Abstract
Major retroperitoneal vessels include the abdominal aorta and its major branches, namely, the common external iliac artery, renal artery, celiac artery, and superior mesenteric artery, as well as the inferior vena cava and its main tributaries, namely, the common external iliac vein and renal vein. Retroperitoneal tumors (RPTs) often invade the major vessels by pushing, wrapping, or even growing into them. For RPTs invading major vessels, surgeons often give up complete resection as they worry about causing damage to vessels infiltrated or wrapped by tumors during the separation. Vascular injury may result in uncontrolled heavy bleeding and is regarded as a contraindication to RPT surgery. With advances in surgical techniques, combined resection of major involved vessels (with or without vascular grafts) in RPT surgery has become an increasingly common avenue to significantly improve the successful rate of tumor resection. Preoperatively comprehensive evaluation of RPTs involving major blood vessels and surrounding organs is essential to a successful operation. B-ultrasound, enhanced CT, and MRI can clearly display the location, size, shape of the tumor, and surrounding organ involvement, particularly the relationship between the tumor and major vessels (such as the abdominal aorta, inferior vena cava, renal artery, iliac artery, superior mesenteric artery, and portal veins); the displacement, compression, or wrapping of blood vessels by the tumor; as well as tumor thrombus within the blood vessels. Preoperative angiography combined with embolization can be used to determine the relationship between the tumor and blood vessels and significantly reduce blood loss intraoperatively by decreasing the blood supply to the tumor.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Retroperitoneal Tumors (RPTs)
- Superior Mesenteric
- Inferior Vena Cava Wall
- Tumor Thrombus
- Left Renal Vein
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Major retroperitoneal vessels include the abdominal aorta and its major branches, namely, the common external iliac artery, renal artery, celiac artery, and superior mesenteric artery, as well as the inferior vena cava and its main tributaries, namely, the common external iliac vein and renal vein. Retroperitoneal tumors (RPTs) often invade the major vessels by pushing, wrapping, or even growing into them. For RPTs invading major vessels, surgeons often give up complete resection as they worry about causing damage to vessels infiltrated or wrapped by tumors during the separation. Vascular injury may result in uncontrolled heavy bleeding and is regarded as a contraindication to RPT surgery. With advances in surgical techniques, combined resection of major involved vessels (with or without vascular grafts) in RPT surgery has become an increasingly common avenue to significantly improve the successful rate of tumor resection. Preoperatively comprehensive evaluation of RPTs involving major blood vessels and surrounding organs is essential to a successful operation. B-ultrasound, enhanced CT, and MRI can clearly display the location, size, shape of the tumor, and surrounding organ involvement, particularly the relationship between the tumor and major vessels (such as the abdominal aorta, inferior vena cava, renal artery, iliac artery, superior mesenteric artery, and portal veins); the displacement, compression, or wrapping of blood vessels by the tumor; as well as tumor thrombus within the blood vessels. Preoperative angiography combined with embolization can be used to determine the relationship between the tumor and blood vessels and significantly reduce blood loss intraoperatively by decreasing the blood supply to the tumor.
Indications for surgery of RPTs involving the major vessels include (a) patients who can tolerate major surgery based on general condition; (b) the tumor without distant metastasis and peritoneal implantation; (c) skilled surgeons; (d) available sufficient blood reserves; and (e) patients who are expected to survive for more than 1 year after tumor resection with high quality of life, according to pathological classification, degree of malignancy, and biological behavior characteristics.
Contraindications include (a) patients who cannot tolerate surgery due to concomitant severe cardiac and pulmonary diseases and coagulation disorders; (b) RPT that grows rapidly and occupies the whole abdomen within 6 months after previous surgery; (c) the tumors with extensively systemic metastasis; (d) limited techniques and equipment; and (e) concomitant invasion of abdominal aorta at the upper segment of the kidney or inferior vena cava at the upper segment, which must be resected.
1 Preoperative Preparation
Preoperative preparation of retroperitoneal tumor surgery includes (a) routine preparation including intestinal preparation and prophylactic use of antibiotics; (b) reservation of sufficient red blood cells, anticoagulants, and plasma; (c) preparation of artificial blood vessels with the diameters matching to the patient’s vessels; and (d) performing central venous catheterization and arterial catheterization monitoring preoperatively.
2 Surgery for Retroperitoneal Tumors Involving Major Abdominal Vessels and Branches
RPTs often involve the abdominal aorta, iliac artery, renal artery, and superior mesenteric artery and may completely enclose or seriously invade the vessels. The separation of the tumor from blood vessels will not only lead to long operation time and heavy bleeding but also tumor residues. Therefore, a correct choice is en bloc resection of the tumor together with the arteries followed by vascular grafts. Since the artery with thick wall is protected by arterial sheath, mild adhesion between RPTs and artery may be separated successfully by careful and patient surgeons with excellent dissecting techniques, while arterial sheath should be usually dissected. For RPTs extensively involving the blood vessels, combined transplantation of the abdominal aorta (and iliac artery) and inferior vena cava (and iliac vein) may be performed. Crawford and Debakey (1956) firstly reported two patients with malignant RPTs who received tumor resection and combined reconstruction of the abdominal aorta and inferior vena cava. Ito et al. have successfully performed combined resection of the abdominal aorta and inferior vena cava and Dacron graft for one patient with retroperitoneal rhabdomyosarcoma invading the bifurcation of the abdominal aorta. Over the past three decades, our team has performed combined transplantation in six patients. According to our experience, as the arterial wall is thick and easy to be separated, we firstly dissociate major blood vessels located at proximal and distal ends of the tumor, wrap #8 Foley catheter to control the bleeding, dissociate the tumor from the area without important structure or identify false capsule of the tumor, then perform the surgery within the capsule to avoid heavy bleeding, and finally separate the capsule from the surface of the blood vessels. Alternatively, sharp separation of the tumor starts from the part easy to separate and far away from major vessels to the part difficult to separate and adjacent to major vessels. The tumor with blood supply should be carefully separated and ligated. It is very difficult and important to identify whether tumor-feeding blood vessels are major retroperitoneal blood vessels, so the separation should be initiated from another direction and gradually moving toward major blood vessels. If it is necessary to resect major vessels, the tumor should be completely resected together with them after blocking their proximal and distal ends, and then the reconstruction of blood vessels is performed before repairing the integrity of reconstructed organs.
The abdominal aorta may be invaded below the level of the renal artery. If the abdominal aorta and bilateral iliac arteries are invaded, the resection of the abdominal aorta and common iliac artery, abdominal aorta anastomosis with artificial blood vessel, external iliac artery anastomosis, and bilateral internal iliac artery ligation should be performed. If the invasion is confined to the abdominal aorta, the abdominal aorta anastomosis and bilateral common iliac artery anastomosis may be performed.
When RPTs or metastatic lymph nodes involve the celiac trunk and/or common hepatic artery, resection of these vessels may be conducted without revascularization; because they are feeding the organs with rich collateral circulation, their removal won’t affect the blood supply to the liver, stomach, and spleen. When the tumors or metastatic lymph nodes involve the proper hepatic artery, en bloc resection of the tumor and metastatic lymph nodes together with proper hepatic artery may be performed without hepatic artery reconstruction; however, the gallbladder should be removed simultaneously, or otherwise ischemic gangrene of the gallbladder may occur. For either celiac artery resection or proper hepatic artery resection, once insufficient blood supply to the liver has been identified intraoperatively, the hepatic artery should be reconstructed.
For RPTs invading the superior mesenteric artery, the resection rate is extremely low. After the excision of the superior mesenteric artery, either direct anastomosis or prosthetic vascular graft or autologous vascular graft is generally considered to be a surgical contraindication, since the intestines can tolerate ischemia for a short period of time during the surgery, and the risk for postoperative thrombosis is high.
3 Surgery for Retroperitoneal Tumors Involving the Inferior Vena Cava
3.1 Surgical Treatment for Retroperitoneal Tumor Accompanied by Inferior Vena Cava Tumor Thrombus
Tumor thrombus is classified into invasive and noninvasive types, of which the invasive type accounts for approximately 12.9–28.5%. The length of the tumor thrombus has no effect on the prognosis. However, the invasion of inferior vena cava wall by tumor thrombus is associated with worse prognosis as an independent factor from lymph node and distant metastasis for overall survival of RPTs (Hardwigsen et al. 2001).
Noninvasive tumor thrombus can be removed after dissecting the inferior vena cava. By contrast, the invaded inferior vena cava or inferior vena cava wall must be removed in patients with invasive tumor thrombus. Usually, it is difficult to surgically remove the tumor thrombus which invades the inferior vena cava above the diaphragm level.
3.2 Resection and Reconstruction of Inferior Vena Cava and Renal Vein During Retroperitoneal Tumor Surgery
RPTs mostly involve the inferior vena cava by pushing, compressing, and wrapping but occasionally invade the inferior vena cava wall, or form tumor thrombus in the lumen, thus leading to chronic obstruction or stenosis. Under this circumstance, varying degrees of collateral circulation may have been established. We have previously reported four patients who underwent complete resection from the superior renal vein (below the hepatic vein) to the bifurcation of the iliac vein and resection of the right kidney. Out of them, three patients received the ligation of left renal vein and only one patient received left renal vein reconstruction (end-to-end anastomosis of the left renal vein with ovarian vein) simultaneously. All patients recovered well with normal renal function without lower limb edema and other complications, of which two patients with leiomyosarcoma in the inferior vena cava had developed complete occlusion of the inferior vena cava preoperatively. However, the ligation of the inferior vena cava below the hepatic plane and above the renal vein plane is often considered to be extremely dangerous, with the mortality rate as high as 90%. The left renal vein has seven venous collateral loops, whereas the right renal vein has only indefinite spermatic vein, ureteral vein, and renal capsular vein. Therefore, generally the venous blood disorder rarely occurs in the ligation of the left renal vein but not the right renal vein. Ligation of the inferior vena cava at the upper segment of the kidney can cause blood stasis of the right kidney which leads to renal dysfunction. If the ligation is necessary, the right nephrectomy must be performed simultaneously to reduce compensatory load of collateral circulation and to eliminate toxins produced by the right kidney with blood stagnation. If collateral circulation of the kidney is poor, one of the following methods may be employed to deal with the difficulty: (a) direct or indirect anastomosis of the renal vein to portal vein or inferior vena cava and (b) autologous renal transplantation and anastomosis of the right renal vein with the iliac vein.
3.3 Simple Repair
Simple repair is indicated for small defect in inferior vena cava wall (typically less than one-fourth of the vascular circumference) or small cracks. Firstly, the proximal and distal ends of the inferior vena cava are blocked with forceps temporarily, and then the gap is continuously sutured and closed with 5–0 Prolene suture, without postoperative anticoagulation.
3.4 Simple Inferior Vena Cava Resection and Stump Ligation
Simple inferior vena cava resection and stump ligation apply to RPTs involving the inferior vena cava below the level of renal vein. If the inferior vena cava below the renal vein is ligated, the blood flow may return through the rich collateral circulation without reconstruction. Keep in mind that the resection of the inferior vena cava should start from the entrance of the renal vein superiorly to the bifurcation of the common iliac vein inferiorly. Simultaneously, the intersection between external iliac vein and internal iliac vein should be reserved in order to prevent pulmonary embolism caused by falling of thromboses formed in the blind-ended vessel. Alternatively, the inferior vena cava and common iliac vein may be resected, and the anastomosis of the internal iliac and external iliac vein is performed, thus allowing compensatory drainage of the lower limb blood through the internal iliac vein.
3.5 Partial Resection of the Inferior Vena Cava and Combined Right Nephrectomy
This operation is indicated for the involvement of both the inferior vena cava and right kidney or complete occlusion of inferior vena cava lumen below the level of hepatic vein. The evidence for combined right nephrectomy is supported by the presence of rich collateral circulation in left renal vein (such as left gonadal vein, left lumbar vein, left suprarenal vein, and the common trunk of left inferior phrenic vein), but not in right renal vein. In animal models, the obstruction of right renal blood flow leads to kidney congestion following ligation of hepatic vein and resection of inferior vena cava. Under this circumstance, large amounts of toxins are produced, resulting in animal death. In contrast, the removal of the right kidney not only eliminates blood stasis and toxins generated from the right kidney but also reduces the load of collateral circulation following the resection of the inferior vena cava. RPTs involving the inferior vena cava mostly lead to chronic obstruction or stenosis of the inferior vena cava, and angiography of the inferior vena cava indicates that various degrees of collateral circulation have been established. At this point, while the inferior vena cava and right kidney are removed, the ligation of the left renal vein close to the inferior vena cava and the conservation of the collateral circulation without vascular reconstruction are performed, because the left renal vein has abundant branches and constant anastomoses connected to the peripheral veins. Alternatively, left renal vein may be anastomosed with ovarian vein to strengthen left renal blood reflux and promote postoperative recovery. During the resection of the inferior vena cava and combined right nephrectomy and/or ligation of left renal vein, attention should be paid to the following points in order to prevent renal failure:
(a) Individual functional test of the left vs. the right kidney is performed preoperatively. The right kidney is removed during the surgery. Before the resection, the left renal vein should be temporarily blocked, and the urine is drained from the bladder. After the bladder is emptied and 20–40 ml of furosemide is intravenously injected, the patient is observed for about 30 min. If several tens to hundreds of milliliters of urine flows out of the bladder after the left renal vein is blocked, the left kidney will be proved to have normal urinary function via collateral venous reflux that has been previously established. Conversely, the anastomosis of the left renal vein with the corresponding veins should be considered if no urine flows out.
(b) The inferior vena cava (above the renal vein) or the left renal vein is blocked. If no congestion or swelling occurs to the left kidney, the right kidney and involved inferior vena cava may be resected.
(c) The residual renal vein pressure is measured intraoperatively, and if greater than 56 cm H2O, left renal vein reconstruction should be performed.
3.6 Partial Resection of the Inferior Vena Cava and Vascular Graft
The reconstruction of the inferior vena cava is required except that the collateral circulation has been established in case of thrombosis or complete occlusion of iliac vein and inferior vena cava below the level of renal vein. The reconstruction of the inferior vena cava or left renal vein must be performed under the following circumstances: (a) radical resection of tumor, with extensive resection scope in retroperitoneum and severe damage to collateral circulation; (b) preoperative patency of the inferior vena cava, regardless of the extent of collateral circulation; and (c) no urine or sudden dramatic reduction in urine output suggesting left kidney reflux disorder during the resection of the inferior vena cava and ligation of left renal vein. The surgical procedures meet the requirements of anatomy and physiology, with little effect on blood circulation, and are suitable for patients with combined inferior vena cava and bilateral renal venous reflux obstruction. An ideal graft material is autologous vein because of better graft patency without inducing foreign body reaction, but it is rarely used to replace inferior vena cava due to smaller caliber and easily collapsed under the increased abdominal pressure. At present, the commonly used graft materials are artificial blood vessels with stents, such as polytetrafluoroethylene (PTFE). However, PTFE has small elasticity coefficient and low compliance and exhibits poor histocompatibility as a foreign body, which may cause varying degrees of transplant rejection and infection. The patency of grafted vessel tends to decline over time, while long-term anticoagulant treatment may result in side effects. After the inferior vena vein is grafted, the anticoagulation and anti-aggregation treatment should be administered using the following regimen: intravenous injection of low-molecular dextran 500 ml/day for 7 days, subcutaneous injection of fraxiparine 0.4 ml/day for 5 days, and oral administration of warfarin 2.5–5 mg/day in the first 3–30 days, with dose adjustment based on weekly detection results of clotting time and prothrombin activity, as well as long-term oral administration of dipyridamole 25 mg (t.i.d.) and enteric-coated aspirin 50 mg (q.d.). To prevent the formation of thrombus in grafts following the reconstruction of the inferior vena cava, many scholars advocate the establishment of temporary arteriovenous fistula within the inguinal region in addition to appropriate anticoagulant drugs, in an attempt to increase the velocity of venous return, thereby reducing thrombosis of the graft.
3.7 Operative Steps for Combined Resection of Inferior Vena Cava, Right Nephrectomy, and Left Renal Vein Ligation
Indications for partial resection of RPTs involving inferior vena cava include (a) a tumor arising from the inferior vena cava; (b) tumor thrombus/thrombosis completely occluding the inferior vena cava; (c) a tumor tightly adhering to or wrapping inferior vena cava, thus making it extremely difficult to separate them; and (d) a tumor significantly invading inferior vena cava wall, leading to tumor residues if not removed.
Endotracheal intubation is performed under general anesthesia. During the surgery, the patient lies in supine position and is turned to the left or right when appropriate.
-
1.
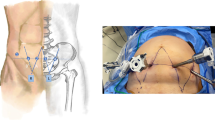
The midline incision is generally made in the upper quadrant of the abdomen, which may arise from the xiphoid superiorly to the pubic symphysis inferiorly. For patients who have previously received RPT resection, the original incision should be preferred (Fig. 13.1). If it cannot meet the needs for exposure during the surgery, the original incision may extend to both ends, or additional transverse incision may be made laterally on the basis of vertical incision (Fig. 13.2).
-
2.
When cutting the abdomen, scars of the original incision may be encountered, which cause mutual integration of normal layers of abdominal wall, so the bleeding should be carefully controlled to identify intra-abdominal structures and avoid excessive resection of tissues. If necessary, the abdominal wall is firstly cut from the ends of the original incision without scars to gain access into the abdominal cavity, where the abdominal contents are often not adhered to the abdominal wall. Then the adhesions of the omentum, intestinal tract, or other tissues with abdominal wall below the incision should be carefully separated (Fig. 13.3).
-
3.
The large RPTs often cause extensive adhesions, especially in cases with previous operation. Usually, the separation of adhesions is performed in the following order: anterior abdominal wall → bilateral abdominal wall → intestine → blood vessels. In the separation process, surgeons should check the presence of important structures such as major blood vessels, ureter, and nerves within the resected tissue (Fig. 13.4) by repeat palpation so as to avoid accidental injury. For tumors that have grown to large size or for a long time, the bleeding should be completely controlled in the separation process, as they extensively adhere to the inner surface of the abdominal wall, with plenty of collateral circulation.
-
4.
Large tumors themselves have extremely rich blood supply, with relatively engorged blood vessels on the surface, and can cause deformation of the abdominal organs by compressing them, e.g., making the liver deform into sheetlike pattern (Fig. 13.5).
-
5.
When separating the adhesion between the tumor and liver, it should be noted that the liver surface at hepatic portal anteriorly and caudate lobes posteriorly can be encountered. As the inferior vena cava is located medially and posteriorly (Fig. 13.6), special attention should be paid to avoid accidental injury. The inferior vena cava and the intersection between external iliac vein and internal iliac posterior to caudate lobe should be carefully dissociated, which is “crisscross” shaped and pushed right upward by the tumor to become flattened and tightly attached to the tumor (Fig. 13.7).
-
6.
In order to verify whether the flat structure is a blood vessel, the surgeon stretches a finger pulp to press the blood vessel and slide along the vascular direction. If the lumen is rapidly filled, the flat structure may be judged as a blood vessel. With this method, the surgeon may also check the direction of blood flow of inferior vena cava (Fig. 13.8).
-
7.
For RPTs invading major blood vessels, dissociation of blood vessels is a major mission of the surgery. There are lots of techniques suitable for separating the inferior vena cava from the tumor surface, including sharp dissection of external margin of the inferior vena cava using a blade (Fig. 13.9) or sharp separation with scissors (Fig. 13.10). The same method also applies to the separation of the medial margin of the blood vessels (Fig. 13.11).
-
8.
When the dissociation of RPTs involving major blood vessels is completed or discontinued, the normal blood vessels with unaffected superior and inferior ends should be identified as soon as possible. Then, the inferior vena cava is wrapped by a blockage band preferably above the tumor before separation (Fig.13.12).
-
9.
The inferior end of the involved inferior vena cava should be also dissociated with sharp dissection rather than blunt dissection, which easily causes damage to fragile vein wall (Fig. 13.13). Long-term invasion of the inferior vena cava or other major vessels by large RPTs makes it extremely difficult to identify the vessel boundaries during the surgery.
-
10.
The dissociation should be performed posterior to the right inferior part of the tumor, equivalent to the right lower quadrant, close to the lower segment of the inferior vena cava. Then the inferior vena cava is ligated and resected, slightly above the bifurcation between the left and right common iliac veins (Fig. 13.14). From this point, the inferior vena cava and iliac vein are gradually separating from the tumor relatively easily. The purpose of resecting the lower end of the inferior vena cava involved by the tumor as the first step is to reduce the volume of circulation at the proximal segment of the inferior vena cava, thereby facilitating the subsequent separation of the upper segment of the inferior vena cava.
-
11.
After resection of its lower end, the inferior vena cava may be lifted up together with the tumor from the lower right side and continuously dissociated toward the cranial direction from the rear (Fig. 13.15). When the dissociation proceeds to the right superior to the tumor, the right kidney should be removed as scheduled. Specifically, inferior vena cava is tightly adhered to right renal vein branches and cannot be separated; moreover, right renal artery is also tightly adhered to and invaded by the tumor. For this reason, the dissociation direction shifts from the tumor to the external superior portion of the right kidney adipose capsule (Fig. 13.16), and then en bloc removal of the tumor and the right kidney is performed.
-
12.
When the intersection between the renal vein and inferior vena cava is pushed by the tumor toward the right superior and closely attached to the tumor, the inferior vena cava should be cut off again and the broken ends should be sutured (Fig. 13.17). Then the residual ends of the left renal vein and inferior vena cava above the renal level become visible, together with the residual ends of the inferior vena cava connected to the right renal vein (Fig.13.18). After the left renal vein is cut, split renal function test is conducted using the method described in this chapter. If the results suggest normal function of the left kidney, the left renal vein and inferior vena cava above the renal level should be separated from the tumor.
-
13.
After cutting the inferior vena cava, the tumor together with the right kidney may be lifted forward to expose the right renal artery at renal hilum and cut near the apex, and then the broken ends are sutured and ligated (Fig. 13.19).
-
14.
Now, the separation shifts to the left inferior portion of the tumor. The tumor is lifted forward to carefully separate the small space between the tumor and abdominal aorta. The artery that bifurcates into small branches to provide blood supply for the tumor should be ligated (Fig. 13.20). As the exposure becomes more and more difficult when the separation continues to the posterior portion of the tumor, surgeons may place their fingers to feel the structure and perform separation and dissection with LigaSure while ensuring the safety of major blood vessels (Fig. 13.21).
-
15.
After complete dissociation of the abdominal aorta, separate the left superior portion of the tumor. Now, the left renal vein has been dissociated, and the renal artery located posterior to the tumor should be separated from the abdominal aorta. The loose tissue within the left upper abdomen that adheres to the tumor is separated from the tumor with combined blunt and sharp dissection, and the blood vessels that are encountered in the separation process are ligated (Fig. 13.22). Finally, the tumor is completely removed after separating a little adhesion between the posterior portion of the tumor and abdominal aorta (Fig. 13.23).
-
16.
After gaining access into the peritoneum, wound bleeding is controlled, the intestine is recovered to normal anatomic position, the device and gauze are counted accurately, the drainage catheter is placed, and the incision is sutured layer by layer (Fig. 13.24).
4 Resection and Reconstruction of Portal Vein and Superior Mesenteric Vein
Under normal circumstances, if the resected segment of the portal vein is less than 3 cm in length, end-to-end anastomosis is applicable, or if it is more than 3 cm in length, vascular grafts should be performed. In our hospital, four cases who received the resection of portal vein and three cases who received the resection of superior mesenteric vein have undergone end-to-end or end-to-side anastomosis, repair, and reconstruction. For one case of leiomyosarcoma, tumor removal failed after laparotomy in other hospitals, and the patient was referred to our hospital for a second operation. The tumor was wrapping and closely attached to the portal vein. In the separation process, the portal vein was ruptured; the tumor was removed together with portal vein. Then end-to-side anastomosis of the proximally residual end of the portal vein with the main branches of the superior mesenteric vein is performed, followed by reconstruction of portal vein and superior mesenteric vein. The patient recovered well postoperatively with no recurrence during 1-year follow-up. It should be noted that during the resection and reconstruction of the portal vein and superior mesenteric vein, vascular occlusion period should be within 30–60 min. After vascular anastomosis, the mesenteric root should be dissociated to establish a tension-free anastomosis.
5 Postoperative Management
Patients with RPTs involving the major vessels should be closely monitored for at least 24–48 h postoperatively. Particular attention should be paid to maintain effective blood volume in order to guarantee myocardial perfusion.
Adequate oxygen is delivered and mechanical ventilation should be maintained if necessary. The blood gas should be monitored for at least 48 h. After the removal of mechanical ventilation, the patient should be urged to practice deep breathing, periodically turned over and percussed on the back, and, simultaneously, encouraged to cough and excrete sputum with the assistance of the clinician. Analgesics are administered to the patients who dare not cough due to pain.
Closely observe the occurrence of internal bleeding. Infuse low molecular dextran for 7–10 days to reduce thrombus formation. Patients in hypercoagulable state may be treated with heparin for 1–2 days. Monitor the occurrence of acidosis for timely correction. Continue administration of broad-spectrum antibiotics.
6 Postoperative Complication
Lower extremity edema is a common complication following the surgery of major retroperitoneal blood vessels, mostly of which is transient. It may occur in patients who undergo inferior vena cava ligation or vascular reconstruction. In addition to the obstruction of blood flow, extensive resection of retroperitoneal lymphatics is another factor responsible for lower extremity edema. The edema may be relieved by raising symptomatic limb, wearing elastic stockings, and administering drugs that can promote edema absorption.
Chylous fistula is relatively common in patients who undergo inferior vena cava resection, which is difficult to treat due to the following reasons: (a) the resection causes venous reflux obstruction and subsequently increased pressure inside the lymphatic vessels and (b) the damaged lymphatic vessels are not properly ligated. Caution should be taken to avoid this complication. Patients developing chylous fistula should be treated with intensive nutrition, enough water and protein, and adequate drainage. Chylous fistula may be gradually closed if it is small in size or be treated operatively if it remains open for more than 1 month (Zhan et al. 2013).
References
Crawford ES, Debakey ME. Wide excision including involved aorta and vena cava and replacement with aortic homograft for retroperitoneal malignant tumors; report of two cases. Cancer. 1956;9(6):1085–91.
Hardwigsen J, Baque P, Crespy B, et al. Resection of the inferior vena cava for neoplasms with or without prosthetic replacement: a 14 patient series. Ann Surg. 2001;223(2):242–9.
Zhan Q, Deng XX, Han B, et al. Robotic-assisted pancreatic resection: a report of 47 cases. Int J Med Rob Comput Assisted Surg. 2013;9(1):44–51.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Luo, CH., Lv, X., Miao, C. (2018). Surgery for Retroperitoneal Tumors Involving Major Abdominal Vessels. In: Luo, CH. (eds) Retroperitoneal Tumors. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1167-6_13
Download citation
DOI: https://doi.org/10.1007/978-94-024-1167-6_13
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1165-2
Online ISBN: 978-94-024-1167-6
eBook Packages: MedicineMedicine (R0)