotomies at the S1-S2 level may place the superior gluteal vessels at particular risk [55]. A Cobb elevator can be used to deepen the osteotomy and score the posterior cortex. Throughout the creation of the osteotomy, hemostasis is maintained with silk ligatures, monopolar and bipolar electrocautery, Floseal and Surgicel, epinephrine-soaked sponges, and bone wax. Prior to closure, a Gore-Tex patch may be placed ventral to the sacrum but dorsal to the rectum and left in place until the second stage, at which point it will serve as a landmark for the safe anterior extent of the dissection.
En bloc total sacrectomy: Following anterior approach and exposure, a spinal needle is placed into the L5/S1 disc space (for tumors that do not extend cephalad to S1), and correct level is confirmed on a lateral radiograph. As noted above, the L5 nerve roots and internal iliac vessels (in particular, the artery on the right) should be identified and protected in the course of dissection anterior to the L5/S1 junction and sacral ala. Discectomy is performed at this level with curettes, Kerrison rongeurs, and endplate elevators, extending dorsally to the posterior annulus. Using a high-speed burr, longitudinal osteotomies are created through the anterior cortices of the posterior ilia bilaterally. The resultant troughs are packed with bone wax for hemostasis; osteotomies will be completed posteriorly during the second-stage procedure. The location of the osteotomies with respect to the sacroiliac joints is dictated by tumor size and margin considerations; for small, midline sacral tumors, the osteotomies may even be created within the sacral ala, thereby preserving the sacroiliac joints.
Coverage with a pedicled rectus abdominis flap is standard practice at our institution. Following completion of the anterior osteotomy, the VRAM flap is harvested by the plastic surgeon. Typically, the flap, once raised, is dunked into the pelvis and secured with a Prolene suture to assist in subsequent orientation. Blake drains are placed within the anterior pelvis. The patient is taken to the intensive care unit for further resuscitation between stages. Vena cava filter is typically placed on the first postoperative day, to obviate the need for chemical DVT prophylaxis. Additionally, we obtain a pelvic CT scan between stages for assessment of the osteotomy level. The posterior procedure is performed 3–5 days later.
5.2.2 Posterior Approach
The patient is placed prone, in a position of neutral lumbar lordosis, and a midline incision is made. For large masses with dorsal extension into the superficial soft tissues, a skin paddle may be removed with the specimen; biopsy tracts should also be ellipsed out with the incision. Dissection proceeds through fascia to the spinous process of L5. Dissection proceeds laterally, elevating the paraspinal musculature proximally, and more distally and laterally, elevating or transecting the gluteus maximus to expose the ilium. Medially, the sacral ala is exposed, as are the adjacent transverse process and lamina of L5. After removal of spinous processes with a Leksell rongeur, laminectomy just cephalad to the level of the planned sacral osteotomy is performed with a diamond-tipped burr and Leksell and Kerrison rongeurs. The dorsal aspect of the dura is dissected free of adhesions to the overlying lamina with a Stevens scissors and Penfield 4. The laminectomy may be widened laterally with a small osteotome. The bilateral nerve roots at L5, S1, and any additional caudal levels to be preserved are exposed laterally and mobilized with a Kerrison rongeur and Stevens tenotomy scissors. The thecal sac is then circumferentially exposed at the level of the planned resection, just caudal to the axilla of the exiting nerve roots, and a right-angled clamp is passed ventral to the dura. The thecal sac is ligated with 2–0 silk suture and clips and then sharply transected. Later in the procedure, after removal of the mass, the transected end of the thecal sac should be covered with dural sealant as well.
Dissection is carried laterally and caudally, elevating the gluteus maximus musculature; a Versa-Trac self-retaining retractor is helpful in maintaining exposure. The extent of lateral exposure will be dictated, in part, by the soft tissue extension of the tumor. Dissection proceeds caudally along the lateral borders of the sacrum, exposing and identifying the piriformis, as well as the sacrotuberous and sacrospinous ligaments. Sizeable traversing vessels are typically encountered about these structures and will need to be ligated; the use of a bipolar sealer (such as the Aquamantys) can be of benefit here. The piriformis and ligaments are subsequently divided, allowing access into the pelvic cavity through the greater sciatic foramen. Now, using blunt dissection, a plane may be developed ventral to the tumor and dorsal to the rectum. The sciatic nerve should be identified and protected throughout. Dissection should be continued caudally until the tip of the coccyx is palpable.
En bloc partial sacrectomy: An intraoperative radiograph is made to assess the correct level for completion of the sacral osteotomy. A high-speed burr and Cobb elevator are used to complete the osteotomy in the midline. As the osteotomy is continued laterally, nerve roots cephalad to the level of resection should be identified, dissected free from overlying bone with a high-speed burr, and protected. The osteotomy is eventually completed laterally into the left and right sciatic foramina. The mass is now lifted caudally, carefully elevating it off of the adjacent rectum; a Gore-Tex patch, if placed ventral to the sacrum during the first stage, will serve to identify the safe plane between the sacrum and rectum. Nerve roots passing through the tumor are sacrificed. Vessels extending into the specimen are clamped and ligated. Remaining soft tissue attachments to the pelvic floor are transected, the specimen is passed off, and hemostasis of the resection bed is obtained.
En bloc total sacrectomy: Posterior exposure is performed as detailed above. The thecal sac is ligated just cephalad to the L5/S1 disc space after laminectomy is performed at this level. The posterior L5/S1 discectomy is completed. The bilateral iliac osteotomies, initiated during the anterior approach, are completed posteriorly using a high-speed burr and Cobb elevator. The sacrum and mass are lifted out of the wound, as described above.
Spinopelvic Reconstruction: As discussed in greater detail above, the extent of sacral resection impacts the degree of biomechanical instability imparted and therefore the need for spinopelvic fixation. Total sacrectomy warrants spinopelvic reconstruction. Multiple techniques for reconstruction of the bony defect after sacrectomy have been reported. Dickey et al. [5] described a triangular construct in which two segments of fibular autograft are oriented in an inverted “V,” such that the apex is “docked” into the inferior endplate of L5 and each limb is buttressed against the inner table of the ilium, along the iliopectineal line. The authors note that the oval “receptacles” in ilium need to be fashioned, utilizing a high-speed burr, during the anterior approach. This construct allows for the structural bone graft to be placed along the force transmission lines between the acetabulum and lumbar spine. As discussed in greater detail below, vascularized fibular grafts can be used in a similar fashion.
Instrumentation is then performed: first, a wide osteotome is used to remove the dorsal cortex of the posterior superior iliac spines, which may be morselized for use as bone graft. Two iliac screws are placed on each side; orienting the pedicle probe toward the ipsilateral greater trochanter helps with proper placement. Standard pedicle screws are placed bilaterally at L4 and L5. A total of four lumbo-pelvic rods are used: on each side, rods are fixed to the heads of the L4, L5, and proximal iliac screws. Rod-to-rod connectors are used to incorporate two additional, parallel rods, one on each side. A horizontal transiliac rod is fixed to the two distal iliac screws. In cadaveric biomechanical testing, this dual-rod, double iliac screw construct has been shown to be stiffer than constructs involving single-rod, single iliac screws, or Galveston rods (in which the distal end of the spinopelvic rod itself is driven between the inner and outer tables of the pelvis) [56]. Cross-link connectors may be used proximally between the parallel lumbo-pelvic rods and distally between the lumbo-pelvic and transiliac rods, to provide additional stability. Burr decortication of the lumbar posterior elements is performed, and morselized autograft and allograft bone is laid down to promote fusion. We routinely make use of calcium phosphate scaffold graft as well.
Additionally, our practice is to perform limited spinopelvic reconstruction for partial sacrectomies cephalad to the S4 foramina, especially in cases of neoadjuvant radiation, which we feel significantly retards the fusion process. In these cases, L5-ilium instrumentation is performed with single rods and double iliac screws bilaterally and a horizontal transiliac rod. Complete exposure and thorough decortication of the remnant sacroiliac joints will increase the probability of fusion.
Vascularized autograft reconstruction: Though not routinely performed at our institution, the use of vascularized fibular autografts in reconstruction after total sacrectomy has been described. Choudry et al. [57] reported on the use of bilateral vascularized fibular grafts, fashioned into an inverted V at the spinopelvic junction as described above; the pedicles are anastomosed with the internal iliac vessels (presumably prohibiting intraoperative iliac ligation). In this study, bony fusion was observed on CT scan at 6 months. Anastomosis of free fibular grafts into the deep inferior epigastric pedicle of the mobilized VRAM flap has also been described [58]. This “vertical rectus abdominis musculocutaneous flow-through flap” technique has the advantage of permitting ligation of the internal iliac vessels during anterior approach. These authors noted arthrodesis on imaging within 3 months of surgery. It should be noted that the added complexity of this procedure, which in some cases required the use of a saphenous vein graft to increase fibular pedicle length, necessitated a three-stage procedure in some cases.
Computer-assisted navigation: Intraoperative CT-guided navigation is now routinely used at our institution. Following anterior sacral exposure during the first-stage procedure, the anterior iliac crest is exposed through a small longitudinal incision. The navigation system tracker is affixed to two Steinman pins driven through the iliac crest, between the inner and outer tables. Intraoperative CT scan is performed. Navigation subsequently aids in identification of the correct osteotomy level and assessment of anterior-posterior depth during creation of the osteotomy. During the posterior procedure, the tracker is affixed to one of the lumbar spinous processes, and CT scan is performed after laminectomy and nerve root exposure. Navigation is then used to confirm that the posterior osteotomy is being performed at the same level as the anterior osteotomy.
5.2.3 Rebar Technique for Reconstruction After Curettage of Proximal Sacral Tumors
In select circumstances, benign tumors (i.e., GCTs) within the proximal sacrum, treated with curettage of the S1 body, may be amenable to reconstruction with an anterior “rebar” technique and limited posterior spinopelvic stabilization. We prefer, in these cases, to perform the posterior intralesional resection and spinopelvic instrumentation as a first stage. Laminectomies of the proximal sacrum and L5 are performed, the filum terminale and sacral nerve roots are dissected and protected, and a Gore-Tex patch is placed ventral to the nervous structures; residual, inaccessible anterior tumor is left for completion of resection in the second stage. As compared with that for a total sacrectomy, less extensive instrumentation is required: single, bilateral L5-ileum spinal rods, with a single horizontal trans-iliac rod, are sufficient. The second stage involves a standard transperitoneal approach and resection of the remnant anterior tumor and S1 body. A high-speed burr, curettes, and Kerrison rongeurs are used to excise the tumor in a piecemeal fashion. If the posterior approach was performed previously, the Gore-Tex patch, placed ventral to the sacral nerve roots, will be encountered and will signify the dorsal extent of safe dissection.
Reconstruction commences with the placement of one or two large fragment screws into both the inferior endplate of L5 and exposed superior aspect of S2. The screws may be placed in a retrograde or antegrade fashion to maximize cortical purchase, such that either the heads or the tips are exposed within the defect. Antibiotic-laden bone cement is then introduced into the defect and molded around the exposed screws. A Gore-Tex patch or segment of Esmarch tourniquet may be utilized as a temporary dorsal “backstop,” preventing damage to the underlying nerve roots as the wet cement cures.
5.3 Posterior-Only Approach for Total Sacrectomy
While staged anterior-posterior approaches are the preferred option for total sacrectomy at our institution, other authors have advocated for posterior-only approaches. Good oncologic outcomes with low rates of bowel or vascular injury have been reported with posterior-only total sacrectomy [17, 20]. The use of radiopaque gauze, packed anteriorly to the sacrum, from above the iliac crests and through the greater sciatic foramina, has been described as a method of tamponading anterior bleeding and displacing the iliac vasculature and pelvic viscera away from the osteotomy sites [17]. Disadvantages of a posterior-only approach include blind dissection of the rectum off of the anterior surface of the sacrum and inability to ligate the internal iliac vasculature as a method of decreasing blood loss. Additionally, it is impossible to raise a rectus abdominis flap from the posterior-only approach, though proponents have argued that preservation of the gluteal vessels (and internal iliac system as a whole) decreases soft tissue ischemia and allows for successful closure with gluteus maximus myocutaneous flaps [20]. Potential contraindications to posterior-only total sacrectomy include direct tumor invasion of the rectum or iliac vessels or tumor extension cephalad to the L5/S1 disc space [20].
Our preference is for staged anterior-posterior approaches for both total and partial sacrectomies, especially for malignant tumors in which curative resection is the goal. In addition to the aforementioned benefits of decreased blood loss (after internal iliac ligation) and safer dissection about the rectum and pelvic vasculature, we feel that negative margins can be more reliably obtained from a combined approach. Tumor visualization is best when approached anteriorly, and “scoring” the anterior cortical osteotomy during the first stage prevents subsequent “lifting up” and splintering of the anterior cortex, which can occur in posterior-only osteotomies and which can result in less precise margins.
6 Complications
Complications following sacrectomy are common, including surgical site infection, hardware failure, vascular or visceral injury, and CSF leak and pseudomeningocele formation; postoperative bowel, bladder, and sexual dysfunctions are typically expected, as discussed above, following sacrifice of the upper sacral nerve roots. Incidence of major complications is likely increased in total and high partial sacrectomies: 85% vs. 29% for resections above vs. below S2 in one review [59]. In a review of 46 patients undergoing partial or total sacrectomy, Sciubba et al. [60] reported postoperative infection in 39% of patients. Identified risk factors included prior lumbosacral surgery and number of surgeons scrubbed into the case, regardless of length of operation. While Staph. aureus was the most frequently cultured organism, the relatively high rate of gram negative infection led the authors to posit that proximity to the rectum, especially in the context of bowel (or bladder) dysfunction, might increase the risk of surgical site infection after sacrectomy. Interestingly, another study of postoperative complications described two delayed rectal perforations, both within 2–3 weeks of sacrectomy, thought to result from bowel ischemia in the setting of internal iliac ligation [51]. This series, reporting on complications in 34 patients with primary sacral tumors, identified five infections, three cases of postoperative foot drop (likely resulting from L5 nerve root injury), one case of cutaneous CSF fistula formation, and three perioperative deaths, highlighting the significant risks associated with these surgeries. Hardware failure occurred after total sacrectomy in 16% of patients included in a systematic review [33], though our experience suggests that the use of adjuvant high-dose radiation significantly increases the risk of postoperative fracture (up to 57% [9]), as well as nonunion and hardware failure. Radiation likely increases the risk of infection as well. A recent report from our institution identified wound infections in 10 of 60 patients (17%) undergoing resection of spinal chordomas after neoadjuvant radiation and in 7 of 58 patients (12%) undergoing resection with adjuvant radiation alone [24].
References
Sugar O. How the sacrum got its name. JAMA. 1987;257(15):2061–3.
Drake RL, Vogl W, Mitchell AW, Tibbitts R, Richardson P, Horn A. Gray’s anatomy for students. Philadelphia: Churchill Livingstone; 2010.
Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, Weber KL, Suki D, Gallia GL, Garonzik I, Gokaslan ZL. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine. 2005;3(2):111–22.
van Wulfften Palthe OD, Houdek MT, Rose PS, Yaszemski MJ, Sim FH, Boland PJ, Healey JH, Hornicek FJ, Schwab JH. How does the level of nerve root resection in en bloc Sacrectomy influence patient-reported outcomes? Clin Orthop Relat Res. 2017;475(3):607–16.
Dickey ID, Hugate RR Jr, Fuchs B, Yaszemski MJ, Sim FH. Reconstruction after total sacrectomy: early experience with a new surgical technique. Clin Orthop Relat Res. 2005;438:42–50.
Gunterberg B. Effects of major resection of the sacrum Oinical studies on urogenital and Anorectal function and a biomechanical study on pelvic strength. Acta Orthop Scand. 1976;47(sup162):1–38.
Hugate RR Jr, Dickey ID, Phimolsarnti R, Yaszemski MJ, Sim FH. Mechanical effects of partial sacrectomy: when is reconstruction necessary? Clin Orthop Relat Res. 2006;450:82–8.
Todd LT Jr, Yaszemski MJ, Currier BL, Fuchs B, Kim CW, Sim FH. Bowel and bladder function after major sacral resection. Clin Orthop Relat Res. 2002;397:36–9.
Osler P, Bredella MA, Hess KA, Janssen SJ, Park CJ, Chen YL, DeLaney TF, Hornicek FJ, Schwab JH. Sacral insufficiency fractures are common after high-dose radiation for sacral Chordomas treated with or without surgery. Clin Orthop Relat Res. 2016;474(3):766–72.
Ruggieri P, Angelini A, Ussia G, Montalti M, Mercuri M. Surgical margins and local control in resection of sacral chordomas. Clin Orthop Relat Res. 2010;468(11):2939–47.
Guo Y, Yadav R. Improving function after total sacrectomy by using a lumbar-sacral corset. Am J Phys Med Rehabil. 2002;81(1):72–6.
Schwab JH, Healey JH, Rose P, Casas-Ganem J, Boland PJ. The surgical management of sacral chordomas. Spine. 2009;34(24):2700–4.
Moran D, Zadnik PL, Taylor T, Groves ML, Yurter A, Wolinsky JP, Witham TF, Bydon A, Gokaslan ZL, Sciubba DM. Maintenance of bowel, bladder, and motor functions after sacrectomy. Spine J. 2015;15(2):222–9.
Gunterberg B, Petersén I. Sexual function after major resections of the sacrum with bilateral or unilateral sacrifice of sacral nerves. Fertil Steril. 1976;27(10):1146–53.
Gunterberg B, Kewenter J, Petersen I, Stener B. Anorectal function after major resections of the sacrum with bilateral or unilateral sacrifice of sacral nerves. Br J Surg. 1976;63(7):546–54.
Phukan R, Herzog T, Boland PJ, Healey J, Rose P, Sim FH, Yazsemski M, Hess K, Osler P, DeLaney TF, Chen YL. How does the level of sacral resection for primary malignant bone tumors affect physical and mental health, pain, mobility, incontinence, and sexual function? Clin Orthop Relat Res. 2016;474(3):687–96.
Asavamongkolkul A, Waikakul S. Wide resection of sacral chordoma via a posterior approach. Int Orthop. 2012;36(3):607–12.
Sebro R, DeLaney TF, Hornicek F, Schwab J, Choy E, Nielsen GP, Rosenthal DI. Frequency and risk factors for additional lesions in the axial spine in subjects with Chordoma: indications for screening. Spine (Phila Pa 1976). 2016;42(1):E37–40.
Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine. Cancer. 2000;88(9):2122–34.
Clarke MJ, Dasenbrock H, Bydon A, Sciubba DM, McGirt MJ, Hsieh PC, Yassari R, Gokaslan ZL, Wolinsky JP. Posterior-only approach for en bloc sacrectomy: clinical outcomes in 36 consecutive patients. Neurosurgery. 2012;71(2):357–64.
Deutsch H, Mummaneni PV, Haid RW, Rodts GE, Ondra SL. Benign sacral tumors. Neurosurg Focus. 2003;15(2):1–3.
Sundaresan N. Chordomas. Clin Orthop Relat Res. 1986;204:135–42.
Hanna SA, Aston WJS, Briggs TWR, Cannon SR, Saifuddin A. Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res. 2008;466(9):2217–23.
Rotondo RL, Folkert W, Liebsch NJ, Chen YLE, Pedlow FX, Schwab JH, Rosenberg AE, Nielsen GP, Szymonifka J, Ferreira AE, Hornicek FJ. High-dose proton-based radiation therapy in the management of spine chordomas: outcomes and clinicopathological prognostic factors. J Neurosurg Spine. 2015;23(6):788–97.
Young VA, Curtis KM, Temple HT, Eismont FJ, DeLaney TF, Hornicek FJ. Characteristics and patterns of metastatic disease from Chordoma. Sarcoma. 2015;2015:517657.
Yonemoto T, Tatezaki SI, Takenouchi T, Ishii T, Satoh T, Moriya H. The surgical management of sacrococcygeal chordoma. Cancer. 1999;85(4):878–83.
DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Dean S, Yeap BY, McManus P, Rosenberg AE, Nielsen GP, Harmon DC, Spiro IJ. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74(3):732–9.
DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Weyman EA, Yeap BY, Depauw N, Nielsen GP, Harmon DC, Yoon SS, Chen YL. Long-term results of phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110(2):115–22.
Park L, DeLaney TF, Liebsch NJ, Hornicek FJ, Goldberg S, Mankin H, Rosenberg AE, Rosenthal DI, Suit HD. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys. 2006;65(5):1514–21.
Chen YL, Liebsch N, Kobayashi W, Goldberg S, Kirsch D, Calkins G, Childs S, Schwab J, Hornicek F, DeLaney T. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine. 2013;38(15):E930–6.
Kabolizadeh P, Chen YL, Liebsch N, Hornicek F, Schwab J, Choy E, Rosenthal D, Niemierko A, DeLaney TF. Updated outcome and analysis of tumor response in Mobile spine and sacral Chordoma treated with definitive high dose photon/proton radiotherapy. Int J Radiat Oncol Biol Phys. 2016;97:254–62.
Stuckey RM, Marco RA. Chondrosarcoma of the mobile spine and sacrum. Sarcoma. 2011;2011:274281.
Bederman SS, Shah KN, Hassan JM, Hoang BH, Kiester PD, Bhatia NN. Surgical techniques for spinopelvic reconstruction following total sacrectomy: a systematic review. Eur Spine J. 2014;23(2):305–19.
Bergh P, Gunterberg B, Meis-Kindblom JM, Kindblom LG. Prognostic factors and outcome of pelvic, sacral, and spinal chondrosarcomas. Cancer. 2001;91(7):1201–12.
Hsieh PC, Xu R, Sciubba DM, McGirt MJ, Nelson C, Witham TF, Wolinksy JP, Gokaslan ZL. Long-term clinical outcomes following en bloc resections for sacral chordomas and chondrosarcomas: a series of twenty consecutive patients. Spine. 2009;34(20):2233–9.
Ozaki T, Flege S, Liljenqvist U, Hillmann A, Delling G, Salzer-Kuntschik M, Jürgens H, Kotz R, Winkelmann W, Bielack SS. Osteosarcoma of the spine. Cancer. 2002;94(4):1069–77.
Schoenfeld AJ, Hornicek FJ, Pedlow FX, Kobayashi W, Garcia RT, DeLaney TF, Springfield D, Mankin HJ, Schwab JH. Osteosarcoma of the spine: experience in 26 patients treated at the Massachusetts General Hospital. Spine J. 2010;10(8):708–14.
Dozois EJ, Privitera A, Holubar SD, Aldrete JF, Sim FH, Rose PS, Walsh MF, Bower TC, Leibovich BC, Nelson H, Larson DW. High sacrectomy for locally recurrent rectal cancer: can long-term survival be achieved? J Surg Oncol. 2011;103(2):105–9.
Wells BJ, Stotland P, Ko MA, Al-Sukhni W, Wunder J, Ferguson P, Lipa J, Last L, Smith AJ, Swallow CJ. Results of an aggressive approach to resection of locally recurrent rectal cancer. Ann Surg Oncol. 2007;14(2):390–5.
Zacherl J, Schiessel R, Windhager R, Herbst F, Karner-Hanusch J, Kotz R, Jakesz R, Teleky B. Abdominosacral resection of recurrent rectal cancer in the sacrum. Dis Colon Rectum. 1999;42(8):1035–9.
Melton GB, Paty PB, Boland PJ, Healey JH, Savatta SG, Casas-Ganem JE, Guillem JG, Weiser MR, Cohen AM, Minsky BD, Wong WD. Sacral resection for recurrent rectal cancer: analysis of morbidity and treatment results. Dis Colon Rectum. 2006;49(8):1099–107.
Leggon RE, Zlotecki R, Reith J, Scarborough MT. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004;423:196–207.
Turcotte RE, Sim FH, Unni KK. Giant cell tumor of the sacrum. Clin Orthop Relat Res. 1993;291:215–21.
Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone. J Bone Joint Surg Am. 1970;52(4):619–64.
Gottfried ON, Schmidt MH, Stevens EA. Embolization of sacral tumors. Neurosurg Focus. 2003;15(2):1–4.
Hosalkar HS, Jones KJ, King JJ, Lackman RD. Serial arterial embolization for large sacral giant-cell tumors: mid-to long-term results. Spine. 2007;32(10):1107–15.
Lin PP, Guzel VB, Moura MF, Wallace S, Benjamin RS, Weber KL, Morello FA, Gokaslan ZL, Yasko AW. Long-term follow-up of patients with giant cell tumor of the sacrum treated with selective arterial embolization. Cancer. 2002;95(6):1317–25.
Raskin KA, Schwab JH, Mankin HJ, Springfield DS, Hornicek FJ. Giant cell tumor of bone. J Am Acad Orthop Surg Glob Res Rev. 2013;21(2):118–26.
Miles WK, Chang DW, Kroll SS, Miller MJ, Langstein HN, Reece GP, Evans GR, Robb GL. Reconstruction of large sacral defects following total sacrectomy. Plast Reconstr Surg. 2000;105(7):2387–94.
Abhinav K, Shaaban M, Raymond T, Oke T, Gullan R, Montgomery ACV. Primary reconstruction of pelvic floor defects following sacrectomy using Permacol™ graft. Eur J Surg Oncol. 2009;35(4):439–43.
Zileli M, Hoscoskun C, Brastianos P, Sabah D. Surgical treatment of primary sacral tumors: complications associated with sacrectomy. Neurosurg Focus. 2003;15(5):1–8.
Yang HL, Chen KW, Wang GL, Lu J, Ji YM, Liu JY, Wu GZ, Gu Y, Sun ZY. Pre-operative transarterial embolization for treatment of primary sacral tumors. J Clin Neurosci. 2010;17(10):1280–5.
Tang X, Guo W, Yang R, Tang S, Dong S. Use of aortic balloon occlusion to decrease blood loss during sacral tumor resection. J Bone Joint Surg Am. 2010;92(8):1747–53.
Yang L, Chong-qi T, Hai-bo S, Lan Z, Tian-fu Y, Hong D, Fu-xing P. Applying the abdominal aortic-balloon occluding combine with blood pressure sensor of dorsal artery of foot to control bleeding during the pelvic and sacrum tumors surgery. J Surg Oncol. 2008;97(7):626–8.
Zoccali C, Skoch J, Patel A, Walter CM, Maykowski P, Baaj AA. The surgical neurovascular anatomy relating to partial and complete sacral and sacroiliac resections: a cadaveric, anatomic study. Eur Spine J. 2015;24(5):1109–13.
Mindea SA, Chinthakunta S, Moldavsky M, Gudipally M, Khalil S. Biomechanical comparison of spinopelvic reconstruction techniques in the setting of total sacrectomy. Spine. 2012;37(26):E1622–7.
Choudry UH, Moran SL, Karacor Z. Functional reconstruction of the pelvic ring with simultaneous bilateral free fibular flaps following total sacral resection. Ann Plast Surg. 2006;57(6):673–6.
Garvey PB, Clemens MW, Rhines LD, Sacks JM. Vertical rectus abdominis musculocutaneous flow-through flap to a free fibula flap for total sacrectomy reconstruction. Microsurgery. 2013;33(1):32–8.
Devin C, Chong PY, Holt GE, Feurer I, Gonzalez A, Merchant N, Schwartz HS. Level-adjusted perioperative risk of sacral amputations. J Surg Oncol. 2006;94(3):203–11.
Sciubba DM, Nelson C, Gok B, McGirt MJ, McLoughlin GS, Noggle JC, Wolinsky JP, Witham TF, Bydon A, Gokaslan ZL. Evaluation of factors associated with postoperative infection following sacral tumor resection: clinical article. J Neurosurg Spine. 2008;9(6):593–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature B.V.
About this chapter
Cite this chapter
Hornicek, F.J. (2020). Two-Stage Total Sacrectomy. In: Guo, W., Hornicek, F., Sim, F. (eds) Surgery of the Pelvic and Sacral Tumor. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1945-0_26
Download citation
DOI: https://doi.org/10.1007/978-94-024-1945-0_26
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1943-6
Online ISBN: 978-94-024-1945-0
eBook Packages: MedicineMedicine (R0)